Abstract
Microvessel density (MVD), an indicator of angiogenesis, has been proposed to predict prognosis of patients with renal cell carcinoma (RCC), but its ability to predict survival of patients with RCC remains controversial. The present study sought to address this question rigorously by systematically reviewing the literature on MVD and RCC prognosis. We identified relevant studies in PubMed, EMBASE and the Cochrane Library, and two reviewers independently assessed study quality and extracted relevant data to compare survival based on MVD stratification in patients with RCC. We identified 15 studies that satisfied the inclusion criteria; eight studies assessed MVD in surgical samples by immunohistochemistry to label factor VIII; four studies, by immunohistochemistry to label CD34; two studies, CD31; and one study, CD105. Survival meta-analysis was performed using data pooled from 10 studies: five based on factor VIII, two based on CD34, two based on CD31 and one based on CD105. The overall survival hazard ratio describing the relationship between MVD and survival in all 10 pooled studies was 0.964 (95% CI: 0.873-1.065), while the individual hazard ratios for pooled studies based on factor VIII were 1.673 (95% CI: 0.860-3.252); CD34, 0.903 (95% CI: 0.853-0.956); and CD31, 0.926 (95% CI: 0.868-0.989). The corresponding result for the sole trial based on CD105 was 0.1759 (95% CI: 0.036-0.856). These findings suggest that MVD is not reliably associated with survival time of patients with RCC, which may reflect the need to take into account whether the microvasculature is differentiated or not. MVD as currently calculated may not be an ideal prognostic factor for patients with RCC.
Keywords: Microvessel density, renal cell carcinoma, prognosis, meta-analysis
Introduction
Renal cell carcinoma (RCC) is one of the most common malignant tumors of the adult kidney [1]. Nearly 30% of patients with RCC who undergo radical nephrectomy surgery develop metastatic disease [2], for which overall 5-year survival is only about 9% [3]. The risk of recurrence is relatively high during the 3-5 years after radical surgery [4]. These characteristics of RCC highlight the importance of developing a reliable prognostic factor to guide clinical decisions [5]. The prognostic factors most frequently used in RCC are tumor grade and nuclear stage, but these factors are widely considered less than reliable. For example, several studies have demonstrated that RCC classified as low grade and low stage can metastasize. Recent developments in cytogenetics and molecular biology have opened new possibilities for developing better prognostic tools.
Angiogenesis, which refers to the proliferation and sprouting of existing blood vessels close to the tumor, is crucial for malignant cancers like to proliferate and progress [6]. Once a tumor reaches 1-2 mm in size, its further growth requires angiogenesis, and the resulting newly formed blood vessels may carry tumor cells through the circulation, leading to metastasis [7]. Different tumor types show different levels of angiogenic activity, which correlates with development of RCC, breast cancer and non-small cell lung carcinoma [8-10].
Microvessel density (MVD) is the parameter most frequently used to quantify intratumoral angiogenesis. Blood microvessels are most often identified by immunohistochemical staining to label factor VIII antigen (FVIII Ag or von Willebrand’s factor), CD31, PECAM-1, CD34, or occasionally CD105 or type IV collagen. FVIII and CD31 are pan-endothelial cell markers associated not only with newly formed microvessels, but also with existing vessels in the tumor. CD34 is often expressed in the pericytes of blood vessels but rarely in normal vessel endothelium or lymphatic endothelium, marking it a good marker of the maturation of immature vessels. CD105, in contrast, is preferentially expressed by activated endothelial cells during angiogenesis, making it more precise than pan-endothelial cell markers for identifying tumor angiogenesis. To minimize subjectivity during MVD quantification, density is calculated using Chalkley count and computerized image analysis.
Although much remains unknown about the mechanisms of angiogenesis [11], MVD has been shown to predict prognosis for various malignant tumors, including lung cancer [7], breast cancer [12], and colorectal cancer [13]. However, whether MVD can predict the prognosis of patients with RCC is controversial. Many retrospective studies have reported MVD to be inversely related to survival in RCC [14-18], but other studies have come to different conclusions [19-23]. Such a relationship is plausible given that RCC progression depends on dense vascularization and arterivenous fistula development.
To address this question definitively, we systematically reviewed the literature on MVD and RCC prognosis in order to meta-analyze data for as large a sample as possible.
Methods
Publication selection
This systematic review was performed according to a predetermined protocol. To be included, studies had to be (a) original research published in English or Chinese that (b) analyzed the relationship between MVD and overall survival (OS) in patients with RCC without other major diseases, in whom (c) MVD was measured in the primary tumor. We searched the PubMed, EMBASE and Cochrane Library databases in September 2013 using the following key words: neovascularization, microvessel density, renal cell carcinoma, prognostic, prognosis and survival. Prospective or retrospective abstracts that described the evaluation of survival in a cohort were selected for further consideration. Reviews, studies involving cell culture and animal models of RCC, and other types of publication were excluded from our review. Reference lists in relevant studies were also manually searched.
In order to exclude duplicate data, we carefully examined the names of all authors and different research centers for each study. When two or more publications reported on the same patient population, only the most recent or complete study was included in the review.
Data extraction and methodological assessment
Articles selected for full-text analysis were evaluated independently by two reviewers, who selected the final set for inclusion. Disagreements were resolved by consensus. The following data were retrieved from studies using a standardized form: authors, publication year, source of patients, number of patients, study design, median duration of follow-up, blinding, number of readers, antibody used to label microvasculature, method of measuring MVD from the labelling data, cut-off value for classifying MVD as high, estimated hazard ratio (HR) and survival data (Table 1).
Table 1.
Main characteristics and results of studies evaluating the MVD and survival
| First author | Year | Patients source | Study design | N | Blinded reading | Readers (n) | Mode of reading | Follow-up (years) | Anti-body | Cut-off | HR estimation | Survival analysis | Results |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Iakovlev [36] | 2012 | Canada | Retro. | 57 | Yes | ? | Automated | 3.25 | CD34 | Median | ND | DFS | Negative |
| Ren [35] | 2011 | China | Retro. | 128 | Yes | 2 | Optical | ? | CD34 | Median | HR | OS | Negative |
| Yao a [34] | 2007 | China | Retro. | 78 | ? | ? | Automated | ? | CD31 | Median | HR | OS | Negative |
| Yao b [34] | 2007 | China | Retro. | 78 | ? | ? | Automated | ? | CD34 | Median | HR | OS | Negative |
| Kawata [33] | 2004 | Japan | Retro. | 17 | ? | ? | Automated | 3.17 | FVIII | Median | RR | OS | Negative |
| Yagasaki [32] | 2003 | Japan | Retro. | 84 | ? | ? | Optical | 2.75 | CD105 | Median | HR | OS | Negative |
| Sabo [17] | 2001 | Israel | Retro. | 49 | ? | ? | Automated | ? | CD34 | > 10% | ND | OS | Negative |
| Song [31] | 2001 | Korea | Retro. | 50 | ? | 2 | Optical | 8 | CD31 | > 10 vessels | Surv. curves | OS | Positive |
| Yoshino [30] | 2000 | Japan | Retro. | 96 | Yes | 2 | Optical | > 5 | FVIII | Median | ND | OS | Positive |
| Nativ [20] | 1998 | Israel | Retro. | 36 | ? | ? | Optical | 8.1 | FVIII | Median | Events | OS | Positive |
| Yoshino [29] | 1998 | Japan | Retro. | 62 | ? | ? | Optical | ? | FVIII | Median | Events | OS | Positive |
| Gelb [28] | 1997 | USA | Retro. | 52 | ? | ? | Optical | 9.08 | FVIII | Median | ND | OS | Negative |
| Imazano [27] | 1997 | Japan | Retro. | 133 | ? | ? | ? | 3.33 | FVIII | ? | HR | OS | Negative |
| Delahunt [26] | 1997 | New Zealand | Retro. | 150 | ? | 3 | Optical | ? | FVIII | > 40 vessels/HPF | ND | OS | Negative |
| Yoshino [21] | 1995 | Japan | Retro. | 45 | Yes | ? | Optical | 2.17 | FVIII | Median | Events | OS, DFS (ND) | Positive |
MVD, micro vessel density; Retro, retrospective study; HPF, high power field; HR, hazard ratio; ND, no data; OS, overall survival; DFS, disease-free survival; Positive MVD, being significantly unfavorable prognostic factor for overall survival; Negative MVD, being significantly favorable or no significant prognostic factor for overall survival.
Study quality was qualitatively assessed because although quality assessment scales have been defined for other tumors, no such scale has been defined for RCC.
Statistical methods
Results from included studies were dichotomized by declaring a study “positive” when it reported that high MVD predicted poor OS, or “negative” when high MVD was not reported to predict poor OS or when it was reported to predict better OS. When continuous data were reported for MVD and OS, we used the Spearman rank correlation coefficient to examine the correlation between the two variables. The nonparametric Mann-Whit-ney test was used to determine the significance of differences in discrete variables. All statistical analyses were performed using STATA version 11.0 (Stata Corporation, College Station, TX). All analyses were considered significant when the two-sided P-value was less than 0.05.
We used the HR to assess the influence of MVD on the OS of patients with RCC. The HR and its variance were estimated using the following methods depending on the data provided in each study: the HR point estimate, the log-rank statistic or its P-value, or the O-E statistic (the difference between numbers of observed and expected events) or its variance. Whenever possible, the HR was estimated from these two methods, which was considered the most accurate method. In studies reporting survival data only in the form of survival curves, survival rates were extracted at specified times to calculate the estimated HR and its variance. Since this method assumes a constant rate of patient censoring during follow-up [24], two reviewers independently read the curves.
We used Peto’s method to combine HRs estimated from individual studies into an overall HR [25]. We tested pooled studies for heterogeneity using chi-squared tests. If heterogeneity was not significant, we planned to perform meta-analysis using a fixed-effects model; otherwise, we planned to use a random-effects model.
Results
Study selection and characteristics
Our database searches turned up a total of 146 studies about MVD and RCC. Of these, 105 were excluded based on the title or abstract as irrelevant to our study objectives or as not involving human patients. Full-text review of the remaining studies identified 15 that met the inclusion criteria [17,20,21,26-36] (Figure 1).
Figure 1.
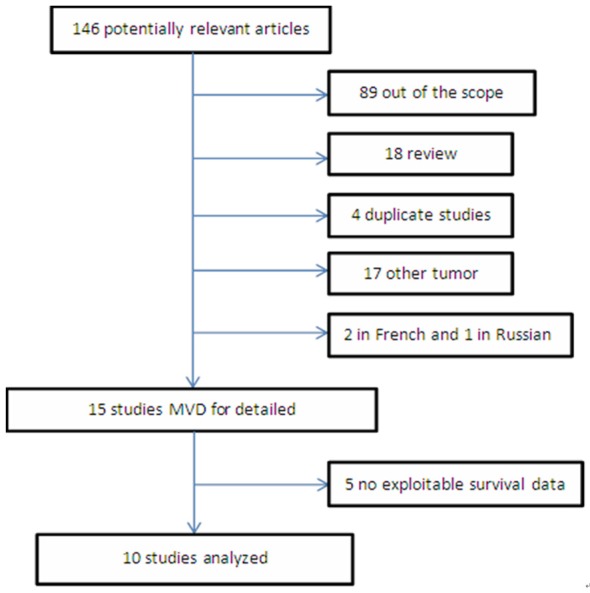
Flow chart of study selection and meta-analysis.
The included studies, all of which were retrospective, involved a total of 1115 patients, with each study involving between 17 and 150 (median, 62). Patients were diagnosed with RCC but not any other cancer, including squamous cell carcinoma. MVD was measured by immunohistochemistry using antibodies against FVIII (8 studies), CD34 (4), CD31 (2) or CD105 (1). Most studies defined the median MVD to be the cut-off for classifying MVD as low or high, whereas a few studies used other methods.
One study reported survival data in a format that we could not use for meta-analysis [36] and four [17,26,28,30] failed to report detailed OS data, preventing us from calculating HR. Thus the remaining 10 studies were included in the final meta-analysis.
Studies results reports and meta-analysis
Of the 15 included studies, 5 studies involving 289 patients were “positive”, meaning that they reported high MVD as predicting poor OS. Of these 5 studies, 4 were included in the final meta-analysis, while one was excluded for lack of data [30]. The remaining 10 studies, involving 826 patients, were “negative”, meaning that they did not report high MVD to be significantly associated with poor OS. In fact, two studies reported high MVD to be associated with better OS. Of these 10 studies, 6 reported HRs directly, so they were included in the meta-analysis.
Of the 10 studies included in the meta-analysis, HRs were reported directly in 6, whereas they had to be calculated from the relevant data in 3 or estimated from survival curve graphs in one. Since the 10 studies showed significant heterogeneity (P < 0.001), we merged HRs from individual studies using a random-effects model. The combined HR for high MVD as a predictor of poor OS was 0.964 (95% CI, 0.873 to 1.065), suggesting no significant association.
Next we stratified the studies in the meta-analysis according to the antigen used to determine MVD. The HRs were as follows for the different antigens: FVIII, 1.673 (95% CI: 0.860-3.252); CD34, 0.903 (95% CI: 0.853-0.956); CD31, 0.926 (95% CI: 0.868-0.989); and CD105, 0.175 (95% CI: 0.036-0.856) (Figure 2).
Figure 2.
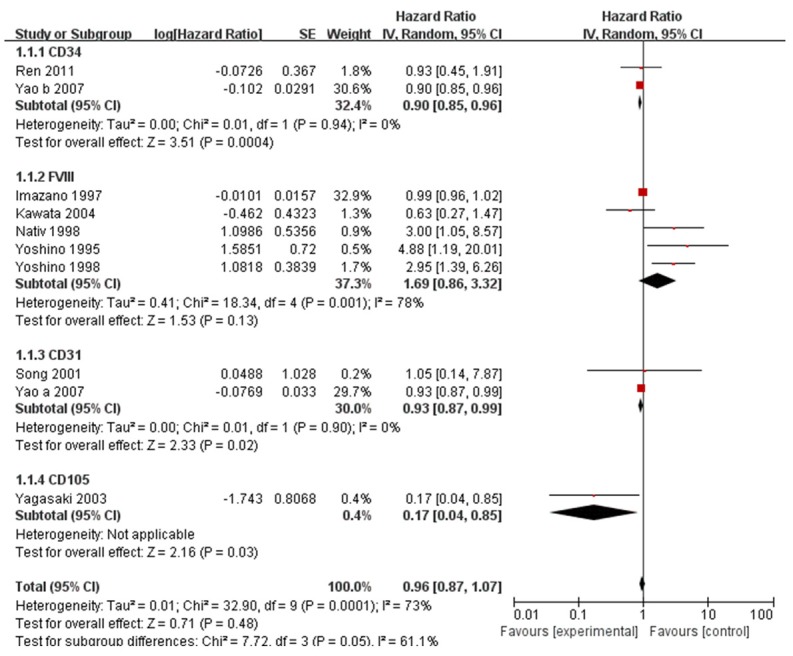
Meta-analysis of studies assessing the relationship of overall survival of RCC patients with MVD measured based on labelling of CD34, CD31, FVIII, or CD105. HR > 1 indicates an association between higher MVD and poor overall survival. HRs were estimated using a DerSimonian and Laird random-effects model.
Studies in the meta-analysis failed to report adequate data for assessing possible associations of MVD with histopathology type or disease stage.
No significant publication bias was detected using the tests of Egger et al. [37] (P = 0.684, Figure 3) or of Begg and Mazumdar [38] (P = 0.858; Figure 4).
Figure 3.
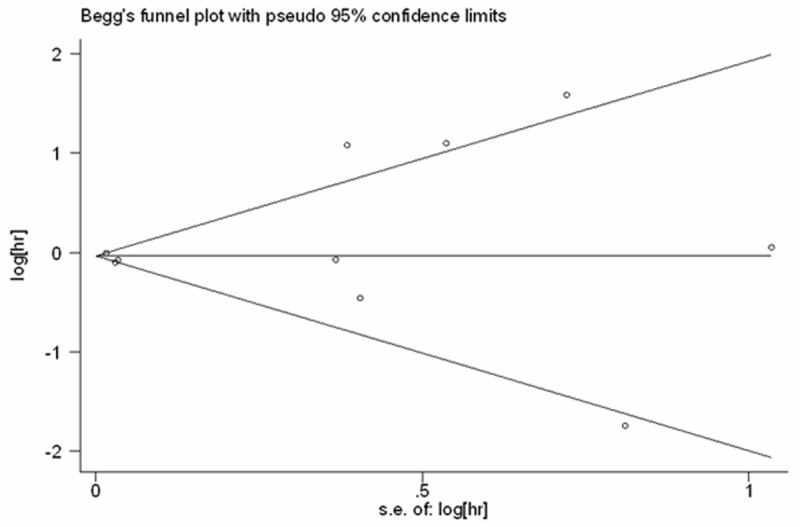
Begg’s funnel plot to assess bias among the 10 studies in the meta-analysis.
Figure 4.
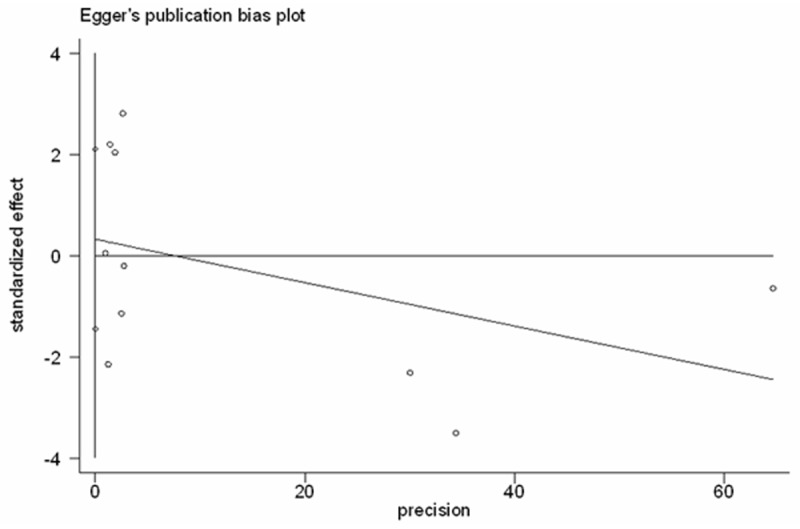
Egger’s funnel plot to assess publication bias among the 10 studies in the meta-analysis.
Discussion
This meta-analysis suggests that MVD, a well-established marker of angiogenesis, is not associated with OS of patients with RCC. Stratified meta-analysis of studies that measured MVD using different antibodies to detect microvasculature supports these results. The HR linking high MVD and poor OS was statistically significant for studies in which CD34 or CD31 was labelled, but the 95% confidence interval was too close to 1 for us to conclude clinical significance. The HR was also significant for the sole study in which CD105 was labeled, but this finding would have to be reproduced in other studies.
MVD is an accepted prognostic factor in lung cancer [7,39-41], breast cancer [42] and colorectal cancer [13]. However, we found no significant association between MVD and OS in patients with RCC. While this result may be genuine, it may also be an artifact of differences in microvessel type. Yao et al. [34] identified two types of microvessels in RCC: undifferentiated microvessels (CD31+/CD34-) and differentiated ones (CD34+). The authors found that a higher proportion of undifferentiated microvessels was associated with greater malignancy and poorer prognosis, while a higher proportion of differentiated microvessels was associated with lower malignancy and better prognosis. Those authors concluded that undifferentiated MVD is an independent prognostic factor in patients with RCC. The studies in our meta-analysis did not report separate results for undifferentiated and differentiated microvessels, so their results probably represent aggregate analysis of both types. Given our negative findings of MVD as a prognostic indicator, future studies should focus specifically on whether undifferentiated microvessels influence OS.
We detected highly significant heterogeneity among the 10 studies included in the meta-analysis. This likely reflects differences in the baseline characteristics of patients (including age, histopathology type, tumor size, disease stage), adjuvant therapy, length of follow-up, and antibody used to assess MVD among the studies. Indeed the mere fact that different labelling antibodies were used is probably enough to cause significant differences in MVD. Weidner et al. [43] chose anti-FVIII antibody to mark primarily the endothelia of mature blood vessels and partly cross-label lymphatic vessels, while other authors chose antibodies against the antiplatelet adhesion molecules CD31 or CD34. CD31 is chosen more frequently than FVIII because the monoclonal anti-CD31 antibody JC-70 can also label immature vessels. On the other hand, the anti-CD31 antibody can react moderately with fibroblasts and plasmacytes, and CD31 is not usually expressed at high levels. In fact, Uzzan et al. [42] reported that CD31-labelling can fail to detect antigen in up to 20% of conventional fixed breast specimens. CD34, while its characteristics are similar to those of CD31, is associated with a lower rate of false negatives [44] and it labels mainly pericytes that surround mature blood vessels. Thus no single antibody is ideal for determining MVD. This suggests the usefulness of combining multiple antibodies for higher accuracy [45], yet none of the studies in our meta-analysis did so.
Another significant source of variation among the studies in our meta-analysis was the method used to determine MVD in each sample, as well as the threshold used to classify MVD values as high or low. Different authors defined MVD as the mean value from multiple measurements of duplicate samples, the mean value from different areas of the same sample, the highest value among 3 or more MVD determinations in different fields of the same area, or the highest value from different areas of the same sample. Most studies used median MVD as the cut-off for classifying MVD as high or low, while the remaining studies used other methods. Future studies should aim to standardize MVD assessment.
The limitations of this meta-analysis are due primarily to limitations within the individual studies. The numerous sources of heterogeneity outlined above increase the risk of selection bias. Some studies did not include blinding, raising the risk of reporting bias. Most studies did not report data at the level of individual participants, forcing us to use aggregate data. All the included studies were observational, highlighting the need for randomized controlled trials and prospective studies in this area.
In conclusion, despite these limitations, our meta-analysis strongly suggests that MVD is not a reliable predictor of OS in patients with RCC. Future studies should examine whether more specific aspects of MVD may have prognostic value, such as the undifferentiated microvessel density or the MVD associated with particular histopathology types or disease stages.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 31370951 and 3117090). We are indebted to the authors of the primary studies included in this meta-analysis; without their contributions, this work would not have been possible.
Disclosure of conflict of interest
None.
References
- 1.Eble JN, Sauter G, Epstein JI, et al. Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs. Lyon: IARC Press; 2004. [Google Scholar]
- 2.Cohen HT, McGovern FJ. Renal-cell carcinoma. N Engl J Med. 2005;353:2477–90. doi: 10.1056/NEJMra043172. [DOI] [PubMed] [Google Scholar]
- 3.Weiss RH, Lin PY. Kidney cancer: identification of novel targets for therapy. Kidney Int. 2006;69:224–32. doi: 10.1038/sj.ki.5000065. [DOI] [PubMed] [Google Scholar]
- 4.Lam JS, Shvarts O, Leppert JT, Pantuck AJ, Figlin RA, Belldegrun AS. Postoperative surveillance protocol for patients with localized and locally advanced renal cell carcinoma based on a validated prognostic nomogram and risk group stratification system. J Urol. 2005;174:466–72. doi: 10.1097/01.ju.0000165572.38887.da. [DOI] [PubMed] [Google Scholar]
- 5.Kirkali Z, Lekili M. Renal cell carcinoma: new prognostic factors? Curr Opin Urol. 2003;13:433–8. doi: 10.1097/00042307-200311000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1997;82:4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- 7.Meert AP, Paesmans M, Martin B, Delmotte P, Berghmans T, Verdebout JM, Lafitte JJ, Mascaux C, Sculier JP. The role of microvessel density on the survival of patients with lung cancer: a systematic review of the literature with meta-analysis. Br J Cancer. 2002;87:694–701. doi: 10.1038/sj.bjc.6600551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nicol D, Hii SI, Walsh M, Teh B, Thompson L, Kennett C, Gotley D. Vascular endothelial growth factor expression is increased in renal cell carcinoma. J Urol. 1997;157:1482–6. [PubMed] [Google Scholar]
- 9.Horak ER, Leek R, Klenk N, LeJeune S, Smith K, Stuart N, Greenall M, Stepniewska K, Harris AL. Angiogenesis, assessed by plattelet/endothelial cell adhesion molecule antibodies, as an indicator of node metastasis and survival in breast cancer. Lancet. 1992;340:1120–4. doi: 10.1016/0140-6736(92)93150-l. [DOI] [PubMed] [Google Scholar]
- 10.Macchiarini P, Fontainini G, Hardın MJ, Squartini F, Angeletti CA. Relation of neovascularization to metastasis of non-small cell lung cancer. Lancet. 1992;340:145–6. doi: 10.1016/0140-6736(92)93217-b. [DOI] [PubMed] [Google Scholar]
- 11.Yilmazer D, Han U, Onal B. A comparison of the vascular density of VEGF expression with microvascular density determined with CD34 and CD31 staining and conventional prognostic markers in renal cell carcinoma. Int Urol Nephrol. 2007;39:691–8. doi: 10.1007/s11255-006-9123-4. [DOI] [PubMed] [Google Scholar]
- 12.Uzzan B, Nicolas P, Cucherat M, Perret GY. Microvessel density as a prognostic factor in women with breast cancer: a systematic review of the literature and meta-analysis. Cancer Res. 2004;64:2941–55. doi: 10.1158/0008-5472.can-03-1957. [DOI] [PubMed] [Google Scholar]
- 13.Des Guetz G, Uzzan B, Nicolas P, Cucherat M, Morere JF, Benamouzig R, Breau JL, Perret GY. Microvessel density and VEGF expression are prognostic factors in colorectal cancer. Meta-analysis of the literature. Br J Cancer. 2006;94:1823–32. doi: 10.1038/sj.bjc.6603176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anastassiou G, Duensing S, Steinhoff G, Zorn U, Grosse J, Dallmann I, Kirchner H, Ganser A, Atzpodien J. Platelet endothelial cell adhesion molecule-1 (PECAM-1): a potential prognostic marker involved in leukocyte infiltration of renal cell carcinoma. Oncology. 1996;53:127–32. doi: 10.1159/000227548. [DOI] [PubMed] [Google Scholar]
- 15.Imao T, Egawa M, Takashima H, Koshida K, Namiki M. Inverse correlation of microvessel density with metastasis and prognosis in renal cell carcinoma. Int J Urol. 2004;11:948–53. doi: 10.1111/j.1442-2042.2004.00931.x. [DOI] [PubMed] [Google Scholar]
- 16.Rioux-Leclercq N, Epstein JI, Bansard JY, Turlin B, Patard JJ, Manunta A, Chan T, Ramee MP, Lobel B, Moulinoux JP. Clinical significance of cell proliferation, microvessel density, and CD44 adhesion molecule expression in renal cell carcinoma. Hum Pathol. 2001;32:1209–15. doi: 10.1053/hupa.2001.28957. [DOI] [PubMed] [Google Scholar]
- 17.Sabo E, Boltenko A, SovaY , Stein A, Kleinhaus S SovaY. Microscopic analysis and significance of vascular architectural complexity in renal cell carcinoma. Clin Cancer Res. 2001;7:533–7. [PubMed] [Google Scholar]
- 18.Schraml P, Struckmann K, Hatz F, Sonnet S, Kully C, Gasser T, Sauter G, Mihatsch MJ, Moch H. VHL mutations and their correlation with tumour cell proliferation, microvessel density, and patient prognosis in clear cell renal cell carcinoma. J Pathol. 2002;196:186–93. doi: 10.1002/path.1034. [DOI] [PubMed] [Google Scholar]
- 19.Joo HJ, Oh DK, Kim YS, Lee KB, Kim SJ. Increased expression of caveolin-1 and microvessel density correlates with metastasis and poor prognosis in clear cell renal cell carcinoma. BJU Int. 2004;93:291–6. doi: 10.1111/j.1464-410x.2004.04604.x. [DOI] [PubMed] [Google Scholar]
- 20.Nativ O, Sabo E, Reiss A, Wald M, Madjar S, Moskovitz B. Clinical significance of tumor angiogenesis in patients with localized renal cell carcinoma. Urology. 1998;51:693–6. doi: 10.1016/s0090-4295(98)00019-3. [DOI] [PubMed] [Google Scholar]
- 21.Yoshino S, Kato M, Okada K. Prognostic significance of microvessel count in low stage renal cell carcinoma. Int J Urol. 1995;2:156–60. doi: 10.1111/j.1442-2042.1995.tb00445.x. [DOI] [PubMed] [Google Scholar]
- 22.MacLennan GT, Bostwick DG. Microvessel density in renal cell carcinoma: lack of prognostic significance. Urology. 1995;46:27–30. doi: 10.1016/S0090-4295(99)80153-8. [DOI] [PubMed] [Google Scholar]
- 23.Minardi D, Lucarini G, Mazzucchelli R, Milanese G, Natali D, Galosi AB, Montironi R, Biagini G, Muzzonigro G. Prognostic role of Fuhrman grade and vascular endothelial growth factor in pT1a clear cell carcinoma in partial nephrectomy specimens. J Urol. 2005;174:1208–12. doi: 10.1097/01.ju.0000173078.57871.2d. [DOI] [PubMed] [Google Scholar]
- 24.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–34. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 25.Yusuf S, Peto R, Lewis J, Collins R, Sleight P. Beta blockade during and after myocardial infarction: an overview of the randomized trials. Prog Cardiovasc Dis. 1985;27:335–71. doi: 10.1016/s0033-0620(85)80003-7. [DOI] [PubMed] [Google Scholar]
- 26.Delahunt B, Bethwaite PB, Thornton A. Prognostic significance of microscopic vascularity for clear cell renal cell carcinoma. Br J Urol. 1997;80:401–4. doi: 10.1046/j.1464-410x.1997.00374.x. [DOI] [PubMed] [Google Scholar]
- 27.Imazano Y, Takebayashi Y, Nishiyama K, Akiba S, Miyadera K, Yamada Y, Akiyama S, Ohi Y. Correlation between thymidine phosphorylase expression and prognosis in human renal cell carcinoma. J. Clin. Oncol. 1997;15:2570–8. doi: 10.1200/JCO.1997.15.7.2570. [DOI] [PubMed] [Google Scholar]
- 28.Gelb AB, Sudilovsky D, Wu CD, Weiss LM, Medeiros LJ. Appraisal of intratumoral microvessel density, MIB-1 score, DNA content, and p53 protein expression as prognostic indicators in patients with locally confined renal cell carcinoma. Cancer. 1997;80:1768–75. doi: 10.1002/(sici)1097-0142(19971101)80:9<1768::aid-cncr11>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 29.Yoshino S, Kato M, Okada K. Evaluation of the prognostic significance of microvessel count and tumor size in renal cell carcinoma. Int J Urol. 1998;5:119–23. doi: 10.1111/j.1442-2042.1998.tb00258.x. [DOI] [PubMed] [Google Scholar]
- 30.Yoshino S, Kato M, Okada K. Clinical significance of angiogenesis, proliferation and apoptosis in renal cell carcinoma. Anticancer Res. 2000;20:591–4. [PubMed] [Google Scholar]
- 31.Song KH, Song J, Jeong GB, Kim JM, Jung SH, Song J. Vascular endothelial growth factor - its relation to neovascularization and their significance as prognostic factors in renal cell carcinoma. Yonsei Med J. 2001;42:539–46. doi: 10.3349/ymj.2001.42.5.539. [DOI] [PubMed] [Google Scholar]
- 32.Yagasaki H, Kawata N, Takimoto Y, Nemoto N. Histopathological analysis of angiogenic factors in renal cell carcinoma. Int J Urol. 2003;10:220–7. doi: 10.1046/j.0919-8172.2003.00608.x. [DOI] [PubMed] [Google Scholar]
- 33.Kawata N, Yagasaki H, Hirakata H, Nagane Y, Morita K, Sugimoto S, Igarashi T, Takimoto Y. The impact of angiogenesis on the prognosis of advanced renal cell carcinomas. Hinyokika Kiyo. 2004;50:157–63. [PubMed] [Google Scholar]
- 34.Yao X, Qian CN, Zhang ZF, Tan MH, Kort EJ, Yang XJ, Resau JH, Teh BT. Two distinct types of blood vessels in clear cell renal cell carcinoma have contrasting prognostic implications. Clin Cancer Res. 2007;13:161–9. doi: 10.1158/1078-0432.CCR-06-0774. [DOI] [PubMed] [Google Scholar]
- 35.Ren J, Liu H, Yan L, Tian S, Li D, Xu Z. Microvessel density and heparanase over-expression in clear cell renal cell cancer: correlations and prognostic significances. World J Surg Oncol. 2011;9:158. doi: 10.1186/1477-7819-9-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iakovlev VV, Gabril M, Dubinski W, Scorilas A, Youssef YM, Faragalla H, Kovacs K, Rotondo F, Metias S, Arsanious A, Plotkin A, Girgis AH, Streutker CJ, Yousef GM. Microvascular density as an independent predictor of clinical outcome in renal cell carcinoma: an automated image analysis study. Lab Invest. 2012;92:46–56. doi: 10.1038/labinvest.2011.153. [DOI] [PubMed] [Google Scholar]
- 37.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101. [PubMed] [Google Scholar]
- 39.Delmotte P, Martin B, Paesmans M, Berghmans T, Mascaux C, Meert AP, Steels E, Verdebout JM, Lafitte JJ, Sculier JP. VEGF and survival of patients with lung cancer: a systematic literature review and meta-analysis. Rev Mal Respir. 2002;19:577–84. [PubMed] [Google Scholar]
- 40.Zhan P, Wang J, Lv XJ, Wang Q, Qiu LX, Lin XQ, Yu LK, Song Y. Prognostic value of vascular endothelial growth factor expression in patients with lung cancer: a systematic review with meta-analysis. J Thorac Oncol. 2009;4:1094–103. doi: 10.1097/JTO.0b013e3181a97e31. [DOI] [PubMed] [Google Scholar]
- 41.Trivella M, Pezzella F, Pastorino U, Harris AL, Altman DG Prognosis In Lung Cancer (PILC) Collaborative Study Group. Microvessel density as a prognostic factor in non-smallcell lung carcinoma: a meta-analysis of individual patient data. Lancet Oncol. 2007;8:488–99. doi: 10.1016/S1470-2045(07)70145-6. [DOI] [PubMed] [Google Scholar]
- 42.Uzzan B, Nicolas P, Cucherat M, Perret GY. Microvessel density as a prognostic factor in women with breast cancer: a systematic review of the literature and meta-analysis. Cancer Res. 2004;64:2941–55. doi: 10.1158/0008-5472.can-03-1957. [DOI] [PubMed] [Google Scholar]
- 43.Weidner N, Folkman J, Pozza F, Bevilacqua P, Allred EN, Moore DH, Meli S, Gasparini G. Tumor angiogenesis: a new significant and independent prognostic indicator in early-stage breast carcinoma. J Natl Cancer Inst. 1992;84:1875–87. doi: 10.1093/jnci/84.24.1875. [DOI] [PubMed] [Google Scholar]
- 44.Leek RD. The prognostic role of angiogenesis in breast cancer. Anticancer Res. 2001;21:4325–32. [PubMed] [Google Scholar]
- 45.Wang J, Li K, Wang B, Bi J. Lymphatic microvessel density as a prognostic factor in non-small cell lung carcinoma: a meta-analysis of the literature. Mol Biol Rep. 2012;39:5331–38. doi: 10.1007/s11033-011-1332-y. [DOI] [PubMed] [Google Scholar]


