Abstract
MicroRNA (miRNA) is a small, non-coding RNAs and it could post-transcriptionally related gene expression by negatively regulating the stability or translational efficiency of their target genes. Previous studies have reported the antineoplastic effect of microRNA-29b (miR-29b) in several kinds of cancers. The aim of this study was to investigate the potential role of miR-29b in human osteosarcoma pathogenesis. Quantitative reverse transcription-polymerase chain reaction (qRT-PCR) was performed to determine the expression level of miR-29b in 20 osteosarcoma specimens and adjacent normal bone tissues. The proliferation, apoptosis, migration and invasion were employed to detect the effect of miR-29b on the osteosarcoma cell line MG63. The results showed that miR-29b expression was relatively decreased in osteosarcoma specimens compared with adjacent normal tissues. Overexpression of miR-29b suppressed MG63 cell proliferation, migration and invasion. Meanwhile miR-29b could induce apoptosis of MG63. Besides, miR-29b directly targets VEGF and over-expression of miR-29b led to down-regulation of VEGF protein level, In conclusions, miR-29b may play an important role in osteosarcoma progression, which might negatively regulate the expression of VEGF and suppresses proliferation and induces apoptosis of MG63 cell line.
Keywords: micro RNA, osteosarcoma, miR-29b, vascular endothelial growth factor
Introduction
It is reported that osteosarcoma is the most common primary malignant tumor of the bone, occurring most frequently in children and adolescents [1]. Usually osteosarcoma develops in the long bones of the body, such as the femur, the tibia or the humerus in about four out of five patients [2]. However, clinically evident metastatic disease is present in 10-20% of patients at diagnosis. Despite aggressive treatment, more than one third of patients develop recurrent high-grade osteosarcomas, with metastatic disease typically affecting the lungs, pleura, the heart and bone itself, so that the 5-year survival rates are still not greater than 60% [3]. Current treatments including surgical intervention and chemotherapy have a moderate effect on early stage cases, but are less effective for more advanced cases. The understanding of the molecular pathways of carcinogenesis or progression would be helpful in improving diagnosis, therapy, and prevention of the disease [4].
MicroRNAs (miRNAs) are short, highly conserved small non-coding RNA molecules of about 22 nucleotides in length that regulate gene expression by binding to the 3’ untranslated region (3’-UTR) of the complementary mRNA sequence, resulting in translational repression and gene silencing [5]. MicroRNAs execute pivotal roles in physiological and pathological process including development, differentiation, metabolism, immunity, cell proliferation and apoptosis [6,7]. Hundreds of altered expressions of miRNAs are found to be closely associated with the formation, invasion and progression of most kinds of human cancers, and about 50% of miRNAs genes are found to be located in cancer related genomic regions [7,8]. In these processes, miRNAs may function as oncogenes or tumor suppressors, and the former ones are often up-regulated, whereas the latter are down-regulated in cancers.
One of the most important miRNA species that were involved in tumorigenesis is miR-29b MiRNA-29 (MiR-29) family includes miR-29a, -29b1, -29b2 and -29c. Human miR-29a and -29b1 are processed from an intron located on chromosome 7 and miR-29b2 and -29c are from chromosome 1. They are highly conserved in human, mouse and rat [9]. Since miR-29b1 and -29b2 have identical sequences, they are collectively referred to as miR-29b. The associations between miR-29b and several kinds of diseases have been reported [10]. MiR-29 is significantly down-regulated during different kinds of cancer, and extensive interest has been focused on the mechanism by which miR-29b inhibits tumorigenesis. For example, Kwiecinski et al. have identified the growth factors PDGF-B, PDGF-C, IGF-I and VEGF-A as target genes of miR-29 [11].
At present, two present studies showed that miR-29b was significantly down-regulated in osteosarcoma tissues [12,13]; however, the exact role of miR-29b in osteosarcoma remains unclear. Here, we investigated the expression of miR-29b in osteosarcoma as well as assessed the effect of miR-29b on biological behaviors including cell proliferation, apoptosis, migration and invasion of osteosarcoma cells. The association between miR-29b and VEGF expression was also detected in this study.
Materials and methods
Ethics statement
The study was approved by the Ethics Committee of Third Xiangya Hospital, Central South University. We obtained written informed consent from all participants involved in our study.
Tissue samples
Osteosarcoma tissue and control samples were obtained from 15 patients from Jan 2013 to Feb. 2013 in Third Xiangya Hospital, Central South University. Briefly, 15 samples (from 7 males and 8 females; 42.32 ± 7.22 and 45.63 ± 8.12 years old, respectively) of osteosarcoma tissues were obtained from patients who underwent surgical resection. Non-tumourous tissue more than 3 cm away from tumors was selected from these patients and used as controls. None of the patients received preoperative treatment, such as radiation therapy or chemotherapy.
RNA isolation
Total RNA was extracted from tumor tissue and adjacent normal mucosa by homogenizing tissue in Trizol reagent (Invitrogen, California, USA) according to the manufacturer’s protocol. Total RNA was extracted from 107 to 108 osteosarcoma cancer cells growing in culture. RNA was extracted from fresh specimens after pulverizing the tumors with a stainless steel mortar and pestle that were chilled on dry ice. The integrity of the RNA was assessed by denaturing agarose gel electrophoresis.
Quantitative real-time RT-PCR
The expression level of miR-29b in each osteosarcoma sample, normal tissue, or cancer cell line sample was quantified by real-time reverse transcriptase PCR (RT-PCR) using TaqMan MicroRNA Assays (Applied Biosystems) and StepOnePlus real-time PCR system (Applied Biosystems). The expression level of hsa-miR-126 in each sample was normalized by expression level of U6. The ΔΔCt method was used to calculate the fold-change.
Cell lines and culture conditions
Human osteosarcoma cell line MG-63 was obtained from the Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences and grown in RPMI 1640 medium (Life Technologies) with 10% fetal bovine serum (FBS, Gibco), 100 IU/mL Penicillin and 100 μg/mL Streptomycin in a humidified atmosphere of 5% CO2 at 37°C.
Cell transfection
Exponentially growing cells (1.5 × 105) were seeded in 12-well plates 12 h before transfection and were transfected with 30 nM miR-29b precursor or the negative control (Ambion, Austin, TX, USA) using the X-treme GENE transfection reagent (Roche Applied Science, Indianapolis, IN, USA) according to the manufacturer’s instructions. Transfection efficiency was monitored by RT-qPCR at 48 hr after transfection.
Cell proliferation assay
After transfection, cells were trypsinised, counted, and seeded onto 96-well plates at a density of 5 × 103 cells/well. And then, cell proliferation was measured using the BrdU proliferation assay as previously reported. Briefly, 24 h after transfection, cells were cultured in triplicate at 5 × 103 cells per well in 96-well plates the day before BrdU incubation (Ribobio, Guangzhou, China). After BrdU labeling, the cells were treated with 100 mL of 1 × Apollo reaction cocktail, stained with 100 mL of Hoechst 33342 (5 mg/mL) and visualized under a fluorescence microscope (Olympus, Japan). The percentage of ErdU positive cells was defined as the proliferation rate. Data were obtained from three independent experiments and presented as mean ± standard deviation (SD).
Cell apoptosis assay
At 48 h post-transfection, the MG65 cells were harvested and resuspended in phosphate-buffered saline (PBS) and then fixed in ethanol at -20°C overnight. The cells were washed with PBS and resuspended in staining solution (50 mg/ml propidium iodide, 1 mg/ml RNase A and 0.1% Triton X-100 in PBS). The stained cells (1 × 105) were then analyzed for apoptosis with an FACScalibur; Becton Dickinson Flow Cytometer (PT. Madagasi Brosa Inc., Batang Hari, Propinsi Sumatera Utara, Indonesia). Early apoptotic cells were defined as the population that was PI negative and Annexin V-FITC positive, while late apoptotic cells were PI positive and Annexin V-FITC positive. The total apoptotic rate was calculated as the early apoptotic rate plus the late apoptotic rate. Annexin V-PE/7-AAD Apoptosis Detection Kit (KeyGEN, Nanjing, China) was used for the apoptosis assay of lentivirus vector transfected cells, similar to the above protocols. Each experiment was performed in triplicate, and data were presented as mean ± SD.
Cell migration and invasion assays
Migration assay was conducted with Transwell inserts with 8.0 mm pore size membrane (24-well format, Corning, New York, USA). To measure invasion ability of MG63 cells, the previously mentioned inserts were pre-coated with Matrigel matrix (BD Science, Sparks, MD, USA). The cells (1 × 105) were resuspended in serum-free medium and seeded to the upper chamber. The lower chambers were filled with complete culture medium containing 10% FBS. After incubation at 37°C for 24 h, the migrated cells present on the lower side of the membrane were fixed, stained and counted. Each experiment was performed in triplicate.
Western blot
Protein samples were resolved on NuPAGE 4-12% Bis Tris gels (Invitrogen) and transferred to PVDF membranes (Roche). Membranes were blocked in 5% skim milk/TBST and probed with either anti-tubulin rat polyclonal antibody (1:1,000, Abcam ab6161-100), anti-β-actin mouse monoclonal antibody (1:10,000, Abcam ab6276-100), anti NRP-2 (C-9) mouse polyclonal antibody (Santa Cruz Biotechnology sc-13117) or anti-DOHH (C-19) goat polyclonal antibody (1:1,000, Santa Cruz Biotechnology sc-55157). Detection was performed with horseradish peroxidase-linked anti-rat-IgG (1:10,000; ab6734-1; Abcam), antimouse-IgG (1:10,000; NA931 V; GE Healthcare) and anti-sheep/goat-IgG (1:10,000; AB324P; Chemicon) secondary antibodies with ECL Plus detection reagent and ECL-Hyperfilm (GE Healthcare).
Statistical analysis
Data were expressed as the means ± SD from at least 3 separate experiments. Statistical analyses were performed using Student’s two-tailed ttest. Differences with P valuess of less than 0.05 are considered significant.
Results
MiRNA-29b was down-regulated in osteosarcoma tissues
To analyze miR-29b expression in osteosarcoma tissues, total RNA of 15 osteosarcoma cases and adjacent normal bone tissues were extracted and the level of miR-29b was detected. The results showed that miR-29b in osteosarcoma tissues (1.98 ± 0.53) significantly decreased compared with normal tissues (5.42 ± 0.84, P < 0.01) (Figure 1).
Figure 1.
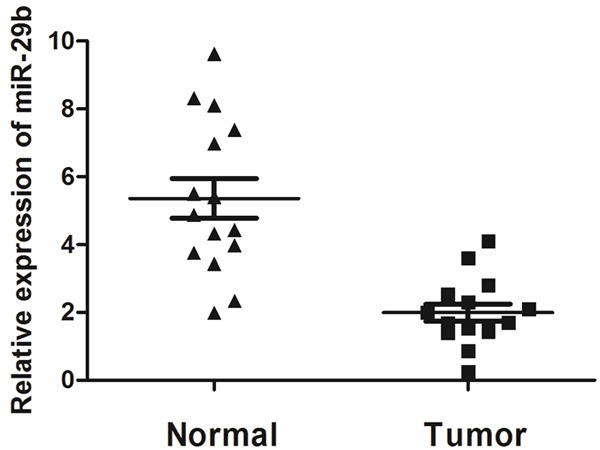
MiR-29b was significantly decreased in osteosarcoma tissues. The mRNA expression of miR-107 was measured by qRT-PCR in osteosarcoma tissues (Cancer tissue) and adjacent normal breast tissue (Normal tissue). Graph represents the 2-ΔΔCt values + SD, P < 0.01.
Effects of miR-29b over-expression on cell growth
In order to assess the effects of miR-29b on osteosarcoma cell growth, the miR-29b precursor was transfected into MG63 cells and cell growth at various post transfection time points was examined. MiR-29b precursor was found to up-regulate miR-29b expression (Figure 2A) and significantly inhibited proliferation in cells post transfection (Data showed in Figure 2B and 2C) three days after transfection.
Figure 2.
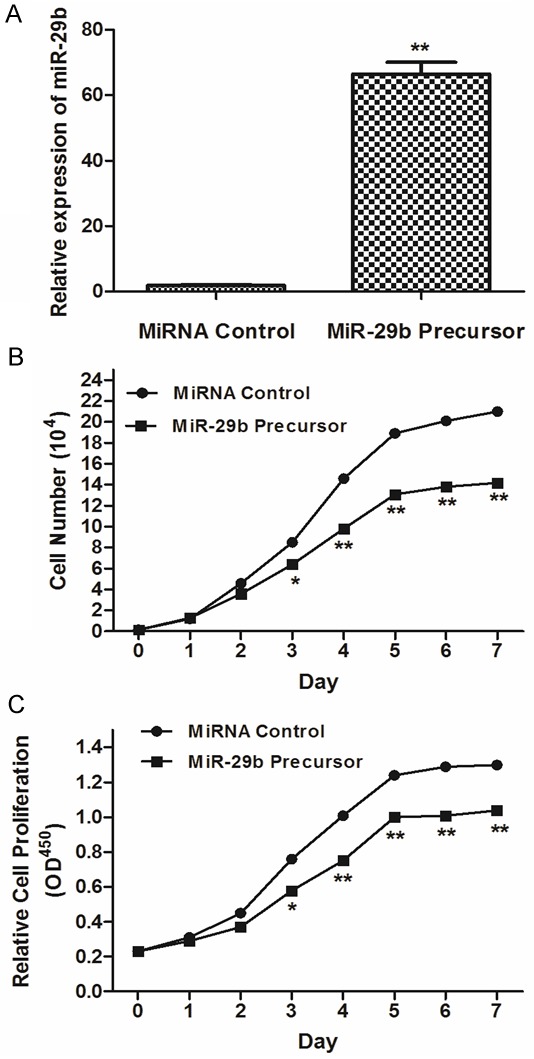
Overexpression of miR-29b inhibits osteosarcoma cell proliferation. A: Expression of miR-16 was determined in MG63 cells after miR-29b precursor transfection compared to controls. B: The growth curve of MG63 cells after miR-29b precursor transfection compared to controls. C: The cell proliferative potential (BrdU) was determined in MG63 cells transfected with miR-29b precursor or negative control (Ctrl). A450 absorption was assayed after transfection for 24 hr.
Influence of miR-29b on cell apoptosis of osteosarcoma cells
To examine the effect of miR-29b on cell apoptosis, we performed apoptosis assays using the Annexin V-FITC/PI staining method. Our results demonstrated that over-expression of miR-29b (miR-29b precursor treated) could induce cell apoptosis obviously compared with negative controls in four MG63 cell lines (Figure 3A, P < 0.05). Besides, advanced studies showed that both late and early apoptosis were induced by miR-29b (Early apoptosis: 9.1% ± 1.2% vs. 2.0% ± 1.2%; late apoptosis: 7.0% ± 0.1% vs. 1.6% ± 0.3%).
Figure 3.
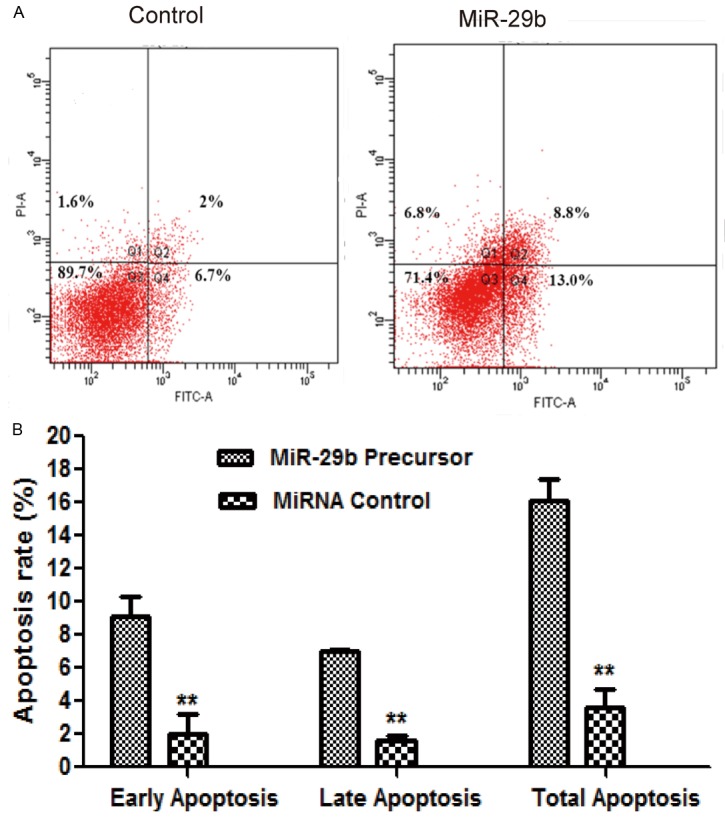
MiR-29b induced apoptosis of MG63 Cells. MG63 cells were plated and transfected with negative control or miRNA-29b mimics. After transfection for 72 hours, cells were collected and stained with Annexin-V/PI. The percentages of early (low right quadrant) and late apoptotic cells (upper right quadrant) were assessed by flow cytometry. A: Flow cytometry plot. B: Percentages of early and late apoptotic cells.
MiR-29b inhibited MG63 cell migration and invasion
A potential role of miR-29b on the MG63 cell migration and invasion was also investigated. Our results indicated that MG63 cells transfected with miR-29b precursor showed a significantly decreased migration and invasion capability, compared with the MG63 cells treated with miRNA control (Figure 4A and 4B, P < 0.05).
Figure 4.
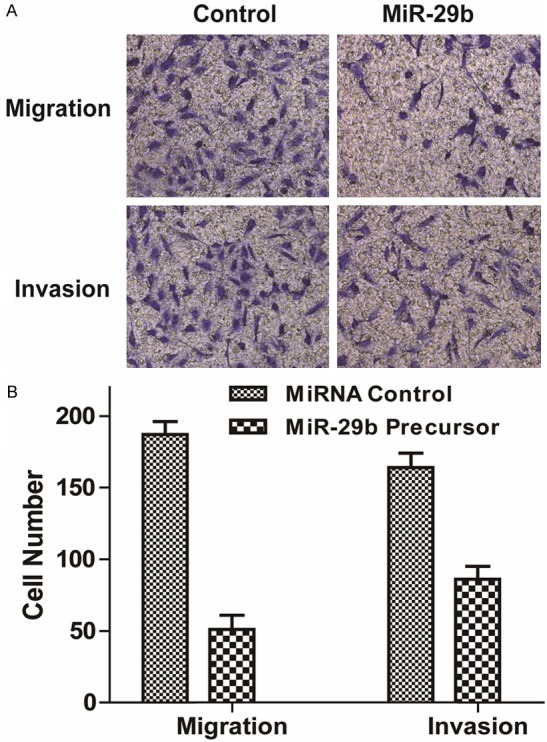
MiR-29b inhibited migration and invasion of MG63 cells. Cell migration and invasion ability was analyzed by transwell chamber assay 18 h after miR-29b or NC transfection. A: Representative images of crystal violet stained MG63 cells transfected with miR-29b or NC. B: Quantification of the migratory cells by solubilization of crystal violet. Data represented mean + SD, P < 0.01.
MiR-29b down-regulates VEGF in osteosarcoma cells
We further determined the expression of VEGF protein by Western blot in MG63 cells transfected with miR-29b precursor. Our data showed that the expression of VEGF protein was significantly decreased in MG63 cells after transfected with miR-29b precursor (Figure 5A and 5B, P < 0.001). These results suggested that miR-29b inhibits VEGF translation in osteosarcoma cancer cells.
Figure 5.
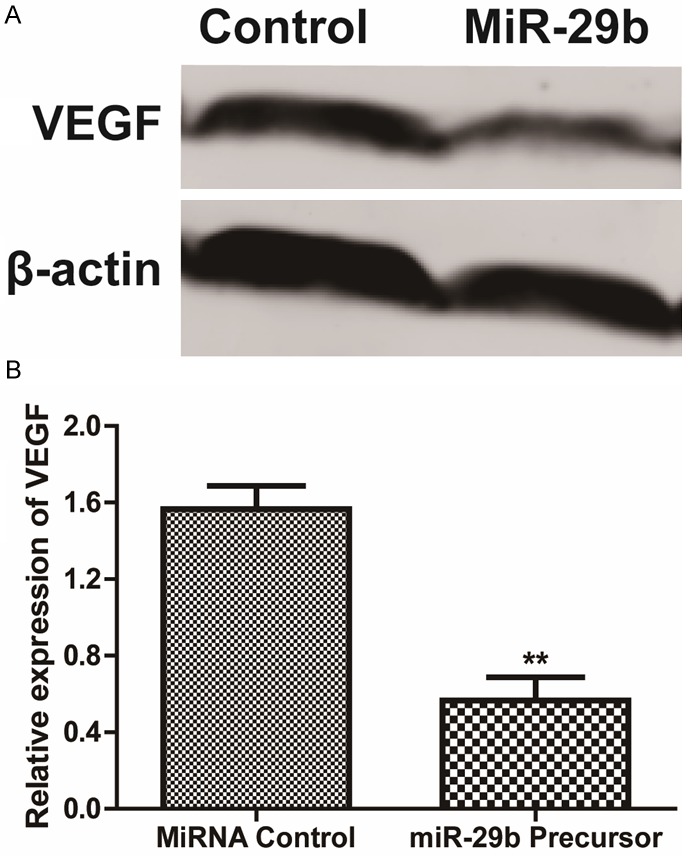
MiR-29b inhibited VEGF expression. A: Western blotting analysis of VEGF protein level. β-actin was used as a loading control. B: The expression level of VEGF in MG63 cells, normalized by β-actin expression, P < 0.05.
Discussion
Emerging evidence has highlighted the crucial impact of miRNA dysregulation on tumourigenesis of human carcinomas. MiRNAs, as a class small regulatory RNA molecules that repress gene expression of their mRNA targets in a sequence specific manner, have been identified as key regulators in a wide variety of oncogenic processes, such as cell proliferation, angiogenesis, cellular differentiation, invasion and metastasis, and can function as either tumor suppressors or oncogenes [12].
In this study, our data revealed that miR-29b greatly inhibited cellular proliferation and induced apoptosis of osteosarcoma cells. Furthermore, up-regulation of miR-29b could inhibit MG63 cancer cell migration and invasion and it suggested that miR-29b may contribute to metastasis in osteosarcoma. The anti-cancer effect of miR-29b might from its ability of inhibiting the expression of VEGF.
In several previous studies, several different studies have reported the abnormal expressed miRNAs in osteosarcoma s. For example, Dai et al showed that seven of them (hsa-miR-451, hsa-miR-1290, hsa-miR-765, hsa-miR-483-5p, hsa-miR-513a-5p, hsa-miR-129-5p and hsa-miR-31) were up-regulated and the other six (hsa-miR-29b, hsa-miR-197, has-let-7b, hsa-miR-324-5p, hsa-let-7i and hsa-miR-484) were down-regulated in osteosarcoma tissues. Zhou et al also miRNA carried out a microarray analysis with a discovery cohort with 12 pairs age-, sex- and tumor stage-matched chemoresistant and control osteosarcoma patients. We have demonstrated in this study that miR-33a is up-regulated in chemoresistant osteosarcoma and that the miR-33a level is negatively correlated with the TWIST protein level in osteosarcoma [14]. Besides, the results of Kobayashi showed that 108 miRNA were commonly expressed in 24 samples of osteosarcoma tissue and subjected to a cell proliferation assay. Besides, found that inhibition of 5 let-7 family miRNA (hsa-let-7a, b, f, g and i) significantly suppressed the proliferation of osteosarcoma cells [15]. The expression of miR-29b was also reported in microarray analysis of osteosarcoma. Jones et al reported that the signature includes high expression of miR-181a, miR-181b, and miR-181c as well as reduced expression of miR-16, miR-29b, and miR-142-5p. It was also demonstrated that miR-181b and miR-29b exhibit restricted expression to distinct cell populations in the tumor tissue [13].
Among the plethora of miRNAs, dysregulation of miR-29b was found in plenty of human cancers [16]. Down-regulation of miR-29b in several different kinds of cancer has been reported by several groups [17-19]. Tan et al reported that the miR-29 family (miR-29a, -29b, and -29c), which negatively regulates DNMT3A and DNMT3B, was examined in association with the Wnt/β-catenin signaling pathway. A positive correlation between the expression of WIF-1 and that of MiR-29s was observed in lung cancer tissues. Methylation-specific PCR and Western blotting indicated that miR-29s positively regulate WIF-1 expression by inhibiting the methylation of its promoter. Furthermore, miR-29 overexpression downregulated β-catenin expression, inhibited cell proliferation and induced apoptosis. The expression of miR-29a and miR-29b was partially regulated by DNMT3A and DNMT3B in a positive feedback loop [20].
VEGF is a pivotal mediator of inflammation and angiogenesis, processes intimately involved in tissue repair. Recent studies indicate that VEGF is a target of proteases that are present in the microenvironment of human chronic non-healing wounds [21]. Previous studies about the association of VEGF and osteosarcoma have been reported and VEGF might promote proliferation, migration and invasion of osteosarcoma [22,23]. In a meta-analysis with 12 studies including a total of 559 osteosarcoma patients, the results showed that compared with osteosarcoma patients with low or negative VEGF expression, patients with high VEGF expression were obviously associated with lower disease-free survival (OR = 0.25, 95% CI 0.11-0.58, P = 0.001, I (2) = 56.4%). In addition, patients with high VEGF expression were obviously associated with lower overall survival (OR = 0.22, 95% CI 0.13-0.35, P < 0.001, I (2) = 0.0%). It suggest that VEGF expression is an effective biomarker of prognosis in patients with osteosarcoma and maybe a potential therapeutic target of osteosarcoma [24]. In this study, VEGF was a tumor-associated mRNA targeted by miR-29b. In addition, the conserved sites of VEGF were predicted by multiple miRNA target prediction algorithms, and these miRNA-mRNA target pairs have potential high binding ability and thermal stability. Our in vitro experiments confirmed that these miRNAs suppress the expression of endogenous VEGF. The associations between miR-29b and VEGF have been reported by several studies. Li et al showed that their study confirmed that miR-29b regulated the expression of MCL1 (myeloid cell leukemia sequence 1), MMP2 (encoding matrix metalloproteinase 2), VEGFA (vascular endothelial growth factor A) and ITGB1 (integrin β1) genes by directly binding to their 3’-UTRs (untranslated regions). Moreover, they identified that there was an inverse correlation between miR-29b and its target genes in subjects with PE [25]. Taken together, these findings support a novel role for miR-29b in invasion, apoptosis and Proliferation of osteosarcoma cells, and miR-29b may become a new potential therapeutic target for osteosarcoma. However, only in vitro studies were conducted in this study, we would conduct several more studies to detect the influence of miR-29b of VEGF expression in vivo.
In conclusion, down-regulation of miR-29ais level is a frequent event in osteosarcoma. Moreover, tumor-suppressive miR-29b directly regulates VEGF, a molecular chaperone involved in the tumorigenesis of osteosarcoma. Restoration of miR-29b inhibited cancer cell proliferation, migration and invasion and induces apoptosis. It suggested that the miR-29b-VEGF pathway contributes to the pathogenesis of osteosarcoma. Therefore, miR-29b could be a promising therapeutic target for osteosarcoma treatment in the future.
Disclosure of conflict of interest
None.
References
- 1.Vijayamurugan N, Bakhshi S. Review of management issues in relapsed osteosarcoma. Expert Rev Anticancer Ther. 2014;14:151–161. doi: 10.1586/14737140.2014.863453. [DOI] [PubMed] [Google Scholar]
- 2.Farcas N, Arzi B, Verstraete FJ. Oral and maxillofacial osteosarcoma in dogs: A review. Vet Comp Oncol. 2014;12:169–80. doi: 10.1111/j.1476-5829.2012.00352.x. [DOI] [PubMed] [Google Scholar]
- 3.van den Berg H, Schreuder WH, de Lange J. Osteosarcoma: A comparison of jaw versus nonjaw localizations and review of the literature. Sarcoma. 2013;2013:316123. doi: 10.1155/2013/316123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abbo O, Pinnagoda K, Micol LA, Beck-Popovic M, Joseph JM. Osteosarcoma metastasis causing ileo-ileal intussusception. World J Surg Oncol. 2013;11:188. doi: 10.1186/1477-7819-11-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun K, Lai EC. Adult-specific functions of animal micrornas. Nat Rev Genet. 2013;14:535–548. doi: 10.1038/nrg3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hulsmans M, Holvoet P. Microrna-containing microvesicles regulating inflammation in association with atherosclerotic disease. Cardiovasc Res. 2013;100:7–18. doi: 10.1093/cvr/cvt161. [DOI] [PubMed] [Google Scholar]
- 7.Swaminathan S, Murray DD, Kelleher AD. Mirnas and hiv: Unforeseen determinants of host-pathogen interaction. Immunol Rev. 2013;254:265–280. doi: 10.1111/imr.12077. [DOI] [PubMed] [Google Scholar]
- 8.Hodzic J, Giovannetti E, Diosdado B, Adema AD, Peters GJ. Regulation of deoxycytidine kinase expression and sensitivity to gemcitabine by micro-rna 330 and promoter methylation in cancer cells. Nucleosides Nucleotides Nucleic Acids. 2011;30:1214–1222. doi: 10.1080/15257770.2011.629271. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Ghazwani M, Li J, Sun M, Stolz DB, He F, Fan J, Xie W, Li S. Mir-29b inhibits collagen maturation in hepatic stellate cells through down-regulating the expression of hsp47 and lysyl oxidase. Biochem Biophys Res Commun. 2014;446:940–4. doi: 10.1016/j.bbrc.2014.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen B, Li H, Zeng X, Yang P, Liu X, Zhao X, Liang S. Roles of microrna on cancer cell metabolism. J Transl Med. 2012;10:228. doi: 10.1186/1479-5876-10-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwiecinski M, Elfimova N, Noetel A, Tox U, Steffen HM, Hacker U, Nischt R, Dienes HP, Odenthal M. Expression of platelet-derived growth factor-c and insulin-like growth factor i in hepatic stellate cells is inhibited by mir-29. Lab Invest. 2012;92:978–987. doi: 10.1038/labinvest.2012.70. [DOI] [PubMed] [Google Scholar]
- 12.Dai N, Zhong ZY, Cun YP, Qing Y, Chen C, Jiang P, Li MX, Wang D. Alteration of the microrna expression profile in human osteosarcoma cells transfected with ape1 sirna. Neoplasma. 2013;60:384–394. doi: 10.4149/neo_2013_050. [DOI] [PubMed] [Google Scholar]
- 13.Jones KB, Salah Z, Del Mare S, Galasso M, Gaudio E, Nuovo GJ, Lovat F, LeBlanc K, Palatini J, Randall RL, Volinia S, Stein GS, Croce CM, Lian JB, Aqeilan R. Mirna signatures associate with pathogenesis and progression of osteosarcoma. Cancer Res. 2012;72:1865–1877. doi: 10.1158/0008-5472.CAN-11-2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou Y, Huang Z, Wu S, Zang X, Liu M, Shi J. Mir-33a is up-regulated in chemoresistant osteosarcoma and promotes osteosarcoma cell resistance to cisplatin by down-regulating twist. J Exp Clin Cancer Res. 2014;33:12. doi: 10.1186/1756-9966-33-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobayashi E, Satow R, Ono M, Masuda M, Honda K, Sakuma T, Kawai A, Morioka H, Toyama Y, Yamada T. Microrna expression and functional profiles of osteosarcoma. Oncology. 2014;86:94–103. doi: 10.1159/000357408. [DOI] [PubMed] [Google Scholar]
- 16.Jiang H, Zhang G, Wu JH, Jiang CP. Diverse roles of mir-29 in cancer (review) Oncol Rep. 2014;31:1509–1516. doi: 10.3892/or.2014.3036. [DOI] [PubMed] [Google Scholar]
- 17.Sandhu R, Rivenbark AG, Mackler RM, Livasy CA, Coleman WB. Dysregulation of microrna expression drives aberrant DNA hypermethylation in basal-like breast cancer. Int J Oncol. 2014;44:563–572. doi: 10.3892/ijo.2013.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poudyal D, Cui X, Le PM, Hofseth AB, Windust A, Nagarkatti M, Nagarkatti PS, Schetter AJ, Harris CC, Hofseth LJ. A key role of microrna-29b for the suppression of colon cancer cell migration by american ginseng. PLoS One. 2013;8:e75034. doi: 10.1371/journal.pone.0075034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dai F, Zhang Y, Zhu X, Shan N, Chen Y. The anti-chemoresistant effect and mechanism of muc1 aptamer-mir-29b chimera in ovarian cancer. Gynecol Oncol. 2013;131:451–459. doi: 10.1016/j.ygyno.2013.07.112. [DOI] [PubMed] [Google Scholar]
- 20.Tan M, Wu J, Cai Y. Suppression of wnt signaling by the mir-29 family is mediated by demethylation of wif-1 in non-small-cell lung cancer. Biochem Biophys Res Commun. 2013;438:673–679. doi: 10.1016/j.bbrc.2013.07.123. [DOI] [PubMed] [Google Scholar]
- 21.Yang L, Engeland CG, Cheng B. Social isolation impairs oral palatal wound healing in sprague-dawley rats: A role for mir-29 and mir-203 via vegf suppression. PLoS One. 2013;8:e72359. doi: 10.1371/journal.pone.0072359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhuang Y, Wei M. Impact of vascular endothelial growth factor expression on overall survival in patients with osteosarcoma: A meta-analysis. Tumour Biol. 2014;35:1745–1749. doi: 10.1007/s13277-014-1692-8. [DOI] [PubMed] [Google Scholar]
- 23.Liu Y, Zheng Q, Wu H, Guo X, Li J, Hao S. Rapamycin increases pcreb, bcl-2, and vegf-a through erk under normoxia. Acta Biochim Biophys Sin (Shanghai) 2013;45:259–267. doi: 10.1093/abbs/gmt002. [DOI] [PubMed] [Google Scholar]
- 24.Chen D, Zhang YJ, Zhu KW, Wang WC. A systematic review of vascular endothelial growth factor expression as a biomarker of prognosis in patients with osteosarcoma. Tumour Biol. 2013;34:1895–1899. doi: 10.1007/s13277-013-0733-z. [DOI] [PubMed] [Google Scholar]
- 25.Li P, Guo W, Du L, Zhao J, Wang Y, Liu L, Hu Y, Hou Y. Microrna-29b contributes to pre-eclampsia through its effects on apoptosis, invasion and angiogenesis of trophoblast cells. Clin Sci (Lond) 2013;124:27–40. doi: 10.1042/CS20120121. [DOI] [PubMed] [Google Scholar]


