Abstract
Postoperative cognitive dysfunction (POCD) is a decline in cognitive performance after a surgery with anaesthesia. The exact reasons of surgery and/or anaesthesia resulting in POCD are unclear. The aim of this study is to investigate the effects of different concentration and duration time of isoflurane anaesthesia on cognitive performance and cellular mechanisms involved in learning and memory function. In present work, young adult male C57BL/6 mice (age: 8 weeks) were anaesthetized by different concentration isoflurane in 100% oxygen for different duration time (Mice in group I1 received 0.7% isoflurane 0.5 h, mice in I2 received 0.7% isoflurane 2 h, mice in I3 received 1.4% isoflurane 2 h, and mice in I4 received 1.4% isoflurane 4 h). Non-anaesthetized mice served as control group (I0). Spatial learning was assessed at 10 days post-anesthesia in Morris water maze (MWM). Hippocampal protein expressions of activated caspase 3, NMDA receptor subunit NR2B, and extracellular-signal regulated kinase (ERK) 1/2 were evaluated 24 hours and 2 weeks post anesthesia. Protein expression of activated caspase3 was detected acute elevated in I3 (24 h post-anesthesia) and acute and long-term elevated in I4 (24 hours and 2 weeks post-anesthesia). There was no significant difference between I1, I2 and control group. Protein expressions of NR2B showed an acute and long-term increasement in I1 and I2, decreasement in I4, and an acute decline, then returned to normal in I3 compared to control group. The ratio of phosopho-ERK1/2 to total-ERK showed an acute increasement in I1 and I2, then came to normal 2 weeks post anesthesia compared to control group, meanwhile, we detected an acute and long-term decline in I3 and I4. In MWM test, mice in I1 and I2 showed cognitive improvement, mice in I3 showed similar to control group, while mice in I4 demonstrated cognitive impairment, which were approximately corresponding to the changes of protein expression of NR2B and activation of ERK1/2. The present data suggested the following: (1) Isoflurane may cause neurotoxicity by inducing caspase activation and apoptosis with the anesthetic concentration increased and duration prolonged. (2) Low concentration of isoflurane in 2 hours can induce a hippocampus-specific elevation of NR2B subunit composition and ratio of p-ERK1/2 to total ERK1/2, produce hippocampal-dependent cognitive improvement. While high concentration of isoflurane exceeding 4 hours may induce a decline of NR2B and ratio of pERK1/2 to ERK1/2, then result in cognitive impairment.
Keywords: Isoflurane, postoperative cognitive dysfunction (POCD), apoptosis, NR2B receptor, extracellular signal-regulated kinase (ERK)
Introduction
Postoperative cognitive dysfunction (POCD) is a deterioration of cognitive performance after anesthesia (and/or surgery) presenting as impaired memory or concentration. Accu-mulating evidence showed that patients experiencing POCD had an increasing risk of death in the first year after surgery [1,2]. Although elderly patients are at the greatest risk of developing POCD [3], POCD is also present in young patients receiving non-cardiac surgery. Monk et al. [1] demonstrated that 36.6% of young patients (18-39 years old) and 30.4% of middle-aged patients (40-59 years old) showed POCD at hospital discharge, with symptoms continuing 5.7% and 5.6%, respectively, at 3 months after surgery. Steinmetz et al. [4] suggested that there were close relationship between POCD and inability to previous job. Anesthesia and surgery were reported as important risk factors of POCD [5-8]. It is well known that general anaesthesia interferes with memory function. But contradictory results have been reported. Some investigators reported anesthesia-induced cognitive impairments in rats [9,11], whereas others described cognitive improvements following anesthetic exposure in rodents [9,12,13]. The exact mechnism is still unclear. Thus this study was designed to elucidate the role of inhalation anesthetics in cognitive function. Inhalation anesthetics, such as isoflurane, modulate NMDA-type glutamate receptors to produce analgesic and anesthetic actions in the central nervous system (CNS) [14-19]. Due to the critical role of NMDA receptors in learning and memory processes [20], these receptors may play a role in anesthesia-induced cognitive deficits in brain. We anaesthetized mice with isoflurane and investigated cognitive performance, and expression levels of activated caspase3, NMDA receptor subunit NR2B and downstream signaling pathways ERK1/2 in hippocampal brains 24 hours and 2 weeks post anaesthesia.
Materials and methods
Animals
All mice were housed separately under standard laboratory conditions (12:12 light/dark cycle, 22°C, 60% humidity) and had free access to tap water and standard mouse chow. Prior to the investigations the mice were allowed to habituate to their new surroundings for at least three weeks after having been transferred from the breeder. The animal protocol was approved by the Standing Committee on Animals at Shanghai Tongji Hospital.
Anesthesia
Young male adult C57BL/6 mice received anesthetic isoflurane plus 100% oxygen during anesthesia. Sixty mice were randomly assigned to five groups (N = 12): (1) anesthesia groups were composed of four groups including I1, I2, I3, and I4. Mice in I1 group were exposed to 0.7% isoflurane (0.5 minimum alveolar concentration, MAC) 0.5 h, I2 exposed to 0.7% isoflurane 2 h, I3 exposed to 1.4% isoflurane (1MAC) 2 h, I4 exposed to 1.4% isflurane 4 h (2) control group (I0) received no isoflurane. The anesthetic and oxygen concentrations were measured continuously (GE Datex-Ohmeda, Tewksbury, MA). The temperature of the anesthetizing chamber was controlled to maintain at 37 ± 0.5°C with a heating pad under the chamber. After recovery all mice were returned to their home cages.There was no mortality during or after anesthesia.
Morris water maze
10 days post anesthesia, eight mice of each group were tested in Morris water maze. A round steel pool, 122 cm in diameter and 60 cm in height, was filled with water to a height of 1.0 cm above the top of a 10 cm diameter platform. The pool was covered with a blue curtain and was located in an isolated room with four visual cues on the wall of the pool. Water was kept at 20°C and opacified with titanium dioxide. The mice were tested in the Morris water maze (MWM) four times per day for 4 days [21]. Each day, animals were given four trials. The animal was placed randomly at each of four starting points (north, south, east, and west) and allowed 60 s to find the hidden platform and stay on it for 15 s. If the animal did not locate the platform within 60 s, it was gently guided to the platform and allowed to stay on it for 15 s. After each trial, the animal was placed in a cage and kept warm with an infrared heating lamp.
A video tracking system recorded the swimming motions of the animals, and the data were analyzed using motion-detection software for the MWM (Shanghai Mobile Datum Information Technology Co., Ltd.). At the end of the reference training, the platform was removed from the pool and the mouse was placed in the opposite quadrant. Each mouse was allowed to swim for 120 s, and the number of times the mouse swam across the platform area was recorded (numbers of cross platform).
Antibodies and immunoblotting
Caspase3, NR2B, pERK1/2, and ERK1/2 (all polyclonal antibodies at ratio 1:1000 from Abcam USA) and β-actin (1:3000, Sigma) were used. Animals were terminated by decapitation 24 h post-anesthesia, and after behavioral testing (2 weeks post-anesthesia). Control group was terminated at the same times. Animals bilateral hippocampi were quickly removed and dissected at 4°C in saline and frozen in liquid nitrogen within 3 min of decapitation, and stored at -80°C. Samples (25 μg protein) were resolved on 8% sodium dodecyl sulfate (SDS)-polyacrylamide gels and transferred to nitrocellulose membranes. Membranes were blocked in 5% dry milk solution for 1 h and then incubated overnight at 4°C with anti-Caspase3, NR2B, pERK1/2 and ERK1/2 in the same 5% dry milk solution. Membranes were rinsed in Tween-TBS (TTBS) buffer and incubated in alkaline phosphatase-conjugated secondary antibodies (1:5000, Burlingame, CA) for 1 h at room temperature. The membranes were washed in TTBS by using the enhanced chemiluminescence method. Quantification of band density was performed using AlphaView Software3.4. Data were normalized to β-actin.
Statistical analysis
Data were expressed as mean ± SD. Statistical comparisons between experimental and control groups were made using one-way analysis of variance (ANOVA) with least Significant Difference (LSD) post hoc testing. The escape latency and swimming speed were analyzed by one-way analysis of variance with repeated measures. A statistical software package for the Social Sciences (SPSS) 14.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. P-values for significance used were P < 0.05.
Resuts
Isoflurane anesthesia induced spatial learning changes 10 days post anesthesia
The mice were tested 10 days after anesthesia (N = 8). A comparison of the time that each mouse took to reach the platform during reference training (escape latency) were recorded. We found that 0.5MAC isoflurane anesthetic exposure decreased the escape latency in I1 and I2 groups as compared to those in the control group (P < 0.05), while 1MAC isoflurane prolonged the escape latency in I4 compared to those in the control group (P < 0.05)(Figure 1A). A comparison of the times that each mouse crossed the location of the absent platform at the end of reference training (numbers of cross platform) indicated that anesthesia groups I1 and I2 increased the platform crossing times while I4 group decreased those as compared with the control group (Figure 1B). There was no difference between the I3 and control group. There was no significant difference in mouse swimming speed between the mice in the isoflurane anesthetic groups and the mice in the control group (data not shown). These data suggest that the different concentration and duration of isoflurane exposure in mice may induce cognitive change in the mice approximately 2 weeks after anesthesia.
Figure 1.
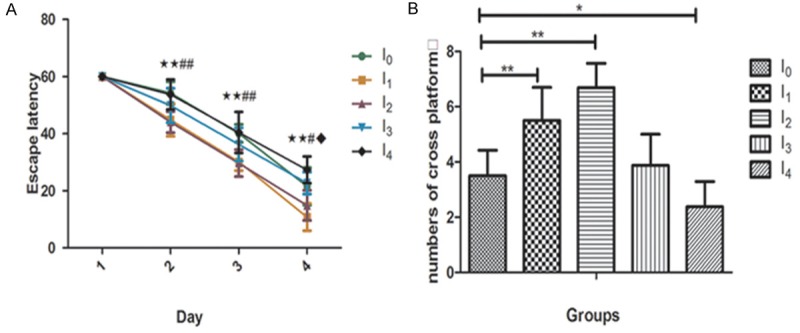
Isoflurane anesthesia induced spatial learning changes 2 weeks post anesthesia. Animals were exposed to 0.5MAC or 1MAC isoflurane for different time. 10 days later the Morris Water Maze was performed. A. The escape latency in the I1 and I2 group decreased significantly, I4 group increased compared with the control group I0 ( (I1 VS I0 ★p < 0.05, ★★p < 0.01; I2 VS I0 ﹟p < 0.05, ﹟﹟p < 0.01; I4 VS I0 ◆p < 0.05, ◆◆p < 0.01). B. I1 and I2 group increased numbers of cross platform significantly, while I4 group decreased compared with the control group. There was no difference between group I3 and control group (*p < 0.05, **p < 0.01).
Given the findings that exposures to isoflurane in young adult mice might induce cognitive change, we next investigated the underlying mechanisms.
Effect of isoflurane anesthesia on caspase 3 activation in the hippocampus of mouse brain
Accumulating studies suggest that anesthetic neurotoxicity may result from caspase3 activation in the CNS [22,23]. In our study, to determine caspase 3 activation levels of isoflurane anesthetic exposure, animals were terminated by decapitation 24 hours post-anesthesia (N = 4) (Fgure 2A) and after behavorial testing (2 weeks post-anesthesia) (N = 4) (Figure 2B). Hippocampal tissues from experimental groups were subjected to western blot analysis with antibodies against caspase3. Representative blots for hippocampus are shown. Caspase 3 activation was measured by caspase 3 fragment (17 kD) quantification. 24 h post-anesthesia, caspase 3 was detected elevated in I3 and I4 (p < 0.01) compared with the control group. 2 weeks post anesthesia caspase3 was still increased in I4 group compared to the control mice (p < 0.01). There were no significant difference between other experimental groups and control group at 24 h and 2 weeks post anesthesia.
Figure 2.
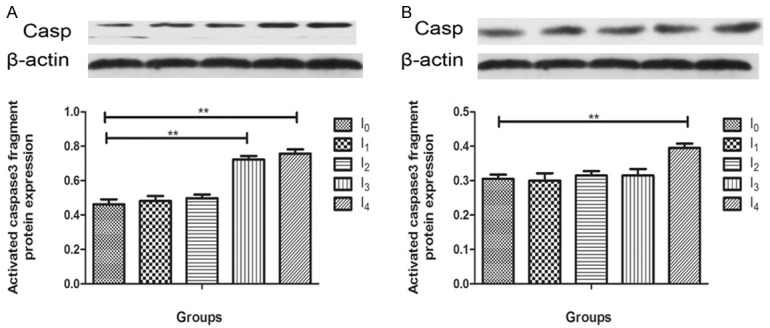
Effect of Isoflurane anesthesia on early and late caspase 3 activation in the hippocampus of mouse brain. A. 24 hours post anesthesia, I3 and I4 group induced caspase-3 cleavage (activation) compared with control group (P < 0.01). I1 and I2 group caused a lesser degree of caspase-3 activation compared with control group (P > 0.05). B. 2 weeks post anesthesia, I4 group induced caspase-3 activation clearly (P < 0.05). There was no diffenence between the rest groups. There was no significant difference in amounts of β-actin [Data are presented as means, error bars represent SD, One-way ANOVA and least Significant Difference (LSD) were performed, *p < 0.05, **p < 0.01].
Effect of isoflurane anesthesia on protein expression levels of NMDA receptor subunits NR2B in the mouse hippocampal brain
In Figure 3, hippocampal brain samples from different groups mice were immunoblotted for NMDA receptor subunits NR2B to determine changes in protein expression as a result of anesthesia exposure 24 hours (N = 4) and 2 weeks post-anesthesia (N = 4). We found that the mice treated with 0.5MAC anesthesia in I1 and I2 demonstrated an acute increase 24 hours post anesthesia (p < 0.01, Figure 3A) and a long-term increase at 2 weeks post-anesthesia compared to naïve mice (p < 0.01, Figure 3B). We also found that the mice treated with 1MAC anesthesia in I3 (p < 0.05) and I4 (p < 0.01) groups indicated an acute decrease in NR2B protein expression levels (Figure 3A), while a long-term decrease in I4 2 weeks post anesthesia compared to naïve mouse in the hippocampus (p < 0.05, Figure 3B).
Figure 3.
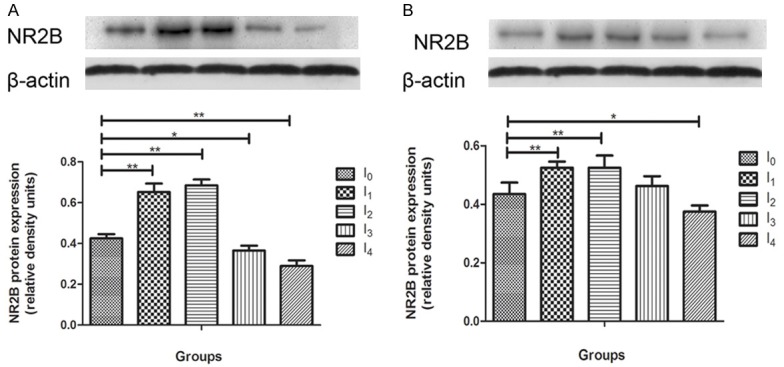
Effect of isoflurane anesthesia on early and late protein expression of NMDA receptor subunits NR2B in the hippocampus of mouse brain. A. 24 hours post anesthesia, I1 and I2 group increased NR2B protein expression significantly (P < 0.05), I3 and I4 group decreased compared with control group (P < 0.05). B. 2 weeks post anesthesia, I1 and I2 group increased NR2B protein expression continuously, I4 dereased continously compared with control group. There was no difference between group I3 and control group [Data are presented as means, error bars represent SD, One-way ANOVA and LSD analysis were performed, *p < 0.05, **p < 0.01].
Effect of isoflurane anesthesia on ERK1/2 activation in the mouse hippocampal brain
Activation of ERK1/2, a downstream kinase of NMDA receptors, is required for hippocampal dependent spatial learning. Hippocampal brain samples from all gruops were immunoblotted for phospho-ERK1/2 and total ERK1/2 to determine changes in activation of ERK1/2, the ratio of phospho- to total-ERK, following isoflurane anesthesia at early (N = 4) and long-term time point (N = 4). An increase in ratio of phosphor- to total-ERK was detected in 0.5MAC anesthesia treated groups I1 and I2 (p < 0.01), meanwhile, a decrease was detected in 1MAC ansthesia treated groups I3 and I4 (p < 0.01) compared to the control group 24 hours post aneshesia Figure 4A). The decrease trend in 1MAC anesthesia treated groups I3 (p < 0.05) and I4 (p < 0.01) continued till 2 weeks post-anesthesia. While the mice in 0.5MAC groups (I1 and I2) became normal as control group (Figure 4B).
Figure 4.
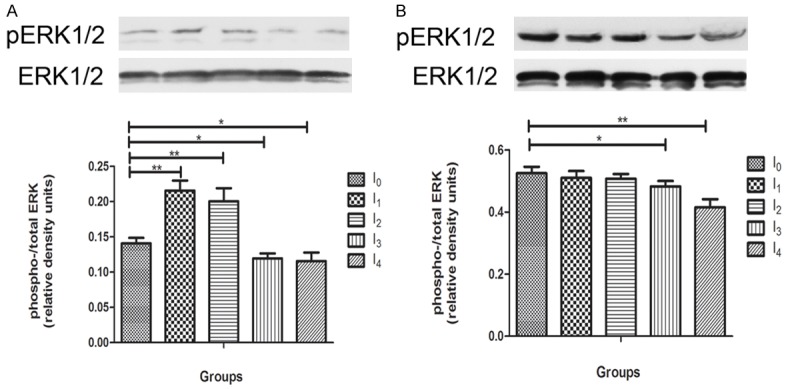
Effect of isoflurane anesthesia on ERK1/2 activation in the hippocampus of mouse brain. Animals were terminated at 24 hours post-anesthesia (A) and 2 weeks post-anesthesia (B) for antibodies against phospho-ERK1/2 and ERK1/2, followed by quantification of the ratio of phospho-ERK over total ERK to determine ERK activation. A. I1 and I2 groups increased ERK activation significantly compared with control group (P < 0.01), I3 and I4 groups decreased ERK activation compared with control group (P < 0.05). B. At 2 weeks post anesthesia, I3 and I4 decreased ERK activation continuously (P < 0.05). There were no difference between group I1, I2 and control group. [Data are presented as means, error bars represent SD. One-way ANOVA and LSD analysis were performed, *p < 0.05, **p < 0.01].
Dicussion
There are conflicting opinions about the effect of anesthetic inhalation on cognitive function. Previous studies suggested cognitive impairment following exposure to anesthetic inhalation, while recently other studies report cognitive improvements following anesthetic exposure. The spatial reference memory version of MWM is a standard task used to assess hippocampal-dependent spatial learning in rodents [21]. Spatial acquisition and retention was assessed using a water maze navigational task. In this study, MWM was adopted to assess spatial learning and memory of mice. We found that, compared with the control group, the mice receiving 0.5MAC isoflurane 0.5 or 2 hours performed cognitively better, the mice receiving 1MAC isoflurane 2 hours performed cognitive similar, the mice receiving 1MAC isoflurane 4 hours performed worse. This indicated that isoflurane may affect spatial learning and memmory with a concentration- and duration-dependent manner.
It is well known that the hippocampus plays a vital role in learning and memory processes and is a known target for the modulatory actions of drugs. There may be an interaction between neural apoptosis and anesthetic insult that result in cognitive dysfunction. Xie et al. [22,23] found that isoflurane may cause neurotoxicity by inducing caspase activation and apoptosis in H4 human neuroglioma cells and in the brain tissues of 5 month-old WT mice. Zhang et al. [24] suggested isoflurane, but not desflurane, induced reactive oxygen species (ROS) accumulation, which then facilitated opening of mitochondrial permeability transition pore (mPTP). Opening of mPTP could cause decreases in levels of mitochondrial membrane potential (MMP), and consequently reduction in adensine-5’-triphosphate (ATP) levels, leading to neurotoxicity (e.g., caspase 3 activation) and finally impairment of learning and memory. In this study, no change was detected 24 hours post-anesthesia in activated caspase 3 in the mice exposed to 0.5MAC isoflurane for 0.5 or 2 hours. However, an increase in activated caspase 3 was detected 24 hours post-anesthesia in the mice exposed to 1MAC isoflurane 2 or 4 hours. The latter increased continously until 2 weeks post-anesthesia. Thus, we concluded that with the anesthetic concentration increased and duration prolonged, isoflurane may cause neurotoxicity by inducing caspase activation and apoptosis.
In our study, it suggested that with the increasement of isoflurane concentration and duration time, caspase3 was elevated significantly, meaning increased aoptosis , but in behavioral test, the group exposed to 1MAC anesthesia 2 h was not impaired in spacial memory ability compared to control group. This means neural apoptosis may cause side effect on learning and memory. While there are many factors in the process of leaning and memory, and neural apoptosis was not a decisive factor. Due to the critical role of NMDA receptors in learning and memory processes [20], these receptors may play a role in anesthesia-induced cognitive deficits. Previously, in cortical neurons, NMDA-gated currents mediated by NR2B-containing receptors were more sensitive to isoflurane than currents mediated by NR2A-containing receptors [25]. NR2B subunit plays a key role in synaptic plasticity, synaptogenesis, excitotoxicity, memory acquisition and learning. Genetic overexpression of the gene encoding NR2B in adult fore-brains led to mice with facilitated LTP induction and improved learning and memory in a variety of behaviourl tasks, whereas a hippocampal NR2B deficit impaired spatial learning [26]. Rammes and Zhao repored that high levels of NMDA receptor subunit NR2B protein expression corresponded to improved spatial learning performance in 4-5 month-old mice [13,27]. Our findings found the two groups receiving 0.5MAC isoflurane 0.5 h or 2 h detected obvious increasement of NMDA receptor subunit NR2B protein expression at early and late time points corresponding to better behavioral performance. While the group receiving 1MAC isoflurane 2 h or 4 h were detected marked reduction of NR2B protein expression at early time point, and the 4 h group continued lower NR2B protein expression till 2 weeks post anesthesia.
Extracellular signal-regulated protein kinase 1/2 (ERK1/2) belong to subfamily of MAPKs, a prototype of MAPKs, they are activated via phosphorylation on Thr202 and Tyr204. The NMDAR have been demonstrated to positively regulate ERK1/2 phosphorylation. It has also been reported that NMDA receptor-dependent ERK activation is mainly mediated by NR2B-containing receptors [28]. Once activated, ERK1/2 translocate from the cytosol to the nucleus to activate specific transcription factors, leading to inducible gene expression. A noticeable role of active ERK1/2 is its strong regulation of gene expression. As an information superhighway between the surface receptor and the nucleus, ERK1/2 effectively link environmental inputs to genomic responses. Through regulating specific sets of transcriptional events, ERK1/2 modulate expression levels and thus functions of various key synaptic proteins. This process influences many forms of synaptic plasticity, including LTP and LTD, cellular models of learning and memory. It was reported that the ERK1/2 protein expression in hippocampus was closely related with hippocampus dependent special learning and memory mask such as MWM [29,30]. Our findings identify an increasement induced by 0.5MAC isoflurane treated groups (I1 and I2) and a reduction caused by 1MAC isoflurane treated groups (I3 and I4) in hippocampal ratio of p-ERK/ERK at early time (24 hours post anesthesia). Two weeks later, there was no difference between 0.5MAC isoflurane treated groups and control group. Meanwhile, 1MAC isoflurane treated groups were still detected lower ratio of p-ERK/ERK. From our results, we considered that the chang of activation of ERK induced by 0.5MAC anesthesia continued not as long as the NR2B. The effect of anesthesia on activation of ERK gradually came to normal. As for 1MAC groups we speculated that they may also be in the process of recovery especially the I3 given that the behavioral performance.
Taken together, our findings suggested low concentration of isoflurane (0.5MAC) exposure in 2 hours did not induce caspase3 activation and neural apopotosis significantly, while induced increasement of hippocampal NMDA receptor subunit NR2B protein expression and ERK1/2 activation contributing to the improvement of spatial learning performance. However, high concentration of isoflurane(1MAC) exposure in 2 hours can cause caspase3 activation and neural apopotosis obviously, meanwhile, inhibted hippocampal NMDA receptor subunit NR2B protein expression and ERK activation at early time point (24 h post anesthesia), then NR2B became normal 2 weeks post anesthesia resulting in no cognitive impairment. Further-more, high concentration of isoflurane exposure exceeding 4 hours may induce caspase3 activation, inhibit NR2B protein expression and ERK1/2 activation for 2 weeks since anesthesia, corresponding to the impairment of spatial learning performance. Thus our study indicate that isoflurane may affect spatial learning and memmory with a concentration- and duration-dependent manner resulting from multipule reasons including neural apotosis, changes of NR2B receptor and its downstream signaling pathways ERK1/2 in hippocampus.
Acknowledgements
This work was supported by operating research grants from The National Nature Scienc Fundation of China (81171297, 81200934).
Disclosure of conflict of interest
None.
References
- 1.Monk TG, Weldon BC, Garvan CW, Dede DE, van der Aa MT, Heilman KM, Gravenstein JS. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008;108:18–30. doi: 10.1097/01.anes.0000296071.19434.1e. [DOI] [PubMed] [Google Scholar]
- 2.Turnbull IR, Wlzorek JJ, Osborne D, Hotchkiss RS, Coopersmith CM, Buchman TG. Effects of age on mortality and antibiotic efficacy in cecal ligation and puncture. Shock. 2003;19:310–3. doi: 10.1097/00024382-200304000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Moller JT, Cluitmans P, Rasmussen LS, Houx P, Rasmussen H, Canet J, Rabbitt P, Jolles J, Larsen K, Hanning CD, Langeron O, Johnson T, Lauven PM, Kristensen PA, Biedler A, van Beem H, Fraidakis O, Silverstein JH, Beneken JE, Gravenstein JS. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. Lancet. 1998;351:857–61. doi: 10.1016/s0140-6736(97)07382-0. [DOI] [PubMed] [Google Scholar]
- 4.Steinmetz J, Christensen KB, Lund T, Lohse N, Rasmussen LS. Long-term consequences of postoperative cognitive dysfunction. Anesthesiology. 2009;110:548–55. doi: 10.1097/ALN.0b013e318195b569. [DOI] [PubMed] [Google Scholar]
- 5.Canet J, Raeder J, Rasmussen LS, Enlund M, Kuipers HM, Hanning CD, Jolles J, Korttila K, Siersma VD, Dodds C, Abildstrom H, Sneyd JR, Vila P, Johnson T, Muñoz Corsini L, Silverstein JH, Nielsen IK, Moller JT ISPOCD2 investigators. Cognitive dysfunction after minor surgery in the elderly. Acta Anaesthesiol Scand. 2003;47:1204–10. doi: 10.1046/j.1399-6576.2003.00238.x. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez RA, Tellier A, Grabowski J, Fazekas A, Turek M, Miller D, Wherrett C, Villeneuve PJ, Giachino A. Cognitive dysfunction after total knee arthroplasty: effects of intraoperative cerebral embolization and postoperative complications. J Arthroplasty. 2005;20:763–71. doi: 10.1016/j.arth.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Steinmetz J, Christensen KB, Lund T, Lohse N, Rasmussen LS ISPOCD Group. Long-term Consequences of Postoperative Cognitive Dysfunction. Anesthesiology. 2009;110:548–55. doi: 10.1097/ALN.0b013e318195b569. [DOI] [PubMed] [Google Scholar]
- 8.Hudson AE, Hemmings HC Jr. Are anaesthetics toxic to the brain? Br J Anaesth. 2011;107:30–7. doi: 10.1093/bja/aer122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Culley DJ, Baxter M, Yukhananov R, Crosby G. The memory effects of general anesthesia persist for weeks in young and aged rats. Anesth Analg. 2003;96:1004–9. doi: 10.1213/01.ANE.0000052712.67573.12. [DOI] [PubMed] [Google Scholar]
- 10.Culley DJ, Baxter MG, Crosby CA, Yukhananov R, Crosby G. Impaired acquisition of spatial memory 2 weeks after isoflurane and isoflurane-nitrous oxide anesthesia in aged rats. Anesth Analg. 2004;99:1393–7. doi: 10.1213/01.ANE.0000135408.14319.CC. [DOI] [PubMed] [Google Scholar]
- 11.Culley DJ, Baxter MG, Yukhananov R, Crosby G. Long-term impairment of acquisition of a spatial memory task following isoflurane-nitrous oxide anesthesia in rats. Anesthesiology. 2004;100:309–14. doi: 10.1097/00000542-200402000-00020. [DOI] [PubMed] [Google Scholar]
- 12.Komatsu H, Nogaya J, Anabuki D, Yokono S, Kinoshita H, Shirakawa Y, Ogli K. Memory facilitation by posttraining exposure to halothane, enflurane, and isoflurane in ddN mice. Anesth Analg. 1993;76:609–12. doi: 10.1213/00000539-199303000-00028. [DOI] [PubMed] [Google Scholar]
- 13.Rammes G, Starker LK, Haseneder R, Berkmann J, Plack A, Zieglgansberger W, Ohl F, Kochs EF, Blobner M. Isoflurane anaesthesia reversibly improves cognitive function and long-term potentiation (LTP) via an up-regulation in NMDA receptor 2B subunit expression. Neuropharmacology. 2009;56:626–36. doi: 10.1016/j.neuropharm.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Eger EI 2nd, Liao M, Laster MJ, Won A, Popovich J, Raines DE, Solt K, Dutton RC, Cobos FV 2nd, Sonner JM. Contrasting roles of the N-methyl-D-aspartate receptor in the production of immobilization by conventional and aromatic anesthetics. Anesth Analg. 2006;102:1397–406. doi: 10.1213/01.ANE.0000219019.91281.51. [DOI] [PubMed] [Google Scholar]
- 15.Hara K, Eger EI 2nd, Laster MJ, Harris RA. Nonhalogenated alkanes cyclopropane and butane affect neurotransmitter-gated ion channel and G-protein-coupled receptors: differential actions on GABAA and glycine receptors. Anesthesiology. 2002;97:1512–20. doi: 10.1097/00000542-200212000-00025. [DOI] [PubMed] [Google Scholar]
- 16.Hoffman WE, Pelligrino D, Werner C, Kochs E, Albrecht RF, Schulte am Esch J. Ketamine decreases plasma catecholamines and improves outcome from incomplete cerebral ischemia in rats. Anesthesiology. 1992;76:755–62. doi: 10.1097/00000542-199205000-00014. [DOI] [PubMed] [Google Scholar]
- 17.Raines DE, Claycomb RJ, Scheller M, Forman SA. Nonhalogenated alkane anesthetics fail to potentiate agonist actions on two ligand-gated ion channels. Anesthesiology. 2001;95:470–7. doi: 10.1097/00000542-200108000-00032. [DOI] [PubMed] [Google Scholar]
- 18.Solt K, Johansson JS, Raines DE. Kinetics of anesthetic-induced conformational transitions in a four-alpha-helix bundle protein. Biochemistry. 2006;45:1435–41. doi: 10.1021/bi052206o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamakura T, Harris RA. Effects of gaseous anesthetics nitrous oxide and xenon on ligand-gated ion channels. Comparison with isoflurane and ethanol. Anesthesiology. 2000;93:1095–101. doi: 10.1097/00000542-200010000-00034. [DOI] [PubMed] [Google Scholar]
- 20.Morgado-Bernal I. Learning and memory consolidation: linking molecular and behavioral data. Neuroscience. 2011;176:12–9. doi: 10.1016/j.neuroscience.2010.12.056. [DOI] [PubMed] [Google Scholar]
- 21.Morris RG, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature. 1986;319:774–6. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- 22.Xie Z, Dong Y, Maeda U, Alfille P, Culley DJ, Crosby G, Tanzi RE. The common inhalation anesthetic isoflurane induces apoptosis and increases amyloid beta protein levels. Anesthesiology. 2006;104:988–94. doi: 10.1097/00000542-200605000-00015. [DOI] [PubMed] [Google Scholar]
- 23.Xie Z, Culley DJ, Dong Y, Zhang G, Zhang B, Moir RD, Frosch MP, Crosby G, Tanzi RE. The common inhalation anesthetic isoflurane induces caspase activation and increases amyloid beta-protein level in vivo. Ann Neurol. 2008;64:618–27. doi: 10.1002/ana.21548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y, Xu Z, Wang H, Dong Y, Shi HN, Culley DJ, Crosby G, Marcantonio ER, Tanzi RE, Xie Z. Anesthetics isoflurane and desflurane differently affect mitochondrial function, learning, and memory. Ann Neurol. 2012;71:687–98. doi: 10.1002/ana.23536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ming Z, Griffith BL, Breese GR, Mueller RA, Criswell HE. Changes in the effect of isoflurane on N-methyl-D-aspartic acid-gated currents in cultured cerebral cortical neurons with time in culture: evidence for subunit specificity. Anesthesiology. 2002;97:856–67. doi: 10.1097/00000542-200210000-00017. [DOI] [PubMed] [Google Scholar]
- 26.Clayton DA, Mesches MH, Alvarez E, Bickford PC, Browning MD. A Hippocampal NR2B Deficit Can Mimic Age-Related Changes in Long-Term Potentiation and Spatial Learning in the Fischer 344 Rat. J Neurosci. 2002;22:3628–37. doi: 10.1523/JNEUROSCI.22-09-03628.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao X, Rosenke R, Kronemann D, Brim B, Das SR, Dunah AW, Magnusson KR. The effects of aging on N-methyl-D-aspartate receptor subunits in the synaptic membrane and relationships to long-term spatial memory. Neuroscience. 2009;162:933–45. doi: 10.1016/j.neuroscience.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krapivinsky G, Krapivinsky L, Manasian Y, Ivanov A, Tyzio R, Pellegrino C, Ben Ari Y, Clapham DE, Medina I. The NMDA receptor is coupled to the ERK pathway by a direct interaction between NR2B and RasGRF1. Neuron. 2003;40:775–84. doi: 10.1016/s0896-6273(03)00645-7. [DOI] [PubMed] [Google Scholar]
- 29.Atkins CM, Selcher JC, Petraitis JJ, Trzaskos JM, Sweatt JD. The MAPK cascade is required for mammalian associativ learning. Nat Neurosci. 1998;1:602–9. doi: 10.1038/2836. [DOI] [PubMed] [Google Scholar]
- 30.Selcher JC, Atkins CM, Trzaskos JM, Paylor R, Sweatt JD. A necessity for MAP kinase activation in mammalian spatial learning. Learn Mem. 1999;6:478–90. doi: 10.1101/lm.6.5.478. [DOI] [PMC free article] [PubMed] [Google Scholar]


