Abstract
B7-H1, an important member of the B7-CD28 super family, has been reported to play an important role in regulation of T-cell mediated anti-tumor response, and also has effect in the biological characteristics of the tumor cells themselves. The bulk of data indicate that cancer immunotherapy targeting the molecule B7-H1 recently has sparked growing interest. We have previously reported that higher expression of B7-H1 in human gastric cancer significantly associated with tumor size, invasion, nodal metastasis, survival and the density of infiltrating Foxp3+ Tregs. In the present study, we used tissue microarray to further study B7-H1 expression in human esophageal cancer tissues and its clinical significance. We found that positive membranous B7-H1 expression could be found in some human esophageal cancer cell lines, and both membranous/cytoplasm and nuclear staining of B7-H1 could be found in esophageal cancer tissues. We demonstrated that the membranous/cytoplasm B7-H1 expression in human esophageal cancer tissues was significantly correlated with tumor invasion depth (P = 0.0261), whereas it was not correlated with patient’s gender, age, tumor size, nodal metastasis, distant metastasis and TNM stage. The survival analysis showed that the overall survival of the patients with positive B7-H1 membrane/cytoplasm expression was significantly poorer than that of the patients with negative B7-H1 membrane/cytoplasm expression (Hazard ratio = 2.157, 95% CI: 1.017-4.577, P = 0.0452). Moreover, we also found that the nuclear B7-H1 expression in human esophageal cancer tissues was significantly correlated with tumor invasion depth (P = 0.0331), whereas it was not correlated with other parameters. The log-rank survival analysis showed that there was no statistically significant difference in prognosis between the patients with positive nuclear B7-H1 staining and the patients with negative nuclear B7-H1 staining (P = 0.6755). Thus, our data showed that B7-H1 can serve as a prognostic predictor for human esophageal cancer, and also could be an important therapeutic target for the immune therapy against this malignancy.
Keywords: B7-H1, esophageal cancer, tissue microarray, survival
Introduction
The esophageal cancer is one of the most common types of human cancer in the world, especially with a high incidence in southern and eastern Africa, western and northern China, parts of South America, and Japan [1-3]. The incidence rates of esophageal cancer are three to four times higher in men than women, and the mortality rates of esophageal cancer are the fifth and eighth in men and women, respectively [4]. According to the histological classification, the squamous cell carcinoma and the adenocarcinoma are the two main types of human esophageal cancer [5], and the squamous cell carcinoma represents 90% of all esophageal cancer cases worldwide. As of now, the overall 5-year survival rate of esophageal cancer still remains very poor, due to its malignant characteristics and the majority of patients are with advanced stages at the time of diagnosis.
As we know, the activation of T-cell not only needs the signal presented by TCR-MHC, but also the signal delivered by co-stimulatory molecule, such as CD28/B7-1, CD28/B7-2 and PD-1/B7-H1, etc. B7-H1 (also named PD-L1 or CD274), is an important member of the B7 family, which can interact with its receptor, PD-1 (programmed death-1, a CD28 family member), inhibit T-cell activation, maintain the exhaustion of T cell, impairing cytokine production, and induce the apoptosis of effector T cells [6,7]. Previous studies have shown that, B7-H1 is broadly distributed in various tissues and cell types, including T cells, B cells, dendritic cells, nature killer cells, activated vascular endothelial cells, mesenchymal stem cells and cultured bone marrow-derived mast cells, even at the human maternal-fetal interface, which may provide protection the fetus against the maternal immune system [8]. In addition, B7-H1 expression has also been found to be up-regulated in many human solid tumors, including liver, ovary, colorectal, lung, pancreatic, gastric, kidney, breast cancers, and etc. [9]. And the cancer cell expressed B7-H1 could provide direct tumor protection, inhibit T cell mediated anti-tumor immunity, and finally lead to cancer immune tolerance and escape [10,11]. Our previous study showed that positive staining of B7-H1 could be found in 42.2% gastric cancer tissues, and B7-H1 expression level was significantly associated with tumor size, invasion, nodal metastasis, and overall survival [12]. And we further confirmed that B7-H1 expression in human gastric cancer tissues significantly associated with the infiltrating density of Foxp3+ Tregs, and the expression level of another inhibitory co-stimulatory molecule B7-H4 [13].
Ohigashi et al. [14] reported that B7-H1 expression could be found in esophageal cancer tissues by using immunohistochemistry in frozen section of this malignancy, and revealed that higher expression of B7-H1 predicted poorer survival of esophageal cancer patients. In the present study, we further studied B7-H1 expression in human esophageal cancer cell lines, and also used tissue microarray to investigate the B7-H1 expression pattern in human esophageal cancer tissues as well as cancer cell lines, and to analyze its clinical significance.
Materials and methods
Patients and tissue microarray
Formalin-fixed, paraffin-embedded esophageal cancer tissue samples were collected from 99 patients who underwent surgical resection between February 2005 and May 2006 in our hospital (76 men and 23 women; median age at diagnosis was 59 years). In addition, 4 normal tissues from the non-malignant portion of esophagus were resected from surgery and used as controls. No patients received pre-operative chemotherapy or radiotherapy. All tumor tissues were confirmed as the esophageal squamous cell carcinoma by using hematoxylin and eosin (H&E) staining after surgical resection. The clinical parameters of the patients are shown in Table 1. Among all the patients, the survival data of 56 patients were available. Then, the paraffin blocks of 99 cases of esophageal cancer tissues were used in the construction of tissue microarray. In brief, the H&E-stained standard slides were reviewed from each section of esophageal cancer tissues, and a representative tumor region and the corresponding formalin-fixed paraffin-embedded tissue block were selected for use in the tissue microarray. The viable invasive carcinoma tissue (epithelial cells) and surrounding tumor stroma from central parts within the tumors were carefully selected and marked on the H&E slides, and then were sampled for the tissue microarray block which was assembled using a tissue-arraying instrument (Beecher Instruments, Silver Springs, MD, USA).
Table 1.
Correlation between clinical parameters and B7-H1 expression on membrane and cytoplasm of cancer cells
| Clinical parameters | Cases | B7-H1 immunostaining score | P-value | ||
|---|---|---|---|---|---|
|
| |||||
| H-score = 0 | H-score > 0 | χ2 | |||
| Gender | |||||
| Male | 76 | 14 | 62 | 0.3590 | 0.5491 |
| Female | 23 | 3 | 20 | ||
| Age (years) | |||||
| < 60 | 51 | 10 | 41 | 0.4389 | 0.5077 |
| ≥ 60 | 48 | 7 | 41 | ||
| Tumor size (cm) | |||||
| < 3.5 | 35 | 7 | 28 | 0.3045 | 0.5811 |
| ≥ 3.5 | 64 | 10 | 54 | ||
| Tumor invasion depth (T) | |||||
| T1 + T2 | 35 | 10 | 25 | 4.947 | 0.0261 |
| T3 + T4 | 64 | 7 | 57 | ||
| Nodal metastasis (N) | |||||
| Yes | 34 | 6 | 28 | 0.0082 | 0.9277 |
| No | 65 | 11 | 54 | ||
| Distant metastasis (M) | |||||
| Yes | 6 | 0 | 6 | 1.324 | 0.2498 |
| No | 93 | 17 | 76 | ||
| TNM stage | |||||
| I | 5 | 3 | 2 | 0.9872 | 0.3204 |
| II | 57 | 7 | 50 | ||
| III | 31 | 7 | 24 | ||
| IV | 6 | 0 | 6 | ||
Values in bold signify P < 0.05.
Antibodies and major regents
Rabbit anti-human B7-H1 monoclonal antibody (NBP1-03220) was purchased from Novus Biologicals (Littleton, CO, USA). PE-conjugated mouse anti-human B7-H1 monoclonal antibody was purchased from BD Pharmingen (BD Biosciences, San Jose, CA, USA). PE-conjugated mouse IgG1 Isotype control was purchased from R&D Systems Inc. (Minnneapolis, USA). The horseradish peroxidase (HRP)-labeled goat anti-mouse/rabbit secondary antibody used in immunohistochemistry was purchased from Dako (Glostrup, Denmark). The cell culture medium and supplements were purchased from HyClone (Thermo, Waltham, USA).
Cell lines and cell culture
Human esophageal cancer cell lines TE-1, Eca-109 and Eca-9706 were obtained from Chinese Academy of Sciences, Shanghai Institutes for Biological Sciences. TE-1 and Eca-9706 were cultured in DMEM, and Eca-109 was cultured in RPMI1640, respectively, and supplemented with 10% FBS. The cell lines were incubated at standard culture conditions (5% CO2, 37°C).
Flow cytometry analysis of B7-H1 expression in esophageal cancer cell lines
Esophageal cancer cell lines TE-1, Eca-109 and Eca-9706 were examined for membranous B7-H1 expression by using flow cytometry analysis. The cells of those three cell lines were collected after cultivation, and were incubated with PE-labeled mouse anti-human B7-H1 monoclonal antibody for 30 min at room temperature, then washed twice in PBS, and then analyzed by the BD FACSCanto II flow cytometry (BD Biosciences, San Jose, CA, USA).
Immunohistochemistry
The paraffin-embedded esophageal cancer tissue microarray block was cut into 3-µm-thick section. A standard immunohistochemical technique was performed using a Ventana BenchMark XT immunostainer (Ventana Medical Systems, Tucson, USA) with the B7-H1 antibody (NBP1-03220, Novus) at a dilution in 1:200. Heat epitope retrieval provided by the immunostainer was done for 30 min.
Evaluation of immunohistochemical staining
All slides were examined independently by two senior pathologists who were not informed of patients’ clinical parameters. First, the membranous and cytoplasm B7-H1 immunostaining densities were assessed according to the H-score method described by our previous reports [15,16]: H-score = (% tumor cells unstained x0) + (% tumor cells stained weak x1) + (% tumor cells stained moderate x2) + (% tumor cells stained strong x3). The H-scores ranged from 0 (100% negative tumor cells) to 300 (100% strong staining tumor cells). Results from the two pathologists were averaged and used in the statistical analysis. Second, the nuclear staining of B7-H1 was considered as positive if there is any B7-H1 staining in the nucleus of esophageal cancer cells.
Statistical analyses
Statistical analysis was performed using the GraphPad Prism 5.0 software package (GraphPad Software, Inc., San Diego, USA). Paired Student’s t-test, the Wilcoxon signed rank test or the survival analysis were used where appropriate. A P-value of < 0.05 was deemed significant.
Results
B7-H1 expression in human esophageal cancer cell lines and tissues
As shown in Figure 1, we demonstrated that positive membrane B7-H1 expression could be found in human esophageal cancer cell lines TE-1 and Eca-109, while it was weakly expressed on esophageal cancer cell line Eca-9706. By using the automatic immunohistochemical staining system Benchmarker XT, we found 82 cases in all 99 patients representing positive membranous/cytoplasm B7-H1 staining (Figure 2A), and 79 cases in all 99 patients representing positive nuclear B7-H1 staining (red arrow, Figure 3A).
Figure 1.
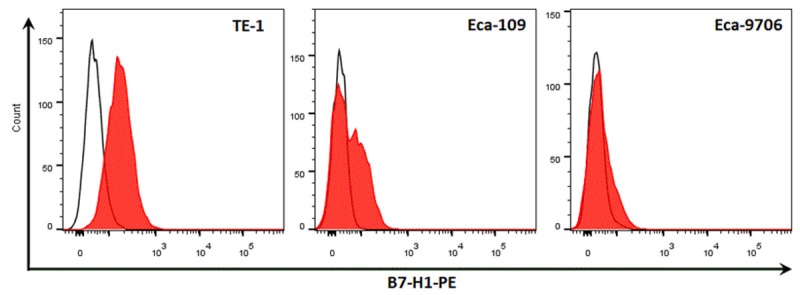
Flow analysis of membrane B7-H1 expression in human esophageal cancer cell lines. We analyze membranous B7-H1 expression in human esophageal cancer cell line by using fluorescence immuno-staining and flow analysis, and we found that membranous B7-H1 expression could be found in TE-1 and Eca-109, while it’s was very weakly expressed on Eca-9706. The continuous lines indicate Isotype controls, and the red shadings indicate specific membranous staining of B7-H1.
Figure 2.
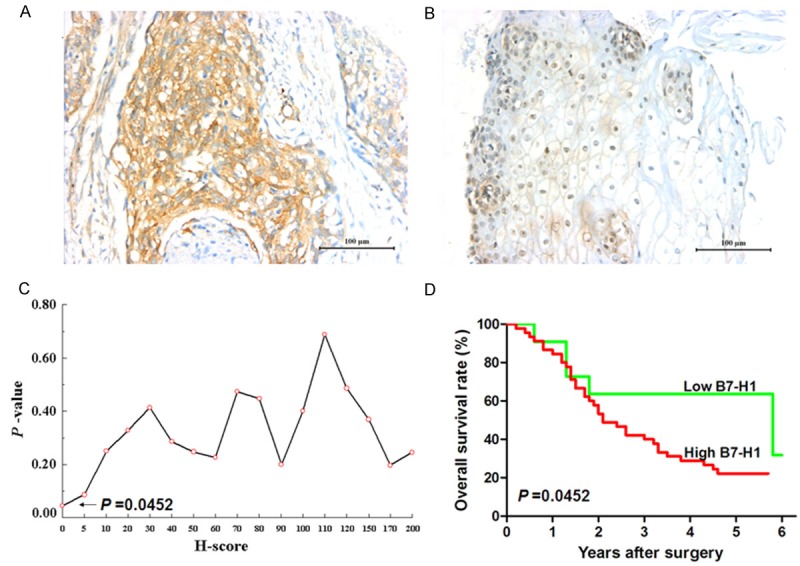
Survival analysis of membrane/cytoplasm B7-H1 expression in human esophageal cancer tissues. A. Positive membrane/cytoplasm B7-H1 expression on esophageal cancer tissue. B. Weakly expression of B7-H1 in epithelial cells of adjacent normal esophageal tissue. Scale bar=100 μm. C. The minimum P-value seek in the log-rank survival analysis of membrane/cytoplasm B7-H1 expression in human esophageal cancer tissues was performed, and when the cutoff value of H-score = 0 was selected, the minimal P-value = 0.0452 was found. D. The log-rank survival analysis was performed when H-score = 0, Hazard Ratio = 2.157, 95% CI: 1.017~4.577, P = 0.0452.
Figure 3.
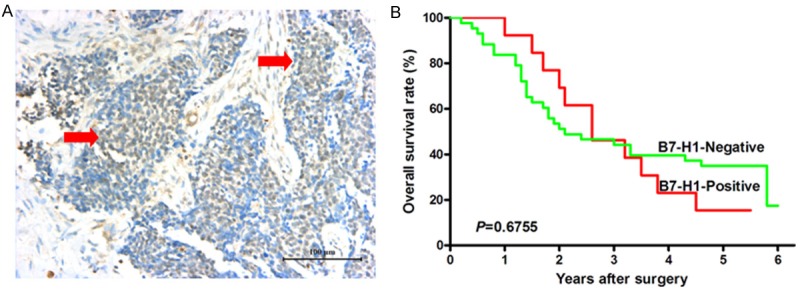
Survival analysis of B7-H1 expression in nuclei of cancer cells in human esophageal cancer tissues. A. Positive B7-H1 expression in nuclei of cancer cells in esophageal cancer tissue, scale bar = 100 μm. B. The log-rank survival analysis showed that there was no statistically significant difference in prognosis between the patients with positive nuclear B7-H1 staining and the patients with negative nuclear B7-H1 staining, P = 0.6755.
B7-H1 expression in relation to patient’s clinical parameters and survival
The correlation between patients’ clinical parameters and B7-H1 expression on membrane and cytoplasm of cancer cells is shown in Table 1. It demonstrated that membrane and cytoplasm B7-H1 expression in human esophageal cancer tissues was significantly correlated with tumor invasion depth (P = 0.0261), whereas it was not correlated with patient’s gender, age, tumor size, nodal metastasis, distant metastasis and TNM stage. When the H-score cut-off value = 0 was selected (Figure 2C), the survival analysis showed that the overall survival of the patients with positive B7-H1 membrane/cytoplasm expression was significantly poorer than that of the patients with negative B7-H1 membrane/cytoplasm expression (Hazard ratio = 2.157, 95% CI: 1.017-4.577, P = 0.0452, Figure 2D). Moreover, as shown in Table 2, we also analyzed the correlation between patients’ clinical parameters and B7-H1 expression in nuclei of esophageal cancer cells, and it also demonstrated that nuclear B7-H1 expression in human esophageal cancer tissues was significantly correlated with tumor invasion depth (P = 0.0331), whereas it was not correlated with patient’s gender, age, tumor size, nodal metastasis, distant metastasis and TNM stage. The log-rank survival analysis showed that there was no statistically significant difference in prognosis between the patients with positive nuclear B7-H1 staining and the patients with negative nuclear B7-H1 staining (Figure 3B).
Table 2.
Correlation between clinical parameters and B7-H1 expression in nuclei of cancer cells
| Clinical parameters | Cases | B7-H1 staining | P-value | ||
|---|---|---|---|---|---|
|
| |||||
| Positive | Negative | χ2 | |||
| Gender | |||||
| Male | 76 | 61 | 15 | 0.0439 | 0.8340 |
| Female | 23 | 18 | 5 | ||
| Age (years) | |||||
| < 60 | 51 | 43 | 8 | 1.3310 | 0.2487 |
| ≥ 60 | 48 | 36 | 12 | ||
| Tumor size (cm) | |||||
| < 3.5 | 35 | 29 | 6 | 0.3143 | 0.5751 |
| ≥ 3.5 | 64 | 50 | 14 | ||
| Tumor invasion depth (T) | |||||
| T1 + T2 | 35 | 32 | 3 | 4.5430 | 0.0331 |
| T3 + T4 | 64 | 47 | 17 | ||
| Nodal metastasis (N) | |||||
| Yes | 34 | 29 | 5 | 0.9704 | 0.3246 |
| No | 65 | 50 | 15 | ||
| Distant metastasis (M) | |||||
| Yes | 6 | 5 | 1 | 0.0495 | 0.8239 |
| No | 93 | 74 | 19 | ||
| TNM stage | |||||
| I | 5 | 5 | 0 | 0.0143 | 0.9049 |
| II | 57 | 44 | 13 | ||
| III | 31 | 25 | 6 | ||
| IV | 6 | 5 | 1 | ||
Values in bold signify P < 0.05.
Discussion
B7-H1 expression has been found in various human cancers, and its expression level significantly associated with patients’ prognoses and a series of clinico-pathological parameters of the patients, as well as densities of infiltrating immune cell subsets, such as lung cancer [17,18], gastric cancer [12,13], pancreatic carcinoma [19,20], breast cancer [21-23], ovarian cancer [24,25], bladder cancer [26,27], renal cell carcinoma [28], liver cancer [29], cervical cancer [30], and etc. Although Ohigashi and his colleagues reported that B7-H1 expression could be found in esophageal cancer tissues by using immunohistochemistry in frozen section of human esophageal cancer tissues, and revealed that higher expression of B7-H1 predicted poorer survival of esophageal cancer patients [14]. In the present study, we further studied B7-H1 expression in human esophageal cancer cell lines, and also used tissue microarray to investigate the B7-H1 expression pattern in human esophageal cancer tissues and to analyze its clinical significance. Our results confirmed that membranous B7-H1 expression could be found in TE-1 cell line, and also could be found in esophageal cancer tissues by using tissue microarray and immunohistochemistry. We also showed the B7-H1 expression level in human esophageal cancer tissue was significantly associated with patient’s prognosis and tumor invasion depth.
We have previously reported that higher expression of B7-H1 in human gastric cancer significantly associated with tumor size, invasion, lymph node metastasis and survival time of the patients [12]. In addition, we also reported that higher expression of B7-H1 in human colorectal cancer tissues significantly associated with densities of infiltrating T cells subsets, and also associated with the expansion of regulatory T cells [31]. However, recent accumulated evidence also indicated that the molecules from B7 family, such B7-H1 and B7-H3, could not only highly express on tumor cells, have a critical role in regulation of T-cell mediated anti-tumor response, but also have effect in the biological characteristics of the tumor cells themselves [32,33]. Ghebeh et al. [34] reported that B7-H1 expression in breast cancer is strongly associated with high proliferative Ki-67-expressing tumor cells, suggesting that B7-H1 expression on cancer cells roles importantly in cancer cell proliferation and cancer progression. Shi et al. [32] also confirmed that B7-H1 expression in human colorectal cancer is significantly associated with patient’s prognosis and is involved in the regulation of the proliferation and the invasion of colorectal cancer cells. Interestingly, B7-H1 could also serve as an anti-apoptotic receptor on cancer cells, which is a novel mechanism indicating that cancer cells can use a receptor on immune cells as a ligand to induce resistance to therapy [35].
Moreover, Cao et al. [33,36] recently found that skin-specific over-expression of B7-H1 could induce carcinogenesis of squamous cell carcinoma, and in the B7-H1 transgenic mice, B7-H1 transgenic-derived keratinocytes and squamous cell carcinoma exhibited a significant reduction of E-cadherin, and an elevated expression of the transcription factors Slug and Twist, suggesting that B7-H1 over-expression in keratinocytes promotes the epithelial-mesenchymal transition and accelerates carcinogenesis. As we know, the epithelial-mesenchymal transition is a key step toward cancer metastasis, and is also an identified phenotypes of tumor invasion and metastasis in human esophageal cancer [37,38]. In addition, the cells underwent epithelial-mesenchymal transition also showed some phenotypes of stem cells [39]. Mani et al. [39] reported that the induction of an epithelial-mesenchymal transition in immortalized human mammary epithelial cells contributed to the acquisition of mesenchymal traits and in the expression of stem-cell markers. Thus, in future, it’s of great importance for us to study the correlation between up-regulation of B7-H1 expression and reduced E-cadherin and/or enhanced Slug and Twist transcription factors in human esophageal squamous cell carcinoma, and to elucidate detailed mechanism of B7-H1 over-expression in the induction of epithelial-mesenchymal transition of cancer cells, cancer invasion and metastasis.
The cancer cell expressed B7-H1 also linked between chemotherapy and cancer immunoresistance. Zhang et al. [40] showed that some chemopreventive agents could up-regulated surface B7-H1 expression on human breast cancer cell lines, and then promoted B7-H1-mediated T cell apoptosis. Previous studies demonstrated that B7-H1, expressed on tumor cells, can interact with PD-1 to reverse a signal, which results tumor cells resistance to apoptosis induction by both immune effectors and pro-apoptotic drugs [35]. Strikingly, Ghebeh et al [23] also reported that doxorubicin could down-regulate the surface B7-H1 expression and up-regulate the nuclear B7-H1 expression, suggesting an important role of B7-H1 in anti-apoptosis of cancer cells by the means of nuclear transportation of this co-stimulatory molecule. In our present study, we also found B7-H1 staining in nuclei of esophageal cancer cells, and the ratio of patients with B7-H1 staining in nuclei in the group with advanced T stage was significantly higher than that in the group with early T stage, which showed that the nuclear transportation of B7-H1 expression promote the invasion of esophageal cancer cells, but the detailed mechanism merits further investigations in future.
Acknowledgements
We thank senior pathologists Changqing Lu, Cao Wu, Yan Tan and Jun Xie (Department of Pathology, the Third Affiliated Hospital of Soochow University, Changzhou, Jiangsu, China) for their expert suggestions and technical assistances. This work was supported by grants from the National Natural Science Foundation of China (No. 81301960 and 81171653), and the Innovative Talents Training Project of Changzhou Health Bureau.
Disclosure of conflict of interest
None.
References
- 1.Schottenfeld D. Epidemiology of cancer of the esophagus. Semin Oncol. 1984;11:92–100. [PubMed] [Google Scholar]
- 2.Yang CS. Research on esophageal cancer in China: a review. Cancer Res. 1980;40:2633–2644. [PubMed] [Google Scholar]
- 3.Stoner GD, Gupta A. Etiology and chemoprevention of esophageal squamous cell carcinoma. Carcinogenesis. 2001;22:1737–1746. doi: 10.1093/carcin/22.11.1737. [DOI] [PubMed] [Google Scholar]
- 4.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 5.Chen L, Luo G, Tan Y, Wei J, Wu C, Zheng L, Zhang X, Xu N. Immunolocalisation of tissue factor in esophageal cancer is correlated with intratumoral angiogenesis and prognosis of the patient. Acta Histochem. 2010;112:233–239. doi: 10.1016/j.acthis.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 6.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 7.Ceeraz S, Nowak EC, Noelle RJ. B7 family checkpoint regulators in immune regulation and disease. Trends Immunol. 2013;34:556–563. doi: 10.1016/j.it.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petroff MG, Chen L, Phillips TA, Azzola D, Sedlmayr P, Hunt JS. B7 family molecules are favorably positioned at the human maternal-fetal interface. Biol Reprod. 2003;68:1496–1504. doi: 10.1095/biolreprod.102.010058. [DOI] [PubMed] [Google Scholar]
- 9.Seliger B, Quandt D. The expression, function, and clinical relevance of B7 family members in cancer. Cancer Immunol Immunother. 2012;61:1327–1341. doi: 10.1007/s00262-012-1293-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8:467–477. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 11.Afreen S, Dermime S. The immunoinhibitory B7-H1 molecule as a potential target in cancer: killing many birds with one stone. Hematol Oncol Stem Cell Ther. 2014;7:1–17. doi: 10.1016/j.hemonc.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 12.Wu C, Zhu Y, Jiang J, Zhao J, Zhang XG, Xu N. Immunohistochemical localization of programmed death-1 ligand-1 (PD-L1) in gastric carcinoma and its clinical significance. Acta Histochem. 2006;108:19–24. doi: 10.1016/j.acthis.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Geng Y, Wang H, Lu C, Li Q, Xu B, Jiang J, Wu C. Expression of costimulatory molecules B7-H1, B7-H4 and Foxp3 Tregs in gastric cancer and its clinical significance. Int J Clin Oncol. 2014 May 9; doi: 10.1007/s10147-014-0701-7. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 14.Ohigashi Y, Sho M, Yamada Y, Tsurui Y, Hamada K, Ikeda N, Mizuno T, Yoriki R, Kashizuka H, Yane K, Tsushima F, Otsuki N, Yagita H, Azuma M, Nakajima Y. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res. 2005;11:2947–2953. doi: 10.1158/1078-0432.CCR-04-1469. [DOI] [PubMed] [Google Scholar]
- 15.Chen L, Di D, Luo G, Zheng L, Tan Y, Zhang X, Xu N. Immunochemical staining of MT2-MMP correlates positively to angiogenesis of human esophageal cancer. Anticancer Res. 2010;30:4363–4368. [PubMed] [Google Scholar]
- 16.Chen L, Sun J, Wu H, Zhou S, Tan Y, Tan M, Shan B, Lu B, Zhang X. B7-H4 expression associates with cancer progression and predicts patient’s survival in human esophageal squamous cell carcinoma. Cancer Immunol Immunother. 2011;60:1047–1055. doi: 10.1007/s00262-011-1017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Konishi J, Yamazaki K, Azuma M, Kinoshita I, Dosaka-Akita H, Nishimura M. B7-H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clin Cancer Res. 2004;10:5094–5100. doi: 10.1158/1078-0432.CCR-04-0428. [DOI] [PubMed] [Google Scholar]
- 18.Mu CY, Huang JA, Chen Y, Chen C, Zhang XG. High expression of PD-L1 in lung cancer may contribute to poor prognosis and tumor cells immune escape through suppressing tumor infiltrating dendritic cells maturation. Med Oncol. 2011;28:682–688. doi: 10.1007/s12032-010-9515-2. [DOI] [PubMed] [Google Scholar]
- 19.Wang L, Ma Q, Chen X, Guo K, Li J, Zhang M. Clinical significance of B7-H1 and B7-1 expressions in pancreatic carcinoma. World J Surg. 2010;34:1059–1065. doi: 10.1007/s00268-010-0448-x. [DOI] [PubMed] [Google Scholar]
- 20.Nomi T, Sho M, Akahori T, Hamada K, Kubo A, Kanehiro H, Nakamura S, Enomoto K, Yagita H, Azuma M, Nakajima Y. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res. 2007;13:2151–2157. doi: 10.1158/1078-0432.CCR-06-2746. [DOI] [PubMed] [Google Scholar]
- 21.Ghebeh H, Mohammed S, Al-Omair A, Qattan A, Lehe C, Al-Qudaihi G, Elkum N, Alshabanah M, Bin Amer S, Tulbah A, Ajarim D, Al-Tweigeri T, Dermime S. The B7-H1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: correlation with important high-risk prognostic factors. Neoplasia. 2006;8:190–198. doi: 10.1593/neo.05733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghebeh H, Barhoush E, Tulbah A, Elkum N, Al-Tweigeri T, Dermime S. FOXP3+ Tregs and B7-H1+/PD-1+ T lymphocytes co-infiltrate the tumor tissues of high-risk breast cancer patients: Implication for immunotherapy. BMC Cancer. 2008;8:57. doi: 10.1186/1471-2407-8-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghebeh H, Lehe C, Barhoush E, Al-Romaih K, Tulbah A, Al-Alwan M, Hendrayani SF, Manogaran P, Alaiya A, Al-Tweigeri T, Aboussekhra A, Dermime S. Doxorubicin downregulates cell surface B7-H1 expression and upregulates its nuclear expression in breast cancer cells: role of B7-H1 as an anti-apoptotic molecule. Breast Cancer Res. 2010;12:R48. doi: 10.1186/bcr2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, Higuchi T, Yagi H, Takakura K, Minato N, Honjo T, Fujii S. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A. 2007;104:3360–3365. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abiko K, Mandai M, Hamanishi J, Yoshioka Y, Matsumura N, Baba T, Yamaguchi K, Murakami R, Yamamoto A, Kharma B, Kosaka K, Konishi I. PD-L1 on tumor cells is induced in ascites and promotes peritoneal dissemination of ovarian cancer through CTL dysfunction. Clin Cancer Res. 2013;19:1363–1374. doi: 10.1158/1078-0432.CCR-12-2199. [DOI] [PubMed] [Google Scholar]
- 26.Wang YH, Cao YW, Yang XC, Niu HT, Sun LJ, Wang XS, Liu J. Effect of TLR4 and B7-H1 on immune escape of urothelial bladder cancer and its clinical significance. Asian Pac J Cancer Prev. 2014;15:1321–1326. doi: 10.7314/apjcp.2014.15.3.1321. [DOI] [PubMed] [Google Scholar]
- 27.Boorjian SA, Sheinin Y, Crispen PL, Farmer SA, Lohse CM, Kuntz SM, Leibovich BC, Kwon ED, Frank I. T-cell coregulatory molecule expression in urothelial cell carcinoma: clinicopathologic correlations and association with survival. Clin Cancer Res. 2008;14:4800–4808. doi: 10.1158/1078-0432.CCR-08-0731. [DOI] [PubMed] [Google Scholar]
- 28.Thompson RH, Gillett MD, Cheville JC, Lohse CM, Dong H, Webster WS, Krejci KG, Lobo JR, Sengupta S, Chen L, Zincke H, Blute ML, Strome SE, Leibovich BC, Kwon ED. Costimulatory B7-H1 in renal cell carcinoma patients: Indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci U S A. 2004;101:17174–17179. doi: 10.1073/pnas.0406351101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao Q, Wang XY, Qiu SJ, Yamato I, Sho M, Nakajima Y, Zhou J, Li BZ, Shi YH, Xiao YS, Xu Y, Fan J. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res. 2009;15:971–979. doi: 10.1158/1078-0432.CCR-08-1608. [DOI] [PubMed] [Google Scholar]
- 30.Karim R, Jordanova ES, Piersma SJ, Kenter GG, Chen L, Boer JM, Melief CJ, van der Burg SH. Tumor-expressed B7-H1 and B7-DC in relation to PD-1+ T-cell infiltration and survival of patients with cervical carcinoma. Clin Cancer Res. 2009;15:6341–6347. doi: 10.1158/1078-0432.CCR-09-1652. [DOI] [PubMed] [Google Scholar]
- 31.Hua D, Sun J, Mao Y, Chen LJ, Wu YY, Zhang XG. B7-H1 expression is associated with expansion of regulatory T cells in colorectal carcinoma. World J Gastroenterol. 2012;18:971–978. doi: 10.3748/wjg.v18.i9.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi SJ, Wang LJ, Wang GD, Guo ZY, Wei M, Meng YL, Yang AG, Wen WH. B7-H1 expression is associated with poor prognosis in colorectal carcinoma and regulates the proliferation and invasion of HCT116 colorectal cancer cells. PLoS One. 2013;8:e76012. doi: 10.1371/journal.pone.0076012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cao Y, Zhang L, Kamimura Y, Ritprajak P, Hashiguchi M, Hirose S, Azuma M. B7-H1 overexpression regulates epithelial-mesenchymal transition and accelerates carcinogenesis in skin. Cancer Res. 2011;71:1235–1243. doi: 10.1158/0008-5472.CAN-10-2217. [DOI] [PubMed] [Google Scholar]
- 34.Ghebeh H, Tulbah A, Mohammed S, Elkum N, Bin Amer SM, Al-Tweigeri T, Dermime S. Expression of B7-H1 in breast cancer patients is strongly associated with high proliferative Ki-67-expressing tumor cells. Int J Cancer. 2007;121:751–758. doi: 10.1002/ijc.22703. [DOI] [PubMed] [Google Scholar]
- 35.Azuma T, Yao S, Zhu G, Flies AS, Flies SJ, Chen L. B7-H1 is a ubiquitous antiapoptotic receptor on cancer cells. Blood. 2008;111:3635–3643. doi: 10.1182/blood-2007-11-123141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cao Y, Zhang L, Ritprajak P, Tsushima F, Youngnak- Piboonratanakit P, Kamimura Y, Hashiguchi M, Azuma M. Immunoregulatory molecule B7-H1 (CD274) contributes to skin carcinogenesis. Cancer Res. 2011;71:4737–4741. doi: 10.1158/0008-5472.CAN-11-0527. [DOI] [PubMed] [Google Scholar]
- 37.Kudo-Saito C, Shirako H, Takeuchi T, Kawakami Y. Cancer metastasis is accelerated through immunosuppression during Snail-induced EMT of cancer cells. Cancer Cell. 2009;15:195–206. doi: 10.1016/j.ccr.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 38.Sung CO, Park CK, Kim SH. Classification of epithelial-mesenchymal transition phenotypes in esophageal squamous cell carcinoma is strongly associated with patient prognosis. Mod Pathol. 2011;24:1060–1068. doi: 10.1038/modpathol.2011.59. [DOI] [PubMed] [Google Scholar]
- 39.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang P, Su DM, Liang M, Fu J. Chemopreventive agents induce programmed death-1-ligand 1 (PD-L1) surface expression in breast cancer cells and promote PD-L1-mediated T cell apoptosis. Mol Immunol. 2008;45:1470–1476. doi: 10.1016/j.molimm.2007.08.013. [DOI] [PubMed] [Google Scholar]


