Abstract
CD117 (C-kit) is thought to play an important role in tumourigenesis. There are limited data in the literature concerning C-kit expression in retinoblastoma. To date, no immunohistochemical studies have been performed to assess the possible association of C-kit with vascular endothelial growth factor (VEGF) in retinoblastoma. This study was designed to investigate C-kit and VEGF immunoexpression in retinoblastoma, their relationship with prognostic parameters as well as the correlation between them. A prospective immunohistochemical study was conducted on 56 retinoblastoma cases. Patients who had received preoperative chemotherapy were excluded. Positive C-kit and VEGF immunoreactivity was observed in 48.2% and 76.8% of retinoblastoma cases respectively. No C-kit immunostaining was seen in the adjacent uninvolved retina. However, VEGF expression was detected within its vasculature. Retinoblastomas with combined pattern of tumour growth revealed a highly significant positive C-kit expression (P = 0.002) compared to cases with endophytic or exophytic growths. Also, positive C-kit expression was statistically higher in cases with optic nerve invasion (P = 0.001) and choroidal invasion (P ≤ 0.01) compared to negative cases. A highly significant positive VEGF expression was detected in cases with optic nerve invasion (P = 0.013) compared to negative cases. Moreover, a highly significant positive correlation was detected between C-kit and VEGF expression (P = 0.006). C-kit is a feature of more aggressive retinoblastomas, with increased expression in tumours spreading beyond the retina. Moreover, VEGF is vastly expressed in retinoblastoma and is associated with optic nerve invasion. Both C-kit and VEGF may represent potential therapeutic targets for retinoblastomas.
Keywords: CD117, VEGF, retinoblastoma, immunohistochemistry
Introduction
Retinoblastoma is a malignant neoplasm composed of embryonic tumour cells from retinoblasts of neuroepithelial origin. It is the most common intraocular tumour of childhood, with a relative incidence of 3% of all pediatric tumours [1]. In well-developed countries, retinoblastoma is rarely a life-threatening condition because of early diagnosis, but in underdeveloped and developing countries, clinical diagnosis is made in advanced stages, and the mortality rate remains high [2].
Prognosis is affected by many risk factors, the most important of which is the extent of invasion of the retinoblastoma into ocular coats and the optic nerve [3]. Very small tumours sometimes may be treated effectively using laser therapy or cryotherapy. However, larger tumours often require removal by enucleation of the affected eye. When retinoblastoma is treated in the early stages by enucleation, the cure rates approach 95% [4-6]. However in addition to the loss of vision, this therapeutic approach may leave the child with a facial deformity that worsens throughout life [7]. More advanced disease may require radiotherapy or chemotherapy in addition to enucleation. Both of these additional therapeutic regimes increase the probability that the surviving child will develop additional malignancies later in life [8]. Because of concerns about the significant morbidity and potential mortality associated with current therapies in the treatment of retinoblastoma, newer therapeutic modalities are being investigated [9].
CD117, also known as proto-oncogene C-kit or tyrosine-protein kinase Kit or Mast/stem cell growth factor receptor (SCFR), is a protein that in humans is encoded by the KIT gene [10]. C-kit proto-oncogene is located in the long arm of chromosome 4 and encodes a 145 kDa transmembrane tyrosine kinase protein that acts as a type III receptor [11]. When tyrosine kinase receptor binds its ligand SCF (stem cell factor), it transduces signals important in a variety of normal physiological and pathogenic processes, including cell survival and proliferation, migration, and differentiation [12].
Mutation of the C-kit gene results in a constitutive activation of the C-kit protein, and it has been well documented in many tumours, such gastrointestinal stromal tumours (GIST) and myelodysplastic syndrome [13]. Response to targeted therapy by the tyrosine kinase receptor inhibitor, imatinib mesylate (STI-571, Gleevec), in KIT-overexpressing malignancies such as GISTs has driven the desire to identify other tumour types which may also be candidates for such a therapeutic approach [14]. To the best of our knowledge, there are limited data in the literature (only two studies) on C-kit immunohistochemical expression in retinoblastoma [11,15].
SCF, the ligand for the C-kit receptor, has been implicated in the regulation of neoplastic angiogenesis. SCF is produced by several types of tumor cells and stimulates mast cell migration, proliferation and degranulation. Mast cells accumulate within and around solid tumours and can release many angiogenic factors, including vascular endothelial growth factor (VEGF) [16]. To date, no immunohistochemical studies have been performed to assess the possible association between C-kit and VEGF in retinoblastoma.
This study was designed to evaluate C-kit and VEGF immunohistochemical expression in retinoblastoma after enucleation relating the results with clinicopathological prognostic factors as well as finding a correlation between these two tumor markers.
Materials and methods
Study population
A prospective case study was conducted in Ocular Oncology clinic, Ophthalmology and Pathology departments, Ain Shams University Hospital in the period between January 2009 and January 2013. The study included 56 children newly diagnosed as retinoblastoma with advanced disease in at least one eye.
All children were subjected to full history taking, including data on age, sex, the nature of complaint (its onset, course and duration), family history and consanguinity, any previous investigations, and any lines of treatment. Fundus examination using the indirect ophthalmoscope with scleral indentation was performed under general anesthesia with full pupillary dilatation. Fundus photography using fundus camera (GENESIS D, KOWA MEDICALS, Japan) was made and the clinical findings were documented. Staging was recorded according to international classification of retinoblastoma [17]. Ocular ultrasonography was performed to determine tumour dimensions and to confirm the presence of intraocular calcification. Computed tomography of the orbits and brain was conducted to detect any intracranial extension and trilateral retinoblastoma. A magnetic resonance imaging was not obtained as a routine method of examination except in cases with no visualization of the optic nerve head during clinical examination.
Enucleation was done for eyes with an extensive tumour filling more than half of the globe or tumour in anterior segment with or without rubeotic glaucoma.
Following enucleation and histopathological analysis of the specimens, postoperative systemic chemotherapy for 3-6 cycles [vincristine (1.5 mg/m2), etoposide (200 mg/m2) and carboplatin (560 mg/m2)] was given for cases with combined massive choroidal invasion and post-laminar optic nerve infiltration to avoid extra ocular relapse (protocol of our institution). Also proper treatment was given for the less advanced eye in those patients with bilateral disease. Follow up of all patients was done every month postoperatively by ophthalmological examination and every six months by magnetic resonance imaging of the orbit and brain to detect early tumour recurrence.
Patients who had received preoperative adjunctive treatments, such as chemotherapy were not included in the study since these treatments could influence the interpretation of immunohistochemistry.
Ethics statement
All patients who participated in this study signed a written, informed consent before surgery. The study was approved by the Research Ethical Committee at Faculty of Medicine, Ain Shams University.
Histopathological analysis
Hematoxylin and Eosin stained slides were examined for the histopathologic diagnosis and cell differentiation status. The specimens were classified as well differentiated (presence of foci of Flexner-Wintersteiner rosettes or fleurettes), poorly differentiated (absence of these structures) or moderately differentiated (presence of isolated Homer-Wright rosettes) [18]. Optic nerve invasion was considered present only if tumour cells could be identified beyond the lamina cribrosa, and choroidal invasion was considered present when tumour cells were seen to infiltrate through Bruch’s membrane. Minimal choroidal invasion was considered present when tumour cells had destroyed Bruch’s membrane without invading the choroid to depth, with a maximum of three microscopic cell clusters and massive choroidal invasion indicating any choroidal involvement that was not minimal [11].
Immunohistochemical staining
Four micrometer sections of formalin-fixed and paraffin-embedded tissue samples of the studied cases were prepared. Immunohistochemical (IHC) staining was performed using primary antibodies; the rabbit polyclonal C-kit antibody; clone A4502 (Dako Cytomation; Dako Canada, Mississauga, Ontario, Canada) at a dilution of 1:30 and rabbit polyclonal VEGF antibody; Cat. #RB-9031-R7 (Thermo Fisher Scientific Inc., Fremont, CA) at a dilution of 1:200. Avidin-Biotin immunoperoxidase complex technique was used according to Hsu et al. [19] by applying the super sensitive detection kit (Biogenex, CA, USA). The prepared tissue sections were fixed on poly-L-lysine coated slides overnight at 37°C. They were deparaffinized and rehydrated through graded alcohol series. Then, sections in case of C-kit were bathed in a 10-3 m sodium citrate buffer (pH 6.0) after bringing the solution to a boil in a pressure cooker and boiled for 20 min while maintaining the pressure. The slides in case of VEGF were placed in a target retrieval solution with high pH (pH 9.9), and antigen retrieval was performed overnight at 40°C.
After quenching in 3% hydrogen peroxide and blocking for 5 min, the sections were incubated overnight at 4°C for c-kit and for one hour for VEGF. Biotinylated anti-mouse immunoglobulin and streptavidin conjugated to horseradish peroxidase were then added. Finally, 3, 3’-diaminobenzidine as the substrate or chromogen was used to form an insoluble brown product. Hematoxylin was used as counterstain for C-kit and VEGF. Sections of GIST and angiosarcoma were used as positive control for C-kit and VEGF respectively. Negative controls had primary antibody replaced by buffer.
Interpretation of immunohistochemical staining
Immunohistochemical analysis of C-kit and VEGF was performed by a single blinded pathologist (one of the authors) without any prior knowledge of the clinicopathological data. C-kit was evaluated for cytoplasmic and membranous expression and VEGF for cytoplasmic expression. Analysis was performed using computerized Image Analyzing Software (Special SIS starter. version 3.2, Olympus, Germany) connected to an Olympus microscope (model BX51, Olympus Japan).
C-kit was evaluated according to the percentage of positively stained tumour cells in at least five areas at a magnification of 400x using a scoring method utilized previously [11]. Samples were considered positive when more than 30% of the cells presented distinct positive immunostaining.
VEGF staining was also evaluated on the basis of the percentage of positive cells, and classified as follows: more than 10% of the cells staining were graded as positive. No detectable staining or < 10% of cells staining was graded as negative [20].
Statistical analysis
All data were coded and statistically analyzed using the SPSS version 13.0. Continuous variables are expressed as mean and standard deviation. Description of qualitative variables was in the form of numbers and percentages. Chi-square and Fisher’s exact tests were used to compare qualitative variable. The level P ≤ 0.05 was considered the cut-off value for significance. Differences were considered highly significant when P ≤ 0.01. Pearson’s correlation coefficient (r) was performed to test the correlation between C-kit and VEGF expressions and different clinicopathological variables. Event-free survival was estimated by using Kaplan-Meier method. Log rank test was used to compare time-to-event variables by levels of a factor variable. Event was defined as death caused by the tumour, associated with treatment or due to second malignancies. Follow up time was calculated from date of enucleation to the date of last contact with each patient.
Results
Clinicopathological features
The study included 24 boys (42.9%) and 32 girls (57.1%). The mean age at diagnosis was 20.94 months ± 11.75 SD (range: 3-48 mon-ths). Positive family history of the disease was found in three patients (5.3%). First degree consanguineous marriage between the parents was reported in four patients (7.1%). Leucocoria was the most common presentation in 50 patients (89.3%). Out of the remaining 6 patients (10.7%), two patients suffered from strabismus, two patients had proptosis and in the last two patients, the tumour was accidentally discovered during routine examination. Bilateral disease was found in seven patients (12.5%) and unilateral in 49 patients (87.5%). Fifty six eyes were in group E according to the international classification of retinoblastoma (all unilateral cases and the advanced eyes in bilateral cases) for which enucleation was indicated. Two out of the remaining seven eyes of the bilateral cases were in group A for which they received local therapy and five eyes were in group B for which chemoreduction and focal consolidation laser therapy was given. A combined exophytic and endophytic pattern of growth was seen in 31 (55.4%) patients, and an endophytic pattern was seen in 21 (37.5%) patients while the exophytic pattern was seen infrequently in 4 (7.1%) patients. Moderately differentiated tumors were the most frequent type; 27/56 (48.2%), followed by poorly differentiated tumours; 20/56 (35.7%) and well differentiated tumours; 9/56 (16.1%). Thirty five cases (62.5%) displayed spread to the choroid and 26 (46.4%) to the optic nerve (post-laminar not spreading to the surgical margin) (Figure 1). Clinical and pathological data for the studied cases are represented in Table 1.
Figure 1.
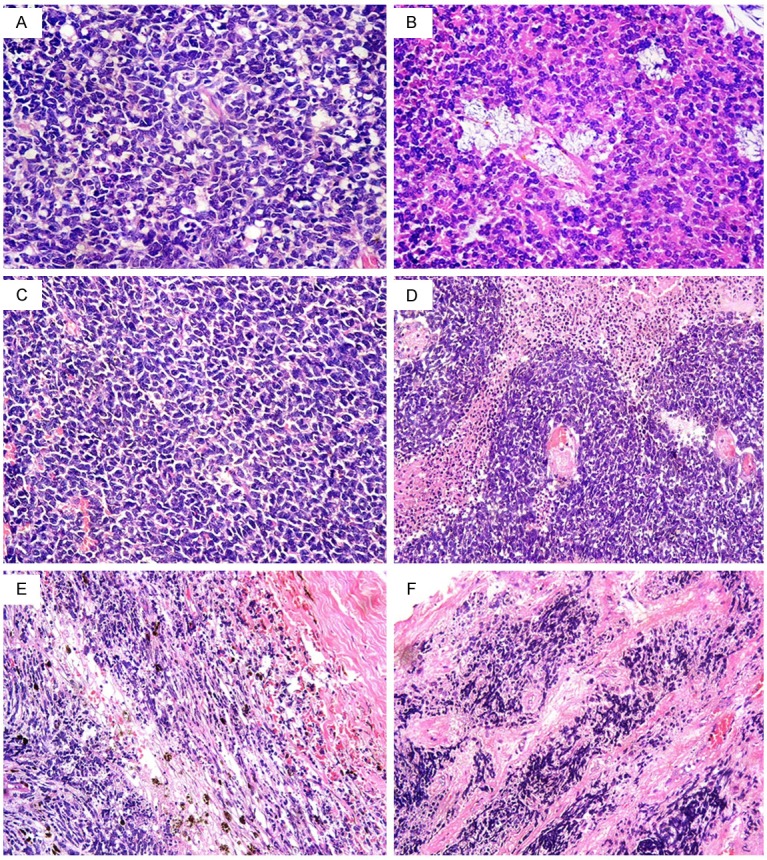
Retinoblastoma: (A) Well differentiated tumour with foci of Flexner-Wintersteiner rosettes, (B) Well differentiated tumour with foci of Flexner-Wintersteiner rosettes and fleurettes, (C) Poorly differentiated tumour, (D) Area of retinoblastoma illustrating the sleeve pattern of growth around central blood vessels. The three cuffs of viable neoplastic cells are well demarcated from the surrounding necrotic cells, (E) Massive invasion of the choroid, (F) Post laminar optic nerve invasion [hematoxylin-eosin, original magnification, (A-C) × 400; (D-F) × 200].
Table 1.
Clinicopathological characteristics in children with retinoblastoma (n = 56)
| Retinoblastoma cases | |
|---|---|
|
| |
| Variable | n (%) |
| Mean age (months) ± (SD) | 20.94 ± 11.75 |
| Sex | |
| ● Boys | 24 (42.9%) |
| ● Girls | 32 (57.1%) |
| Tumour laterality | |
| ● Unilateral | 49 (87.5%) |
| Right eye | 16 (28.6%) |
| Left eye | 33 (58.9%) |
| ● Bilateral | 7 (12.5%) |
| Pattern of tumour growth | |
| ● Endophytic | 21 (37.5%) |
| ● Exophytic | 4 (7.1%) |
| ● Mixed | 31 (55.4%) |
| Degree of differentiation | |
| ● Well differentiated | 9 (16.1%) |
| ● Moderately differentiated | 27 (48.2%) |
| ● Poorly differentiated | 20 (35.7%) |
| Choroidal invasion | |
| ● Negative | 21 (37.5%) |
| ● Positive | 35 (62.5%) |
| Minimal | 24/35 (68.6%) |
| Massive | 11/35 (31.4%) |
| Optic nerve invasion | |
| ● Negative | 30 (53.6%) |
| ● Positive | 26 (46.4%) |
| Extent of Invasion | |
| ● No invasion beyond retina | 14 (25.0%) |
| ● Choroidal invasion only | 16 (28.5%) |
| ● Optic nerve invasion only | 7 (12.5%) |
| ● Both choroidal and optic nerve invasion | 19 (34.0%) |
n: number of cases.
Immunohistochemical expression of C-kit in retinoblastoma
Positive C-kit immunoreactivity was observed in 48.2% (27/56) of retinoblastoma cases. All the positive samples exhibited cytoplasmic staining with membranous accentuation (Figure 2). No immunostaining was seen in the adjacent uninvolved retina. Regarding the pattern of growth of retinoblastomas, all the four exophytic tumours (100%) as well as 58.1% (18/31) of tumours with mixed growth pattern showed positive C-kit expression compared with only 23.8% (5/21) of endophytic tumors. A highly significant positive expression was detected in cases with combined pattern of growth (P = 0.002) (Table 2).
Figure 2.
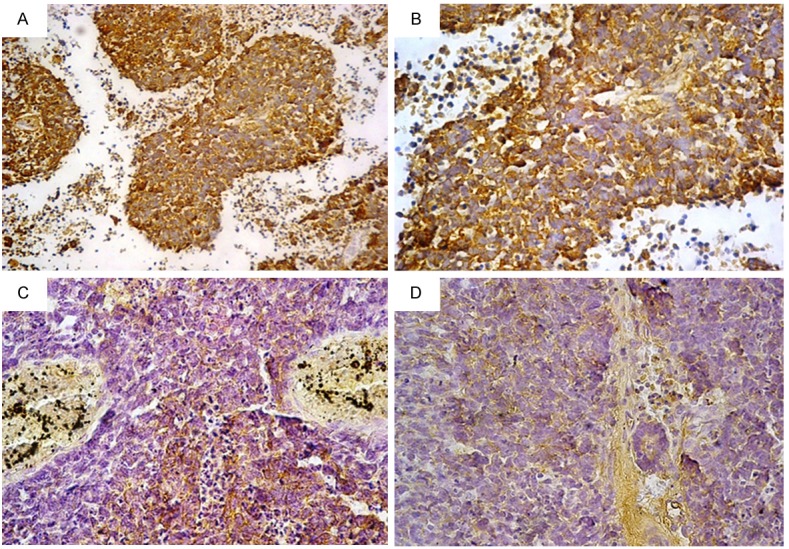
C-kit expression in retinoblastoma: (A) Strong cytoplasmic positivity with membranous accentuation, (B) The same field with higher magnification power, (C) Moderate positivity, (D) Weak positivity [Immunohistochemistry, original magnification, (A) × 200; (B-D) × 400].
Table 2.
C-kit and VEGF immunohistochemical expression and different clinicopathological variables in retinoblastoma cases (n = 56)
| C-kit expression | VEGF expression | ||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Variable | n (%) | Negative n = 29 (51.8%) | Positive n = 27 (48.2%) | P-value | Negative n = 13 (23.2%) | Positive n = 43 (76.8%) | P-value |
| Pattern of growth | |||||||
| Endophyic | 21 (37.5%) | 16 (76.2%) | 5 (23.8%) | 0.002 (HS) | 5 (23.8%) | 16 (67.2%) | 0.51 (NS) |
| Exophytic | 4 (7.1%) | 0 (0.0%) | 4 (100.0%) | 0 (0.0%) | 4 (100.0%) | ||
| Combined | 31 (55.4%) | 13 (41.9%) | 18 (58.1%) | 8 (25.8%) | 23 (74.2%) | ||
| Degree of differentiation | |||||||
| Well & moderately differentiated | 36 (64.3%) | 16 (44.4%) | 20 (55.6%) | 0.98 (NS) | 7 (19.4%) | 29 (80.6%) | 0.26 (NS) |
| Poorly differentiated | 20 (35.7%) | 13 (65.0%) | 7 (35.0%) | 6 (30.0%) | 14 (70.0%) | ||
| Optic nerve invasion | |||||||
| Absent | 30 (53.6%) | 22 (73.3%) | 8 (26.7%) | 0.001 (HS) | 11 (36.7%) | 19 (63.3%) | 0.013* (HS) |
| Present | 26 (46.4%) | 7 (26.9%) | 19 (73.1%) | 2 (7.7%) | 24 (92.3%) | ||
| Choroidal invasion | |||||||
| Absent | 21 (37.5%) | 17 (81.0%) | 4 (19.0%) | ≤ 0.01 (HS) | 5 (23.8%) | 16 (76.2%) | 0.43 (NS) |
| Present | 35 (62.5%) | 12 (34.3%) | 23 (65.7%) | 8 (22.9%) | 27 (77.1%) | ||
n: number of cases, NS: non-significant, HS: highly significant;
fisher’s exact test.
Nineteen out of 26 retinoblastoma cases with optic nerve invasion (73.1%) displayed positivity for C-kit compared with only 26.7% (8/30) of cases without invasion. A highly significant positive C-kit expression was detected in cases with optic nerve invasion (P = 0.001) (Table 2).
Moreover, a highly significant positive C-kit expression was found in cases with choroidal invasion (P ≤ 0.01) where 65.7% (23/35) of retinoblastoma cases with choroidal invasion showed positive C-kit immunoreactivity. However, only 19% (4/21) of cases without choroidal invasion showed positive expression (Table 2). As an additional investigation of this relationship, choroidal invasion was further stratified as “minimal” or “massive”. It was found that 91% (10/11) of cases with massive choroidal invasion displayed positive C-kit expression compared to only 54.2% (13/24) of cases with minimal choroidal invasion. The increased C-kit expression with massive choroidal invasion was shown to be statistically significant (P = 0.05) (Table 3).
Table 3.
C-kit and VEGF immunohistochemical expression and extent of choroidal invasion in retinoblastoma cases (n = 35)
| C-kit expression | VEGF expression | ||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Variable | n (%) | Negative n = 12 | Positive n = 23 | P-value | Negative n = 8 | Positive n = 27 | P-value |
| Extent of choroidal invasion | |||||||
| Minimal | 24 (68.6%) | 11 (45.8%) | 13 (54.2%) | 0.05 (S) | 7 (29.2%) | 17 (70.8%) | 0.38 (NS) |
| Massive | 11 (41.4%) | 1 (9.0%) | 10 (91.0%) | 1 (9.0%) | 10 (91.0%) | ||
n: number of cases, NS: non-significant, S: significant.
To study the relationship between C-kit and the degree of tumour differentiation, the specimens were divided into two groups. Group I included the moderate and well-differentiated tumours while group II was composed of poorly differentiated tumours. In group I, 55.6% (20/36) of the specimens displayed positivity for C-kit, whereas 35% (7/20) of specimens in group II showed positive expression. There was no statistically significant difference between the two groups as regards C-kit expression (P = 0.98) (Table 2).
Immunohistochemical expression of VEGF in retinoblastoma
Positive VEGF immunoreactivity was detected in 76.8% (43/56) of retinoblastoma cases. VEGF immunostaining was noted in the cytoplasm of the neoplastic cells (Figure 3). It was also expressed within vasculature of adjacent uninvolved retina.
Figure 3.
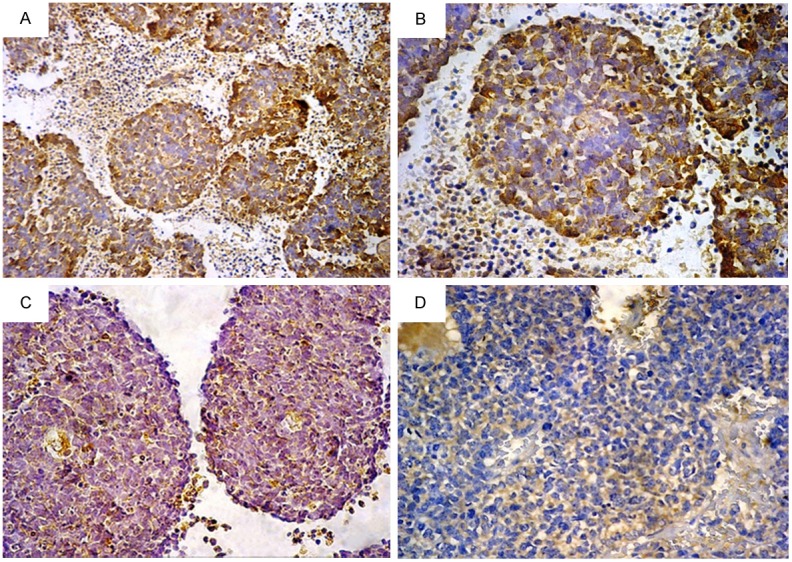
VEGF expression in retinoblastoma: (A) Strong cytoplasmic positivity, (B) The same field with higher magnification power, (C) Moderate positivity, (D) Weak positivity [Immunohistochemistry, original magnification, (A) × 200; (B-D) × 400].
VEGF expression was more frequent in tumours with optic nerve invasion than in those without optic nerve invasion. This finding was statistically highly significant (P = 0.01) (Table 2).
The majority of tumors with choroidal invasion (77.1%) were also positive for VEGF. However, there was no statistically significant difference between cases with negative and positive choroidal invasion (P = 0.43) (Table 2) or cases with massive and minimal choroidal invasion (P = 0.38) (Table 3).
No statistically significant difference was found between various patterns of tumour growth (P = 0.51) or different degrees of tumour differentiation (P = 0.26) (Table 2) regarding VEGF expression.
Immunohistochemical expression of both C-kit and VEGF in retinoblastoma
Comparing the expression levels of C-kit and VEGF in each case, out of 27 retinoblastoma cases with positive C-kit expression, 25 cases (92.6%) also showed VEGF expression. A highly significant difference was found between positive and negative cases (P = 0.01) (Table 4).
Table 4.
C-kit and VEGF immunohistochemical expression in retinoblastoma cases (n = 56)
| VEGF expression | C-kit expression | |||
|---|---|---|---|---|
|
| ||||
| Negative n (%) | Positive n (%) | P-value | ||
| 29 (51.8%) | 27 (48.2%) | |||
| Negative | 13 (23.2%) | 11 (38.0%) | 2 (7.4%) | 0.01 (HS) |
| Positive | 43 (76.8%) | 18 (62.0%) | 25 (92.6%) | |
n: number of cases, HS: highly significant.
Correlation between each of C-kit and VEGF expression and different clinicopathological variables
Pearson’s correlation coefficient was done between each of C-kit expression and VEGF expression and different variables: age, sex, tumor growth pattern, degree of differentiation and presence of choroidal or optic nerve invasion. A highly significant positive correlation was detected between C-kit expression and presence of choroidal invasion (r = 0.53, P ≤ 0.0001) as well as optic nerve invasion (r = 0.46, P = 0.0003). There was also a highly significant positive correlation between VEGF expression and presence of optic nerve invasion (r = 0.34, P = 0.009). Moreover, a highly significant positive correlation was found between C-kit expression and VEGF expression (r = 0.36, P = 0.006) (data are not tabulated).
Relationship between event-free survival and histopathological features
The mean period of follow up was 23.5 months ± 13.2 SD (r: 5-48 months). Two (3.57%) out of 56 retinoblastoma cases died due to extra ocular extension and intracranial metastasis of the disease within 5 and 9 months respectively after enucleation, adjuvant chemotherapy and despite treatment of central nervous system metastasis. These two patients revealed combined choroidal and optic nerve invasion as well as positive C-kit and VEGF expression.
The estimated event-free survival at 4 years for all patients was 96.4%. However, it was 80% for patients with combined massive choroidal and optic nerve invasion and 100% for patients with intraretinal disease and patients with other histopathological features. No significant difference in event-free survival distribution was found between groups (P = 0.1). Figure 4 represented the event-free survival curve.
Figure 4.
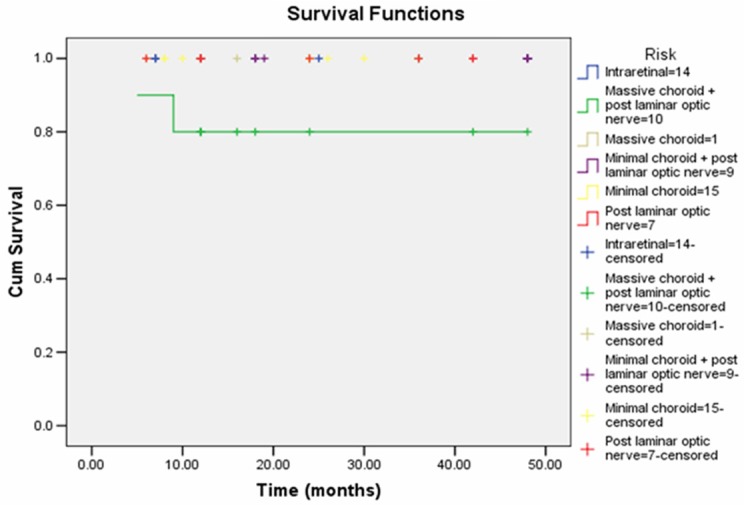
Kaplan-Meier estimates of the proportion of patients survived at 4 years with various histopathological features.
The event-free survival of the cases with positive C-kit expression was 92.6% and it was 100% for the negative cases. No significant difference was found between the two groups using Log rank test (P = 0.13).
The event-free survival of the cases with positive VEGF expression was 95.3% and it was 100% for the negative cases. No significant difference was found between the two groups using Log rank test (P = 0.43).
Discussion
Retinoblastoma is the most common intraocular tumour in childhood [1]. Because of concerns about the significant morbidity and potential mortality associated with current therapies in the treatment of retinoblastoma, newer therapeutic modalities are being investigated [9].
C-kit gene encodes a tyrosine kinase receptor related to platelet-derived growth factor (PDGF)/colony-stimulating factor 1 (CSF-1) [21]. The C-kit pathway has been shown to be involved in a number of physiological and pathological processes, including hematopoiesis, spermatogenesis, melanogenesis, and oncogenesis [22].
Two mechanisms of C-kit activation exist: autocrine and/or paracrine activation by its ligands and ligand-independent activation by mutation. Ligand-dependent activation of C-kit depends on the engagement of its ligand (SCF). The binding of C-kit by SCF induces phosphorylation and activation of the C-kit signal transduction pathway. This type of activation occurs during some normal physiological processes, and during oncogenesis of some cancers [23,24].
In GIST, several groups have studied the clinical implication of the KIT mutation status of exon 11 and were able to establish a possible relationship between these mutations and malignant behaviour. The activating mutations lead into ligand-independent activation of the tyrosine kinase of KIT and have been shown to have a transforming effect in vitro [25,26].
The proto-oncogene C-kit has received widespread attention due to the development of effective novel inhibitory therapeutics such as imatinib mesylate, and the successful treatment of tumours such as GISTs which are known to overexpress the receptor in more than 95% of cases [14].
C-kit expression was studied in pediatric solid tumours, being observed in 100% of synovial sarcomas, 100% of “rhabdoid” rhabdomyosarcomas, 83% of osteosarcomas, 77% of embryonal rhabdomyosarcomas, 71% of Ewing tumours, 55% of neuroblastomas and 52% of Wilms’ tumours [27]. There are limited data in the literature concerning its expression in retinoblastoma.
In the current research, positive C-kit expression was found in 48.2% of the studied retinoblastoma cases. This result raises the possibility that imatinib mesylate could be a therapeutic option in these tumors. Our results are in agreement with Barry et al. [11] who detected positive C-kit immunostaining in 52.38% of retinoblastoma cases. However, Bosch et al. [15] observed positive C-kit expression in only 19.2% of retinoblastomas. Although the same antibody was used as in our work, the dilution in their study was much weaker (1:300). This difference may partly explain the discrepancy between their results and ours. Another explanation would be that the authors used TMA, in which only a small fraction (0.6 mm diameter) of each specimen is used compared with a standard slide. It may be possible that a particular area of a tumour included in the array was negative whereas other areas would be positive.
In the present study, a highly significant correlation was found between increased C-kit expression and pattern of tumour growth, optic nerve invasion as well as choroidal invasion. Parallel to our results, Barry et al. [11] found a strong correlation between C-kit expression and histopathological features of worse prognosis including optic nerve and choroidal invasion. In contrast to Barry et al. [11], we found that cases with massive choroidal invasion displayed a statistically significant increase in C-kit expression compared to cases with minimal invasion.
Our data are in concordance with the data published by Bosch et al. [15] and Barry et al. [11] who revealed no statistical significant relationship between C-kit expression and degree of tumour differentiation.
Retinoblastomas are rapidly growing tumours that frequently outgrow their blood supply resulting in extensive areas of necrosis. The characteristic microscopic features of retinoblastomas include concentric arrangement of proliferating viable cells forming sleeves around blood vessels. At the periphery of the sleeves the cells of the retinoblastoma become necrotic. The growth rate of retinoblastomas is more dependent on the ability of the tumour to induce neovascularization than on the inherent growth rate of the neoplastic cells [28].
Angiogenesis is a critical process in tumour progression that not only provides the growing tumour with required oxygen, nutrients, and growth factors but also provides the circulatory access that allows tumour cells to metastasize [29,30]. Previous studies [31] have reported that angiogenic potential in retinoblastoma correlates with invasive growth and metastasis and that these two factors are associated with poor prognosis. Tumour angiogenesis is regulated by a variety of both positive and negative regulatory molecules [29,32]. When the net effects of the positive regulatory factors overwhelm the effects of the negative factors, the recruitment of preexisting blood vessels and growth of new vessels into tumours occurs, in a process known as the “angiogenic switch” [32]. Among the most important proangiogenic determinants of the angiogenic switch is the level of VEGF expression by tumour cells [29].
VEGF is a glycoprotein that can combine with heparin and has a relative molecular weight of 34-46 KD [33]. It participates in the formation of the vascular tumour stroma [11]. It is known that VEGF messenger RNA is expressed in retinoblastoma neoplastic cell and that VEGF, which is secreted from neoplastic cells, influences nearby endothelial cells and functions as a paracrine mediator [34]. In vitro, studies have shown that VEGF stimulates endothelial cell division and migration [35].
In our study, 76.8% of retinoblastomas showed positive VEGF expression. Our results are consistent with those published by Zhou et al. [36] and Wang et al. [37] who detected positive VEGF immunoreactivity in 72.5% and 64.7% of retinoblastomas respectively. However, Arean et al. [18] found higher rates of expression (98%).
Studies on the prognostic value of VEGF expression in retinoblastomas revealed conflicting results. Concomitant with Zhou et al. [36] we found a significant positive correlation between VEGF expression and optic nerve invasion. Moreover, Yuan S and Song H [38] found that VEGF expression was related to invasion and metastasis. In contrast, Arean et al. [18] observed no significant correlation between VEGF expression and optic nerve invasion. Parallel to Arean et al. [18], the present study found no statistical significant relationship between VEGF and choroidal invasion.
Previous studies concerning the relationship between VEGF expression and degree of tumor differentiation in retinoblastomas revealed discrepancy in the results. Arean et al. [18] reported that of the 7 patients with well-differentiated retinoblastoma, 2 showed focal and weak VEGF staining, whereas the other 5 had strong and diffuse staining. These results differ from those of Kerimogglu et al. [39], who measured angiogenesis (micro vessel density) and found higher levels in poorly differentiated retinoblastomas. Moreover, Zhou et al. [36] stated that VEGF expression was significantly higher in the undifferentiated pattern than in the rosette pattern. In the current research, no statistical correlation could be detected between VEGF and degree of tumor differentiation.
To the best of our knowledge, the current study reports for the first time the direct relationship between C-kit and VEGF immunoreactivity in retinoblastoma. A significant positive correlation was detected between these two proteins in the studied cases. Low oxygen tension regulates VEGF expression by enhancing transcription of VEGF gene and by stabilization of its mRNA. It is now clear that the transcription factor hypoxia-inducible factor (HIF)-1α is the major regulator of VEGF transcription in response to hypoxia [40,41]. Activation of C-Kit by SCF leads to a predominantly HIF-1 α-mediated enhancement of VEGF expression [42]. Boesiger et al. [43] showed that, in response to SCF (the ligand for the C-kit receptor), mouse or human mast cells can rapidly release vascular permeability factor/VEGF by degranulation and can then sustain release by secreting newly synthesized protein. Inhibition of C-Kit signaling with imatinib could have clinically relevant antiangiogenic effects by inhibiting VEGF expression [42]. Angiogenesis inhibition strategies have many advantages when they are compared to other approaches to anti-cancer therapy. Unlike most tumour cells, endothelial cells are readily accessible from the blood circulation and as genetically stable cells they are not likely to develop resistance to cytostatic therapy [44].
Chemoprophylaxis is necessary for patients with tumor extending to the surgical margin of the optic nerve and is likely to be beneficial in preventing metastasis in patients with tumor extending beyond the lamina cribrosa. Chemoprophylaxis was not offered to patients with prelaminar optic nerve disease or isolated choroidal involvement [45,46]. In the present study, chemoprophylaxis was given to 10 cases with combined massive choroidal and post-laminar optic nerve invasion. Two of them developed metastasis and died. No correlation was found between C-kit and VEGF expression and the risk for metastasis and survival of those patients receiving postoperative chemotherapy.
In conclusion, C-kit protein is expressed in nearly half of the retinoblastoma patients. Our paper provides convincing evidence that retinoblastoma should be included in the list of C-kit-expressing cancers. C-kit expression is a feature of more aggressive retinoblastoma, with increased expression in tumours spreading beyond the retina. Results suggest that the C-kit molecular pathway may be important in retinoblastoma growth, and point to its use as a target for therapy with imatinib mesylate. However, further studies for example on the in-vitro effect of C-kit inhibition by imatinib mesylate on retinoblastoma cell lines must be undertaken to test the possibility of treatment of patients with such drug. The present study confirms VEGF expression in retinoblastoma. It is associated with optic nerve invasion. Our data suggest that the use of anti-VEGF therapies in retinoblastoma may be efficacious in targeting immature neovessels within the tumour and reducing tumour progression. Our study demonstrates for the first time the positive relationship between C-kit and VEGF in retinoblastoma. Results suggest that inhibition of C-Kit signaling with imatinib might result in inhibition of tumour angiogenesis. However, further studies are necessary to clarify the mechanisms of C-kit expression in order to be effective in developing therapeutic strategies.
Disclosure of conflict of interest
None.
References
- 1.Dyer MA, Rodriguez-Galindo C, Wilson MW. Use of preclinical models to improve treatment of retinoblastoma. PLoS Med. 2005;2:e332. doi: 10.1371/journal.pmed.0020332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang CY, Chiou TJ, Hwang B, Bai LY, Hsu WM, Hsieh YL. Retinoblastoma in Taiwan: survival rate and prognostic factors. Jpn J Ophthalmol. 2006;50:242–249. doi: 10.1007/s10384-005-0320-y. [DOI] [PubMed] [Google Scholar]
- 3.McLean IW, Burnier M, Zimmerman L, Jakobiec F. Armed Forces Institute of Pathology atlas of tumor pathology: tumors of the eye and ocular adnexa. Washington DC: Armed Forces Institute of Pathology; 1994. [Google Scholar]
- 4.Eng C, Li FP, Abramson DH, Ellsworth RM, Wong FL, Goldman MB, Seddon J, Tarbell N, Boice JD Jr. Mortality from second tumors among long-term survivors of retinoblastoma. J Natl Cancer Inst. 1993;85:1121–1128. doi: 10.1093/jnci/85.14.1121. [DOI] [PubMed] [Google Scholar]
- 5.Advani SH, Rao SR, Iyer RS, Pai SK, Kurkure PA, Nair CN. Pilot study of sequential combination chemotherapy in advanced and recurrent retinoblastoma. Med Pediatr Oncol. 1994;22:125–128. doi: 10.1002/mpo.2950220212. [DOI] [PubMed] [Google Scholar]
- 6.Byrne J, Fears TR, Whitney C, Parry DM. Survival after retinoblastoma: long-term consequences and family history of cancer. Med Pediatr Oncol. 1995;24:160–165. doi: 10.1002/mpo.2950240304. [DOI] [PubMed] [Google Scholar]
- 7.Kaste SC, Chen G, Fontanesi J, Crom DB, Pratt CB. Orbital development in long-term survivors of retinoblastoma. J. Clin. Oncol. 1997;15:1183–1189. doi: 10.1200/JCO.1997.15.3.1183. [DOI] [PubMed] [Google Scholar]
- 8.Krishnakumar S, Sundaram A, Abhyankar D, Krishnamurthy V, Shanmugam MP, Gopal L, Sharma T, Biswas J. Major histocompatibility antigens and antigen-processing molecules in retinoblastoma. Cancer. 2004;100:1059–69. doi: 10.1002/cncr.20062. [DOI] [PubMed] [Google Scholar]
- 9.Benz MS, Scott IU, Murray TG, Kramer D, Toledano S. Complications of systemic chemotherapy as treatment of retinoblastoma. Arch Ophthalmol. 2000;118:577–578. [PubMed] [Google Scholar]
- 10.Andre C, Hampe A, Lachaume P, Martin E, Wang XP, Manus V, Hu WX, Galibert F. Sequence analysis of two genomic regions containing the KIT and the FMS receptor tyrosine kinase genes. Genomics. 1997;39:216–226. doi: 10.1006/geno.1996.4482. [DOI] [PubMed] [Google Scholar]
- 11.Barry RJ, de Moura LR, Marshall JC, Fernandes BF, Orellana ME, Antecka E, Martins C, Burnier MN. Expression of C-kit in retinoblastoma: a potential therapeutic target. Br J Ophthalmol. 2007;91:1532–6. doi: 10.1136/bjo.2007.119651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ashman LK. The biology of stem cell factor and its receptor C-kit. Int J Biochem Cell Biol. 1999;31:1037–51. doi: 10.1016/s1357-2725(99)00076-x. [DOI] [PubMed] [Google Scholar]
- 13.Heinrich MC, Rubin BP, Longley BJ, Fletcher JA. Biology and genetic aspects of gastrointestinal stromal tumors: KIT activation and cytogenetic alterations. Hum Pathol. 2002;33:484–495. doi: 10.1053/hupa.2002.124124. [DOI] [PubMed] [Google Scholar]
- 14.Jones C, Rodriguez-Pinilla M, Lambros M, Bax D, Messahel B, Vujanic GM, Reis-Filho JS, Pritchard-Jones K. c-KIT overexpression, without gene amplification and mutation, in paediatric renal tumours. J Clin Pathol. 2007;60:1226–31. doi: 10.1136/jcp.2007.046441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bösch D, Pache M, Simon R, Schraml P, Glatz K, Mirlacher M, Flammer J, Sauter G, Meyer P. Expression and amplification of therapeutic target genes in retinoblastoma. Graefes Arch Clin Exp Ophthalmol. 2005 Feb;243:156–62. doi: 10.1007/s00417-004-1036-2. [DOI] [PubMed] [Google Scholar]
- 16.Zhang W, Stoica G, Tasca SI, Kelly KA, Meininger CJ. Modulation of tumor angiogenesis by stem cell factor. Cancer Res. 2000;60:6757–62. [PubMed] [Google Scholar]
- 17.Murphree AL. Intraocular retinoblastoma: the case for a new group classification. Ophthalmol Clin N Am. 2005;18:41–53. doi: 10.1016/j.ohc.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Areán C, Orellana ME, Abourbih D, Abreu C, Pifano I, Burnier MN Jr. Expression of vascular endothelial growth factor in retinoblastoma. Arch Ophthalmol. 2010;128:223–9. doi: 10.1001/archophthalmol.2009.386. [DOI] [PubMed] [Google Scholar]
- 19.Hsu SM, Raine L, Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabelled antibody (PAP) procedures. J Histochem cytochem. 1981;29:577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- 20.Potti A, Ganti AK, Foster H, Knox S, Hebert BJ, Tendulkar K, Sholes K, Koch M, Kargas S. Immunohistochemical detection of HER-2/neu, c-kit (CD117) and vascular endothelial growth factor (VEGF) overexpression in soft tissue sarcomas. Anticancer Res. 2004;24:333–7. [PubMed] [Google Scholar]
- 21.Yarden Y, Kuang WJ, Yang-Feng T, Coussens L, Munemitsu S, Dull TJ, Chen E, Schlessinger J, Francke U, Ullrich A. Human proto-oncogene c-KIT: a new cell surface receptor tyrosine kinase for an unidentified ligand. EMBO J. 1987;6:3341–51. doi: 10.1002/j.1460-2075.1987.tb02655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ullrich A, Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990;61:203–12. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- 23.Krystal GW, Hines SJ, Organ CP. Autocrine growth of small cell lung cancer mediated by co-expression of c-KIT and stem cell factor. Cancer Res. 1996;56:370–6. [PubMed] [Google Scholar]
- 24.DiPaola RS, Kuczynski WI, Onodera K, Ratajczak MZ, Hijiya N, Moore J, Gewirtz AM. Evidence for a functional kit receptor in melanoma, breast, and lung carcinoma cells. Cancer Gene. 1997;4:176–82. [PubMed] [Google Scholar]
- 25.Lasota J, Jasinski M, Sarlomo-Rikala M, Miettinen M. Mutations in exon 11 of c-Kit occur preferentially in malignant versus benign gastrointestinal stromal tumors and do not occur in leiomyomas or leiomyosarcomas. Am J Pathol. 1999;154:53–60. doi: 10.1016/S0002-9440(10)65250-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lasota J, Wozniak A, Sarlomo-Rikala M, Rys J, Kordek R, Nassar A, Sobin LH, Miettinen M. Mutations in exons 9 and 13 of KIT gene are rare events in gastrointestinal stromal tumors. A study of 200 cases. Am J Pathol. 2000;157:1091–1095. doi: 10.1016/S0002-9440(10)64623-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smithey BE, Pappo AS, Hill DA. C-kit expression in pediatric solid tumors: a comparative immunohistochemical study. Am J Surg Pathol. 2002;26:486–92. doi: 10.1097/00000478-200204000-00011. [DOI] [PubMed] [Google Scholar]
- 28.Burnier MN, McLean IW, Zimmerman LE, Rosenberg SH. Retinoblastoma. The relationship of proliferating cells to blood vessels. Invest Ophthalmol Vis Sci. 1990;31:2037–40. [PubMed] [Google Scholar]
- 29.Kerbel R, Folkman J. Clinical translation of angiogenesis inhibitors. Nat Rev Cancer. 2002;2:727–39. doi: 10.1038/nrc905. [DOI] [PubMed] [Google Scholar]
- 30.Fidler IJ. The pathogenesis of cancer metastasis: the “seed and soil” hypothesis revisited. Nat Rev Cancer. 2003;3:453–8. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 31.Jockovich ME, Piña Y, Alegret A, Cebulla C, Feuer W, Murray TG. Heterogeneous tumor vasculature in retinoblastoma: implications for vessel targeting therapy. Retina. 2008;28:S81–S86. doi: 10.1097/IAE.0b013e318150d6f0. [DOI] [PubMed] [Google Scholar]
- 32.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–10. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 33.An FQ, Matsuda M, Fujii H, Matsumoto Y. Expression of vascular endothelial growth factor in surgical specimens of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2000;126:153–160. doi: 10.1007/s004320050025. [DOI] [PubMed] [Google Scholar]
- 34.Lee SY, Kim DK, Cho JH, Koh JY, Yoon YH. Inhibitory effect of bevacizumab on the angiogenesis and growth of retinoblastoma. Arch Ophthalmol. 2008;126:953–958. doi: 10.1001/archopht.126.7.953. [DOI] [PubMed] [Google Scholar]
- 35.Stitt AW, Simpson DA, Boocock C, Gardiner TA, Murphy GM, Archer DB. Expression of vascular endothelial growth factor (VEGF) and its receptors is regulatedin eyes with intra-ocular tumours. J Pathol. 1998;186:306–312. doi: 10.1002/(SICI)1096-9896(1998110)186:3<306::AID-PATH183>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 36.Zhou L, Xu J, Kang J. Expression of matrix metalloproteinase-2, matrix metalloproteinase-9 and vascular endothdial growth factor in retinoblastoma and its clinical significance. Yan Ke Xue Bao. 2010;25:62–64. doi: 10.3969/g.issn.1000-4432.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 37.Wang XL, Niu YJ, Ma JM. HIF-1alpha, HPSE and VEGF promote malignant progression of retinoblastoma. Zhonghua Yan Ke Za Zhi. 2010;46:140–4. [PubMed] [Google Scholar]
- 38.Yuan S, Song H. Expression and clinical implication of Matrix Metalloproteinase-1 and Vascular Endothelial Growth Factor in retinoblastoma. Yan Ke Xue Bao. 2010;25:48–51. doi: 10.3969/g.issn.1000-4432.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 39.Kerimoğglu H, Kiratli H, Dinçtürk AA, Söylemezoğlu F, Bilgiç S. Quantitative analysis of proliferation, apoptosis, and angiogenesis in retinoblastoma and their association with the clinicopathologic parameters. Jpn J Ophthalmol. 2003;47:565–571. doi: 10.1016/j.jjo.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 40.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–32. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 41.Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003;9:677–84. doi: 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]
- 42.Litz J, Krystal GW. Imatinib inhibits c-Kit-induced hypoxia-inducible factor-1alpha activity and vascular endothelial growth factor expression in small cell lung cancer cells. Mol Cancer Ther. 2006;5:1415–22. doi: 10.1158/1535-7163.MCT-05-0503. [DOI] [PubMed] [Google Scholar]
- 43.Boesiger J, Tsai M, Maurer M, Tamaguchi M, Brown LF, Claffey KP, Dvorak HF, Galli SJ. Mast cells can secrete vascular permeability factor/vascular endothelial growth factor and exhibit enhanced release after immunoglobulin E-dependent up regulation of Fc epsilon receptor I expression. J Exp Med. 1998;188:1135–1145. doi: 10.1084/jem.188.6.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hasan J, Jayson GC. VEGF antagonists. Expert Opin Biol Ther. 2001;1:703–18. doi: 10.1517/14712598.1.4.703. [DOI] [PubMed] [Google Scholar]
- 45.Honavar SG, Singh AD, Shields CL, Meadows AT, Demrici H, Cater J, Shields J. Post enucleation adjuvant therapy in high-risk retinoblastoma. Arch Ophthalmol. 2002;120:923–931. doi: 10.1001/archopht.120.7.923. [DOI] [PubMed] [Google Scholar]
- 46.Chantada GL, Dunkel IJ, de Dávila MT, Abramson DH. Retinoblastoma patients with high risk ocular pathological features: who needs adjuvant therapy? Br J Ophthalmol. 2004;88:1069–1073. doi: 10.1136/bjo.2003.037044. [DOI] [PMC free article] [PubMed] [Google Scholar]


