Abstract
SPP1, PAI and caveolin-1 are known to be closely associated with tumor progression in several kinds of human tumors. This study aimed to investigate the expression of SPP1, PAI and caveolin-1 in oral squamous cell carcinoma (OSCC), and to evaluate their association with the prognosis in oral carcinoma. Immunohistochemical staining was used to examine the expression of SPP1, PAI and caveolin-1 in 17 normal oral mucosa, 6 oral epithelial dysplasia and 43 OSCC specimens by tissue microarrays. High expression of SPP1, PAI and caveolin-1 was found in OSCC patients, and SPP1 and PAI expression were significantly higher in OSCC than in normal oral mucosa. No significant correlations were found between SPP1, PAI and caveolin-1 expression and clinicopathological factors. Expression of SPP1, PAI and caveolin-1 was also not associated with overall survival. Moreover, SPP1 was closely correlated with PAI, caveolin-1 and Keap1, and PAI had significant correlations with caveolin-1, Keap1 and Nrf2, and caveolin-1 was associated with Keap1 by using the Pearson correlation coefficient test. Our findings suggest that overexpressed SPP1, PAI and caveolin-1 were linked to carcinogenesis and progression, and thus they may serve as potential prognostic factors in OSCC.
Keywords: SPP1, PAI, caveolin-1, oral squamous cell carcinoma, prognosis
Introduction
Oral squamous cell carcinoma (OSCC) is one the most common cancer in the world and there is a possible rise within developing countries, the treatment of OSCC is complex and requires a multidisciplinary approach [1]. Operation associated with chemotherapy and radiotherapy is now recognized as the most effective treatment for most cases of OSCC. Nevertheless, the prognosis for patients suffering from OSCC remains poor, with a 50% 5-year overall survival (OS) rate remains relatively unchanged for the past 3 decades [2]. Because of the continuously increased prevalence of oral cancer and poor survival for patients with oral cancers, to find a reliable biomarker for prediction of progression and prognosis of OSCC is still an important issue.
Metastasis is the major obstacle to the successful treatment of OSCC patients, the genetic mechanisms contributing to behaviors of metastatic cells in OSCC are not well understood. Secreted phosphoprotein 1 (SPP1), firstly found to be secreted by transformed epithelial cells, is involved in a series of physiological and pathophysiological processes, including cell attachment, proliferation, migration, invasion and inflammation [3,4]. Previous studies implicated that SPP1 was up-regulated in a number of cancers including oral cancer. Recently, it was reported to contribute to proliferation, metastasis, angiogenesis and progression [5,6]. However, it has not yet found routine use in the clinic. Pasminogen activator inhibitor 1 (PAI-1), also known as Serpine 1, is the key inhibitor of the plasminogen activation system, and plays an important role in cell signal transduction pathways [7]. PAI-1, as a molecule that is highly expressed in different kinds of cancers including oral cancer, has been shown to have both pro- and anti-tumor effects, including migration, invasion, apoptosis and angiogenesis [8]. However, high expression of PAI-1 is associated with a worse survival in a variety of cancers [9]. Thus, PAI-1 appears to play a pivotal role in tumor growth and may represent a potential therapeutic target for oral cancer.
Caveolin-1, a multifunctional protein, is the main constituent molecule of caveolae and represents a scaffolding molecule for several signaling molecules including epidermal growth factor receptor [10]. Interestingly, previous studies have implicated that a reduced expression of Caveolin-1 was found in cancers including head and neck carcinoma [11]. However, other studies recognize caveolin-1 as a tumor promoter because Caveolin-1 is overexpressed in various kinds of cancers, especially in oral cancer [12,13]. Therefore, it is necessary to uncover the role of Caveolin-1 in carcinogenesis and development of OSCC. Keap1-Nrf2 [Kelch-like ECH-associated protein 1-nuclear factor (erythroid-derived 2)-like 2] system, as a key signaling pathway that regulates transcription of a series of cyto-protective proteins, plays an important role in oxidative stress, inflammation and carcinogenesis [14], including oral cancer [15]. Keap1 is essential for the regulation of Nrf2 activity, both of them enhanced proliferation, drug resistance and could be potent survival factors in cancers [16]. However, the mechanism among SPP1, PAI and caveolin-1, Keap1-Nrf2 system and OSCC is unclear, and the roles of these molecular markers in OSCC have not been clarified.
To date, the expression of SPP1, PAI and caveolin-1 and the relationship between SPP1, PAI and caveolin-1 and clinicopathological data including patient survival in OSCC have not been revealed. The purpose of this study was to investigate the relationship between SPP1, PAI and caveolin-1 and clinicopathological data including patient survival.
Materials and methods
Patient samples
To determine the expression of SPP1, PAI and caveolin-1 in OSCC, we selected 43 formalin-fixed, paraffin-embedded specimens from the Department of Oral Maxillofacial-Head Neck Oncology, School and Hospital of Stomatology Wuhan University. The procedures were performed in accordance with the National Institutes of Health guidelines regarding the use of human tissues. Information about medical history, smoking, and alcohol consumption was collected from a standardized questionnaire. Histological features of OSCC were further classified into well-differentiated, moderately-differentiated, and poorly-differentiated OSCC. Clinical staging and tumor-node-metastasis (TNM) status of OSCCs at initial presentation of the tumor were determined according to the criteria of the International Union Against Cancer. Clinical characteristics of the patient cohorts have been previously described [15]. This study was approved by the Medical Ethics Committee of Hospital of Stomatology Wuhan University, and written informed consent was obtained from each patient.
Tissue microarray construction
For the tissue microarrays (T12-412), we used 43 tumor tissue samples collected between 2008 and 2009, including 19 lymph node metastatic samples. In addition, 17 normal oral mucosa and 6 oral epithelial dysplasia were selected as control group. The cases were selected based on the availability of FFPE tissue blocks with enough tumor tissue for TMA construction. Each normal, epithelial dysplasia and cancer specimen was at least in duplicate. Custom made tissue arrays of formalin-fixed tissues from OSCC mentioned above were constructed with 1.5 mm core from each patient (T12-412) mentioned above.
Immunohistochemistry
Immunohistochemical studies of the human OSCC tissue microarrays were done using the following antibodies: monoclonal mouse anti-human SPP1 (dilution 1:100) from Abcam Biotechnology Inc., (Cambridge, UK), polyclonal rabbit anti-human PAI (1:200) from Epitomics Biotechnology, Inc., (Burlingame, USA), monoclonal mouse anti-human caveolin-1 (dilution 1: 200) from Proteintech Group Inc., (Chicago, USA). Immunohistochemical staining was performed using a peroxidase- labeled streptavidinbiotin technique. Briefly, tissue sections were deparaffinized and rehydrated. Next, sections were incubated in 3% hydrogen peroxide and treated with 10% normal goat serum. Then sections were incubated overnight within primary antibody, followed by second antibody and an avidin-biotin-peroxidase reagent. Diaminobenzidine as well as a counterstaining with haematoxylin resulted in the visualization of the immunostaining. The expression of Keap1 and Nrf2 have been studied in our previously study [15].
Scoring of immunohistochemistry results
Automated image acquisition using an Aperio ScanScope CS scanner (Vista, CA, USA) has been described previously [9]. In brief, monochromatic, high-resolution images which were obtained of each histospot were evaluated on a computer screen for intensity of staining, the images were driven by custom program. The signal intensity of SPP1, PAI and caveolin1 from pixels was measured on scales by Aperio Quantification software (Version 9.1). His-toscore of membrane and nuclear staining was calculated as a percentage of different positive cells using the formula (3+) × 3+ (2+) × 2 + (1+) × 1. Moreover, the biomarker levels were categorized as low or high expression groups) for outcome analyses by using the histoscores distribution.
Hierarchical clustering
In Microsoft excel, the staining scores were converted into scaled values centered on zero, then the Cluster 3.0 with average linkage based on Pearson’s correlation coefficient was used to achieve the hierarchical analysis, and the results were visualized using the Java Tree View 1.0.5. Finally, the clustered data were arranged with markers on the horizontal axis and tissue samples on the vertical axis. Biomarkers with a close relationship are located next to each other.
Statistical analysis
All statistical analyses were performed using GraphPad Prism 5.03 (GraphPad Software, Inc., La Jolla, CA) statistical packages. One-way ANOVA analysis was used to evaluate the difference in protein levels among each group. The correlation of these markers was analyzed by two-tailed Pearson statistics. The survival curves were constructed by the Kaplan-Meier method and compared using the log-rank test. The level of significance was set to P < 0.05. All P values reported were based on two-sided tests. Unless otherwise indicated, values are presented as means ± SEM.
Results
Expression of SPP1, PAI and caveolin-1 in normal oral mucosa, oral epithelial dysplasia and OSCC
To test roles of SPP1, PAI and caveolin-1 in the progression of OSCC, we measured the expression levels of SPP1, PAI and caveolin-1 in a total of 17 normal oral mucosa, 7 oral epithelial dysplasia and 43 OSCC specimens from human tissue arrays. Notably, the expression of SPP1 was mainly located in the cytoplasm of the cancer cells, and the level of SPP1 in OSCC was significant higher compared with the normal oral mucosa (P < 0.05, Figure 1). The PAI exhibited high expression in the cytoplasm of the cancer cells, and PAI levels in OSCC were significantly higher when compared with normal oral mucosa (P < 0.05, Figure 1). In carcinoma, high caveolin-1 expression was seen in the cytoplasm, as seen in Figure 1, there was no significant difference between its levels in OSCC and normal oral mucosa (P > 0.05).
Figure 1.
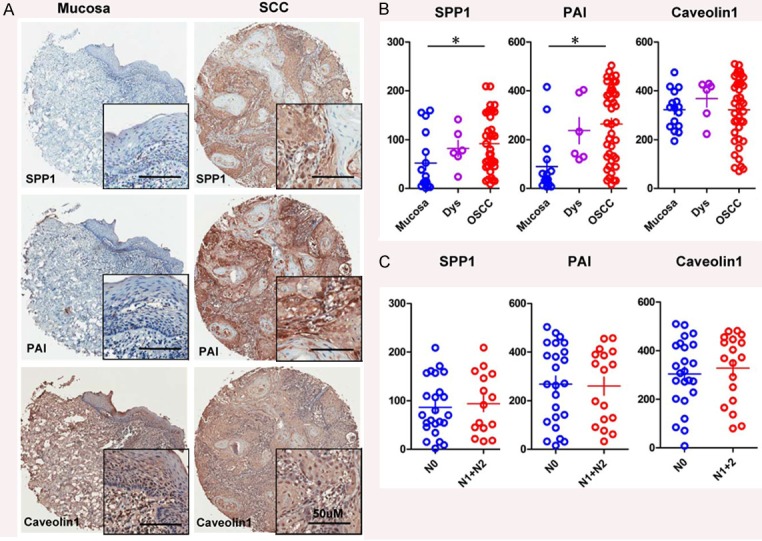
Human OSCC tissue array analysis revealed that SPP1, PAI, and caveolin-1 were overexpressed in human OSCCs: A. Representative immunohistochemical staining (IHC) of SPP1, PAI, caveolin-1 in human oral cancer tissue (right) compared with normal oral mucosa (left) (Scale bars = 50 uM); B. Quantatitive of histoscore of SPP1, PAI, Caveolin-1 expression in normal oral mucosa, oral epithelial dysplasia and human oral cancer, SPP1 levels in OSCC was significantly higher when compared with normal oral mucosa, and PAI levels in OSCC was significantly higher when compared with normal oral mucosa (Mean ± SEM; *P < 0.05; **P < 0.01; ***P < 0.001; One-way ANOVA); C. The expression of SPP1, PAI, and caveolin-1 was not correlated with lymph node status of human oral cancer (Quantification using Aperio nuclear quantification software, and statistics using Graph Pad Prism 5. Mean ± SEM; *, P < 0.05; Mann-Whitney U test).
Correlation between these marker expression, pathologic features, and stage of disease
To determine the correlation between SPP1, PAI and caveolin-1 with clinicopathological features. The results showed that the association of SPP1, PAI and caveolin1 with clinicopathological features, and we could found that SPP1, PAI and caveolin-1 were not significantly correlated with tumor stage (T1 to T3), lymph node status (N0 to N1), pathological grade (GI to GIII) (P > 0.05). Then a large sample of OSCC tissues with follow-up will be collected to further confirm the correlation between SPP1, PAI and caveolin-1 with tumor stage, lymph node status and pathological grade.
Relationships among expression of SPP1, PAI, Caveolin-1, Keap1 and Nrf2
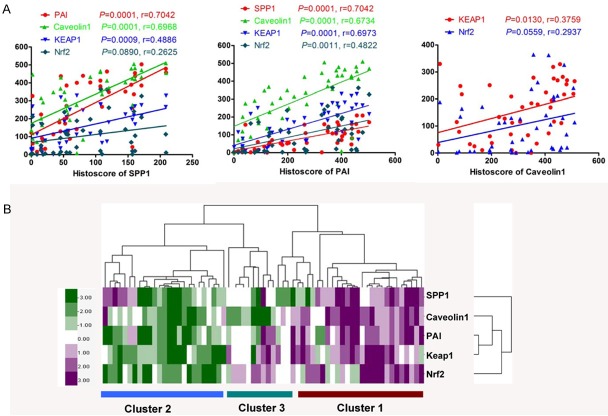
The expression of Keap1 and Nrf2 have been studied in our previously study [15]. In order to access the relationships among expression of SPP1, PAI, caveolin-1, Keap1 and Nrf2, Pearson statistics was used. As summarized in Table 1, SPP1 was found to be closely associated the PAI (P = 0.0001, r = 0.7042), caveolin-1 (P = 0.0001, r = 0. 6968) and Keap1 (P = 0.0009, r = 0.4886), but not Nrf2 (P = 0.0890, r = 0.2625, Figure 3A; Table 1), and PAI was strongly correlated with caveolin-1 (P = 0.0001, r = 0. 6734), Keap1 (P = 0.0001, r = 0. 6973) and Nrf2 (P = 0.0011, r = 0. 4822, Figure 3A; Table 1), and caveolin-1 was associated with Keap1 (P = 0.0130, r = 0. 3759) and Nrf2 (P = 0.0559, r = 0. 2937, Figure 3A; Table 1) by using the Pearson correlation coefficient test. By hierarchical clustering, the expression of tumor associated SPP1, caveolin1, Keap1 and Nrf2 is more close to expression of PAI (Figure 3B).
Table 1.
Pearson correlation coefficient test analyses of the array immunostainings of SPP1, PAI, caveolin-1, Keap1 and Nrf2 in OSCC
| Markers | PAI | Caveolin-1 | Keap1 | Nrf2 |
|---|---|---|---|---|
| SPP1 | P = 0.0001, R = 0.7042 | P = 0.0001, R = 0.6968 | P = 0.0009, R = 0.4886 | P = 0.0890, R = 0.2625 |
| PAI | P = 0.0001, R = 0.6734 | P = 0.0001, R = 0.6973 | P = 0.0011, R = 0.4822 | |
| Caveolin-1 | P = 0.0130, R = 0.3759 | P = 0.0559, R = 0.2937 |
Figure 3.
Correlation of SPP1, PAI and caveolin-1 with Keap1, Nrf2 in human OSCC tissue array: A. SPP1 was found to be closely associated the PAI (P = 0.0001, r = 0.7042), Caveolin-1 (P = 0.0001, r = 0. 6968) and Keap1 (P = 0.0009, r = 0.4886 ), but not Nrf2 (P = 0.0890, r = 0.2625, A, Table 1), and PAI was strongly correlated with caveolin-1 (P = 0.0001, r = 0. 6734), Keap1 (P = 0.0001, r = 0. 6973) and Nrf2 (P = 0.0011, r = 0. 4822, A, Table 1), and caveolin-1 was associated with Keap1 (P = 0.0130, r = 0. 3759) and Nrf2 (P = 0.0559, r = 0. 2937, A, Table 1) in human OSCC tissue array; B. Hierarchical Clustering of Keap1, Nrf2 with SPP1, PAI and caveolin-1 in human OSCC tissue array. Histoscore based on quantification using Aperio quantification software and statistics with Graph Pad Prism5. Mean ± SEM; 2-tailed Pearson correlation statistics.
Association between SPP1, PAI and caveolin-1 expression and OSCC patient outcome using TMA specimens
Furthermore, follow-up information of 43 OSCC patients was available, ranging from 11 months to 40 months (21.6 ± 1.2). At the end of our study, 5 patients were lost during follow up period, 25 patients were alive, 15 patients had recurrence with 13 died of cancer. The 3-year overall survival is 51.2 and disease-free survival rate were 47.1%. Our data indicate that SPP1, PAI and caveolin-1 were over-expressed in OSCC. To determine whether the expression of SPP1, PAI and caveolin-1 was clinically significant, we investigated the association SPP1, PAI and caveolin-1 expression and overall survival. Kaplan-Meier plot analysis for overall survival revealed that over-expressed SPP1 (P = 0.650), PAI (P = 0.650) and caveolin-1 (P = 0.524) were not associated with shorter survival (Figure 2).
Figure 2.
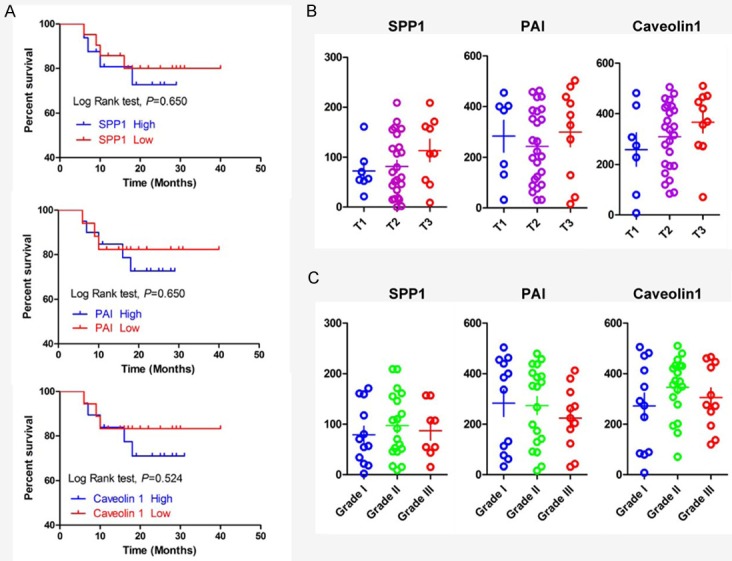
Human OSCC tissue array analysis revealed that SPP1, PAI and caveolin-1 were overexpressed in human OSCCs: A. Overall survival of the OSCC patients with SPP1, PAI and caveolin-1 expression calculated and presented by Kaplan-Meier analysis, and SPP1, PAI and caveolin1 expression were not significantly correlated with overall survival (P > 0.05); B. SPP1, PAI and caveolin-1 were not significantly associated with tumor stage (T1 to T3, P > 0.05), pathological grade (GI to GIII, P > 0.05) (Quantification using Aperio nuclear quantification software, and statistics using Graph Pad Prism 5).
Discussion
The prevalence of oral cancer continuously increased and the poor survival has not been changed in recent 30 years, and the poor prognosis of OSCC is always correlated with metastasis. Therefore, it’s important to find a reliable biomarker for prediction the progression and overall survival and elucidation of the underlying molecular mechanisms are essential to understand the clinical behavior and facilitate the management of OSCC.
In this study, we investigated the expression of SPP1, PAI and caveolin-1 in 17 normal oral mucosa, 7 oral epithelial dysplasia and 43 OSCC specimens by tissue microarrays. Our results showed that SPP1 was mainly located in cytoplasm, and its expression increased progressively from normal oral mucosa to OSCC, and the expression of SPP1 was significantly different between normal oral mucosa and OSCC. Recently, high expression of SPP1 has been reported in many kinds of cancers, including squamous cell carcinoma from various organ sites [17]. In human ovarian carcinoma [18,19], PAI is expressed at higher levels than in normal tissues or compared with adjacent normal tissues. These results are thus consistent with our present study on OSCC and indicate that PAI is involved in the tumor development of certain types of human malignancies. Very little is known, however, about how the role of caveolin-1 does evolve from the early cancer stage to metastases. Although recent studies have reported a reduced expression of caveolin-1 was found in cancers including head and neck carcinoma [11], other studies recognize caveolin-1 as a tumor promoter because Caveolin-1 was over-expressed in various kinds of cancers, especially in oral cancer [12,13]. Consistent with these previous reports, our findings showed that highly expressed caveolin-1 was seen in the cytoplasm, and its expression level was stronger in OSCC than in normal oral mucosa. Our current findings support several previous studies noting that caveolin-1 was an oncogene in OSCC. Therefore, further studies are necessary to elucidate the role of caveolin-1 in carcinogenesis and development of OSCC.
Keap1-Nrf2 system, as a key signaling pathway that regulates transcription of a series of cyto-protective proteins, plays an important role in oxidative stress, inflammation and carcinogenesis [14]. Keap1 is essential for the regulation of Nrf2 activity, both of them enhanced proliferation, drug resistance and could be potent survival factors in cancers [16]. In order to investigate the relationships between SPP1, PAI, caveolin-1 and Keap1-Nrf2 system in OSCC, the Spearman rank correlation coefficient test was applied. Of considerable interest were the correlation between SPP1, PAI, caveolin-1 and Keap1-Nrf2 system, SPP1 was closely associated with PAI, caveolin-1, Keap1 and Nrf2, and PAI was significantly correlated with caveolin-1, Keap1 and Nrf2 in OSCC. Moreover, the finding of strong correlations between caveolin-1 expression with Keap1-Nrf2 system were of much interest, and the correlation between PAI expression with both of Keap1 and Nrf2 was also significantly. These findings, by themselves, suggested that SPP1, PAI and caveolin-1 played important roles together with Keap1-Nrf2 system in the progression of OSCC. In our study, similar to the study in malignant glioma, Nrf2 accumulation in the nucleus could be stimulated by SPP1, and SPP1 increased Nrf2-DNA binding activity resulting in increased HO-1 expression and cell migration [20]. The level of PAI expression was assessed as Nrf2 suppressed TGF-β-mediated signaling in radiation-induced pulmonary fibrosis [21]. In addition, the concentrations of SPP1 in tumor and plasma were not significantly associated, and an inverse correlation between PAI plasma levels and tumor SPP1 was found in advanced non-small-cell lung cancer [22]. Along with our findings in this study, these observations suggest that SPP1, PAI, caveolin-1 and Keap1-Nrf2 system played important roles together in carcinogenesis and progression in OSCC, nevertheless, the regulation mechanisms among them in OSCC need further researches.
Furthermore, we further analyzed the association among the levels of SPP1, PAI and caveolin-1 expression and clinicopathologic factors. In laryngeal and hypopharyngeal cancer, overexpressed SPP1 was closely related with tumor invasion and metastasis [5,17], PAI was correlated with the metastasis and invasion in ovarian carcinoma [18], caveolin-1 was associated with metastasis and worse prognosis in hepatocellular carcinoma [12]. Surprisingly, we could not found that SPP1, PAI and caveolin-1 were significantly correlated with tumor stage, lymph node status and pathological grade. Owing to the small number of tumor specimens, we could not detect any statistically relevant correlations between patients’ clinical data. Of note is that, our analysis showed that SPP1, PAI and caveolin-1 were not correlated with overall survival. In contrast to our current study, SPP1 was suggested as an independent predictor of poor prognosis in intrahepatic cholangiocarcinoma [23], and PAI was significantly associated with overall survival in ovarian cancer [18,19], in hepatocellular carcinoma caveolin-1 was associated with poor prognosis [12]. In non–small-cell lung cancer, SPP1, PAI levels did not correlate with patient outcomes, which was accordance with our results. Importantly, plasma concentrations of SPP1 and PAI were significantly interrelated and significantly decreased after S0003 treatment in chemotherapy- treated patients with advanced non–small-cell lung cancer [22]. In head and neck squamous cell carcinomas, high plasma SPP1 levels were not correlated with poor prognosis [24]. Further studies are necessary to elucidate the correlation between these factors and prognosis because these factors seem to be sequential events correlated with the progression of OSCC. Considering the limited number of samples used in our study, further accumulation of data is required to consolidate the significance of SPP1, PAI and caveolin-1 expression in OSCC, especially as a prognostic marker.
In conclusion, our results showed that the SPP1, PAI and caveolin-1 were over-expressed in OSCC and closely correlated with Keap1-Nrf2 system, suggesting that they played key roles in carcinogenesis and progression in OSCC. These findings raise the possibility of these markers as new targets for OSCC patient’s treatment.
Acknowledgements
This study was supported by the National Natural Science Foundation of China 81072203, 81272963 (ZJS) and the Fundamental Research Funds for the Central Universities (2042014kf0095).
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Sun ZJ, Zhang L, Hall B, Bian Y, Gutkind JS, Kulkarni AB. Chemopreventive and chemotherapeutic actions of mTOR inhibitor in genetically defined head and neck squamous cell carcinoma mouse model. Clin Cancer Res. 2012;18:5304–5313. doi: 10.1158/1078-0432.CCR-12-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnston NI, Gunasekharan VK, Ravindranath A, O’Connell C, Johnston PG, El-Tanani MK. Osteopontin as a target for cancer therapy. Front Biosci. 2008;13:4361–4372. doi: 10.2741/3009. [DOI] [PubMed] [Google Scholar]
- 4.Rangaswami H, Bulbule A, Kundu GC. Osteopontin: role in cell signaling and cancer progression. Trends in cell biology. 2006;16:79–87. doi: 10.1016/j.tcb.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Shevde LA, Das S, Clark DW, Samant RS. Osteopontin: an effector and an effect of tumor metastasis. Curr Mol Med. 2010;10:71–81. doi: 10.2174/156652410791065381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Servais EL, Suzuki K, Colovos C, Rodriguez L, Sima C, Fleisher M, Rusch VW, Sadelain M, Adusumilli PS. An in vivo platform for tumor biomarker assessment. PLoS One. 2011;6:e26722. doi: 10.1371/journal.pone.0026722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Czekay RP, Wilkins-Port CE, Higgins SP, Freytag J, Overstreet JM, Klein RM, Higgins CE, Samarakoon R, Higgins PJ. PAI-1: An Integrator of Cell Signaling and Migration. Int J Cell Biol. 2011;2011:562481. doi: 10.1155/2011/562481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gomes-Giacoia E, Miyake M, Goodison S, Rosser CJ. Targeting plasminogen activator inhibitor-1 inhibits angiogenesis and tumor growth in a human cancer xenograft model. Mol Cancer Ther. 2013;12:2697–2708. doi: 10.1158/1535-7163.MCT-13-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andreasen PA, Egelund R, Petersen HH. The plasminogen activation system in tumor growth, invasion, and metastasis. Cell Mol Life Sci. 2000;57:25–40. doi: 10.1007/s000180050497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orlichenko L, Weller SG, Cao H, Krueger EW, Awoniyi M, Beznoussenko G, Buccione R, McNiven MA. Caveolae mediate growth factor-induced disassembly of adherens junctions to support tumor cell dissociation. Mol Biol Cell. 2009;20:4140–4152. doi: 10.1091/mbc.E08-10-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang H, Su L, Muller S, Tighiouart M, Xu Z, Zhang X, Shin HJ, Hunt J, Sun SY, Shin DM, Chen ZG. Restoration of caveolin-1 expression suppresses growth and metastasis of head and neck squamous cell carcinoma. Br J Cancer. 2008;99:1684–1694. doi: 10.1038/sj.bjc.6604735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang ZB, Cai L, Zheng SG, Xiong Y, Dong JH. Overexpression of caveolin-1 in hepatocellular carcinoma with metastasis and worse prognosis: correlation with vascular endothelial growth factor, microvessel density and unpaired artery. Pathol Oncol Res. 2009;15:495–502. doi: 10.1007/s12253-008-9144-7. [DOI] [PubMed] [Google Scholar]
- 13.Xue J, Chen H, Diao L, Chen X, Xia D. Expression of caveolin-1 in tongue squamous cell carcinoma by quantum dots. Eur J Histochem. 2010;54:e20. doi: 10.4081/ejh.2010.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang XJ, Sun Z, Villeneuve NF, Zhang S, Zhao F, Li Y, Chen W, Yi X, Zheng W, Wondrak GT, Wong PK, Zhang DD. Nrf2 enhances resistance of cancer cells to chemotherapeutic drugs, the dark side of Nrf2. Carcinogenesis. 2008;29:1235–1243. doi: 10.1093/carcin/bgn095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang CF, Zhang L, Ma SR, Zhao ZL, Wang WM, He KF, Zhao YF, Zhang WF, Liu B, Sun ZJ. Clinical significance of Keap1 and Nrf2 in oral squamous cell carcinoma. PLoS One. 2013;8:e83479. doi: 10.1371/journal.pone.0083479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hartikainen JM, Tengstrom M, Kosma VM, Kinnula VL, Mannermaa A, Soini Y. Genetic polymorphisms and protein expression of NRF2 and Sulfiredoxin predict survival outcomes in breast cancer. Cancer Res. 2012;72:5537–5546. doi: 10.1158/0008-5472.CAN-12-1474. [DOI] [PubMed] [Google Scholar]
- 17.Lu JG, Li Y, Li L, Kan X. Overexpression of osteopontin and integrin alpha in laryngeal and hypopharyngeal carcinomas associated with differentiation and metastasis. J Cancer Res Clin Oncol. 2011;137:1613–1618. doi: 10.1007/s00432-011-1024-y. [DOI] [PubMed] [Google Scholar]
- 18.Ren F, Shi H, Zhang G, Zhang R. Expression of deleted in liver cancer 1 and plasminogen activator inhibitor 1 protein in ovarian carcinoma and their clinical significance. J Exp Clinical Cancer Res. 2013;32:60. doi: 10.1186/1756-9966-32-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang W, Ling D, Tan J, Zhang J, Li L. Expression of urokinase plasminogen activator and plasminogen activator inhibitor type-1 in ovarian cancer and its clinical significance. Oncol Rep. 2013;29:637–645. doi: 10.3892/or.2012.2148. [DOI] [PubMed] [Google Scholar]
- 20.Lu DY, Yeh WL, Huang SM, Tang CH, Lin HY, Chou SJ. Osteopontin increases heme oxygenase-1 expression and subsequently induces cell migration and invasion in glioma cells. Neuro Oncol. 2012;14:1367–1378. doi: 10.1093/neuonc/nos262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Travis EL, Rachakonda G, Zhou X, Korhonen K, Sekhar KR, Biswas S, Freeman ML. NRF2 deficiency reduces life span of mice administered thoracic irradiation. Free Radic Biol Med. 2011;51:1175–1183. doi: 10.1016/j.freeradbiomed.2011.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mack PC, Redman MW, Chansky K, Williamson SK, Farneth NC, Lara PN Jr, Franklin WA, Le QT, Crowley JJ, Gandara DR SWOG. Lower osteopontin plasma levels are associated with superior outcomes in advanced non-small-cell lung cancer patients receiving platinum-based chemotherapy: SWOG Study S0003. J. Clin. Oncol. 2008;26:4771–4776. doi: 10.1200/JCO.2008.17.0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sulpice L, Rayar M, Desille M, Turlin B, Fautrel A, Boucher E, Llamas-Gutierrez F, Meunier B, Boudjema K, Clément B, Coulouarn C. Molecular profiling of stroma identifies osteopontin as an independent predictor of poor prognosis in intrahepatic cholangiocarcinoma. Hepatology. 2013;58:1992–2000. doi: 10.1002/hep.26577. [DOI] [PubMed] [Google Scholar]
- 24.Lim AM, Rischin D, Fisher R, Cao H, Kwok K, Truong D, McArthur GA, Young RJ, Giaccia A, Peters L, Le QT. Prognostic significance of plasma osteopontin in patients with locoregionally advanced head and neck squamous cell carcinoma treated on TROG 02.02 phase III trial. Clin Cancer Res. 2012;18:301–307. doi: 10.1158/1078-0432.CCR-11-2295. [DOI] [PMC free article] [PubMed] [Google Scholar]