Abstract
Epithelial-to-mesenchymal transition (EMT) has been shown to play an important role in renal fibrogenesis. Recent studies suggested parathyroid hormone (PTH) could accelerate EMT and subsequent organ fibrosis. However, the precise molecular mechanisms underlying PTH-induced EMT remain unknown. The present study was to investigate whether Wnt/β-catenin signaling pathway is involved in PTH-induced EMT in human renal proximal tubular cells (HK-2 cells) and to determine the profile of gene expression associated with PTH-induced EMT. PTH could induce morphological changes and gene expression characteristic of EMT in cultured HK-2 cells. Suppressing β-catenin expression or DKK1 limited gene expression characteristic of PTH-induced EMT. Based on the PCR array analysis, PTH treatment resulted in the up-regulation of 18 genes and down-regulation of 9 genes compared with the control. The results were further supported by a western blot analysis, which showed the increased Wnt4 protein expression. Wnt4 overexpression also promotes PTH-induced EMT in HK-2 cells. The findings demonstrated that PTH-induced EMT in HK-2 cells is mediated by Wnt/β-catenin signal pathway, and Wnt4 might be a key gene during PTH-induced EMT.
Keywords: Epithelial-to-mesenchymal transition, parathyroid hormone, renal tubular epithelial cell, Wnt/β-catenin signal pathway
Introduction
Chronic kidney disease (CKD) has been a global public health problem, and renal interstitial fibrosis (RIF) is the common characteristic of CKD leading to end-stage renal failure [1]. Many research findings from the pathological changes in human renal diseases and experimental kidney disease models showed that the deterioration of renal function is determined by the extent and severity of tubulointerstitial fibrosis. Though therapeutic interventions retard the progression of renal disease in experimental models and human CKD clinical trials, there is no specific treatment or intervention available to prevent these processes [2-4]. Interstitial fibrosis is essentially a process of myofibroblast proliferation and excessive accumulation of extracellular matrix. The main pathological features are inflammatory-cell infiltration, tubular atrophy, capillary loss and abundant extracellular matrix (ECM) accumulation. The matrix component is synthesized and secreted by fibroblasts. Renal interstitial fibroblasts have three main sources, including renal interstitial fibroblasts, circulating mesenchymal cells and renal tubular epithelial-to-mesenchymal transition (EMT). Recently the role of EMT in tubulointerstitial fibrosis has received much attention [5-7]. The EMT of the renal tubule is regulated by different growth factors, cytokines, hormones and extracellular signals [8,9]. Transforming growth factor-β1 (TGF-β1) is regarded as one of the most important cytokines leading to EMT. As the core factor, TGF-β1 can promote and regulate renal tubular EMT under pathological condition.
Secondary hyperparathyroidism (SHPT) is the common complications in patients with CKD [10]. Biochemical and histological evidence show that elevated levels of parathyroid hormone (PTH) typically occur when the glomerular filtration rate (GFR) is < 70 mL/min. PTH is a polypeptide of 84 amino acids, which initiate signaling pathways by interacting with its receptor to exert its biological activity. In addition to causing renal osteodystrophy, it affects cardiovascular system, nervous system, lipid metabolism, skin and other tissues and organs, and indirectly accelerates the decline in renal function [11-13]. PTH receptors have been found in renal mesangial cells and renal tubular epithelial cells [14]. In the previous study, we have demonstrated that PTH could induce connective tissue growth factor (CTGF) upregulation in renal tubular cells, which strongly suggest that PTH play an important role in the fibrotic process and could induce EMT in HK-2 cells [15,16]. However, the underlying mechanisms remain unknown.
The role of Wnt/β-catenin pathway in RIF gradually comes to be known [17,18]. The expression of Wnt4 could be induced in the murine kidney 48 h after unilateral ureteral obstruction (UUO), and showed a continuous increase of up for 4 weeks [19]. Nonphosphorylated β-catenin level increased in the cytoplasm, accompany with the increased level of fibronectin and α-SMA. Wnt4 is also involved in the pathogenesis of acute renal failure. The increased level of vimentin was observed in the kidney from 56 patients undergoing renal transplantation after 3 months [20]. The entry of β-catenin to nuclear significantly increased and it interacted with the members of the transcription factor LEFI/TCF to promote EMT. Wnt/β-catenin pathway could also damage the basement membrane of renal tubular epithelial cells by regulating the expression of matrix metalloproteinases-7 (MMP-7) to promote cell migration and RIF [21]. These studies have showed the important role of Wnt/β-catenin pathway in the renal tubule. We hypothesized that Wnt/β-catenin pathway might be involved in PTH-induced renal interstitial fibrosis. In the present study, we investigated whether Wnt/β-catenin pathway is involved in PTH-induced EMT in cultured human renal proximal tubular cells.
Materials and methods
Cell culture and treatment
Human kidney proximal tubular cell line HK-2 was obtained from the Institute of Basic Medical Science, Beijing, China. The cells were grown in Dulbecco’s Modified Eagle Medium (DMEM, Gibco, Germany)/F-12 supplemented with 10% fetal bovine serum (FBS), penicillin and streptomycin, and maintained at 37°C in humidified air with 5% CO2. HK-2 cells were incubated with serum-free DMEM/F-12 for 24 h before treatment with PTH (Sigma, USA). The cells were treated with PTH for the indicated period of time with or without dickkopf-related protein 1 (DKK1, Peprotech, USA).
RNA interference
Sequences of β-catenin siRNA were designed and synthesized by GenePharma, (Shanghai, China). β-catenin siRNA contains 4 individual siRNAs. β-catenin-siRNA-1: 5’- CUG CGG AAG AUG GGA UCA ATT -3’ (sense) and 5’- UUG AUC CCA UCU UCC GCA GTT -3’ (antisense). β-catenin-siRNA-2: 5’- GGA GAG UAC AUU UGC UUU ATT -3’ (sense) and 5’- UAA AGC AAA UGU ACU CUC CTT -3’ (antisense). β-catenin-siRNA-3: 5’- CCU CUU UGU AGC UCC UAU ATT -3’ (sense) and 5’- UAU AGG AGC UAC AAA GAG GTT -3’ (antisense). β-catenin-siRNA-4: 5’- GGC UCC AUA UUU CAA CUA ATT -3’ (sense) and 5’- UUA GUU GAA AUA UGG AGC CTT -3’ (antisense). The negative control siRNA was also purchased from GenePharma. The cells were transfected with β-catenin siRNA or negative control siRNA using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instruction.
Plasmid construction and transfection
To generate the Wnt4 expression vector, the open reading frame of human Wnt4 cDNA was cloned into the eukaryotic expression vector pcDNA 3.1. The primers used for the amplification of the open-reading frame of the Wnt4 cDNA were CTTAAGCTTGCCGCCACCATGAGT (forward primer) and TGTGAATTCTCATCGGCACGTGTGCA (reverse primer). For transient transfection, the cells were transfected with pcDNA 3.1 vector or pcDNA 3.1-Wnt4 at about 80% confluence using the Lipofectamine™2000 (Invitrogen, San Diego, CA, USA) reagent according to the manufacturer’s instructions.
Quantitative real-time PCR (qRT-PCR)
Following treatment, cells were harvested and total RNA was immediately extracted using TRIzol reagent (Invitrogen) according to the manufacture’s instructions. For expression analysis of α-SMA and E-cadherin genes, two μg of total RNA was used to synthesize first strand DNA with reverse transcriptase according to the manufacturer’s protocol (Promega). Quantitative RT-PCR (qRT-PCR) was performed with a Green PCR Master Mix Kit (Shanghai, Shine Co., China). Breifly, one microliter of first strand cDNA and gene specific primers were used along with Hostart Fluo-PCR Mix in a 20 μL reaction under the following conditions: pre-denaturation at 95°C for 5 min, 35 cycles of denaturation at 95°C for 10 sec, annealing at 57°C for 15 sec, and extension at 72°C for 20 sec. Each sample was performed in triplicate and was quantified based on the formula 2-ΔC’T. The primer pairs for qRT-PCR are listed in Table 1.
Table 1.
Primer pairs for qRT-PCR
Human Wnt signaling pathway plus RT2 profiler PCR array
For the analysis of Wnt signaling pathway related genes involved in PTH-induced EMT, human Wnt signaling pathway plus RT2 Profiler PCR Array was employed (OIAGEN). cDNA was synthesized using RT2 First Strand Kit (Qiagen) and samples analyzed for expression of 84 genes involved in PTH-induced EMT by RT2 Profiler PCR Array. The data analysis was performed based on the ΔΔCT method to calculate the ΔCt for each pathway and for each gene across two PCR arrays. The fold-change for each gene was calculated as 2-ΔΔCT.
Western blotting
After PTH treatment, cells were lysed in ice-cold RIPA lysis buffer. Lysates were centrifuged at 13200 rpm for 20 min at 4°C to obtain the proteins, and then protein concentration was determined by Lowry assay with modification. The protein lysates were separated by SDS-PAGE and then transferred to PVDF membrane (Millipore). The membranes were blocked with 5% non-fat milk solution for 1 h at room temperature (RT) and incubated in primary antibody dissolved in block solution at 4°C overnight. The proteins were probed by antibody against TGF-β1, E-cadherin, α-smooth muscle actin (α-SMA), β-catenin or GAPDH. After washing, the membrane was incubated with horseradish peroxidase-conjugated secondary antibody corresponding to the primary antibody for 1 h at room temperature, and visualized with using ECL at RT following the washing step. The relative protein levels were determined by densitometry of the bands with Sxlmage software (Shanghai, China).
Immunofluorescence staining
After PTH treatment, cells were fixed and permeated with 4% paraformaldehyde for 10, followed by blocking in 10% normal goat serum for 20 min at RT, and then incubated with the primary antibody for 2 h at 37°C. After washing, the slides were incubated with FITC/TRITC-conjugated secondary antibody, followed by nuclear counterstaining with DAPI for 5 min. Cells were visualized under a fluorescence microscope and photographs were recorded.
Statistical analysis
Data were expressed as mean ± SEM of 3 independent experiments, and t-test and variance analysis were conducted using the statistical software SPSS12.0. Each treatment group was compared with the control group with Dunnett’s t-test, and P-value less than 0.05 was considered significant.
Results
PTH induces morphological changes and gene expression characteristic of EMT in cultured HK-2 cells
The EMT-associated properties of HK-2 cells after PTH treatment were observed under our culture conditions. Stimulation of HK-2 cells with PTH induced a change of morphology consistent with EMT (Figure 1A). PTH also significantly reduced E-cadherin mRNA levels while simultaneously increasing expression of α-SMA in a time-dependent manner (Figure 1B). To confirm these mRNA changes, we assessed the effects of PTH on E-cadherin and α-SMA proinvolved in PTH-induced EMT of HK-2 cells. β-catenin expression was suppressed using siRNA. Two days following the transfection with β-catenin siRNA, the level of β-catenin expression was reduced by ~70% as compared with the cells transfected with negative control siRNA (Figure 2A and 2B). After PTH treatment, control cells expressing negative control siRNA showed decreased expression of E-cadherin and increased expression of α-SMA during EMT. Cells transiently transfected with β-catenin siRNA exhibited increased expression of E-cadherin and decreased expression of α-SMA in contrast to negative control siRNA cells (Figure 2C and 2D), suggesting the activity of Wnt/β-catenin signal pathway may be responsible for PTH-induced EMT. Then, DKK1, an antagonist of the Wnt/β-catenin signaling pathway, was used to further confirm the role of Wnt/β-catenin signal pathway in PTH-induced EMT in HK-2 cells. After PTH treatment, the expression of E-cadherin decreased, and the expression of α-SMA increased. However, the expression of E-cadherin was upregulated amd and α-SMA downregulated in cell treated with PTH plus DKK1, when compared with cells treated with PTH alone, suggesting DKK1 inhibits PTH induction mediated by Wnt/β-catenin signal pathway (Figure 2E and 2F). Taken together, the results indicated that PTH-induced EMT in HK-2 cells is mediated by Wnt/β-catenin signal pathway.
Figure 1.
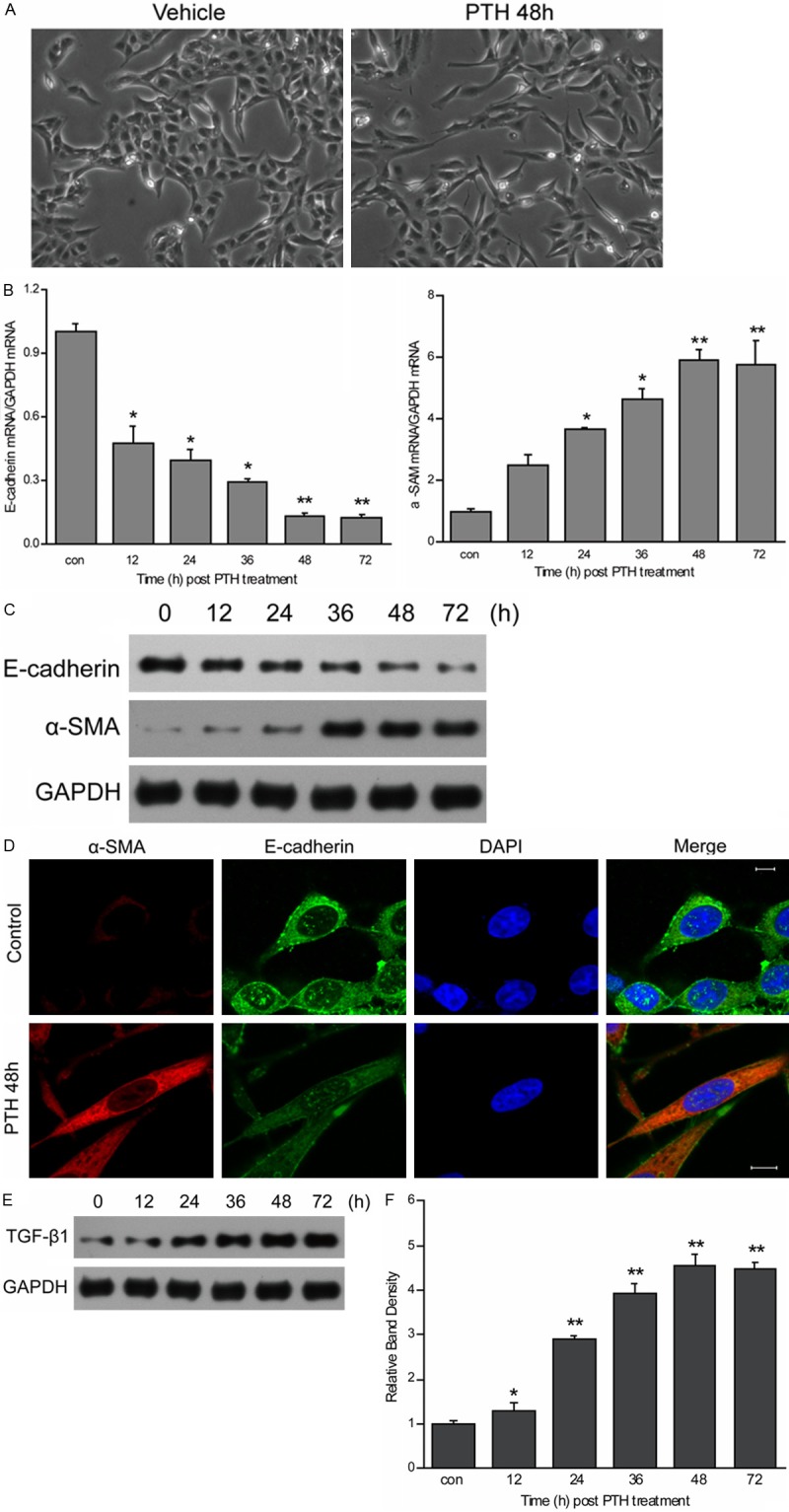
EMT was induced by PTH. (A) HK-2 cells were cultured in medium with or without 10-10 M PTH for 48 h. Cellular morphology was photographed by phase contrast microscope. (B) HK-2 cells were exposed to 10-10 M PTH for 12, 24, 36, 48 and 72 h. The mRNA expression of E-cadherin and α-SMA were quantified by qRT-PCR. GAPDH was used as an internal control. Results are expression as the mean ± SE (*P < 0.05, **P < 0.01 compared with control). (C) HK-2 cells were treated as (B). Cell lysates were prepared, and the protein expression of E-cadherin and α-SMA was detected using western blotting with anti-E-cadherin antibody and anti-α-SMA antibody. GAPDH was used as an internal control. (D) The loss of expression of E-cadherin and the increase of expression of α-SMA were confirmed by immunofluorescence. (E, F) HK-2 cells were treated as B. The protein expression of TGF-β1 was detected by western blotting. GAPDH was used as an internal control. Results are expression as the mean ± SE (*P < 0.05, **P < 0.01 compared with control).
Figure 2.
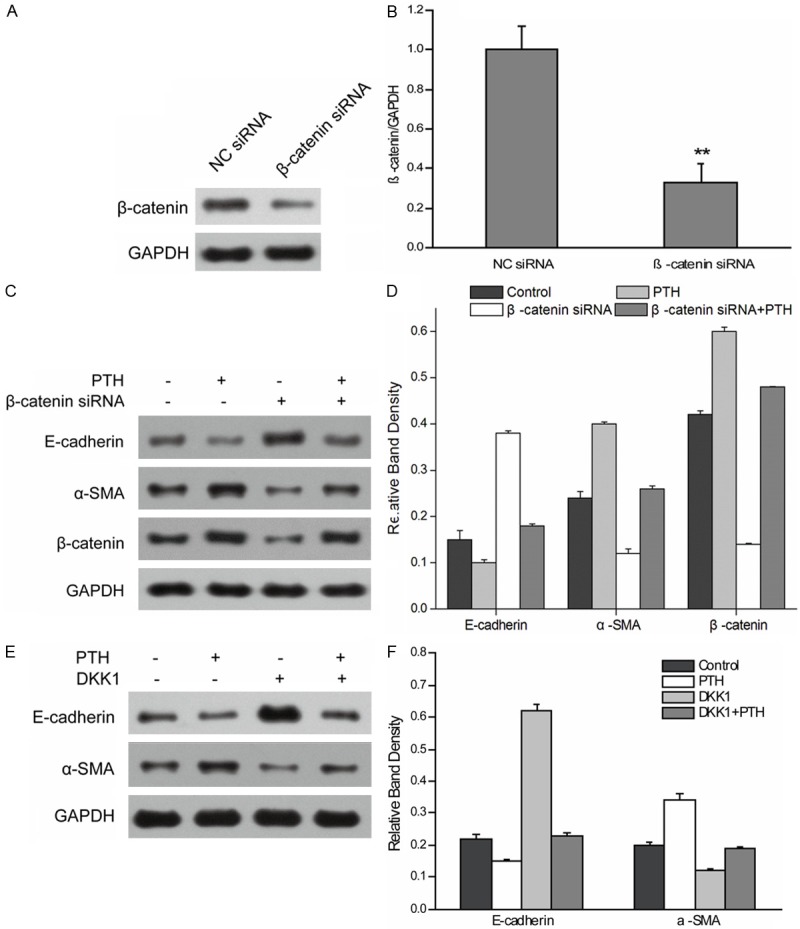
Wnt/β-catenin pathway is required for PTH-induced EMT in HK-2 cells. A, B. HK-2 cells were transfected with β-catenin siRNA or negative control siRNA (NC siRNA). After transfection for 48 h, cells were collected and β-catenin expression was revealed by western blot. GAPDH from the same loading was used as control. Data shown are means ± SEM from three independent cell preparations, **P < 0.01. C, D. Control siRNA and β-catenin siRNA cells were treated without or with PTH for 48 h. Blots were probed with antibodies for E-cadherin, α-SMA, and β-catenin. GAPDH from the same loading was used as control. Results are expression as the mean ± SE. E, F. After treatment for 48 h with PTH with or without DKK1, cells were collected and cell lysates were prepared. The protein expression of E-cadherin and α-SMA was detected using western blotting with anti-E-cadherin antibody and anti-α-SMA antibody. GAPDH was used as an internal control. Results are expression as the mean ± SE.
Wnt/β-catenin pathway related gene expression profile in HK-2 cells after PTH induction
To gain further insight into the role of Wnt/β-catenin in PTH-induced EMT in HK-2 cells, the expression profiles of PTH-treated HK-2 cells were compared to that of control group using human Wnt signaling pathway plus RT2 Profiler PCR Array, which contain 84 genes related to Wnt-mediated signal transduction. Using filtering criteria of a 2.0 or greater fold-change in expression, we analyzed the differentially expressed genes in the two types of cells. Of the 84 genes examined, 27 showed a > 2.0-fold change in expression: 18 up-regulated and 9 down-regulated genes (Table 2). Positive regulators include CSNK2A1, DAAM1, DKK1, FRAT1, FZD3, FZD6, MMP7, MYC, NKD1, RHOA, SFRP1, VANGL2, WISP1, WNT1, WNT10A, WNT16, WNT2B and WNT4, while negative reugulators have APC, FZD5, FZD9, PPARD, SFRP4, SOX17, TCF7, WNT5A and WNT7B.
Table 2.
Wnt/β-catenin pathway related gene expression differences in HK-2 cells after PTH treatment
| # | Gene Bank | Symbol | Gene description | Fold up/down | P value |
|---|---|---|---|---|---|
| 1 | NM_001895 | CSNK2A1 | Casein kinase 2, alpha 1 polypeptide | +2.39 | 0.015011 |
| 2 | NM_014992 | DAAM1 | Disheveled associated activator of morphogenesis 1 | +3.13 | 0.001121 |
| 3 | NM_012242 | DKK1 | Dickkopf WNT signaling pathway inhibitor 1 | +2.27 | 0.008531 |
| 4 | NM_005479 | FRAT1 | Frequently rearranged in advanced T-cell lymphomas 1 | +2.09 | 0.000127 |
| 5 | NM_017412 | FZD3 | Frizzled class receptor 3 | +2.89 | 0.002186 |
| 6 | NM_003506 | FZD6 | Frizzled class receptor 6 | +2.06 | 0.000127 |
| 7 | NM_002423 | MMP7 | Matrix metallopeptidase 7 | +2.20 | 0.000004 |
| 8 | NM_002467 | MYC | V-myc avian myelocytomatosis viral oncogene homolog | +2.64 | 0.021895 |
| 9 | NM_033119 | NKD1 | Naked cuticle homolog 1 | +3.92 | 0.000101 |
| 10 | NM_001664 | RHOA | Ras homolog family member A | +2.56 | 0.013018 |
| 11 | NM_003012 | SFRP1 | Secreted frizzled-related protein 1 | +6.17 | 0.000027 |
| 12 | NM_020335 | VANGL2 | VANGL planar cell polarity protein 2 | +2.20 | 0.000582 |
| 13 | NM_003882 | WISP1 | WNT1 inducible signaling pathway protein 1 | +2.01 | 0.000017 |
| 14 | NM_005430 | WNT1 | Wingless-type MMTV integration site family, member 1 | +2.19 | 0.001214 |
| 15 | NM_025216 | WNT10A | Wingless-type MMTV integration site family, member 10A | +2.45 | 0.008442 |
| 16 | NM_057168 | WNT16 | Wingless-type MMTV integration site family, member 16 | +2.55 | 0.000142 |
| 17 | NM_004185 | WNT2B | Wingless-type MMTV integration site family, member 2B | +2.10 | 0.000392 |
| 18 | NM_030761 | WNT4 | Wingless-type MMTV integration site family, member 4 | +2.08 | 0.000138 |
| 19 | NM_000038 | APC | Adenomatous polyposis coil | -2.41 | 0.000087 |
| 20 | NM_003468 | FZD5 | Frizzled class receptor 5 | -2.38 | 0.000127 |
| 21 | NM_003508 | FZD9 | Frizzled class receptor 9 | -3.24 | 0.001747 |
| 22 | NM_006238 | PPARD | Peroxisome proliferator-activated receptor delta | -2.33 | 0.005087 |
| 23 | NM_003014 | SFRP4 | Secreted frizzled-related protein 4 | -3.89 | 0.005834 |
| 24 | NM_022454 | SOX17 | SRY (sex determining region Y)-box 17 | -2.11 | 0.002714 |
| 25 | NM_003202 | TCF7 | Transcription factor 7 (T-cell specific, HMG-box) | -7.09 | 0.001325 |
| 26 | NM_003392 | WNT5A | Wingless-type MMTV integration site family, member 5A | -2.11 | 0.002027 |
| 27 | NM_058238 | WNT7B | Wingless-type MMTV integration site family, member 7B | -3.50 | 0.001124 |
Overexpression of Wnt4 promote PTH-induced EMT in HK-2 cells
HK-2 cells were transfected with the pcDNA3.1-Wnt for Wnt overexpression. The result showed that the expression of Wnt in cells transfected with pcDNA3.1-Wnt4 is higher than that in the cells transfected with pcDNA3.1 vector. After PTH treatment, the cells expressing pcDNA3.1 vector showed decreased of E-cadherin and increased expression of α-SMA during EMT. The cells transiently transfected with pcDNA3.1-Wnt4 exhibited decreased expression of E-cadherin and increased expression of α-SMA in contrast to the cells expressing pcDNA3.1 vector (Figure 3). The result indicated the up-regulation of Wnt promotes PTH-induced EMT, suggesting Wnt4 is important for PTH-induced EMT mediated by Wnt/β-catenin signaling pathway.
Figure 3.
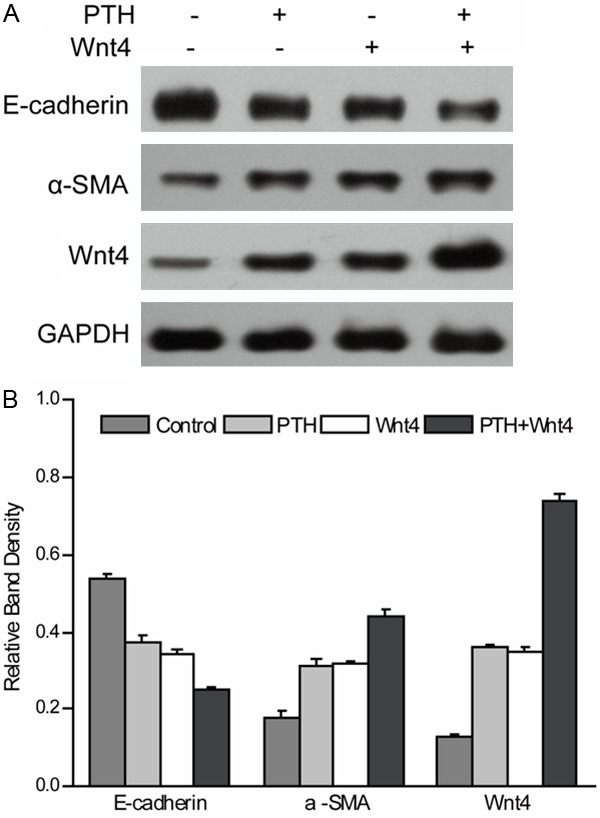
Overexpression of WNT4 promote PTH-induced EMT in HK-2 cell. Cells transfected with pcDNA 3.1 vector or pcDNA 3.1-Wnt4 were treated without or with PTH for 48 h. Blots were probed with antibodies for E-cadherin, α-SMA, and Wnt4. GAPDH from the same loading was used as control. Results are expression as the mean ± SE.
Discussion
A number of investigations into the molecular events of EMT in renal fibrosis have paved the way for the design of improved specific therapies. In the present study, we investigated the role of Wnt/β-catenin pathway in PTH-induced EMT and determined the profile of gene expression associated with PTH-induced EMT, which broaden knowledge of the precise molecular mechanism mediating PTH-induced EMT, which is important for developing strategies to inhibit or reverse EMT in renal fibrosis.
As a conservative transduction pathway in the evolutionary process, the Wnts widely affects many physiological and pathological processes [22]. Wnt ligands could elicit distinct subcellular events based on the environment. Upon binding of the Wnt ligand to its receptor, there are several distinct signal transduction cascades that can be grouped as the canonical Wnt pathway, the Wnt-Ca2+ pathway, and the Wnt planar cell polarity pathway [23-25]. The canonical Wnt pathway (Wnt/β-catenin pathway) is silenced in normal kidney, while it is activated in diseased adult kidney [26]. In Wnt/β-catenin pathway, Wnt binds to its receptors Frizzled and low density lipoprotein receptor-related protein (LRP) that results in activation and phosphorylation of LRP. Activated LRP6 recruits Dishvelled and Axin and then inhibits glycogen synthase kinase-3β (GSK-3β)-mediated phosphorylation and proteosomal degradation of β-catenin. β-catenin accumulates in the nucleus and regulates gene expression through transcription factor T cell factor (TCF) and/or lymphoid enhancer factor (LEF) [27,28].
In the obstructive nephropathy model of renal fibrosis, Nguyen et al. found that obstruction induced during metanephrogenesis disrupts the normal pattern of Wnt-4, -7b and -11 expressions and interferes with the normal transformation process in developing kidneys, by maintaining the mesenchymal component and inducing the transformation of epithelium to mesenchyme [19]. Then Surendran et al. demonstrated that Wnt-dependent β-catenin signaling increases along with increased expression of markers of fibrosis unilateral ureteral obstruction (UUO) [29]. Renal tubular epithelial and interstitial β-catenin is activated under renal pathological condition, which plays a key role in cell-cell adhesion. These results suggest that Wnt proteins may be involved in renal fibrosis by modulating EMT. In addition to participation in renal interstitial fibrosis in obstructive nephropathy, Wnt/β-catenin is also involved in renal fibrosis diseases caused by a variety of kidney transplantation and diabetic nephropathy [30,31]. Our study demonstrated that Wnt/β-catenin pathway is required for PTH-induced EMT, suggesting PTH may promote renal interstitial fibrosis through Wnt/β-catenin pathway.
The Wnts, Fzd receptors and target genes creat a complex network of signaling system, so a comprehensive analysis of the expression of all members of the Wnt/β-catenin pathway is very important to understand the molecular mechanisms. In the unilateral ureteral obstruction model of renal fibrosis, Bienz et al found that the expression levels of Wnt (1, 2, 2b, 3, 3a, 4, 5a, 6, 7a, 7b, 8a, 9a, 16), FZD (3, 10), DKK (1, 3, 4), Twist were upregulated [32]. He et al. showed that, except for Wnt5b, Wnt8b, and Wnt9b, all members of Wnt family genes were upregulated [17]. In this study, we also performed a comprehensive analysis of the expression and regulation of Wnts and their receptors and antagonists in HK-2 cells after PTH treatment. We demonstrated that DKK1, FZD3, MMP7, Wnt1, Wnt16, Wnt2B and Wnt4 were upregulated in HK-2 cells after PTH treatment, which were consistent with the previous studies, suggesting PTH-induced EMT are caused by combined action of these proteins.
Wnt4 is a secreted glycoprotein that is critical for nephrogenesis [33]. In some experimental models, Wnt4 has been demonstrated to plays a role during regeneration process in acute renal failure. On the other hand, during experimental renal injury, some evidence showed that Wnt4 participates in renal fibrosis. Therefore, when will Wnt4 have a protective role or when will induce fibrosis depends on the specific cellular context. The result here showed that the up-regulation of Wnt4 promotes PTH-induced EMT that was consistent with the induction of Wnt4 in fibrosis, indicating that Wnt4 might be a key gene during PTH-induced EMT.
In conclusion, we demonstrated that Wnt/β-catenin pathway is involved in regulating PTH-induced EMT in human renal proximal tubular cells, implicating that Wnt/β-catenin pathway is required for EMT induced by PTH. Wnt4 might be a key gene during PTH-induced EMT. These findings provide significant insights into the role and mechanisms of Wnt/β-catenin signaling in renal fibrogenesis and offer a new strategy in developing therapeutic modalities for the treatment of fibrotic kidney diseases.
Acknowledgements
This study was supported by Chinese Post-doctoral Scientific Foundation (No. 2012M521928) and Shandong Provicial outstanding young scientist fund (No. BS2012YY041).
Disclosure of conflict of interest
None.
References
- 1.Noronha IL, Fujihara CK, Zatz R. The inflammatory component in progressive renal disease - are interventions possible? Nephrol Dial Transplant. 2002;17:363–368. doi: 10.1093/ndt/17.3.363. [DOI] [PubMed] [Google Scholar]
- 2.Fujihara CK, Malheiros DM, Zatz R, Noronha IL. Mycophenolate mofetil attenuates renal injury in the rat remnant kidney. Kidney Int. 1998;54:1510–1519. doi: 10.1046/j.1523-1755.1998.00138.x. [DOI] [PubMed] [Google Scholar]
- 3.Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, Lewis JB, Ritz E, Atkins RC, Rohde R, Raz I Collaborative Study Group. Collaborative Study Group. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 4.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S RENAAL Study Investigators. RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 5.Carew RW, Wang B, Kantharidis P. The role of EMT in renal fibrosis. Cell Tissue Res. 2012;347:103–116. doi: 10.1007/s00441-011-1227-1. [DOI] [PubMed] [Google Scholar]
- 6.Efstratiadis G, Divani M, Katsioulis E, Vergoulas G. Renal fibrosis. Hippokratia. 2009;13:224–9. [PMC free article] [PubMed] [Google Scholar]
- 7.Zeisberg M, Kalluri R. The role of epithelial-to-mesenchymal transition in renal fibrosis. J Mol Med (Berl) 2004;82:175–181. doi: 10.1007/s00109-003-0517-9. [DOI] [PubMed] [Google Scholar]
- 8.Hsieh PF, Liu SF, Lee TC, Huang JS, Yin LT, Chang WT, Chuang LY, Guh JY, Hung MY, Yang YL. The role of IL-7 in renal proximal tubule epithelial cells fibrosis. Mol Immunol. 2012;50:74–82. doi: 10.1016/j.molimm.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Iwano M. EMT and TGF-beta in renal fibrosis. Front Biosci (Schol Ed) 2010;2:229–238. doi: 10.2741/s60. [DOI] [PubMed] [Google Scholar]
- 10.Fukagawa M, Komaba H, Kakuta T. Hyperparathyroidism in chronic kidney disease patients: an update on current pharmacotherapy. Expert Opin Pharmacother. 2013;14:863–871. doi: 10.1517/14656566.2013.783017. [DOI] [PubMed] [Google Scholar]
- 11.Goettsch C, Iwata H, Aikawa E. Parathyroid hormone: critical bridge between bone metabolism and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2014;34:1333–1335. doi: 10.1161/ATVBAHA.114.303637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turner RT, Evans GL, Zhang M, Sibonga JD. Effects of parathyroid hormone on bone formation in a rat model for chronic alcohol abuse. Alcohol Clin Exp Res. 2001;25:667–671. [PubMed] [Google Scholar]
- 13.Harvey S, Fraser RA. Parathyroid hormone: neural and neuroendocrine perspectives. J Endocrinol. 1993;139:353–361. doi: 10.1677/joe.0.1390353. [DOI] [PubMed] [Google Scholar]
- 14.Esbrit P, Santos S, Ortega A, Fernández-Agulló T, Vélez E, Troya S, Garrido P, Peña A, Bover J, Bosch RJ. Parathyroid hormone-related protein as a renal regulating factor. From vessels to glomeruli and tubular epithelium. Am J Nephrol. 2001;21:179–184. doi: 10.1159/000046244. [DOI] [PubMed] [Google Scholar]
- 15.Guo Y, Zhang A, Ding Y, Wang Y, Yuan W. Genistein ameliorates parathyroid hormone-induced epithelial-to-mesenchymal transition and inhibits expression of connective tissue growth factor in human renal proximal tubular cells. Arch Med Sci. 2013;9:724–730. doi: 10.5114/aoms.2013.36929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo Y, Yuan W, Wang L, Shang M, Peng Y. Parathyroid hormone-potentiated connective tissue growth factor expression in human renal proximal tubular cells through activating the MAPK and NF-kappaB signalling pathways. Nephrol Dial Transplant. 2011;26:839–847. doi: 10.1093/ndt/gfq521. [DOI] [PubMed] [Google Scholar]
- 17.He W, Dai C, Li Y, Zeng G, Monga SP, Liu Y. Wnt/beta-catenin signaling promotes renal interstitial fibrosis. J Am Soc Nephrol. 2009;20:765–776. doi: 10.1681/ASN.2008060566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim MK, Maeng YI, Sung WJ, Oh HK, Park JB, Yoon GS, Cho CH, Park KK. The differential expression of TGF-β1, ILK and wnt signaling inducing epithelial to mesenchymal transition in human renal fibrogenesis: an immunohistochemical study. Int J Clin Exp Pathol. 2013;6:1747–1758. [PMC free article] [PubMed] [Google Scholar]
- 19.Nguyen HT, Thomson AA, Kogan BA, Baskin LS, Cunha GR. Expression of the Wnt gene family during late nephrogenesis and complete ureteral obstruction. Lab Invest. 1999;79:647–658. [PubMed] [Google Scholar]
- 20.Hertig A, Verine J, Mougenot B, Jouanneau C, Ouali N, Sebe P, Glotz D, Ancel PY, Rondeau E, Xu-Dubois YC. Risk factors for early epithelial to mesenchymal transition in renal grafts. Am J Transplant. 2006;6:2937–2946. doi: 10.1111/j.1600-6143.2006.01559.x. [DOI] [PubMed] [Google Scholar]
- 21.He W, Tan RJ, Li Y, Wang D, Nie J, Hou FF, Liu Y. Matrix metalloproteinase-7 as a surrogate marker predicts renal Wnt/β-catenin activity in CKD. J Am Soc Nephrol. 2012;23:294–304. doi: 10.1681/ASN.2011050490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller JR. The Wnts. Genome Biol. 2002;3:REVIEWS3001. doi: 10.1186/gb-2001-3-1-reviews3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Staal FJ, Luis TC, Tiemessen MM. WNT signalling in the immune system: WNT is spreading its wings. Nat Rev Immunol. 2008;8:581–593. doi: 10.1038/nri2360. [DOI] [PubMed] [Google Scholar]
- 24.Endo Y, Wolf V, Muraiso K, Kamijo K, Soon L, Uren A, Barshishat-Küpper M, Rubin JS. Wnt-3a-dependent cell motility involves RhoA activation and is specifically regulated by dishevelled-2. J Biol Chem. 2005;280:777–786. doi: 10.1074/jbc.M406391200. [DOI] [PubMed] [Google Scholar]
- 25.Liang H, Coles AH, Zhu Z, Zayas J, Jurecic R, Kang J, Jones SN. Noncanonical Wnt signaling promotes apoptosis in thymocyte development. J Exp Med. 2007;204:3077–3084. doi: 10.1084/jem.20062692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pulkkinen K, Murugan S, Vainio S. Wnt signaling in kidney development and disease. Organogenesis. 2008;4:55–59. doi: 10.4161/org.4.2.5849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giles RH, van Es JH, Clevers H. Caught up in a Wnt storm:Wnt signaling in cancer. Biochim Biophys Acta. 2003;1653:1–24. doi: 10.1016/s0304-419x(03)00005-2. [DOI] [PubMed] [Google Scholar]
- 28.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 29.Surendran K, Schiavi S, Hruska KA. Wnt-dependent beta-catenin signaling is activated after unilateral ureteral obstruction, and recombinant secreted frizzled-related protein 4 alters the progression of renal fibrosis. J Am Soc Nephrol. 2005;16:2373–2384. doi: 10.1681/ASN.2004110949. [DOI] [PubMed] [Google Scholar]
- 30.Ho C, Lee PH, Hsu YC, Wang FS, Huang YT, Lin CL. Sustained Wnt/β-catenin signaling rescues high glucose induction of transforming growth factor-β1-mediated renal fibrosis. Am J Med Sci. 2012;344:374–382. doi: 10.1097/MAJ.0b013e31824369c5. [DOI] [PubMed] [Google Scholar]
- 31.Xiao L, Wang M, Yang S, Liu F, Sun L. A glimpse of the pathogenetic mechanisms of Wnt/β-catenin signaling in diabetic nephropathy. Biomed Res Int. 2013;2013:987064. doi: 10.1155/2013/987064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bienz M. beta-Catenin: a pivot between cell adhesion and Wnt signalling. Curr Biol. 2005;15:R64–7. doi: 10.1016/j.cub.2004.12.058. [DOI] [PubMed] [Google Scholar]
- 33.Surendran K, McCaul SP, Simon TC. A role for Wnt-4 in renal fibrosis. Am J Physiol Renal Physiol. 2002;283:F431–441. doi: 10.1152/ajprenal.0009.2001. [DOI] [PubMed] [Google Scholar]


