Abstract
The actin cytoskeleton is a dynamic structure with actin-binding proteins (ABPs) playing an essential role in the regulation of migration, differentiation and signal transduction in all eukaryotic cells. We examined the relationship between altered expression of four ABPs and clinical parameters in esophageal squamous cell carcinoma (ESCC). To this end, we analyzed 152 formalin-fixed and paraffin-embedded esophageal curative resection specimens by immunohistochemistry for tensin, profilin-1, villin-1 and talin. A molecular predictor model, based on the combined expression of the four proteins, was developed to correlate the expression pattern of the four ABPs with clinical factors and prognosis of ESCC. According to the results, weak significance was found for tensin in lymph node metastasis (P=0.033), and profilin-1 in pTNM stage (P=0.031). However, our four-protein model showed strong correlation with the 5-year overall survival rate (P=0.002). Similarly, Kendall’s tau-b test also showed the relationship between the collective expression pattern of the four ABPs with lymph node metastasis (P=0.005) and pTNM stage (P=0.001). Our results demonstrate that the collective protein expression pattern of four actin-binding proteins could be a biomarker to estimate the prognosis of ESCC patients.
Keywords: Actin-binding protein, a molecular prognostic model, prognosis, esophageal squamous cell carcinoma
Introduction
The actin cytoskeleton is comprised of a large group of proteins that exists in most types of cells, and plays a major role in eukaryotic cellular structure, migration, and endocytosis, and promotes invasion and metastasis of cancer cells [1]. The actin cytoskeleton may promote these processes by: 1) changing the shape of cells by participating in the formation of sheet-like, finger-like or bleb-like pseudopods [2], 2) inducing inside-out and outside-in signaling to regulate cell adhesion [3], and 3) generating force by dynamically assembling an actomyosin network to promote cell movement [4]. Many studies have revealed that the abnormal actin regulation is responsible for the invasion and metastasis of cancer cells [5].
These complex functions require precise regulation through modulation of actin-binding proteins (ABPs). Eukaryotic cells contain a large number of ABPs that regulate the structure and dynamics of the actin cytoskeleton in cells [1]. Tensin, localized at focal adhesions, binds to actin filaments and is involved in signaling pathways. Major binding domains of tensin include: 1) a phosphotyrosine-binding domain that binds the cytoplasmic tail of β-integrin, 2) a Src homology 2 domain that binds FAK, DLC-1, and PTEN, and 3) an N-terminal region related to PP1α [6]. Tensin regulates cell polarization, migration and invasion by protein phosphatase [7]. Profilin-1, a small actin-binding protein, is ubiquitously expressed in all types of cells. In general, profilin-1 binds to three classes of ligands, including actin, proteins containing poly-l-proline (PLP) stretches, and phosphoinositide (PPI)-based lipids [8]. Profilin-1 can promote the velocity of membrane protrusion by facilitating various PLP domain-bearing actin regulators, including N-WASP and Ena/VASP. Therefore, profilin-1 could be an important element in regulating actin dynamics and cell motility [8,9]. Villin-1 belongs to the gelsolin family, including gelsolin, severin, fragmin, and CapG [10]. However, villin-1 is unique in actin-capping, -bundling and -severing in epithelial cells, and influences the polymerization and depolymerization of actin filaments [11]. Talin is a focal adhesion protein that interacts with multiple adhesion molecules, including integrins, vinculin, focal adhesion kinase (FAK) and actin [12]. By binding to the NPXY motif of the integrin β subunit and actin, talin mediates the bidirectional signaling pathway between a cell and the ECM, making talin an important regulator of cell adhesion and motility [13,14].
Current research shows that the development of different cancers, including esophageal squamous cell carcinoma (ESCC), may result in abnormal expression of ABPs [15-17]. We choose to study tensin, villin-1, profilin-1 and talin, all of which are classical ABPs [1]. All have been shown to play an important role of the invasion and metastasis of cancer calls and affect the prognosis of patients in other malignancies [9,18,19]. However, the characteristics of these four proteins in ESCC remain unclear. Since actin-binding proteins synergize with each other to regulate cell function, it is highly possible that the expression pattern of the four-protein combination could accurately reflect tumor status and prognosis.
Materials and methods
Patients and tissue specimens
For the retrospective study, immunohistochemical staining was performed on 152 paraffin-embedded ESCC specimens and adjacent normal esophageal epithelium (16 cases) surgically resected at Shantou Central Hospital from 2000 to 2006. Among these patients, 34 cases were female and 118 cases were male. The median age was 58 years with a range of 31-75 years. Patients were followed up for a maximum period of 148.9 months, and minimum period of 1.3 months, with a median of 33 months. All of the tumors were confirmed as ESCC by the pathologists in Department of Clinical Pathology, the Central Hospital of Shantou City, and the cases were classified according to the seventh edition of the pathologic tumor-node-metastasis (pTNM) classification of the International Union against Cancer. Information about age, gender, stage of disease, and histopathological factors was obtained from medical records. Patient data is summarized in Table 1. This study was approved by the local ethics committee.
Table 1.
Clinicopathological characteristics of patients
| Characteristics | Five-year survival rate (%) |
|---|---|
| Age | |
| 57 or younger (n=75) | 46.7 |
| 58 or older (n=77) | 37.2 |
| Gender | |
| Male (n=114) | 39.7 |
| Female (n=38) | 48.4 |
| Histological grade | |
| Well differentiation (n=44) | 42.8 |
| Moderate differentiation (n=93) | 41.7 |
| Poor differentiation (n=15) | 40.9 |
| Depth of tumor invasion (T) | |
| Muscle (T2, n=8) | 52.5 |
| Serosa (T3, n=143) | 41.7 |
| Surrounding (T4, n=1) | 0 |
| Regional lymph node metastasis | |
| N0 (n=77) | 58.9 |
| N1 (n=75) | 24.3 |
| pTNM stage | |
| I/II (n=81) | 57.0 |
| III/IV (n=71) | 24.3 |
Tissue microarray and immunohistochemistry staining
Tissue microarrays (TMAs) were constructed as previously described [20-22]. 4 μm-thick tissue microarray sections were cut from the TMA blocks, dewaxed in xylene, rehydrated in alcohol, and incubated in 3% hydrogen peroxide for 10 min to block endogenous peroxidase activity. Antigen retrieval was performed by microwave oven heating (10 min) in 0.01 M sodium citrate buffer (pH 6.0). Sections were incubated with 10% normal goat serum in PBS for 15 min at room temperature to block nonspecific binding. Then sections were incubated overnight at 4°C with each of the four primary antibodies: rabbit anti-human tensin polyclonal antibody (sc-28542, 1:500 dilution, Santa Cruz Biotechnology, Inc. Dallas, Texas, USA), rabbit anti-human profilin-1 monoclonal antibody (EPR6304, 1:1000 dilution, Epitomics, Burlingame, California, USA), mouse anti-human villin-1 monoclonal antibody (66096-1-lg, 1:500 dilution, Proteintech Company, Wuhan, Hubei, China) and mouse anti-human Talin monoclonal antibody (sc-365460, 1:200 dilution, Santa Cruz Biotechnology, Inc. Dallas, Texas, USA). Then, bound antibodies were visualized with a PV-9000 2-Step Plus Poly-HRP Anti-Mouse/Rabbit IgG Detection System (ZSGB-BIO, Beijing, China) and a Liquid DAB Substrate Kit (Invitrogen, Carlsbad, CA, USA). Negative controls were prepared by substituting normal mouse or rabbit IgG for the primary antibodies.
Evaluation of immunohistochemical staining
Positive reactions were defined as those showing brown signals in the cell cytoplasm. Each separate tissue was scored on the basis of intensity and cells of positive staining. The intensity of positive staining was scored as follows: 0, negative; 1, weak staining; 2, moderate staining; 3, strong staining. The rate of positive cells was scored over a range of 0-4 as follows: 0, 0-5%; 1, 6-25%; 2, 26-50%; 3, 51-75%; 4, 75%. If the positive staining was homogeneous, a final score was achieved by multiplication of the two scores, producing a total range of 0-12. When the staining was not homogeneous, we scored it as follows: each component was scored independently and summed for the results. For example, a specimen containing 25% tumor cells with moderate intensity (1×2=2), 25% tumor cells with weak intensity (1×1=1). For statistical analysis, to reduce subjective error, every protein was graded by two persons at least. We divided all samples into two groups according to the final score. Negative expression group was set as follows: tensin score <5, profilin-1 <1 score, villin-1 score <1, and talin score ≤4. Positive expression group was set as follows: tensin score ≥5, profilin-1 score ≥1, villin-1 score ≥1, and talin score >4.
Combined protein analysis
To understand the association of tensin, profilin-1, villin-1 and talin expression with the survival of ESCC patients, a Cox proportional hazards regression analysis was used to evaluate the association between biomarker expression and survival of ESCC patients. We then constructed a model to estimate risk by summing the expression level of each biomarker (positive-expression =1, negative-expression =0) multiplied by its regression coefficient [23]. Patients were dichotomized into high- or low-risk groups using the 50th percentile (i.e., median) risk score as a cut-off value.
Statistical analysis
Statistical analyses were performed using SPSS 13.0 for Windows. The Kendall tau-b rank correlation analysis was used to evaluate the association between tensin, profilin-1, villin-1, and talin expression, and clinicopathological parameters including age, gender, differentiation grade, invasive depth, lymph node metastasis and pTNM stage. Cumulative survival time was calculated by the Kaplan-Meier method and analysed by the log-rank test. Univariate and multivariate analyses were based on the Cox proportional hazards regression model. P value less than 0.05 was considered significant.
Results
Actin-binding protein expression in normal esophagus epithelial tissue and ESCC
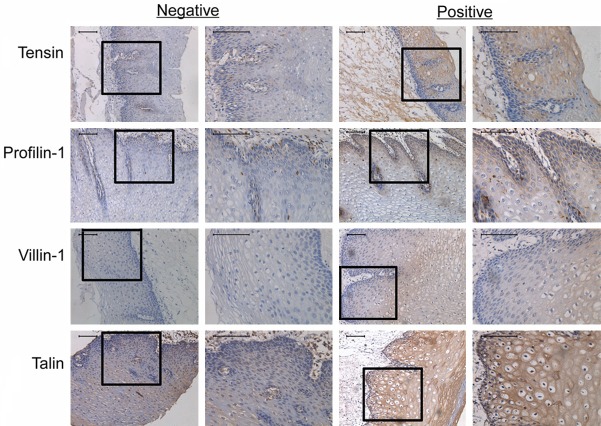
All four proteins were predominantly expressed in the cytoplasm of both normal esophagus epithelial and ESCC tissue (Figures 1 and 2). For normal esophageal epithelial tissue (Figure 1), out of a total of 15 patients, 3 were negative and 12 were positive for tensin expression. Profilin-1 staining was examined in 16 patients, 5 of whom were negative and 11 were positive. In 13 patients, 5 were negative and 8 were positive for villin-1 expression, and in a total of 10 patients, 2 were negative and 8 were positive for talin expression. The positive rates of tensin, profilin-1, villin-1 and talin expression were 80%, 68.8%, 61.5%, and 80%, respectively. In ESCC tissue, the positive intensity was very different from normal tissue (Figure 2). Statistical analysis revealed tensin score ranging from 0 to 7, with most scoring lower than 5. Profilin-1 and villin-1 scores ranged from 0 to 11, most scored lower than 4, and only one patient scored 11. Talin scores were well-distributed over the entire 0 to 12 range. When dividing the 152 cases into negative and positive expression groups, 95% (144/152) were negative for tensin, whereas only 13% (20/152), 12% (18/152), and 13% (20/152) were negative for profilin-1, villin-1, and talin, respectively.
Figure 1.
Expression of tensin, profilin-1, villin-1 and talin in human normal esophageal epithelium. All original micrographs are at the same magnification (400×). The embedded micrographs are at 200×. Scale bars =50 µm.
Figure 2.
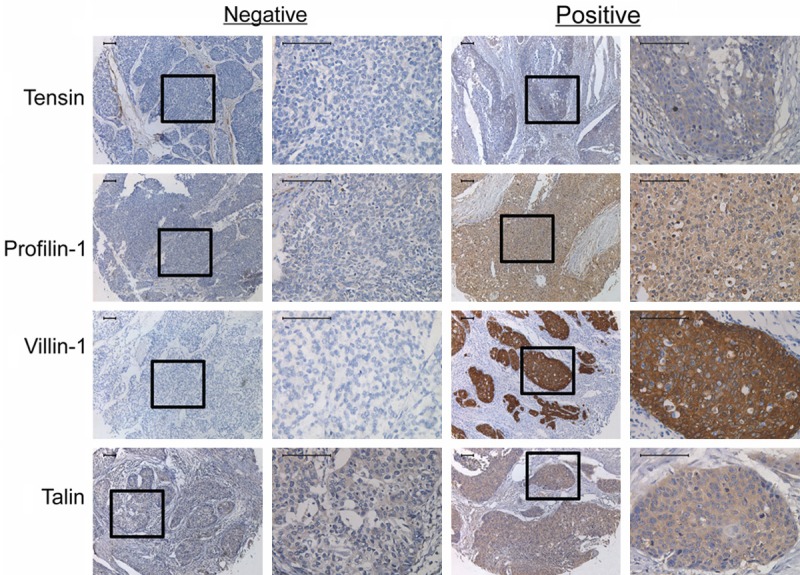
Expression of tensin, profilin-1, villin-1 and talin in human ESCC tissues. All original micrographs are at the same magnification (400×). The embedded micrographs are at 200×. Scale bars = 50 µm.
Actin-binding protein expression and clinical pathological parameters in ESCC
To determine the association of ABPs with clinical pathological parameters, including age, gender, differentiation, invasive depth and pTNM stage, we performed the Kendall’s tall-b test (Table 2). Factors showing significant correlations were lymph node metastasis (P=0.033) with tensin, and pTNM stage (P=0.031) with profilin-1. Neither Villin-1 nor talin showed any significant correlation with clinical parameters (data not shown).
Table 2.
Association of tensin and profilin-1 expression with clinical pathological parameters in ESCC
| Tensina | r, P * | Profilin-1b | r, P | |||
|---|---|---|---|---|---|---|
|
|
|
|||||
| – | + | – | + | |||
| Age (years) | ||||||
| <58 | 73 | 2 | 0.115, 0.276 | 9 | 66 | -0.034, 0.811 |
| ≥58 | 71 | 6 | 11 | 66 | ||
| Gender | ||||||
| Male | 108 | 6 | 0.000, 1.000 | 13 | 101 | -0.090, 0.406 |
| Female | 36 | 2 | 7 | 31 | ||
| Differentiation | ||||||
| G1 | 43 | 1 | 0.117, 0.207 | 3 | 41 | -0.149, 0.063 |
| G2 | 88 | 5 | 13 | 80 | ||
| G3 | 13 | 2 | 4 | 11 | ||
| Invasive depth | ||||||
| T2 | 7 | 1 | -0.078, 0.379 | 1 | 7 | 0.006, 1.000 |
| T3 | 136 | 7 | 19 | 124 | ||
| T4 | 1 | 0 | 0 | 1 | ||
| LN metastasisc | ||||||
| N0 | 76 | 1 | 0.180, 0.033 | 6 | 71 | -0.161, 0.057 |
| N1 | 68 | 7 | 14 | 61 | ||
| pTNM stage | ||||||
| I/II | 79 | 2 | 0.134, 0.147 | 6 | 75 | -0.182, 0.031 |
| III/IV | 65 | 6 | 14 | 57 | ||
The Kendall’s tau-b test;
p value <0.05 was considered significant.
LN: lymph node.
negative (–), score <5; positive (+), score ≥5.
negative (–), score <1; positive (+), score ≥1.
Association between ABPs and patient survival
We used the Kaplan-Meier test to identify the association between the ABPs and patient survival (Figure 3). Negative expression for tensin or villin-1 meant better survival compared to positive expression, while positive expression for profilin-1 or talin meant better survival compared to negative expression. However, all four proteins showed no statistical significance with survival of ESCC patients (tensin, P=0.071; villin-1, P=0.160; profilin-1, P=0.072; talin, P=0.146).
Figure 3.
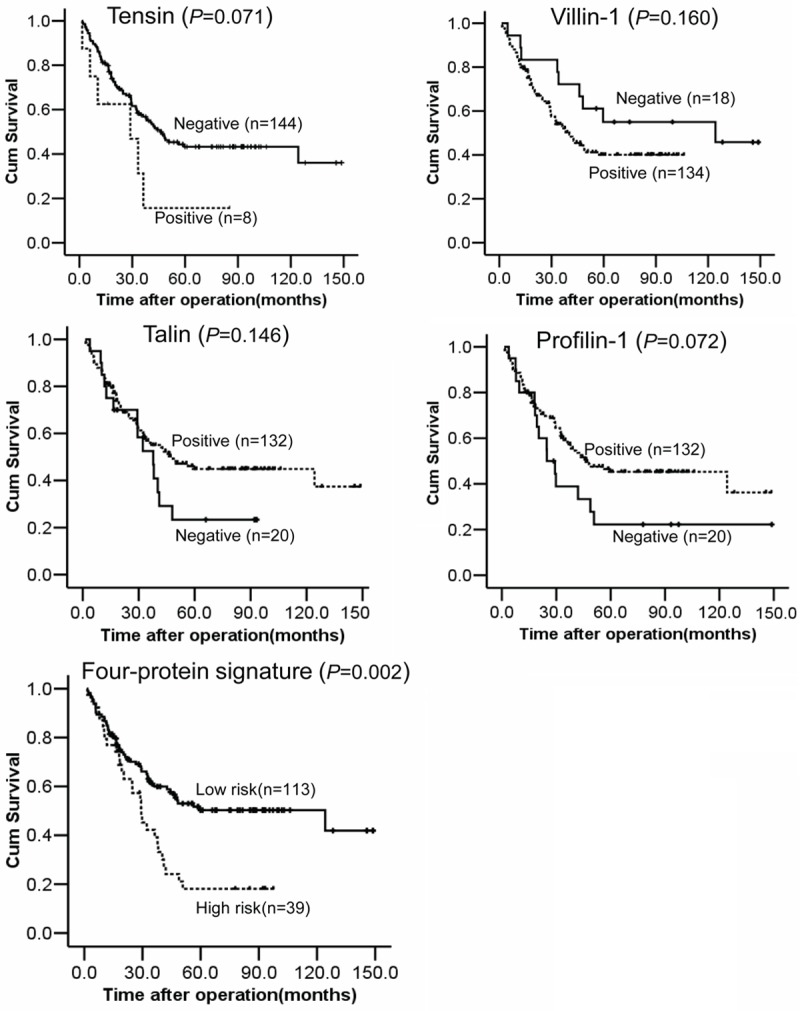
Overall survival of 152 patients with ESCC in relation to tension, villin-1, talin and profiln-1 expression, and the risk level of the four-protein signature. Survival rates were analyzed using the Kaplan-Meier survival test.
Four-protein signature analysis
Our molecular prognostic model was calculated as Y = (β1) × (tensin) + (β2) × (profilin-1) + (β3) × (villin-1) + (β4) × (talin), with Y equal to risk score and βn equal to each protein’s coefficient value from univariate Cox proportional hazards regression analysis. In the model, β1=0.749, β2=0.51, β3=0.519 and β4=0.425. Then 152 patients were dichotomized into a high and low risk group using the 50th percentile (median) cut-off of the combined final score value. Comparison of our four-protein signature analysis, for all 152 patients, showed strong correlation with Kaplan-Meier curves for overall survival (P=0.002), with the low risk score group predicted to have better prognosis than the high risk group (Figure 3). The Kendall’s tau-b test revealed significance for lymph node metastasis (P=0.005) and pTNM classification (P=0.001), whereas other parameters, such as age, gender, differentiation and invasive depth had no significance (Table 3). In the Cox regression models, only lymph node metastasis is an independent prognostic marker for shorter overall, with a relative risk of 2.827 [95.0% CI=1.652-4.542, P=0.000], while the four-protein model did not (data no shown).
Table 3.
Association between four-protein signature risk and clinical pathological parameters in ESCC
| Clinical parameters | Four-protein signature | r, P * | |
|---|---|---|---|
|
| |||
| Low risk | High risk | ||
| Age (years) | |||
| <58 | 60 | 15 | 0.128, 0.139 |
| ≥58 | 53 | 24 | |
| Gender | |||
| Male | 84 | 30 | -0.026, 0.832 |
| Female | 29 | 9 | |
| Differentiation | |||
| G1 | 33 | 11 | 0.073, 0.358 |
| G2 | 72 | 21 | |
| G3 | 8 | 7 | |
| Invasive depth | |||
| T2 | 7 | 1 | 0.51, 0.626 |
| T3 | 105 | 38 | |
| T4 | 1 | 0 | |
| LN metastasis | |||
| N0 | 65 | 12 | 0.234, 0.005 |
| N1 | 48 | 27 | |
| pTNM stage | |||
| I/II | 65 | 12 | 0.265, 0.001 |
| III/IV | 48 | 27 | |
Kendall’s tau-b test;
p value <0.05 was considered significant.
LN: lymph node.
Discussion
Esophageal cancer (EC) has the eighth highest incidence rate (456,000 new cases) and sixth highest mortality rates (400,000 deaths) worldwide, with an estimated 3.2% of total cancer cases, and 4.9% of cause of Cancer-related deaths in 2012, with nearly half of the EC cases and deaths occurring in the Far East, especially in China [24]. There are two main histological types, adenocarcinoma and squamous cell carcinoma [25]. Although the rates of esophageal adenocarcinoma in Western countries are increasing, ESCC is still the leading type of EC in the world [26]. Currently, the diagnosis and prognosis of ESCC still remain difficult. The 5-year overall survival is only 31.0% [27]. So it is vital to identify prognosis markers for ESCC. In our research, we used immunohistochemistry to detect the correlations between tensin, profilin-1, villin-1 and talin, and various clinicopathological parameters in ESCC patients. A molecular predictor model, based on the combined expression of the four ABPs, was also developed to study the association of the four ABPs with clinical factors and the prognosis of ESCC. According to our results, only weak significance could be observed between lymph node metastasis and tensin, and between pTNM stage and profilin-1. However, the four ABPs collectively demonstrate strong significance with 5-year overall survival rate in our four-protein model analysis, and a significant association with lymph node metastasis and pTNM stage, based on Kendall’s tau-b test.
Tensin is a family of multi-domain scaffold proteins that bind the cytoplasmic tail of β-integrins in adhesions that anchor stress fibers in cells [7]. Human clinical sample analysis found that human breast carcinoma, prostate carcinoma, head and neck squamous cell carcinoma, and melanoma have largely reduced expression of tensin [28]. It has also been shown that some cancer cell lines do not express detectable levels of tensin protein relative to normal fibroblasts that have abundant expression [29]. In our study, we find that tensin is mainly expressed in the cytoplasm and the positive frequency of tensin decreases in ESCC, in accord with prior investigators [7]. It is worth noting that lymphatic metastasis is closely related to the positive-expression of tensin (P=0.033), suggesting that tensin may be absent in early stages of ESCC and then become re-expressed, leading to lymphatic metastasis in late stages of cancer, indicative of poor prognosis.
Profilin-1 has been reported to inhibit the migration of tumor cells by reducing cell-cell adhesion, and is down-regulated in several different types of cancer [30-32]. Our study reveals that the prognosis of patients is significantly associated with regional lymph node metastasis and pTNM stage. Profilin-1 is principally expressed in the cytoplasm, and positive staining is more apparent in cancer cells than in normal cells. Profilin-1 staining displayed a similar trend as the survival curve, suggesting a possible association with prognosis, but the correlation between profilin-1 staining and 5-year overall survival did not reach significance, in conformity with prior investigators [30]. This may only indicate that profilin-1 plays a partly negative regulatory role in ESCC. Up-regulation of villin-1 mRNA occurs more frequently in the early recurrence patients as compared to the late recurrence patients, suggesting that the existence of a subtype of villin-1-positive cervical tumors have poor response to radiotherapy [33]. We were unable to detect a significant correlation between villin-1 expression and prognosis, although there is an obvious tendency for higher expression to correspond to poor prognosis.
Talin has an essential role in contact with integrin β subunits and actin filaments [34,35]. It has been reported that talin is associated with the differentiation of cancer, such as in HCC [36]. However, the regulation of talin expression in tumors is still a controversial area. Over-expression of talin-1 can promote prostate cancer cell adhesion, migration and invasion via activation of FAK and AKT signaling [37]. In HCC, levels of talin expression may be connected to the dedifferentiation of HCC and contribute to the higher rate of portal vein invasion. In our current study, cytoplasmic expression of talin in ESCC does not correlate with prognosis (P=0.146).
The above results show that we could not identify a significant relationship between prognosis and any individual ABP. This is likely due to the dynamic nature of the actin cytoskeleton being a complex process involving the co-participation of a variety of ABPs [39] as a general mechanism of actin assembly, WASP family proteins bind Arp2/3 to polymerize G-actin into dendritic actin networks. Then F-actin extends under the regulation of capping proteins and is finally anchored at the membrane in association with myosin-X, IRSp53, Ena/VASP and mDia2, and cross-linked with fascin [40]. In addition, many experiments showed a combined effect of ABPs in determining actin-based motility, and the involvement of multi-ABP mutations in cancer metastasis and invasion [1,41]. Therefore, it is not surprising that prognosis should correlate with combined ABP expression patterns rather than individual ABP expression, as supported by our current study demonstrating the significant correlation of our four ABPs with the prognosis of ESCC. In addition, our data also reveals the significance of the four-protein combination with lymph node metastasis and pTNM classification, further suggesting that multiple ABPs have an effect in prognostic with influencing the lymph node metastasis. In conclusion, the results of our study indicate that the combined expression pattern of actin-binding proteins can be a prognostic marker for patients. However, the specific mechanisms of the interaction between ABPs and ESCC still require further study.
Acknowledgements
We thank Dr. Stanley Li Lin, Department of Pathophysiology, The Key Immunopathology Laboratory of Guangdong Province, Shantou University Medical College, for the assistance in revising the manuscript. This work was supported by grants from the National Research Program to Xu LY (No. 2012CB526600), the National High Technology Research and Development Program of China to Li EM (No. 2012AA02A503 and No. 2012AA02A209), and the Natural Science Foundation of China-Guangdong Joint Fund to Li EM and Xu LY (No. U0932001 and No. U1301227).
Disclosure of conflict of interest
None.
References
- 1.Saarikangas J, Zhao H, Lappalainen P. Regulation of the actin cytoskeleton-plasma membrane interplay by phosphoinositides. Physiol Rev. 2010;90:259–89. doi: 10.1152/physrev.00036.2009. [DOI] [PubMed] [Google Scholar]
- 2.Ridley AJ. Life at the leading edge. Cell. 2011;145:1012–22. doi: 10.1016/j.cell.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 3.Li Z, Delaney MK, O’Brien KA, Du X. Signaling during platelet adhesion and activation. Arterioscler Thromb Vasc Biol. 2010;30:2341–49. doi: 10.1161/ATVBAHA.110.207522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clark K, Langeslag M, Figdor CG, van Leeuwen FN. Myosin II and mechanotransduction: a balancing act. Trends Cell Biol. 2007;17:178–86. doi: 10.1016/j.tcb.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Gross SR. Actin binding proteins: their ups and downs in metastatic life. Cell Adh Migr. 2013;7:199–213. doi: 10.4161/cam.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall EH, Balsbaugh JL, Rose KL, Shabanowitz J, Hunt DF, Brautigan DL. Comprehensive analysis of phosphorylation sites in Tensin1 reveals regulation by p38MAPK. Mol Cell Proteomics. 2010;9:2853–63. doi: 10.1074/mcp.M110.003665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall EH, Daugherty AE, Choi CK, Horwitz AF, Brautigan DL. Tensin1 requires protein phosphatase-1alpha in addition to RhoGAP DLC-1 to control cell polarization, migration, and invasion. J Biol Chem. 2009;284:34713–22. doi: 10.1074/jbc.M109.059592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ding Z, Bae YH, Roy P. Molecular insights on context-specific role of profilin-1 in cell migration. Cell Adh Migr. 2012;6:442–49. doi: 10.4161/cam.21832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bae YH, Ding Z, Zou L, Wells A, Gertler F, Roy P. Loss of profilin-1 expression enhances breast cancer cell motility by Ena/VASP proteins. J Cell Physiol. 2009;219:354–64. doi: 10.1002/jcp.21677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhai L, Zhao P, Panebra A, Guerrerio AL, Khurana S. Tyrosine phosphorylation of villin regulates the organization of the actin cytoskeleton. J Biol Chem. 2001;276:36163–67. doi: 10.1074/jbc.C100418200. [DOI] [PubMed] [Google Scholar]
- 11.Khurana S, George SP. Regulation of cell structure and function by actin-binding proteins: villin’s perspective. Febs Lett. 2008;582:2128–39. doi: 10.1016/j.febslet.2008.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harburger DS, Calderwood DA. Integrin signalling at a glance. J Cell Sci. 2009;122:159–63. doi: 10.1242/jcs.018093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Critchley DR, Gingras AR. Talin at a glance. J Cell Sci. 2008;121:1345–47. doi: 10.1242/jcs.018085. [DOI] [PubMed] [Google Scholar]
- 14.Anthis NJ, Campbell ID. The tail of integrin activation. Trends Biochem Sci. 2011;36:191–98. doi: 10.1016/j.tibs.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang H, Xu L, Xiao D, Xie J, Zeng H, Cai W, Niu Y, Yang Z, Shen Z, Li E. Fascin is a potential biomarker for early-stage oesophageal squamous cell carcinoma. J Clin Pathol. 2006;59:958–64. doi: 10.1136/jcp.2005.032730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cai HX, Yang LC, Song XH, Liu ZR, Chen YB, Dong GK. Expression of paxillin and FAK mRNA and the related clinical significance in esophageal carcinoma. Mol Med Rep. 2012;5:469–72. doi: 10.3892/mmr.2011.664. [DOI] [PubMed] [Google Scholar]
- 17.Xie JJ, Xu LY, Wu ZY, Zhao Q, Xu XE, Wu JY, Huang Q, Li EM. Prognostic implication of ezrin expression in esophageal squamous cell carcinoma. J Surg Oncol. 2011;104:538–43. doi: 10.1002/jso.21909. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura E, Iwakawa M, Furuta R, Ohno T, Satoh T, Nakawatari M, Ishikawa K, Imadome K, Michikawa Y, Tamaki T, Kato S, Kitagawa T, Imai T. Villin1, a novel diagnostic marker for cervical adenocarcinoma. Cancer Biol Ther. 2009;8:1146–53. doi: 10.4161/cbt.8.12.8477. [DOI] [PubMed] [Google Scholar]
- 19.Fang KP, Zhang JL, Ren YH, Qian YB. Talin-1 correlates with reduced invasion and migration in human hepatocellular carcinoma cells. Asian Pac J Cancer Prev. 2014;15:2655–61. doi: 10.7314/apjcp.2014.15.6.2655. [DOI] [PubMed] [Google Scholar]
- 20.Zhao Q, Shen JH, Shen ZY, Wu ZY, Xu XE, Xie JJ, Wu JY, Huang Q, Lu XF, Li EM, Xu LY. Phosphorylation of fascin decreases the risk of poor survival in patients with esophageal squamous cell carcinoma. J Histochem Cytochem. 2010;58:979–88. doi: 10.1369/jhc.2010.955765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun LL, Sun XX, Xu XE, Zhu MX, Wu ZY, Shen JH, Wu JY, Huang Q, Li EM, Xu LY. Overexpression of Jumonji AT-rich interactive domain 1B and PHD finger protein 2 is involved in the progression of esophageal squamous cell carcinoma. Acta Histochem. 2013;115:56–62. doi: 10.1016/j.acthis.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 22.Xu QX, Li EM, Zhang YF, Liao LD, Xu XE, Wu ZY, Shen JH, Xu LY. Overexpression of sigma1 receptor and its positive associations with pathologic TNM classification in esophageal squamous cell carcinoma. J Histochem Cytochem. 2012;60:457–66. doi: 10.1369/0022155412443542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kadara H, Behrens C, Yuan P, Solis L, Liu D, Gu X, Minna JD, Lee JJ, Kim E, Hong WK, Wistuba II, Lotan R. A five-gene and corresponding protein signature for stage-I lung adenocarcinoma prognosis. Clin Cancer Res. 2011;17:1490–501. doi: 10.1158/1078-0432.CCR-10-2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Globocan. Estimate cancer incidence, mortality and prevalence worldwide in 2012. 2012 [Google Scholar]
- 25.Zhang Y. Epidemiology of esophageal cancer. World J Gastroenterol. 2013;19:5598–606. doi: 10.3748/wjg.v19.i34.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 27.Zhang BH, Cheng GY, Xue Q, Gao SG, Sun KL, Wang YG, Mu JW, He J. Clinical outcomes of basaloid squamous cell carcinoma of the esophagus: a retrospective analysis of 142 cases. Asian Pac J Cancer Prev. 2013;14:1889–94. doi: 10.7314/apjcp.2013.14.3.1889. [DOI] [PubMed] [Google Scholar]
- 28.Rhodes DR, Yu J, Shanker K, Deshpande N, Varambally R, Ghosh D, Barrette T, Pandey A, Chinnaiyan AM. ONCOMINE: a cancer microarray database and integrated data-mining platform. Neoplasia. 2004;6:1–6. doi: 10.1016/s1476-5586(04)80047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen H, Ishii A, Wong WK, Chen LB, Lo SH. Molecular characterization of human tensin. Biochem J. 2000;351:403–11. [PMC free article] [PubMed] [Google Scholar]
- 30.Zou L, Jaramillo M, Whaley D, Wells A, Panchapakesa V, Das T, Roy P. Profilin-1 is a negative regulator of mammary carcinoma aggressiveness. Br J Cancer. 2007;97:1361–71. doi: 10.1038/sj.bjc.6604038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Janke J, Schluter K, Jandrig B, Theile M, Kolble K, Arnold W, Grinstein E, Schwartz A, Estevez-Schwarz L, Schlag PM, Jockusch BM, Scher- neck S. Suppression of tumorigenicity in breast cancer cells by the microfilament protein profilin 1. J Exp Med. 2000;191:1675–86. doi: 10.1084/jem.191.10.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gronborg M, Kristiansen TZ, Iwahori A, Chang R, Reddy R, Sato N, Molina H, Jensen ON, Hruban RH, Goggins MG, Maitra A, Pandey A. Biomarker discovery from pancreatic cancer secretome using a differential proteomic approach. Mol Cell Proteomics. 2006;5:157–71. doi: 10.1074/mcp.M500178-MCP200. [DOI] [PubMed] [Google Scholar]
- 33.Xieraili M, Yasen M, Mogushi K, Obulhasim G, Mayinuer A, Aihara A, Tanaka S, Mizushima H, Tanaka H, Arii S. Villin 1 is a predictive factor for the recurrence of high serum alpha-fetoprotein-associated hepatocellular carcinoma after hepatectomy. Cancer Sci. 2012;103:1493–501. doi: 10.1111/j.1349-7006.2012.02315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Critchley DR. Cytoskeletal proteins talin and vinculin in integrin-mediated adhesion. Biochem Soc Trans. 2004;32:831–36. doi: 10.1042/BST0320831. [DOI] [PubMed] [Google Scholar]
- 35.Nayal A, Webb DJ, Horwitz AF. Talin: an emerging focal point of adhesion dynamics. Curr Opin Cell Biol. 2004;16:94–98. doi: 10.1016/j.ceb.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 36.Zhang JL, Qian YB, Zhu LX, Xiong QR. Talin1, a valuable marker for diagnosis and prognostic assessment of human hepatocelluar carcinomas. Asian Pac J Cancer Prev. 2011;12:3265–69. [PubMed] [Google Scholar]
- 37.Sakamoto S, McCann RO, Dhir R, Kyprianou N. Talin1 promotes tumor invasion and metastasis via focal adhesion signaling and anoikis resistance. Cancer Res. 2010;70:1885–95. doi: 10.1158/0008-5472.CAN-09-2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kanamori H, Kawakami T, Effendi K, Yamazaki K, Mori T, Ebinuma H, Masugi Y, Du W, Nagasaka K, Ogiwara A, Kyono Y, Tanabe M, Saito H, Hibi T, Sakamoto M. Identification by differential tissue proteome analysis of talin-1 as a novel molecular marker of progression of hepatocellular carcinoma. Oncology. 2011;80:406–15. doi: 10.1159/000330734. [DOI] [PubMed] [Google Scholar]
- 39.Khurana S, George SP. The role of actin bundling proteins in the assembly of filopodia in epithelial cells. Cell Adh Migr. 2011;5:409–20. doi: 10.4161/cam.5.5.17644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hashimoto Y, Kim DJ, Adams JC. The roles of fascins in health and disease. J Pathol. 2011;224:289–300. doi: 10.1002/path.2894. [DOI] [PubMed] [Google Scholar]
- 41.Goult BT, Zacharchenko T, Bate N, Tsang R, Hey F, Gingras AR, Elliott PR, Roberts GC, Ballestrem C, Critchley DR, Barsukov IL. RIAM and vinculin binding to talin are mutually exclusive and regulate adhesion assembly and turnover. J Biol Chem. 2013;288:8238–49. doi: 10.1074/jbc.M112.438119. [DOI] [PMC free article] [PubMed] [Google Scholar]