Abstract
Background and aims: According to recent findings, some tumor cells function as endothelial progenitor cells to initiate tumor vasculogenesis, known as “vasculogenic mimicry” (VM). Notch1, the key regulator of vasculogenesis and embryonic differentiation, has shown a correlation with a poor prognosis in hepatocellular carcinoma (HCC). We attempted to elucidate the relationship between Notch1 and the vascularization of HCC. Materials and methods: HCC cell lines were assayed for tube formation and low-density lipoprotein (LDL) absorption. The translation level of targets of interest was verified using western blot. Notch1 was silenced in HepG2, BEL-7402 and HCCLM6 using lentivirus shRNA. A hypoxic culture was conducted in an anaerobic culture chamber to induce VM in HepG2. Samples from 53 patients with HCC, i.e., 5 with metastasis and 48 without were tested for Notch1+ cells and CD34 negative plus Periodic Acid-Schiff (PAS) positive structures, respectively. Results: BEL-7402 and HCCLM6 were capable of tube formation and LDL absorption in vitro, while HepG2 was negative for both. Notch1 down-regulation suppressed endothelial marker expression and greatly impaired tube formation. After hypoxic culture, the tube formation capacity of HepG2 was significantly enhanced, along with an increase in Notch1 expression. Notch1 was strongly and profusely expressed in all 5 cases of distant metastasis, while 19 of the 48 cases without metastasis were sparsely positive (P < 0.05). Notch1 positivity was mainly seen in the cytoplasm and nuclei. VM structures were only found in 2 cases from the metastasis group (P < 0.05). Conclusions: HCC is capable of VM. Notch1 might serve as a potential target for VM development in HCC.
Keywords: Notch1, hepatocellular carcinoma, vasculogenesis
Introduction
Hepatocellular carcinoma (HCC) is the second leading cause of cancer-related death with an incidence of 750,000 new cases worldwide each year [1]. Although treatment options are diverse and the selections of individual patients for different treatments have been greatly improved during the last few decades, the overall survival still remains unsatisfying. One of the notorious reasons for such upsetting prognosis is the early metastasis of the tumor, which is not uncommon at the time of diagnosis. HCC is often aggressive in behavior and tends to compromise blood vessels in the vicinity, sending tumor cells to the blood stream and giving birth to distal metastasis and frequent relapse. As investigations continue, the idea of heterogeneity among cancer cells is being widely accepted, and some cell types are believed to be responsible for tumor sustenance, relapse, and metastasis. Various researchers found that several types of aggressive cancers (including HCC) could form peculiar network structures that contained red blood cells, simulating vasculogenesis [2-4]. This phenomenon was called vasculogenic mimicry (VM) and might actively participate in the growth of cancers particularly under hypoxia [5,6]. Since such vessels were in essence constituted by cancer cells, the underlying mechanism of the network formation was inevitably different from the vessels formed by normal endothelial cells, thereby providing explanations for the accumulating reports of the failure of VEGFR targeted drugs [7-9].
Notch signaling pathway is stringently conserved along the road of evolution, from invertebrates to mammals. Once vigorously activated during embryonic development, the Notch signaling pathway remains versatile in fully developed human body. It is composed of 4 transmembrane receptors (Notch-1, -2, -3 and -4) and 5 canonical ligands (Delta like1, 3, 4 and Jagged1, 2). The canonical pathway is activated when the ligands are bound to the extracellular domain of the receptors, followed by proteolytic cleavage and translocation of the intracellular domain into the nucleus, arousing multiple functions such as vasculature formation, stem cells maintenance and differentiation and proliferation of epidermis and endothelium, etc. The Notch signaling pathway is particularly important to vasculature formation in that it promotes vessel sprouting in adulthood as well as angioblast differentiation in embryogenesis [10,11]. Furthermore, it has also been reported that the Notch signaling pathway is involved in the derivation of hepatic stem cells from bone marrow [12]. Recent findings demonstrated Notch1 up-regulation was correlated with carcinogenesis and poor prognosis of HCC [13-16]. However, the underlying mechanisms have so far been dimly understood.
In the present study, we tried to elucidate the relationship between Notch1 and VM in HCC.
Methods and materials
Cell culture
Human hepatocellular carcinoma cell lines HepG2 and BEL-7402 were obtained from the Guangdong Provincial Key Laboratory of Malignant Tumor Epigenetics and Gene Regulation, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University (Guangzhou, China). HCCLM6 was obtained from Zhongshan Hospital, Fudan University (Shanghai, China). BEL-7402 cell line was cultured in RPMI-1640 (Life Technologies, California, USA), while HCCLM6 and HepG2 were cultured in high glucose DMEM (Life Technologies, California, USA). Both culture media for the cell lines were supplemented with 10% fetal bovine serum (Biological Industries, Kibbutz Beit Haemek, Israel) as recommended by the suppliers. Cells were remained in incubator at 37°C with 5% CO2 in humidified atmosphere. To exert oxidative stress, cells of interest were transferred to anaerobic cultivation with 5% CO2 and 1% O2.
Gene silencing
Human Notch1 expression was stably knockdown using recombinant lentivirus shRNA targeting sequence 5’-GGGCUAACAAAGAUAUGCA-3’ (GenePharma, Shanghai, China). After infection, the cells were selected using 1 μg/ml puromycin during cell culture. The effectiveness of gene silencing was verified by Western blot.
Western blot analysis
The cells (2×106/well) were washed twice with ice-cold PBS (phosphate-buffered saline) and lysed on ice in lysis buffer [50 mM Tris (pH 7.4), 150 mM NaCl, 1% NP-40, 0.1% SDS], 1% protease inhibitor phenylmethane sulfonyl fluoride (PMSF; Beyotime Institute of Biotechnology, Shanghai, China). Protein concentration was determined using BCA protein quantification kit (Bocai, Shanghai, China). Whole cell extracts (30 μg) were fractionated by 8% sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride membrane (Millipore, Bedford, Massachusetts, USA). Proteins of interest were revealed with specific antibodies as indicated in 4°C overnight: rabbit anti human Notch1 polyclonal antibody in a final dilution of 1:1000 (Abcam, Cambridge, UK), rabbit anti human VE-Cadherin polyclonal antibody in a final dilution of 1:1000 (Abcam, Cambridge, UK), rabbit anti human vWF polyclonal antibody in dilution of 1:500 (Abcam, Cambridge, UK) and mouse anti-α-tubulin monoclonal antibody in a final dilution of 1:1000 (Cell Signaling Technology, Danvers, MA, USA). Secondary antibody HRP linked anti-rabbit IgG in a final dilution of 1:2000 (Cell Signaling Technology, Danvers, Massachusetts, USA) was subsequently put into use. Specific signals were visualized using ECL chemiluminescent detection kit (Immubilon Western, Millipore, Massachusetts, USA).
Tube formation and low density lipoprotein (LDL) absorption assay
The tumor cells (5×105/well) were seeded onto the six-well plates after matrigel (BD Biosciences, Franklin Lakes, New Jersey, USA) pavement according to the manufacturer’s protocol. 6 hours after seeding, Dil-AC-LDL was added into serum free basic culture media (Dulbecco’s Modified Eagle Medium, Life Technologies, California, USA), incubated for 4 hours. Results were taken every 24 h. For further investigation, cells on matrigel were retrieved using Cell Recovery Solution (BD Biosciences, Franklin Lakes, New Jersey, USA).
Patient samples
We collected 5 paraffin embedded section samples from HCC patients with distant metastasis and 48 cases without metastasis in the Department of Hepatopancreatobiliary Surgery at Sun Yat-sen Memorial Hospital from 2005 to 2009 (Table 1). None of the patients enrolled in this study had received preoperative treatment. For those cases with metastasis, the samples were collected from the orthotopic tumors. All the tissue samples were collected with written informed consent according to the Internal Review and the Ethics Boards of the Sun Yat-sen Memorial Hospital of Sun Yat-sen University. The whole procedure was approved by the ethical committee of the hospital and in accordance with the Declaration of Helsinki.
Table 1.
Clinicopathological characteristics of HCC patients and the expressions of Notch1 and VM structures
| n | gender | TNM staging | differentiation | Notch1 | VM | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||
| F | M | I | II | III | IV | Well | Moderate | Poor | + | - | + | - | ||
| Metastasis | 5 | 1 | 4 | - | - | - | 5 | 0 | 2 | 3 | 5 | 0 | 2 | 3 |
| Non-metastasis | 48 | 8 | 40 | 25 | 4 | 19 | 0 | 10 | 15 | 23 | 19 | 29 | 0 | 48 |
Immunohistochemistry (IHC) and PAS staining
The procedure was carried out as previously described [17]. Monoclonal rabbit anti-human Notch1 in a dilution of 1:200 (Abcam, Cambridge, UK) and monoclonal sheep anti-human CD34 in a dilution of 1.7 μg/ml (R&D systems, Minneapolis, USA) were used as primary antibodies. IgG-HRP anti-rabbit secondary antibody (DAKO, Hamburg, Germany) and IgG-HRP anti-sheep secondary antibody both at a dilution of 1:300 (EarthOx, California, USA) were applied. The slides were detected by DAB and counterstained by hematoxylin (DAKO, Hamburg, Germany). To detect VM pattern structures, periodic acid-Schiff (PAS) staining was applied before hematoxylin counterstaining. All the slides were studied using light microscope by three independent investigators.
Statistical analysis
All data were analyzed using SPSS 13.0. Binominal data were performed using chi-square test and continuity correction was put into use when necessary. Statistical significance was considered when P < 0.05.
Results
HCC cell lines displayed distinct VM capacity in vitro
BEL-7402 and HCCLM6 could form vessel-like structures on matrigel and the cells could also absorb Dil-AC-LDL in the meantime, with human umbilical vein endothelial cells (HUVECs) as positive control while HepG2 could only form clusters on matrigel (Figure 1A, 1C, 1E, 1G represented the tube formation assays performed by BEL-7402, HCCLM6, HepG2 and HUVEC, respectively). Figure 1B, 1D, 1F, 1H showed the Dil-AC-LDL absorption assay in the same field of the 4 cell lines mentioned above, respectively). Even the tube-like structures formed by the 2 cell lines looked slightly different from each other. The tube-like structures of HCCLM6 were more intact and the cell-cell contact was tighter than those of BEL-7402. Compared to monolayer culture, the expression of Notch1 and vWF, VE-cadherin of cells recovered from matrigel was increased (Figure 1I).
Figure 1.
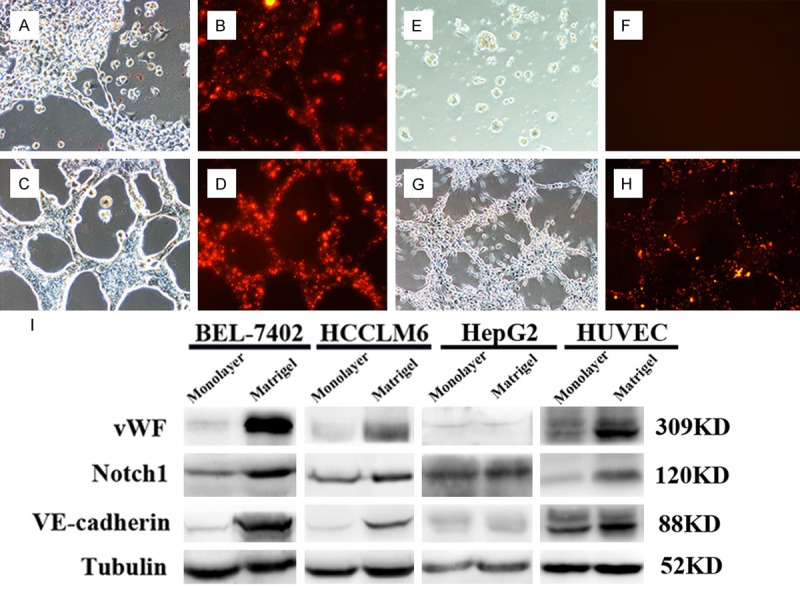
HCC cell lines displayed in vitro endothelial functions. HCC cell lines BEL-7402 (A), HCCLM6 (C) and HepG2 (E) were used to perform the tube formation assay. Tube-like structures were seen in BEL-7402 and HCCLM6 cell lines and they were capable of Dil-AC-LDL absorption (B: BEL-7402, D: HCCLM6), with HUVEC as positive control (G, H). HepG2 merely clustered on matrigel (E) and failed to absorb LDL (F). Western blot showed that the expression of Notch1 and endothelial markers VE-cadherin and vWF were increased when cultured on matrigel, compared to monolayer culture (I).
Notch1 knockdown leads to impairment of VM development
To determine whether Notch1 expression would affect VM development, we performed tube formation assays after inhibition of Notch1 using lentivirus shRNA. After Notch1 depletion, the tube formation capacity of BEL-7402, HCCLM6 and HUVEC was also significantly reduced and VE-cadherin expression decreased correspondingly (Figure 2A the expression of Notch1 and VE-Cadherin were reduced after Notch1 depletion; Figure 2B-G the tube formation capacity of BEL-7402, HCCLM6 and HUVEC was decreased after Notch1 depletion).
Figure 2.
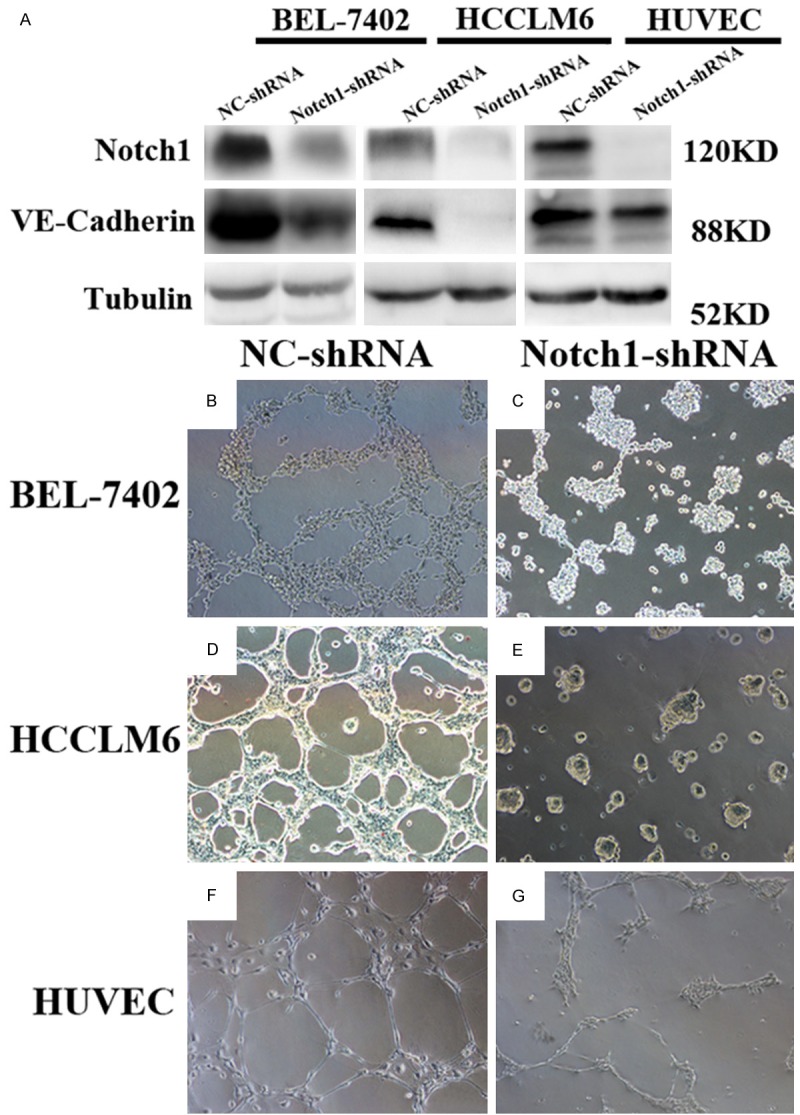
Notch1 knockdown impaired in vitro VM development. After Notch1 was knockdown, endothelial marker VE-cadherin was decreased (A), along with the impaired tube formation ability (B, C. BEL-7402-NC-shRNA, BEL-7402-Notch1-shRNA; D, E. HCCLM6-NC-shRNA, HCCLM6-Notch1-shRNA; F, G. HUVEC-NC-shRNA, HUVEC-Notch1-shRNA).
Endothelial attributes could be induced under hypoxic culture in vitro
To further illustrate the mechanism of VM, we transferred HepG2 into hypoxic culture in endothelial condition culture medium (EGM-2). As a result, after incubating in EGM-2 culture medium and under hypoxic condition for 72 h, HepG2 not only took on a transformation on cellular morphous and increased VE-cadherin expression, but also acquired greater tube formation capacity (Figure 3A the original outlook of HepG2 on monolayer culture; B after 72 h of hypoxic culture, the outlook of HepG2 changed; C the outlook of HUVEC on monolayer culture; D after treated in hypoxia for 72 h, HepG2 acquired enhanced tube formation capacity; E the increase expression of Notch1 and VE-Cadherin of HepG2 after hypoxic culture).
Figure 3.
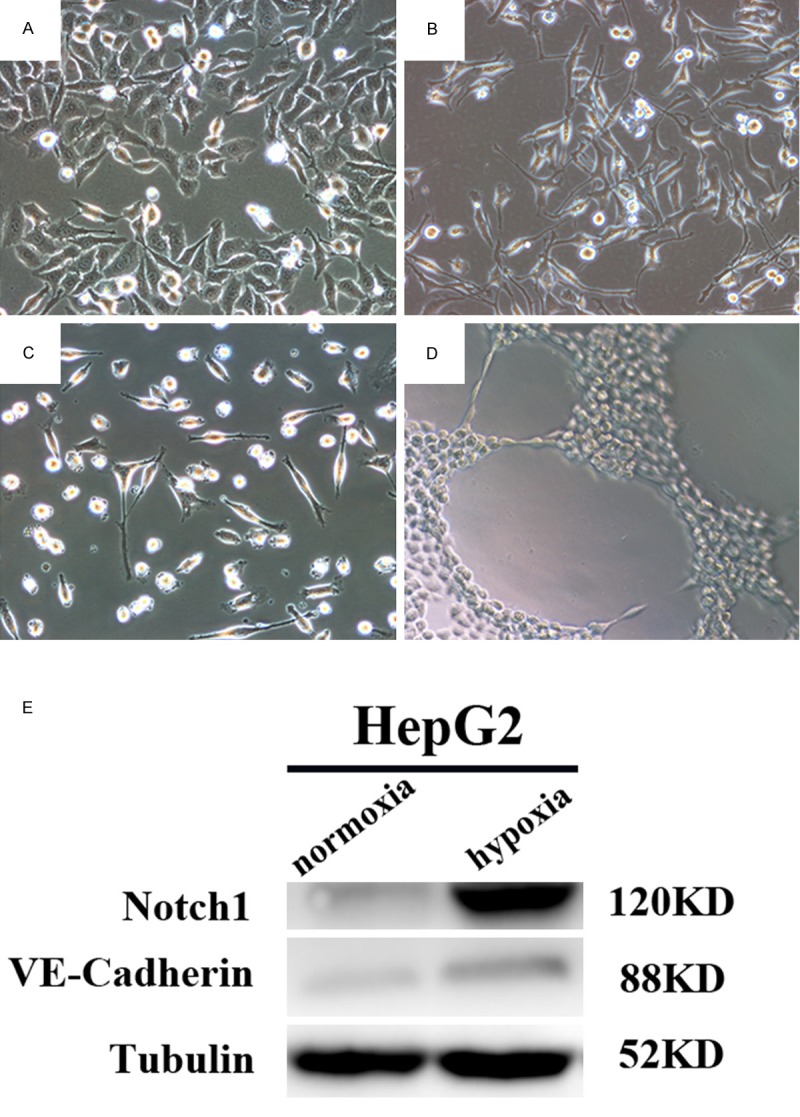
Hypoxia changed the outlook of HepG2 and promoted endothelial functions, which was associated with Notch1 expression. The outlook of HepG2 changed from polygonal (A) to spindle-like morphous (B) after oxidative stress was exerted, much to HUVEC cells resemblance (C). The capacity of tube formation was also enhanced (D). The expression of Notch1 and VE-Cadherin was raised after hypoxic culture (E).
Notch1 positivity was seen in HCC while VM structures were exclusively present in cases with distant metastasis
The primary tumors from HCC cases with distant metastasis were all profusely and strongly positive for Notch1. Its expression was seen in the cytoplasm and some of the nucleus of the cancer cells. These cells invariably distributed in clusters near the tumor mesenchym (Figure 4A). In contrast, Notch1 was much lower in positivity in cases without metastasis and these cases were lack of the VM structures (19/48, Continuity Correction χ2=4.455, P=0.035) (Table 1, Figure 4B). VM structures exclusively appeared in patients with metastasis (2/5) (Figure 4C, 4D). In them, red blood cells could be seen, but no mosaic vessels were found.
Figure 4.
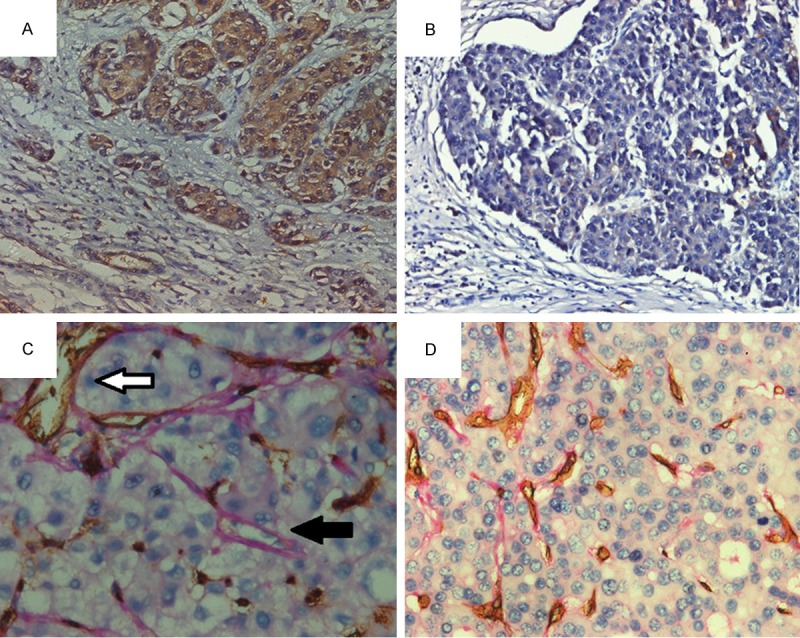
Notch1 and VM expression in HCC patients with metastasis and those without. Notch1 was more commonly and strongly expressed in the primary tumors from HCC with distant metastasis (A) than those without (B), while VM appeared exclusively in cases from the metastasis group (C). Cases without metastasis were negative for VM structures (D). White arrow in C showed CD34+ endothelial cells. Black arrow showed CD34-/PAS+ VM structures, inside which red blood cells could be seen.
Discussion
Vascularization has been universally agreed to be vital for the growth of tumor. It is traditionally believed that new blood vessels developed either by the extension of the already existing blood vessels or from the angioblasts recruited from bone marrow, known as angiogenesis and vasculogenesis respectively. Unrelenting efforts were invested in the pursuit for the ideal anti-angiogenesis therapy and sorafenib was the only drug approved by FDA thus far to treat advanced stage HCC. Although in the first few years there were several reports showing sorafenib significantly improved patients’ survival quality, increasing studies revealed that drug resistance could develop in prolonged administration settings [9,18-23]. Recently, investigators found that the formation of new blood vessels was not necessarily dependent upon angiogenesis or vasculogenesis. Under the selective stress of hypoxia or anti-angiogenic treatment such as sorafenib, some biologically aggressive, poorly differentiated tumors, including HCC, were capable of developing fluid conducting networks, simulating vasculogenesis [2,4,24,25]. Thus came the term vasculogenic mimicry (VM). In order to find out whether HCC cells could develop VM in vitro, we used HCC cell lines BEL-7402, HCCLM6 and HepG2 to perform tube formation and LDL absorption assays. Both BEL-7402 and HCCLM6 started to form tube-like structures in 6 h after seeding, and lasted for 72 h when the loops began to fall apart. Both cell lines were able to absorb LDL after seeding on matrigel. Western blot showed endothelial marker VE-cadherin and vWF was significantly increased when cultured on matrigel than monolayer culture. Although several studies showed that only a small subset of tumor cells in tumor tissues exhibited endothelial features, cell sorting was not necessary before tube formation assay as in the case of the cell lines BEL-7402 and HCCLM6 [26]. First of all, we assumed that since the variety of cell subpopulations from parental tumor tissues were much more diverse than cell lines, subpopulation selection would be inevitable [27]. Furthermore, vasculogenic mimicry is a trait of plasticity, often closely correlated with cell differentiation status. Sure enough, the well differentiated cell line HepG2 failed to form any tube-like structures in vitro. Additionally, immunohistochemical analysis of tumor tissues from HCC patients was performed to further investigate VM in vivo. We divided 53 paraffin embedded samples into 2 groups based on the condition of distant metastasis and performed the CD34/PAS dual staining. In the present study, there were 5 HCC cases with distant metastasis and 48 without distant metastasis. We found that 2 of the 5 cases with distant metastasis were positive for VM structures while those without metastasis failed to show any (P < 0.05). Since cancer patients with distant metastasis do not normally undergo surgical resections unless palliative resection could relieve the symptoms, the cohort of distant metastasis in our study was relatively small. Thus the possible correlation between VM and metastasis still needs further investigations.
The Notch signaling pathway is involved in multiple functions including proliferation regulation, phenotypic homeostasis and cell fate decision during development in various organs [28]. Particularly for the liver, Notch2 played a part in intrahepatic bile duct development [29,30]. However, recent studies showed that Notch1 was correlated with poor survival in HCC as well [13,16]. Apart from the functions mentioned above, the Notch signaling pathway is also critical for endothelial progenitor cells to develop new blood vessels [31,32]. So far as VM is concerned, tumor cells with such capacity bear close resemblance to endothelial progenitor cells phenotypically and functionally. In the present study, we discovered that Notch1 expression was relatively high in BEL-7402 and HCCLM6 but low in HepG2, in line with their tube formation capacity. Additionally, Notch1 suppression in BEL-7402 and HCCLM6 cell lines could lead to down-regulation of VE-cadherin and thereby inhibiting the tube formation ability in vitro. Of note, the proliferation of HUVEC almost came to a halt after Notch1 was knockdown (data not shown), suggesting Notch1 might be vital for the growth of endothelial cells or cells bearing endothelial characteristics. Moreover, patients with distant metastasis expressed Notch1 far more than those without and that VM structures appeared exclusively in the former group (P < 0.05). Interestingly, however, none of the Notch1+ cases from the non-metastasis group manifested any trace of VM, indicating that VM development might be associated with later stage of tumor progression when oxidative stress became more prominent. Based on this assumption, hypoxic culture was performed on HepG2. After hypoxic induction, HepG2 demonstrated greater in vitro tube formation capacity, with Notch1 expression being elevated. Thus, we postulate that the Notch1 associated VM developing capacity might be largely dependent upon the differentiation status of tumor cell itself, the stage of disease progression and the crosstalk between tumor cells and the surrounding niche. Sure enough, the Notch1+ tumor cells were mainly seen in the vicinity of the tumor mesenchyma, indicating that these highly invasive cells interacted actively with tumor mesenchyma. In the light of the findings above, we hypothesized that HCC cells could develop VM, a process correlated with Notch1. What’s more, we previously found that Notch1 might partake in the process of transdifferentiation from poorly differentiated HCC into tumor endothelium [33]. Although it remains largely unknown why in some scenarios tumor cells had to develop into mature endothelium while vasculogenic mimicry could already serve the same purpose, it has been demonstrated that Notch1 might contribute to these processes.
In conclusion, HCC with distant metastasis strongly expressed Notch1 and some could develop VM structures. In vitro evidence further confirmed that Notch1 was correlated with VM development and might serve as a potential target for HCC treatment.
Acknowledgements
This research program was funded by the Special Research Foundation of the National Nature Science Foundation of China (81172068).
Disclosure of conflict of interest
None.
References
- 1.Maluccio M, Covey A. Recent progress in understanding, diagnosing, and treating hepatocellular carcinoma. CA Cancer J Clin. 2012;62:394–399. doi: 10.3322/caac.21161. [DOI] [PubMed] [Google Scholar]
- 2.Guzman G, Cotler SJ, Lin AY, Maniotis AJ, Folberg R. A pilot study of vasculogenic mimicry immunohistochemical expression in hepatocellular carcinoma. Arch Pathol Lab Med. 2007;131:1776–1781. doi: 10.5858/2007-131-1776-apsovm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hendrix MJ, Seftor EA, Hess AR, Seftor RE. Vasculogenic mimicry and tumour-cell plasticity: lessons from melanoma. Nat Rev Cancer. 2003;3:411–421. doi: 10.1038/nrc1092. [DOI] [PubMed] [Google Scholar]
- 4.Chiao MT, Yang YC, Cheng WY, Shen CC, Ko JL. CD133+ glioblastoma stem-like cells induce vascular mimicry in vivo. Curr Neurovasc Res. 2011;8:210–219. doi: 10.2174/156720211796558023. [DOI] [PubMed] [Google Scholar]
- 5.Kirschmann DA, Seftor EA, Hardy KM, Seftor RE, Hendrix MJ. Molecular pathways: vasculogenic mimicry in tumor cells: diagnostic and therapeutic implications. Clin Cancer Res. 2012;18:2726–2732. doi: 10.1158/1078-0432.CCR-11-3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang S, Li M, Zhang D, Xu S, Wang X, Liu Z, Zhao X, Sun B. Hypoxia influences linearly patterned programmed cell necrosis and tumor blood supply patterns formation in melanoma. Lab Invest. 2009;89:575–586. doi: 10.1038/labinvest.2009.20. [DOI] [PubMed] [Google Scholar]
- 7.Chen KF, Chen HL, Tai WT, Feng WC, Hsu CH, Chen PJ, Cheng AL. Activation of phosphatidylinositol 3-kinase/Akt signaling pathway mediates acquired resistance to sorafenib in hepatocellular carcinoma cells. J Pharmacol Exp Ther. 2011;337:155–161. doi: 10.1124/jpet.110.175786. [DOI] [PubMed] [Google Scholar]
- 8.van Malenstein H, Dekervel J, Verslype C, Van Cutsem E, Windmolders P, Nevens F, van Pelt J. Long-term exposure to sorafenib of liver cancer cells induces resistance with epithelial-to-mesenchymal transition, increased invasion and risk of rebound growth. Cancer Lett. 2013;329:74–83. doi: 10.1016/j.canlet.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 9.Villanueva A, Llovet JM. Second-line therapies in hepatocellular carcinoma: emergence of resistance to sorafenib. Clin Cancer Res. 2012;18:1824–1826. doi: 10.1158/1078-0432.CCR-12-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tung JJ, Tattersall IW, Kitajewski J. Tips, stalks, tubes: notch-mediated cell fate determination and mechanisms of tubulogenesis during angiogenesis. Cold Spring Harb Perspect Med. 2012;2:a006601. doi: 10.1101/cshperspect.a006601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herbert SP, Huisken J, Kim TN, Feldman ME, Houseman BT, Wang RA, Shokat KM, Stainier DY. Arterial-venous segregation by selective cell sprouting: an alternative mode of blood vessel formation. Science. 2009;326:294–298. doi: 10.1126/science.1178577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Avital I, Inderbitzin D, Aoki T, Tyan DB, Cohen AH, Ferraresso C, Rozga J, Arnaout WS, Demetriou AA. Isolation, characterization, and transplantation of bone marrow-derived hepatocyte stem cells. Biochem Biophys Res Commun. 2001;288:156–164. doi: 10.1006/bbrc.2001.5712. [DOI] [PubMed] [Google Scholar]
- 13.Ahn S, Hyeon J, Park CK. Notch1 and Notch4 are markers for poor prognosis of hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2013;12:286–294. doi: 10.1016/s1499-3872(13)60046-6. [DOI] [PubMed] [Google Scholar]
- 14.Villanueva A, Alsinet C, Yanger K, Hoshida Y, Zong Y, Toffanin S, Rodriguez-Carunchio L, Solé M, Thung S, Stanger BZ, Llovet JM. Notch signaling is activated in human hepatocellular carcinoma and induces tumor formation in mice. Gastroenterology. 2012;143:1660–1669. doi: 10.1053/j.gastro.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou L, Wang DS, Li QJ, Sun W, Zhang Y, Dou KF. The down-regulation of Notch1 inhibits the invasion and migration of hepatocellular carcinoma cells by inactivating the cyclooxygenase-2/Snail/E-cadherin pathway in vitro. Dig Dis Sci. 2013;58:1016–1025. doi: 10.1007/s10620-012-2434-7. [DOI] [PubMed] [Google Scholar]
- 16.Zhou L, Zhang N, Song W, You N, Li Q, Sun W, Zhang Y, Wang D, Dou K. The significance of Notch1 compared with Notch3 in high metastasis and poor overall survival in hepatocellular carcinoma. PLoS One. 2013;8:e57382. doi: 10.1371/journal.pone.0057382. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Yu XH, Xu LB, Zeng H, Zhang R, Wang J, Liu C. Clinicopathological analysis of 14 patients with combined hepatocellular carcinoma and cholangiocarcinoma. Hepatobiliary Pancreat Dis Int. 2011;10:620–625. doi: 10.1016/s1499-3872(11)60105-7. [DOI] [PubMed] [Google Scholar]
- 18.Chow AK, Ng L, Lam CS, Wong SK, Wan TM, Cheng NS, Yau TC, Poon RT, Pang RW. The Enhanced Metastatic Potential of Hepatocellular Carcinoma (HCC) Cells with Sorafenib Resistance. PLoS One. 2013;8:e78675. doi: 10.1371/journal.pone.0078675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang Y, Zheng T, Song R, Wang J, Yin D, Wang L, Liu H, Tian L, Fang X, Meng X, Jiang H, Liu J, Liu L. Hypoxia-mediated sorafenib resistance can be overcome by EF24 through Von Hippel-Lindau tumor suppressor-dependent HIF-1alpha inhibition in hepatocellular carcinoma. Hepatology. 2013;57:1847–1857. doi: 10.1002/hep.26224. [DOI] [PubMed] [Google Scholar]
- 20.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 21.Xia H, Ooi LL, Hui KM. MicroRNA-216a/217-induced epithelial-mesenchymal transition targets PTEN and SMAD7 to promote drug resistance and recurrence of liver cancer. Hepatology. 2013;58:629–641. doi: 10.1002/hep.26369. [DOI] [PubMed] [Google Scholar]
- 22.Xin HW, Ambe CM, Hari DM, Wiegand GW, Miller TC, Chen JQ, Anderson AJ, Ray S, Mullinax JE, Koizumi T, Langan RC, Burka D, Herrmann MA, Goldsmith PK, Stojadinovic A, Rudloff U, Thorgeirsson SS, Avital I. Label-retaining liver cancer cells are relatively resistant to sorafenib. Gut. 2013;62:1777–86. doi: 10.1136/gutjnl-2012-303261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bruix J, Raoul JL, Sherman M, Mazzaferro V, Bolondi L, Craxi A, Galle PR, Santoro A, Beaugrand M, Sangiovanni A, Porta C, Gerken G, Marrero JA, Nadel A, Shan M, Moscovici M, Voliotis D, Llovet JM. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial. J Hepatol. 2012;57:821–829. doi: 10.1016/j.jhep.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 24.Lai CY, Schwartz BE, Hsu MY. CD133+ melanoma subpopulations contribute to perivascular niche morphogenesis and tumorigenicity through vasculogenic mimicry. Cancer Res. 2012;72:5111–5118. doi: 10.1158/0008-5472.CAN-12-0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu TJ, Sun BC, Zhao XL, Zhao XM, Sun T, Gu Q, Yao Z, Dong XY, Zhao N, Liu N. CD133+ cells with cancer stem cell characteristics associates with vasculogenic mimicry in triple-negative breast cancer. Oncogene. 2013;32:544–553. doi: 10.1038/onc.2012.85. [DOI] [PubMed] [Google Scholar]
- 26.Choi SA, Wang KC, Phi JH, Lee JY, Park CK, Park SH, Kim SK. A distinct subpopulation within CD133 positive brain tumor cells shares characteristics with endothelial progenitor cells. Cancer Lett. 2012;324:221–230. doi: 10.1016/j.canlet.2012.05.026. [DOI] [PubMed] [Google Scholar]
- 27.Gudjonsson T, Villadsen R, Ronnov-Jessen L, Petersen OW. Immortalization protocols used in cell culture models of human breast morphogenesis. Cell Mol Life Sci. 2004;61:2523–2534. doi: 10.1007/s00018-004-4167-z. [DOI] [PubMed] [Google Scholar]
- 28.Andersson ER, Sandberg R, Lendahl U. Notch signaling: simplicity in design, versatility in function. Development. 2011;138:3593–3612. doi: 10.1242/dev.063610. [DOI] [PubMed] [Google Scholar]
- 29.Geisler F, Nagl F, Mazur PK, Lee M, Zimber-Strobl U, Strobl LJ, Radtke F, Schmid RM, Siveke JT. Liver-specific inactivation of Notch2, but not Notch1, compromises intrahepatic bile duct development in mice. Hepatology. 2008;48:607–616. doi: 10.1002/hep.22381. [DOI] [PubMed] [Google Scholar]
- 30.Zong Y, Panikkar A, Xu J, Antoniou A, Raynaud P, Lemaigre F, Stanger BZ. Notch signaling controls liver development by regulating biliary differentiation. Development. 2009;136:1727–1739. doi: 10.1242/dev.029140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kwon SM, Eguchi M, Wada M, Iwami Y, Hozumi K, Iwaguro H, Masuda H, Kawamoto A, Asahara T. Specific Jagged-1 signal from bone marrow microenvironment is required for endothelial progenitor cell development for neovascularization. Circulation. 2008;118:157–165. doi: 10.1161/CIRCULATIONAHA.107.754978. [DOI] [PubMed] [Google Scholar]
- 32.Rodilla V, Villanueva A, Obrador-Hevia A, Robert-Moreno A, Fernández-Majada V, Grilli A, López-Bigas N, Bellora N, Albà MM, Torres F, Duñach M, Sanjuan X, Gonzalez S, Gridley T, Capella G, Bigas A, Espinosa L. Jagged1 is the pathological link between Wnt and Notch pathways in colorectal cancer. Proc Natl Acad Sci U S A. 2009;106:6315–6320. doi: 10.1073/pnas.0813221106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu M, et al. Bmi-1 Up-regulated Rat Oval Cells Generated Poorly Differentiated Hepatocellular Carcinoma and Tumor Endothelial Cells in Vivo. Hepatology. 2013;58:1087A. [Google Scholar]


