Abstract
The purpose of this study was to: 1) examine the effects of hydroxysafflor yellow A (HSYA) on the proliferation, collagen and cytokine synthesis of vascular adventitial fibroblasts as induced by angiotensin II (Ang II) in normal Sprague-Dawley (SD) rats in vitro, and 2) to assess the effects of HSYA on morphological changes and collagen accumulation of vascular adventitia in spontaneously hypertensive rats (SHR) in vivo. In vitro experiment, vascular adventitial fibroblasts from SD rats were isolated, cultured, and divided into control groups, model groups and HSYA groups. Cell morphology of adventitial fibroblasts was assessed using laser confocal microscopy, while cell proliferation with the MTT assay, and collagen synthesis was determined using hydroxyproline chromatometry. Immunocytochemistry and reverse transcription PCR were used for detecting the expression of TGF-β1, MMP-1, α-SMA and NF-κB in adventitial fibroblasts. In vivo experiment, vascular adventitia proliferation and collagen synthesis were analyzed using hematoxylin-eosin and Sirius staining. Our results showed that: 1) in vitro experiment of SD rats, HSYA inhibited proliferative activity and collagen synthesis of adventitial fibroblasts as induced by Ang II, and the inhibitory effects of HSYA on the increased expression of MMP-1, TGF-β1, α-SMA and NF-κB p65 as induced by Ang II were assessed, and 2) in vivo experiment of SHR, histological analysis displayed fewer pathological changes of vascular adventitia in HSYA treatment groups as compared with no HSYA treatment groups, and MMP-1, TGF-β1, α-SMA and NF-κB p65 expression significantly reduced after HSYA treatment (P < 0.05). Our results revealed that HSYA treatment significantly decreased the amount of cytokines and collagen synthesis in vascular adventitia components. This study provides experimental evidence demonstrating that HSYA has the capacity to decrease vascular adventitia proliferation and hyperplasia during vascular remodeling.
Keywords: Hydroxysafflor yellow A, adventitial fibroblasts, proliferation, collagen, angiotensin II
Introduction
Processes involved with vascular remodeling contribute to many cardiovascular, reproductive and ocular diseases. The prevailing belief is that hyperplasia of the vascular intima and tunica media play a major role in modulating vasculature structure and function, while the role of the vascular adventitia has long been neglected. More recent findings, which demonstrate that fibroblasts contribute to the formation of intimal atherosclerotic lesion or vascular restenosis, suggests that the vascular adventitia may serve as a critical regulator of vascular restenosis [1,2]. Moreover, results from work within our laboratory [3,4] revealed that fibroblasts in the adventitia are activated prior to atherosclerotic lesion formation and thus may play an important role in the early process of atherosclerosis. Adventitial fibroblasts are activated during the early stages of injury or stress and secrete large numbers of cytokines, enzymes and chemokines [5,6]; all of which can influence cell migration, proliferation and differentiation [7,8]. In addition, increases in the production and accumulation of collagen and elastin are observed in the adventitia during the development of remodeling following injury or stress.
Angiotensin II (Ang II), an important component of the renin-angioensin system (RAS), can produce hypertension by changing vascular tension and blood flow. In hypertension development within spontaneous hypertensive rats (SHR), the RAS system is activated and generates a large number of Ang II. Ang II can also induce proliferation and hyperplasia of medial smooth muscle cells and the vascular adventitia to cause their migration to the intima [9].
Safflower is a traditional Chinese herbal medicine that shows a remarkable efficacy in the treatment of coronary heart disease through its capacity of preventing arteriosclerosis and inhibiting platelet activation [10-12]. Hydroxysafflor yellow A (HSYA) is a water soluble monomer extracted from safflower that can exert a variety of effects upon the cardiovascular system. For example, HSYA can reduce phenylephrine or KCl-induced vasoconstriction [13], inhibit endothelin release, increase myocardial flow, improve myocardial oxygen consumption, and inhibit myocardial ischemia [14]. However, whether HSYA has the capacity to protect vascular adventitia proliferation and hyperplasia against vascular injury induced by Ang II is not known. In this study, we examined some of the possible mechanisms of HSYA as related to vascular adventitia remodeling and evaluated the potential for treatment of atherosclerosis and hypertension with HSYA.
Materials and methods
Animals and reagents
Male Sprague-Dawley (SD) rats, 9-weeks-old (230-260 g) and 9-week-old spontaneously hypertensive rats (SHR) were used in these experiments. Rats were provided by experimental animal center of Binzhou Medical University. All experimental animals were treated according to guidelines approved by University Committee on the use and care of laboratory animals. Hydroxysafflor yellow A was purchased from the Puzhen Biological Technology Co (Shanghai, China). The hydroxyproline assay kit was purchased from the Jiancheng Co (Nanjing, China). Trizol and the two-step PCR detection kits were purchased from the TaKaRa Co (Dalian, China). Anti-TGF-β1 antibody, anti-α-SMA antibody, anti-MMP-1 antibody, anti-NF-κB p65 antibody for immunocytochemistry were purchased from the Boster Co (Wuhan, China). The light microscope and camera were purchased from the Nikon Corporation (Japan). All other chemicals and reagents were of analytical grade.
Adventitial fibroblasts cultivation and experimental groups of SD rats
The SD rats were euthanized with 3% excessive pentobarbital anesthesia and then sterilized by immersion within 75% alcohol. Under aseptic conditions, abdominal aortas were isolated [15]; the adventitia was stripped and sectioned into small tissue blocks. Tissue blocks were incubated in a humidified atmosphere of 5% CO2 in air at 37°C in DMEM culture medium (containing 15% fetal bovine serum). The medium was changed every two days until the cells coated the bottom of the bottle. The cells were subcultured and the resultant ~2-3 generations of cells, which were cultured on 25-mm cover slips, were used in the experiments. Vascular adventitial fibroblasts were identified by immunohistochemical staining using the primary antibodies vimentin, desmin and SM-a-actin (1:100).
The 2-3 generations of adventitia fibroblasts were cultured in 10% fetal bovine serum (FBS), and seeded at a density of 3 × 104 cells/well in 6-well plates. When cells approached 80% confluence, the culture medium was changed to 1% FBS medium. The fibroblasts were the aliquoted into the following groups: control (no Ang II), model (Ang II 1 × 10-6 mol/L), HSYA1 (HSYA 50 μg/mL plus Ang II 1 × 10-6 mol/L) and HSYA2 (HSYA 100 μg/mL plus Ang II 1 × 10-6 mol/L). All cells were cultured for 24 or 48 h.
MTT assay for observing cell proliferation of SD rats
Vascular adventitial fibroblasts were evaluated for their in vitro proliferation activity with use of the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide) assay. The fibroblast cell lines were harvested during the logarithmic growth phase and seeded in 96-well plates at a density of 1 × 104 cells/mL. The fibroblasts were then divided into the four treatment groups described above and all cells were cultured for 12 h, 24 h, 36 h, 48 h, respectively. At each of the time points, 20 μL of MTT solution (5 mg/mL) was added to each well and the cells were then incubated for an additional 4 h. The culture supernatant was removed and 150 μL of dimethyl sulfoxide was added to each well to fully dissolve the MTT-formazan crystals. Cell growth inhibition was determined by measuring the absorbance (Abs) at λ = 570 nm using a microplate reader and calculated according to the following equation: Growth inhibition = (1 - OD of treated cells/OD of control cells) × 100%.
Hydroxyproline assay technique for determination of cell collagen synthesis of SD rats
The hydroxyproline assay [16] involved extraction of hydroxyproline from collagen and oxidation to pyrrole by chloramine-T, for production of color with para-dimethyl benzaldehyde. The adventitial fibroblast lines were harvested during the logarithmic growth phase and seeded in 24-well plates at a density of 1 × 104 cells/mL (n = 5). A total of 1 mL was added to each well which was then cultured at 37°C in a humidified incubator for 24 h (5% CO2). The culture medium was then changed to 1% FBS medium. The cells were then divided into the four treatment groups, as described above, and treated with either Ang II or HSYA/Ang II for 48 h. A sample from the supernatant was removed for the hydroxyproline assay. The color signals were measured in a spectrophotometer at 550 nm, and compared to a standard curve.
Adventitial fibroblast morphological analysis and immunohistochemical staining of SD rats
The inhibitory effects of HSYA on the increased expression of MMP-1, TGF-β1, α-SMA and NF-κB p65 were assessed. The cells were fixed in 4% paraformaldehyde, and then incubated with primary antibodies in blocking solution overnight at 4°C, washed 3X in PBS, and incubated with the secondary antibody in a 37°C incubator for 30 min. The cells were identified by immunohistochemical staining using the primary antibodies TGF-β1 (1:500), MMP-1 (1:100), α-SMA (1:200) and NF-κB (1:100) with IgG FITC or IgGCy3 (1:200) as the fluorescence labeling goat anti rabbit IgG, nuclear dye (DAPI) as the second antibody. The cells of the control groups were incubated with PBS in the absence of the primary antibody. Laser confocal microscopy was used for fluorescent analysis. The experiment was repeated three times. Ten different horizons of cells were randomly selected, and the rates of positive cell conversions were calculated.
SD rats adventitial fibroblast mRNA extraction and reverse transcription PCR
Reverse transcription PCR was used to determine mRNA expression of TGF-β1, MMP-1, α-SMA and NF-κB in the adventitial fibroblasts of the four groups as described above. Total RNA extraction was performed according to the Trizol reagent kit instructions and subsequently according to the two-step PCR detection kit manual of the TaKaRa Company.
PCR primers were synthesized by the TaKaRa Company (Table 1). A reverse transcription PCR amplification system in a total of 12.5 μl were applied to the following PCR program: 5 min @ 94°C (pre denaturation), 30 s @ 94°C (initial denaturation), 30 s @ 58°C, 1 min @ 72°C, repeated 30 times, and 5 min @ 72°C (amplification). The PCR products were assessed on 1.5% agarose gels. Results of the electrophoresis were scanned by a Gel imaging system for quantitative analysis and photographed.
Table 1.
PCR primers used in this study
| Gene Name | Sequence |
|---|---|
| TGF-β1 | forward: 5’-GTCATAGATTGCATTGTTGC-3’ |
| reverse: 5’-AAGGAGACGGAATACAGGG-3’ | |
| MMP-1 | forward: 5’-GATGGATCCCAAGCCATATATGGACGTTCC-3’ |
| reverse: 5’-TTGGAATTCCGGACTTCATCTCTGTCGG-3’ | |
| α-SMA | forward: 5’-CGAGAAGCTGCTCCAGCTATGTG-3’ |
| reverse: 5’-CTCTCTTGCTCTGCGCTTCGT-3’ | |
| NF-κB | forward: 5’-GAAGAAGCGAGACCT GGAG-3’ |
| reverse: 5’-T CCGGAACACAATGGCCAC-3’ | |
| β-actin | forward: 5’-GGAGATTACTGCCCTGGCTCCTA-3’ |
| reverse: 5’-GACTCATCGTACTCCTGCTTGCTG-3’ |
Animal model and morphological analysis of SHR
A total of 18 males, 9-week-old spontaneously hypertensive rats (SHR) were used as experimental subjects. These rats were randomly divided into the following groups: model (treated daily with normal saline), HSYA1 (treated daily with single dose of 0.5 ml/kg HSYA) and HSYA2 (1 ml/kg HSYA, daily). A separate group of 6 males, 9-week-old SD rats (230 g) treated with normal saline served as a control group. After 48 days of treatment (daily intraperitoneal injections of the drugs described above) rats were sacrificed.
The grades of vascular adventitia proliferation and hyperplasia of SHR were analyzed by hematoxylin-eosin and Sirius staining. Samples from the abdominal aortas were isolated, fixed with 10% paraformaldehyde solution in sterile phosphate-buffered saline and embedded in paraffin wax. Sections were cut at 5 μm using a microtome and deparaffinized tissue sections were subjected to staining for histological examination. The slides were examined by light microscopy and photographed.
SHR vascular adventitia mRNA extraction and reverse transcription PCR
Abdominal aortas of SHR were isolated and the adventitia stripped. Total RNA was extracted from aorta adventitia using Trizol reagent according to the manufacturer’s instructions. RNA was reverse-transcribed into cDNA using reverse transcriptase. PCR was then performed in a final volume of 20 μL using a PCR reagent kit (Takara Co). The β-actin gene was used as an internal control. The cycling program involved preliminary denaturation at 95°C for 10 min, followed by 35 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 1 min, and a final elongation step at 72°C for 5 min.
Statistical analysis
Data were presented as the mean ± SD. For determination of the significant differences, statistical analysis was performed with the one-way ANOVA followed by multiple comparison tests using SPSS software. Values of P < 0.05 were regarded as being statistically significant.
Results
Effect of HSYA on adventitial fibroblasts proliferation and collagen synthesis of SD rats
As shown in Figure 1A, HSYA inhibited proliferative activity as a function of exposure time and concentration. Growth inhibition rates of fibroblasts treated with HSYA for 48 h at concentrations of 50 and 100 μg/ml group were 19.3 and 40.2% of the respective model group (P < 0.05). As shown in Figure 1B, with regard to collagen, as determined by hydroxyproline content, the group receiving Ang II showed greater amounts of hydroxyproline content as compared with controls. However, hydroxyproline content was significantly reduced after HSYA administration (P < 0.05), the collagen expressed as a function of culture duration with statistically significant differences being obtained among each of the four groups of 12 h, 24 h, 36 h and 48 h, and decreased gradually with as a function of increasing concentrations of HSYA.
Figure 1.
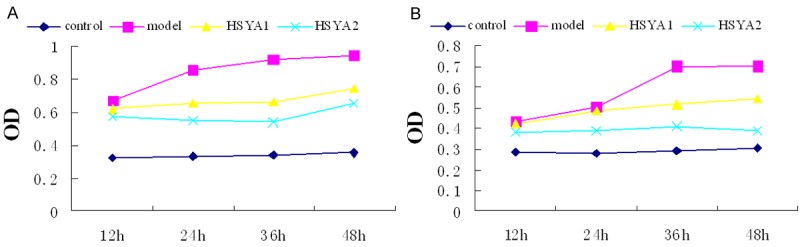
Growth and collagen synthesis curve of fibroblasts in various groups. A: Maximal growth inhibition rates of the HSYA2 group (40.2% of those model group) was observed following 48 h of HYSA treatment using a concentration of 100 μg/ml (P < 0.05). B: Collagen expression decreased as a function of increasing concentrations of HSYA, with maximal effects being obtained in the HSYA2 group (P < 0.05).
HSYA downregulates adventitial fibroblast mRNA expression of SD rats
The PCR reaction was evaluated using each band of agarose gel absorbance and analysis of the grey PCR product was evaluated by a Gel imaging system. The grey values were calculated relative to β-actin. The fibroblasts in model groups cultured with Ang II upregulated TGF-β1, MMP-1, α-SMA and NF-κB mRNA expression compared to control groups, while HSYA groups significantly downregulated mRNA expression compared to model groups. What’s more, there was significant difference between HSYA1 and HSYA2 groups (P < 0.05) (Figure 2A, 2B). These data of expression demonstrated that HSYA can inhibit MMP-1, TGF-β1, α-SMA and NF-κB expression at transcriptional and translational levels.
Figure 2.
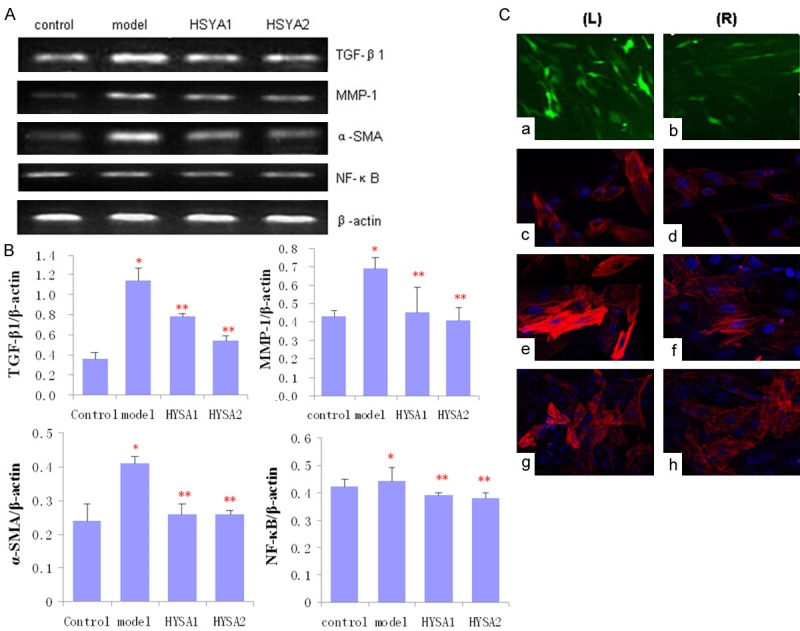
Expression of TGF-β1, MMP-1, α-SMA and NF-κB in adventitial fibroblasts with or without HSYA treatment. A: Significant differences among groups are indicated by RT-PCR. B: Averages of at least three independent experiments (n = 3). Bars indicate the mean ± SD. *P < 0.05 compared to the control (model vs. control, *P < 0.05; HSYA1 and HSYA2 vs. model, **P < 0.05). C: Fibroblasts stained with FITC anti-body (green) indicating intracellular TGF-β1; Fibroblasts stained with Cy3 anti-body (red) indicating intracellular MMP-1; α-SMA and NF-κB (nuclei stained blue (DAPI). Representative photographs of immunofluorescence of fibroblasts induced by Ang II in the absence (left, L) or presence (right, R) of HSYA. a, b: Fibroblasts stained with TGF-β1 (green); c, d: Fibroblasts stained with MMP-1 (red); e, f: Fibroblasts stained with α-SMA (red). g, h: Fibroblasts stained with NF-κB (red) (× 200).
HSYA downregulates MMP-1, TGF-β1, α-SMA and NF-κB expression of SD rats
It was observed that Ang II can upregulate the TGF-β1, MMP-1, α-SMA and NF-κB expression in fibroblasts as determined with immunocytochemistry. However, treating fibroblasts with 50 or 100 μg/mL of HSYA for 48 h resulted in significantly lower levels of TGF-β1, MMP-1 expression. In addition, α-SMA expression, which represents a characteristic marker of myofibroblasts, was also down regulated by HSYA. Results from immunofluorescent assays have indicated that little or very weak intracellular staining of NF-κB was present following HSYA treatment (Figure 2C). With comparing the expression of TGF-β1, MMP-1, α-SMA and NF-κB in the four treatment groups, it appears that HSYA was effective in inhibiting the increase that would normally be present in expression of these cytokines.
Effect of HSYA on the expression of TGF-β1, MMP-1, α-SMA and NF-κB mRNA of vascular adventitia tissue of SHR
The gene expression of TGF-β1, MMP-1, α-SMA and NF-κB were examined in vascular adventitia tissue of SHR using quantitative RT-PCR (Figure 3A, 3B). The model group (SHR treated with normal saline) showed significant increases (P < 0.05) in the levels of TGF-β1, MMP-1, α-SMA and NF-κB mRNA expression as compared to the control group (SD rats treated with normal saline). Treatment of SHR with HSYA significantly decreased mRNA levels compared to the model group (P < 0.05). This effect appeared to be dose-dependent as the higher dose of HSYA was associated with a more substantial reduction in mRNA levels. It is believed that HSYA affected the expression of these genes at the transcriptional and translational levels in the vascular adventitia.
Figure 3.
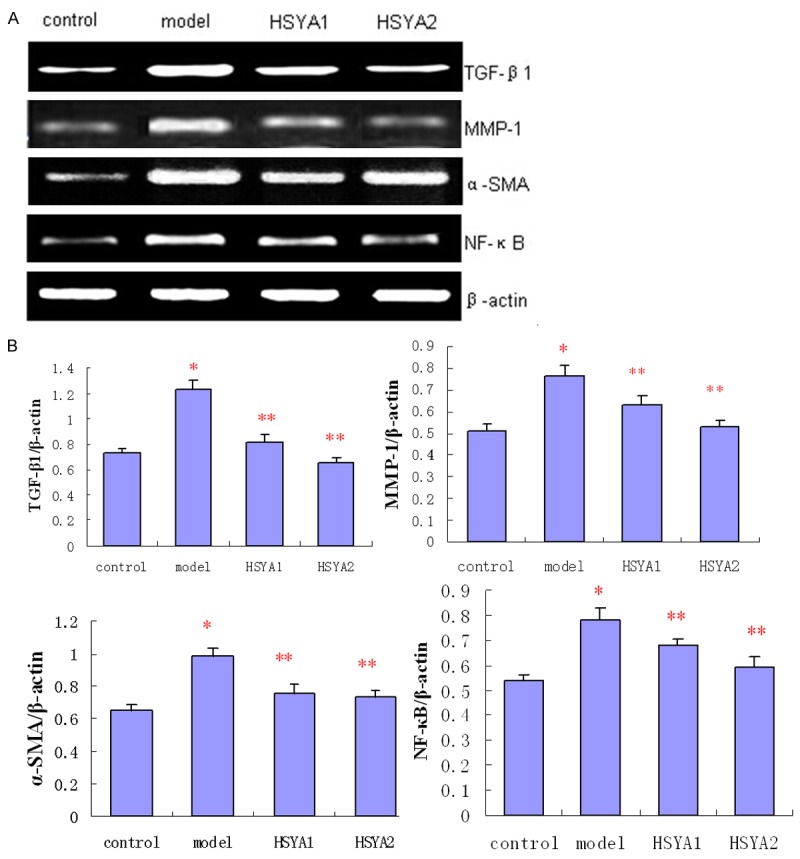
Expression of TGF-β1, MMP-1, α-SMA and NF-κB in vascular adventitia in the presence or absence of HSYA. A: Significant differences in mRNA expression among the four groups are indicated by RT-PCR. Results represent the average of at least six independent experiments (n = 6). B: Bars indicate the mean ± SD. *P < 0.05 compared to the control. (model vs. control, *P < 0.05; HSYA1 and HSYA2 vs. model, **P < 0.05).
SHR vascular adventitia morphological analysis and histochemical staining
Samples from serial sections of the ascending aorta ascending were stained with haematoxylin and eosin in order to observe changes in the tissue morphology. Some sections were stained with red Sirius to identify collagen fibers. A comparative analysis of the adventitia tissue from the groups is presented in Figure 4. The severity of changes varied from slight to moderate. Histological analysis indicated that the HSYA treatment groups displayed fewer pathological changes in vascular adventitia as compared with the model group.
Figure 4.
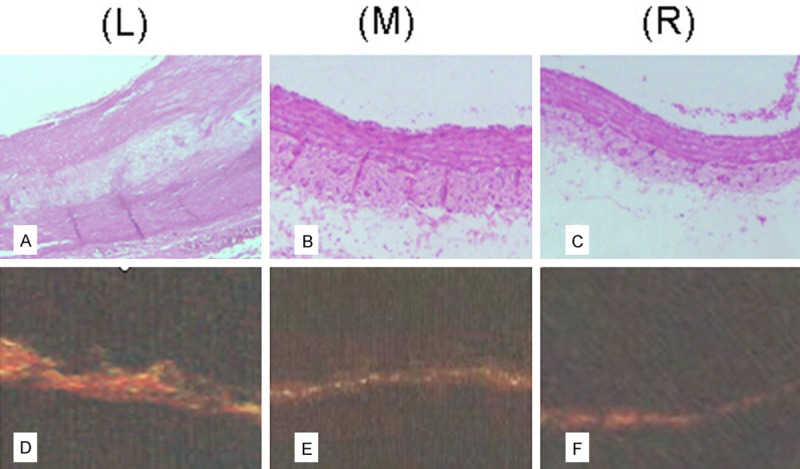
Expression of collagen in vascular adventitia. Representative examples of Hematoxylin-Eosin and Sirius staining: SHR vasculature without HSYA treatment (left, L); SHR vasculature with HSYA treatment (middle, M); SD rat vasculature with normal saline as control (right, R). A: Hematoxylin-Eosin staining showing artery atherosclerotic plaques in vascular walls of SHR without HSYA treatment. B: SHR with HSYA treatment showing fewer pathological changes in vasculature as compared with the SHR without HSYA treatment in the model groups. However, the vasculature, especially adventitia of SHR without HSYA treatment, remained thicker than that of SD rats in control groups. C: Vasculature of SD rats treated with normal saline in the control group showing thinner walls than that of SHR (× 100). D-F: Vasculature was stained with Sirius red to identify collagen fibers. Photographs show the distribution of collagen in the three groups described above (L, M, R), Collagen fibers were found mainly to exist in the vascular adventitia. Collagen I was the main type present, but a small amount of collagen III was also observed. SHR with or without HSYA treatment showed greater amounts of collagen content in the vascular adventitia than that of control SD rats. However, collagen content was significantly reduced after HSYA treatment of SHR (P < 0.05).
Discussion
Cardiovascular diseases are a leading cause of mortality. Vascular remodeling represents a major component of cardiovascular diseases due to its association with atherosclerosis and hypertension [17,18]. Recently, the vascular adventitia has been recognized as playing an important role in vascular remodeling [19], in particular, the vascular adventitial fibroblasts [20]. Adventitial fibroblasts are activated during the early stages of vascular remodeling and change their phenotype into myofibroblasts. These myofibroblasts show characteristics of smooth muscle with smooth muscle α-actin (α-SMA) in plasma and migrate toward the neointimal lumen where they are involved in proliferation of vascular restenosis. Activated adventitial fibroblast exhibit a number of specific histological, biochemical, and functional characteristics involved with the development of remodeling. These include the expressions of TGF-β [21,22], MMPs and NF-κB, all of which contribute to regulating vascular proliferation responses. TGF-β1 represents one of the most important cytokines in this regard as it appears to promote vascular proliferation, migration and to increase the production and accumulation of collagen and elastin in the adventitia during remodeling [23]. MMP-1 is an extracellular matrix protein hydrolysis enzyme, which participates in regulating vascular remodeling and cell migration. Results from a number of studies have shown that intimal hyperplasia and vascular remodeling is dependent on the role of MMPs [24,25]. Zhang et al. [26] reported that TGF-β via activation of the NF-κB pathway induces the production of MMP-9. This MMP-9, through the degradation of extracellular matrix, results in myofibroblast migration. Inhibition of MMPs activity can significantly inhibit the occurrence of restenosis.
Activation of the nuclear transcription factor NF-κB can affect a variety of gene expressions and regulations. Many promoter regions of genes encode cytokines containing transcription factors for NF-κB binding sites, such as IL-1β and TNF-α. As a result, NF-κB activation can lead to an increase in the expression of a number cytokines. Brand K [27] demonstrated that lesions accompanying atherosclerosis were associated with the presence of the activated form of NF-κB. Chun-Yan S [28] reported that Hydroxysafflor Yellow A inhibited NF-κB activation, thus altering the expression of these inflammatory cytokines. Ang II represents an important physiological component in this process by regulating vascular tension and blood flow, and promoting cell growth and proliferation. Secretion of Ang II occurs at local tissue sites in response to vascular injury and has been shown to induce a proliferation of vascular adventitial fibroblasts [9]. Ang II can also stimulate vascular fibroblasts secreting collagen, thereby increasing TGF-β1 expression which then further increases the expression of fibroblast collagen. In this way, Ang II can work by strengthening the expressions of other growth factors [29].
Safflower is a traditional Chinese herbal medicine and has remarkable efficacy in treating coronary heart disease, arteriosclerosis and cerebral infarction. HSYA is a water soluble monomer component extracted from safflower and shows a number of protective effects upon vascular tissue damage. For example, HYSA decreases blood pressure, improves organ blood flow, inhibits blood clots and reduces inflammation [30,31]. Other effects of HYSA have been reported by Zang et al. [10] who found that HSYA inhibits the platelet activating factor that combines with specific platelet receptors on the cell membranes, and can inhibit the pindolol-β receptor specific binding capacity of ventricular muscle cells located in the membrane [11]. Other reports that HSYA could significantly reduce blood pressure and heart rate, which may be related to activation of cell membrane BKCa and K-ATP channels [12]. Taken together, these results suggest that HSYA can affect cell membranes and thus, transmembrane signal transduction. The fact that HSYA is water-soluble and therefore will not readily pass through cell membranes further supports a role for the cell membrane to serve as a likely target for HSYA. At this site, one potential effect of HSYA can involve the blocking of transmembrane signal transduction. Further work will be required to confirm this, as well as other potential mechanisms of HYSA action.
To the best of our knowledge there are no reports regarding the potential for HSYA to protect vascular remodeling against adventitia proliferation and hyperplasia as induced by Ang II. Based upon the results of the present experiment, it is clear that a number of bioactive substances are released from vascular adventitial fibroblasts by Ang II induced proliferation. These substances, which include cytokines or chemokines, may not only activate the proliferation of vascular adventitial fibroblasts but also participate in the hyperplasia of vascular remodeling. The exact role of HSYA in the repair of vascular remodeling remains unclear. Some notable effects observed in the present experiment include a decrease in some cytokines and gene expression and inhibition of vascular adventitial hyperplasia as induced by Ang II.
Additional work will be critical to ascertain the exact mechanisms of restoration and angiogenesis resulting from HYSA and to identify ways of promoting beneficial angiogenesis while inhibiting vascular adventitial hyperplasia. This study provides a foundation not only for future work on these mechanism but also for the identification of effective management in the prevention and treatment of vascular adventitia remodeling diseases of the cardiovascular system as well as in retinal and placental sites of injury.
Acknowledgements
This work was supported by the Science Plan Projects of University in Shandong (J10LF61), National Nature Science Foundation of China (NSFC81370730), Nature Science Foundation from Shandong Province (ZR2011HQ006, ZR2011HL064) and Science Development Plan from Yantai City (2011216).
Disclosure of conflict of interest
None.
References
- 1.Yoshikawa M, Nakamura K, Nagase S, Sakuragi S, Kusano KF, Matsubara H, Ohe T. Effects of combined treatment with angiotensin II type 1 receptor blocker and statin on stent restenosis. J Cardiovasc Pharmacol. 2009;53:179–186. doi: 10.1097/FJC.0b013e318199f30b. [DOI] [PubMed] [Google Scholar]
- 2.Siow RC, Mallawaarachchi CM, Weissberg PL. Migration of adventitial myofibroblasts following vascular balloon injury: insights from in vivo gene transfer to rat carotid arteries. Cardiovasc Res. 2003;59:212–221. doi: 10.1016/s0008-6363(03)00292-x. [DOI] [PubMed] [Google Scholar]
- 3.Xu F, Ji J, Li L, Chen R, Hu W. Activation of adventitial fibroblasts contributes to the early development of atherosclerosis: a novel hypothesis that complements the “Response-to-Injury Hypothesis” and the “Inflammation Hypothesis”. Med Hypotheses. 2007;69:908–912. doi: 10.1016/j.mehy.2007.01.062. [DOI] [PubMed] [Google Scholar]
- 4.Xu F, Ji J, Li L, Chen R, Hu WC. Adventitial fibroblasts are activated in the early stages of atherosclerosis in the apolipoprotein E knockout mouse. Biochem Biophys Res Commun. 2007;352:681–688. doi: 10.1016/j.bbrc.2006.11.073. [DOI] [PubMed] [Google Scholar]
- 5.Guo W, Shan B, Klingsberg RC, Qin X, Lasky JA. Abrogation of TGF-beta1-induced fibroblast-myofibroblast differentiation by histone deacetylase inhibition. Am J Physiol Lung Cell Mol Physiol. 2009;297:L864–870. doi: 10.1152/ajplung.00128.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu Y, Xu Q. Adventitial biology: differentiation and function. Arterioscler Thromb Vasc Biol. 2011;31:1523–1529. doi: 10.1161/ATVBAHA.110.221176. [DOI] [PubMed] [Google Scholar]
- 7.Ait-Oufella H, Taleb S, Mallat Z, Tedgui A. Recent advances on the role of cytokines in atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31:969–979. doi: 10.1161/ATVBAHA.110.207415. [DOI] [PubMed] [Google Scholar]
- 8.Yuan WD, Liu W, Li JM, Li XY, Sun XH, Xu F, Man XJ, Fu Q. Effects of BMSCs interactions with adventitial fibroblasts in transdifferentiation and ultrastructure processes. Int J Clin Exp Pathol. 2014;7:3957–3965. [PMC free article] [PubMed] [Google Scholar]
- 9.An SJ, Boyd R, Wang Y, Qiu XF, Wang HD. Endothelin-1 expression invascular adventitial fibroblasts. Am J Physiol Heart Circ Physiol. 2006;290:H700–708. doi: 10.1152/ajpheart.00326.2005. [DOI] [PubMed] [Google Scholar]
- 10.Zang BX, Jin M, Si N, Zhang Y, Wu W, Piao YZ. Antagonistic effect of hydroxysafflor yellow A on the platelet activating factor receptor. Yao Xue Xue Bao. 2002;37:696–699. [PubMed] [Google Scholar]
- 11.Zang BX, Jin M, Li JR. Effect of safflor yellow and hydroxysafflor yellow A on pindolol-β receptor specific binding capacity. Cardiovasc Pulm Dis. 2008;27:301–303. [Google Scholar]
- 12.Nie PH, Zhang L, Zhang WH, Rong WF, Zhi JM. The effects of hydroxysafflor yellow A on blood pressure and cardiac function. J Ethnopharmacol. 2012;139:746–750. doi: 10.1016/j.jep.2011.11.054. [DOI] [PubMed] [Google Scholar]
- 13.Zhang L, Nie PH, Zhang GH, Rong WF, Zhi JM. Endothelium-independent vasodilation effect of hydroxysafflor yellow A in thoracic aorta of Wistar rats. J Med Plants Res. 2011;5:2187–2191. [Google Scholar]
- 14.Ji DB, Zhang LY, Li CL, Ye J, Zhu HB. Effect of Hydroxysafflor yellow A on human umbilical vein endothelial cells under hypoxia. Vascul Pharmacol. 2009;50:137–145. doi: 10.1016/j.vph.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 15.Zhu DL, Herembert T, Marche P. Increased proliferation of adventitial fibroblasts form spontaneously hypertensive rat aorta. J Hypertens. 1991;9:1161–1168. [PubMed] [Google Scholar]
- 16.Fu Q, Tao Y, Piao H, Du MR, Li DJ. Trophoblasts and Decidual Stromal Cells Regulate Decidual NK Cell Functions Via Interaction between Collagen and LAIR-1. Am J Reprod Immunol. 2014;71:368–378. doi: 10.1111/aji.12211. [DOI] [PubMed] [Google Scholar]
- 17.Schulze-Bauer CA, Regitnig P, Holzapfel GA. Mechanics of the human femoral adventitia including the highpressure response. Am J Physiol Heart Circ Physiol. 2002;282:H2427–2440. doi: 10.1152/ajpheart.00397.2001. [DOI] [PubMed] [Google Scholar]
- 18.Kantachuvesiri S, Fleming S, Peters J, Peters B, Brooker G, Lammie AG, Mcgrath I, Kotelevtsey Y, Mullins JJ. Controlled hypertension, a transgenic toggle switch reveals differential mechanisms underlying vascular disease. J Biol Chem. 2001;276:36727–36733. doi: 10.1074/jbc.M103296200. [DOI] [PubMed] [Google Scholar]
- 19.McGrath JC, Deighan C, Briones AM, Shafaroudi MM, McBride M, Adler J, Arribas SM, Vila E, Daly CJ. New aspects of vascular remodelling: the involvement of all vascular cell types. Exp Physiol. 2005;90:469–475. doi: 10.1113/expphysiol.2005.030130. [DOI] [PubMed] [Google Scholar]
- 20.Sartore S, Chiavegato A, Faggin E, Franch R, Puato M, Ausoni S, Pauletto P. Contribution of adventitial fibroblasts to neointima formation and vascular remodeling: from innocent bystander to active participant. Circ Res. 2001;89:1111–1121. doi: 10.1161/hh2401.100844. [DOI] [PubMed] [Google Scholar]
- 21.Wang Z, Chen C, Finger SN, Kwajah S, Jung M, Schwarz HS, Wanson N, Lareu FF, Raghunath M. Suberoylanilide hydroxamic acid: A potential epigenetic therapeutic agent for lungfibrosis? Eur Respir J. 2009;34:145–155. doi: 10.1183/09031936.00084808. [DOI] [PubMed] [Google Scholar]
- 22.Ryan ST, Koteliansky VE, Gotwals PJ, Lindner V. Transforming growth factor-beta-dependent events in vascular remodeling following arterial injury. J Vasc Res. 2003;40:37–46. doi: 10.1159/000068937. [DOI] [PubMed] [Google Scholar]
- 23.Siow RC, Churchman AT. Adventitial growth factor signalling and vascular remodelling: potential of perivascular gene transfer from the outside-in. Cardiovasc Res. 2007;75:659–668. doi: 10.1016/j.cardiores.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 24.Raffetto JD, Khalil RA. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem Pharmacol. 2008;75:346–359. doi: 10.1016/j.bcp.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amalinei C, Caruntu ID, Balan RA. Biology of metalloproteinases. Rom J Morphol Embryol. 2007;48:323–334. [PubMed] [Google Scholar]
- 26.Zhang H, Wang ZW, Wu HB, Li Z, Li LC, Hu XP, Ren ZL, Li BJ, Hu ZP. Transforming growth factor-β1 induces matrix metalloproteinase-9 expression in rat vascular smooth muscle cells via ROS-dependent ERK-NF-κB pathways. Mol Cell Biochem. 2013;375:11–21. doi: 10.1007/s11010-012-1512-7. [DOI] [PubMed] [Google Scholar]
- 27.Brand K, Page S, Rogler G, Bartsch A. Brandl R, Knuechel R, Page M, Kaltschmidt C, Baeuerle PA and Neumeier D. Activated t ranscription factor nuclear factor-kappa B is present in the atherosclerotic lesion. J Clin Invest. 1996;97:1715–1722. doi: 10.1172/JCI118598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun CY, Pei CQ, Zang BX, Wang L, Jin M. The ability of Hydroxysafflor Yellow A to attenuate Lipopolysaccharide-induced pulmonary inflammation injury in mice. Phytother Res. 2010;24:1788–1795. doi: 10.1002/ptr.3166. [DOI] [PubMed] [Google Scholar]
- 29.Wang W, Huang XR, Canlas E, Oka K, Truong LD, Deng C, Bhowmick NA, Ju WJ, Bottinger EP, Lan HY. Essential role of Smad 3 in angiotensinII-induced vascular fibrosis. Circ Res. 2006;98:1032–1039. doi: 10.1161/01.RES.0000218782.52610.dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu YN, Zhou ZM, Chen P. Evidence that hydroxysafflor yellow A protects the heart against ischemia-reperfusion injury by inhibiting mitoehondrial permeability transition pore opening. Clin Exp Pharmacol Physiol. 2008;35:211–216. doi: 10.1111/j.1440-1681.2007.04814.x. [DOI] [PubMed] [Google Scholar]
- 31.Zhu HB, Zhang L, Wang ZH, Tian JW, Fu FH, Liu K, Li CL. Therapeutic effects of hydroxysafflor yellow A on focal cerebral ischemic injury in rats and its primary mechanisms. J Asian Nat Prod Res. 2005;7:607–613. doi: 10.1080/10286020310001625120. [DOI] [PubMed] [Google Scholar]


