Abstract
Lysyl oxidase (LOX) has been reported to regulate tumor metastasis and has been found to involve in modification of extracellular matrix (ECM) in the context of tumorigenesis. The aim of this study is to determine the prognostic significance of LOX in non-small cell lung cancer (NSCLC) patients and to examine the correlation between LOX expression and ECM remodeling-associated MMP2/MMP9 in NSCLC tissues. The mRNA expression of LOX, MMP2 and MMP9 was investigated by quantitative real-time reverse transcriptase-polymerase chain reaction (qRT-PCR) in 30 NSCLC patients. The protein expression of LOX was measured by immunohistochemistry (IHC) in 110 paraffin-embedded tissues with NSCLC and the protein expression of MMP2/MMP9 was measured by in 30 NSCLC patients. The correlation between LOX expression and clinical parameters and MMP2/MMP9 was analyzed by appropriate statistics. The Kaplan-Meier method, univariate and multivariate regression analysis was used to analyze the correlation between LOX expression and overall survival (OS). The relative mRNA expression or protein expression of LOX were significantly higher in NSCLC tumor tissues than in the corresponding noncancerous tissues (P < 0.05). High LOX expression was significantly associated with MMP2, MMP9, tumor size, lymph node metastasis, pathological stage and OS (P < 0.05). Univariate and multivariate analysis showed that LOX was an independent prognostic factor for OS. Our results indicate that LOX may play a role in the metastasis of NSCLC by promoting MMP2/MMP9 expression. LOX expression is an independent prognostic factor in OS in NSCLC.
Keywords: LOX, NSCLC, prognosis, ECM
Introduction
Non-small cell lung cancer (NSCLC), the most common type of lung cancer, is nowadays the leading cause of cancer-related death worldwide [1]. Despite the recent advances in treatment of NSCLC, 5-year survival rate of lung cancer was approximately lower than 15% [2] and the most common cause of all cancer deaths are the result of local invasion or distant metastases, rather than the primary tumors. Therefore, it is of great significance to make a better understanding of the molecular biology of NSCLC invasion and metastasis, and to identify novel molecular markers for the improvement of clinical management of patients with NSCLC.
Mounting evidences supports the view that tumor microenvironment plays a critical role in tumorigenesis [3,4]. Although there is a wide range of biological functions of tumor microenvironment in cancer, an important characteristic is the remodeling and stiffening of the extracellular matrix (ECM) [5,6]. Nowadays, interest in the role of lysyl oxidase (LOX) in modulating of cancer ECM has grown increasingly [7,8]. LOX, an extracellular copper-dependent amine oxidase, catalyzes the exchange of an amine to aldehyde group on a peptidyl lysine, producing hydrogen peroxide (H2O2) and ammonia as by-products of catalytic activity. The function of LOX is the covalent cross-linking of collagens or elastin to increase ECM tensile strength. Its expression is essential for normal connective tissue function, embryonic development and wound healing [9,10]. Additionally, aberrant LOX expression or enzymatic activity is also involved in carcinogenesis and cancer progression [8]. Both increased and decreased LOX expressions have been reported in different cancer cell lines and primary tumors [11], which suggest the function of LOX may depend on the type of cancer or the specific tumor microenvironment. However, accumulating evidences indicated LOX is prone to promote tumor progression and metastasis by regulating collagen crosslinking and stiffness in breast cancer [7], colorectal cancer [12] and lung cancer [13].
Matrix metalloproteinases (MMPs), which represent the most prominent family of proteinases associated with tumorigenesis, are a family of zinc-dependent endopeptidases [14]. MMP-mediated ECM degradation has also been shown to lead to cancer cell invasion and metastasis. Although the direct mechanism has not yet been elucidated, there is evidence to indicate that LOX-mediated ECM crosslinking may function in synergy with MMPs to remodeling of the ECM to promote cancer metastasis [15]. Previously, we have demonstrated LOX over expression is strongly linked to poor progression free survival in NSCLC [16]. However, the relationship between the LOX expression and overall survival (OS) with NSCLC has not been investigated. Furthermore, the possibility that LOX might have a role in patients with NSCLC by promoting MMPs has not been investigated.
In the present study, using immunohistochemistry (IHC) staining, we observed high LOX expression was significantly associated with poor OS in patients with NSCLC and multivariate analysis showed that high LOX expression was an independent predictor of OS. Meanwhile, we found that the expression of LOX in primary NSCLC was positively correlated with MMP2 and MMP9.
Materials and methods
Patients and specimens
For quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR) analysis, we collected 30 paired fresh NSCLC tumor tissue samples and corresponding adjacent noncancerous tissue samples from patients who underwent surgery between 2010 and 2011. In addition, a total of 119 patients who underwent surgery for histologically verified NSCLC at the Department of Thoracic Surgery, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, between 2007 and 2009 were enrolled in this study. This study was approved by The Ethics Committee of Tongji Hospital, Tongji Medical College and written informed consent was obtained from each patient. TNM classification was performed according to 7th edition of AJCC TNM classification. None of these patients received any anticancer therapy prior to sample collection. Of these patients, 9 were excluded due to death within 60 days of surgery to reduce the confounding variable of perioperative mortality. The 60-day cut-point was used in our study as it has been used to exclude postoperative mortality in several previous studies in NSCLC [17,18]. The pathologic classifi cation of each sample was confi rmed by a review of hematoxylin-and-eosin-stained sections. The clinicopathological characteristics of the included 110 patients are summarized in Table 1.
Table 1.
Relationship between LOX expression and clinicopathological characteristics of NSCLC patients
| Characteristics | Total cases(n = 110) | LOX protein | P value | |
|---|---|---|---|---|
|
|
||||
| + | - | |||
| Age | ||||
| ≤65 | 86 | 56 | 30 | 0.327 |
| >65 | 24 | 13 | 11 | |
| Gender | ||||
| Male | 75 | 47 | 28 | 0.985 |
| Female | 35 | 22 | 13 | |
| Pathological type | ||||
| Squamous | 41 | 26 | 15 | 0.909 |
| Adenocarcinoma | 69 | 43 | 26 | |
| Differentiation | ||||
| Well | 37 | 23 | 14 | 0.930 |
| Moderate + Poor | 73 | 46 | 27 | |
| Tumor size (cm) | ||||
| ≤3 | 42 | 20 | 22 | 0.010 |
| >3 | 68 | 49 | 19 | |
| Lymph node involvement | ||||
| No | 48 | 24 | 24 | 0.015 |
| Yes | 62 | 45 | 17 | |
| TNM stage | ||||
| I | 25 | 8 | 17 | 0.001 |
| II | 44 | 32 | 12 | |
| III | 41 | 29 | 12 | |
RNA isolation, reverse transcription and qRT-PCR
Total RNA of NSCLC and paired noncancerous tissues were extracted using Trizol (Invitrogen, Carlsbad, USA). The concentration of RNA was measured by a spectrophotometer (ND 2000, Nanodrop Inc, Wilmington, Del). One micrograms of RNA was used for reverse transcription. RNA was reverse-transcribed using SuperScript First Strand cDNA System (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. LOX, MMP2, MMP9 and β-actin genes were amplified in a fluorescence reader ABI Stepone system (Applied Biosystems, Foster City, CA, USA). The amplification was carried out in a total volume of 10 μl containing 5 μl Fast SYBR Green Master Mix (Applied Biosystems, Foster City, CA, USA), 3 μl sterile water, 1 μl cDNA and 0.5 μl of each primer. Cycling conditions were as follows: initial enzyme activation at 95°C for 20 s, followed by 40 cycles of denature at 95°C for 3 s, anneal/extend at 60°C for 30 s. The primer sequences for LOX gene were: 5’-CCTGGTTCCTGAATCTGACT-3’ (forward) and 5’-CTTCAGAACACCAGGCACTG-3’ (reverse); the primer sequences for MMP2 gene were: 5’-CTTCTTCCCTCGCAAGCC-3’ (forward) and 5’-ATGGATTCGAGAAAACCG-3’ (reverse); the primer sequences for MMP9 gene were 5’-ACGCAGACATCGTCATCC-3’ (forward) and 5’-AACCGAGTTGAACCACG-3’ (reverse); and the primer sequences for β-actin gene were: 5’-GCAAATGCTTCTAGGCGGAC-3’ (forward) and 5’-GCTGTCACCTTCACCGTTCC-3’ (reverse). The relative expression of the mRNA was calculated with the following formula: Ratio = 2-ΔCt, in which ΔCt = (Ct target gene - Ct β-actin). Each experiment was carried out in triplicate.
Immunohistochemistry
All specimens were fixed with 4% formaldehyde, dehydrated, embedded and cut into 4 μm serial sections. Staining of paraffin-embedded sections was done as previously described [16]. Briefly, the sections were heated in a microwave oven in citrate buffer (pH = 6.0) for 15 min at 95°C and was then cooled to room temperature. Endogenous peroxidase activity was blocked by incubation in 3% hydrogen peroxide for 20 min at room temperature. After washing with PBS, non-specific binding sites were blocked with normal goat serum for 30 min at room temperature. The sections were then incubated overnight at 4°C with primary antibody (rabbit monoclonal anti-LOX, 1:400, Novus Biological, Inc., Littleton, CO, USA; rabbit polyclonal anti-MMP2, 1:50, Boster, China; rabbit polyclonal anti-MMP9, 1:50, Boster Biological Technology, Wuhan, China). After washing with PBS, sections were incubated with secondary antibodies for 30 minutes at 37°C. The sections were then washed three times with PBS and the sections were visualized with diaminnobenzidine-tetrahydrochloride (DAB kit, Zhonshan Goldenbridge Biological Technology Co., LTD, Beijing, China). Finally, the sections were counterstained with hematoxylin and dehydrated. Negative controls were performed with PBS under the same experimental conditions.
Immunohistochemical staining was assessed independently by two investigators who were blinded to all clinicopathological features. Five different fields (×400) were randomly examined. The immunoreactivity score method to evaluate the immunostaining results has been previously described [16]. Stain intensity is as follows: no staining (score 0), weak staining (score 1), moderate staining (score 2), or strong staining (score 3). Staining area is as follows: less than 10% (score 1), 11%-50% (score 2), 51%-80% (score 3), 81%-100% (score 4). Points for staining intensity and percentage of positive tumor cells were added and the overall score were grouped into four categories: negative (≤10% of tumor cells stained positive, regardless of intensity), weak expression (score 3), moderate expression (score 4 to 5), and strong expression (score 6 to 7). Moderate and strong expression was rated as positive, while weak expression was rated as negative for analysis.
Statistical analysis
Statistical analysis was performed using SPSS statistical software 11.0 for windows. Data were presented as mean ± standard deviation. Difference/correlations between two groups were assessed by student’s t test, x2 tests, and Pearson’s correlation test. Survival curves were calculated using the Kaplan-Meier method and the statistical significance was assessed using the log-rank test. Multivariate survival analysis based on the Cox proportional hazard model was carried out to identify the significant independent prognostic factors. Differences at P < 0.05 were considered to be statistically significant.
Results
Expression of LOX mRNA by qRT-PCR in NSCLC tissues
Our qRT-PCR results showed that LOX mRNA expression was upregulated in 25 of 30 NSCLC samples compared with corresponding adjacent noncancerous tissues. The mean expression value of mRNA in NSCLC tissues was significantly higher than the value in paired normal tissues (Figure 1A).
Figure 1.
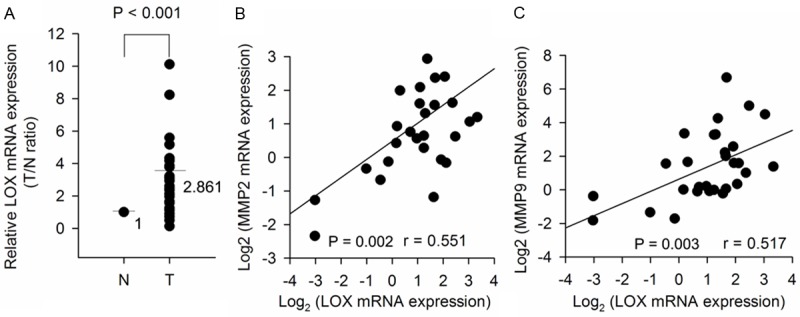
LOX and MMP2/MMP9 are positively expressed in resected NSCLC tumors. Total RNA extracted from 30 pairs of matched NSCLC samples was used for qRT-PCR analysis of mRNA expression. A. Upregulation of LOX mRNA expression in NSCLC tissues. The results were normalized to β-actin expression and expressed as fold change in tumor compared with matched N (P < 0.001). B. Positive correlation between LOX mRNA and MMP2 mRNA in NSCLC tissues (P = 0.002). C. Positive correlation between LOX mRNA and MMP9 mRNA in NSCLC tissues (P = 0.003). N: non-tumor tissue; T: tumor tissue.
Correlation of LOX expression with clinicopathlogical characteristics
In agree with our previous study [16], we have observed that LOX protein expression was localized in cytoplasm of tumor cells (Figure 2), and positive LOX protein expression in tumor tissues (22/30, 73.3%) of NSCLC was significant higher than that in paired normal tissues (10/30, 33.3%, P = 0.002). In order to analyze the correlation between LOX and clinicopathlogical characteristics, IHC staining was performed in 110 archived paraffin-embedded NSCLC samples. As shown in Table 1, LOX protein was positively correlated with tumor size (P = 0.010), lymph node involvement (P = 0.015), and TNM stage (P = 0.001); however, no significant associations were detected for LOX expression with patient age, sex, histological grade (all P>0.05).
Figure 2.
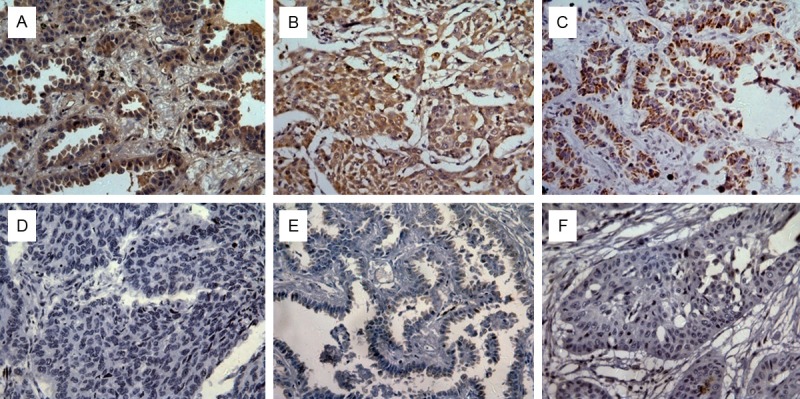
Cytoplasm localization of LOX, MMP2 and MMP9 in NSCLC. Immunohistochemistry was performed to examine the expression of LOX, MMP2 and MMP9 protein in tumor tissues (×400). A. Positive expression of LOX protein in NSCLC. B. Positive expression of MMP2 in NSCLC. C. Positive expression of MMP9 in NSCLC. D. Negative expression of LOX protein in NSCLC. E. Negative expression of MMP2 protein in NSCLC. F. Negative expression of MMP9 protein in NSCLC.
Relationship between the expressions of LOX and MMP2/MMP9 in NSCLC
To determine the relationship between LOX and MMP2/MMP9, we analyzed 30 pairs of NSCLC clinical specimens. The expression of LOX mRNA positively correlated with that of MMP2 mRNA, and that of MMP9 mRNA levels (Figure 1B and 1C). The similar results were also observed between LOX protein and MMP2/MMP9 protein by IHC staining, which indicates that the increase in LOX protein expression is in keeping with the increase of MMP2/MMP9 protein expression in NSCLC samples (Table 2).
Table 2.
Correlation between LOX and MMP2/MMP9 protein in NSCLC tumor tissues
| MMP2 expression | rspearman | P value | MMP9 expression | rspearman | P value | |||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Positive (n = 26) | Negative (n = 4) | Positive (n = 21) | Negative (n = 9) | |||||
| LOX expression | ||||||||
| Positive (n = 24) | 23 | 1 | 0.539 | 0.002 | 19 | 5 | 0.400 | 0.028 |
| Negative (n = 6) | 3 | 3 | 2 | 4 | ||||
Prognostic significance of LOX expression in NSCLC
To investigate the prognostic significance of LOX protein in NSCLC has an impact on patients overall survival (OS), we performed survival analysis using a Kaplan-Meier method with a log-rank test. Kaplan-Meier analysis indicated that LOX over-expression was significantly associated with poorer OS in NSCLC patients (Figure 3). Therefore, the over-expression of LOX may affect the prognosis of NSCLC patients. To evaluate whether overexpression of LOX could be an independent risk factor for poor prognosis in NSCLC, conventional clinicopathological characteristics and LOX protein level were assessed by Cox’s univariate and multivariate hazard regression model (Table 3). In the univariate analysis, lymph node involvement, TNM stage and LOX protein level were significantly associated with poorer OS in NSCLC patients. In the multivariate analysis, LOX protein level, together with lymph node involvement, TNM stage was an independent prognostic factor for OS in NSCLC.
Figure 3.
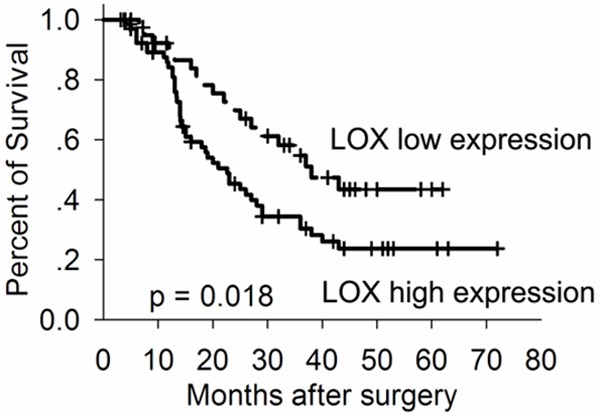
Kaplan-Meier survival curves of overall survival in NSCLC patients based on LOX expression. Patients with high LOX expression had poorer overall survival rate as compared to those with low LOX expression.
Table 3.
Univariate analysis and multivariate analyses of prognostic factors for overall survival in NSCLC patients
| Variable | Category | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| LOX | Positive/negative | 1.890 | 1.101-3.244 | 0.021 | 1.886 | 1.066-3.337 | 0.029 |
| Age | >65 years/≤65 years | 1.112 | 0.622-1.988 | 0.719 | 1.211 | 0.642-2.282 | 0.555 |
| Gender | Female/male | 0.784 | 0.454-1.355 | 0.383 | 0.767 | 0.405-1.454 | 0.416 |
| Pathological type | ADC/SCC | 1.337 | 0.772-2.313 | 0.300 | 1.252 | 0.698-2.247 | 0.451 |
| Differentiation | Moderate + Poor/well | 1.251 | 0.736-2.128 | 0.409 | 1.682 | 0.876-3.230 | 0.118 |
| Tumor size | >3 cm/≤3 cm | 1.380 | 0.825-2.307 | 0.220 | 1.166 | 0.670-2.028 | 0.587 |
| Lymph node involvement | Yes/No | 2.422 | 1.442-4.065 | 0.001 | 2.609 | 1.488-4.574 | 0.001 |
| TNM stage | II+III/I | 1.958 | 1.160-3.303 | 0.012 | 2.783 | 1.570-4.932 | < 0.001 |
HR: hazard ratio; ADC: Adenocarcinoma; SCC: Squamous cell cancer.
Discussion
Experimental evidences point at LOX as a potential target for cancer therapy [7,12,13]. However, the role of the extracellular matrix-remodeling enzyme in solid tumor progression and survival is not fully understood. Therefore, the present work is an attempt to shed light into the involvement of LOX in NSCLC. With this purpose, we investigated the correlation between LOX expression in NSCLC and clinicopathological data. Moreover, we evaluated the correlation between LOX expression and ECM remodeling-associated MMP2/MMP9 expression and the prognostic value of LOX in NSCLC.
Recently, strong evidence has come forth for the role of LOX as a metastasis promoter [8]. In vivo and in vitro studies have shown that up-regulation of LOX increased cancer cell invasion and metastases in breast cancer [19] and colorectal cancer [12]. In addition to local effects on invasion, secreted LOX has been shown to accumulate within the lungs and to contribute to the formation of the premetastatic niche by stimulating cross-linking collagen IV in the basement membrane and promoting the recruitment of bone marrow derived cells that stimulate angiogenesis [15]. In the present study, we showed an evident overexpression of LOX in NSCLC tumor tissues by qRT-PCR and IHC when compared to adjacent normal tissues. Furthermore, analysis of the relationship between LOX protein expression in NSCLC tissues and clinical characteristics of patients revealed that high LOX expression was significantly correlated with prognosis-related features, including tumor size, lymph node involvement, and TNM stage. However, the impact of LOX in NSCLC is still unclear because decreased LOX expression has been reported to be with advancing tumor stage in patients with bronchogenic carcinoma. One possible explanation of this paradox may be the small sample size included in that study. Our findings are in accordance to the work of other groups [20-23], which shown LOX is associated with tumor progression. Thus, it is likely that LOX may play a role in the development of NSCLC.
In order to evaluate the effect of LOX expression on the prognosis of the patients with NSCLC, NSCLC tissues from patients with survival data were analyzed by IHC for LOX expression. The Kaplan-Meier analysis indicated that post-operative OS period of the LOX positive group was notably shorter than that of the negative group. Cox model univariate analysis and multivariate analysis proved that LOX high expression was an independent factor affecting prognosis, which demonstrated our proposal that LOX plays an important role in NSCLC development and progression. The pro-tumor effect has also been found in esophageal squamous cell carcinoma [24], gastric cancer [21] and oral and oropharyngeal squamous cell carcinoma [22] where LOX was shown to be an independent prognostic factor for OS. In contrast, previous publications also reported the anti-tumor activity of LOX in animal and in human cells [25,26]. This paradox may be accounted for the existence of multiple forms of LOX proteins. Actually, LOX is synthesized as a 50-kD pro-enzyme, secreted into the extracellular environment. The pro-enzyme is cleaved extracellular by bone morphogenetic protein-1 (BMP-1) into the mature 32-kD LOX protein and an 18-kD pro-peptide (LOX-PP) [27]. Recent evidence indicates that it is not the mature LOX protein that suppresses the neoplastic transformation, but rather the LOX-PP [28,29]. Further studies are required to investigate the potential mechanism of LOX and LOX-PP in the development of cancer.
ECM dysregulation and remodeling are essential for the progression of the neoplastic process and are characteristics of solid tumor [14]. Thus, the identification of biomarkers that influence ECM dysregulation and remodeling is emergence as it may provides avenues for practical therapeutic intervention. The expression of LOX, an extracellular matrix-remodeling enzyme, was found to be signifi cantly associated with MMP2/MMP9 expression in a variety of human cancers. Erler et al. have reported that the activation of MMP2 is strongly correlated with LOX expression in breast cancer [15]. It has also been shown that LOX secreted by hypoxic epithelial ovarian cancer cells may contribute to cell migration and invasion by upregulating the expression of MMP2 and MMP9 [20]. Similar conclusions have been drawn by Yang et al. in study of cervical cancer cells [30], which suggests inhibition the enzyme activity of LOX by β-aminopropionitrile reduces the expression of MMP9. Taken together, these results show that LOX may promotes ECM remodeling-associated MMP2 and MMP9 expression in tumor. Although the direct mechanism between LOX and matrix metalloproteinases has not yet been elucidated, our present study reported the correlation between LOX and MMP2/MMP9 for the first time. However, considering the complicated intracellular and extracellular function of LOX, the critical importance of LOX in ECM remodeling in tumor microenvironment needs to be further explored.
In conclusion, our results indicate that LOX overexpression in NSCLC may play a pivotal role in tumor progression, and LOX expression was significantly correlated with both MMP2/MMP9 and serves as an independent biomarker for poor survival. According to our results, LOX may be an important target for the therapy of NSCLC. Further investigations are necessary to clarify and understand the mechanisms of LOX in ECM remodeling in NSCLC.
Acknowledgements
The work is supported by the Natural Science Foundation of Hubei Province (No. 2011CDB561).
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, Cooper D, Gansler T, Lerro C, Fedewa S, Lin C, Leach C, Cannady RS, Cho H, Scoppa S, Hachey M, Kirch R, Jemal A, Ward E. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62:220–241. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 3.Gribben J, Rosenwald A, Gascoyne R, Lenz G. Targeting the microenvironment. Leuk Lymphoma. 2010;51(Suppl 1):34–40. doi: 10.3109/10428194.2010.500072. [DOI] [PubMed] [Google Scholar]
- 4.Deep G, Agarwal R. Targeting tumor microenvironment with silibinin: promise and potential for a translational cancer chemopreventive strategy. Curr Cancer Drug Targets. 2013;13:486–499. doi: 10.2174/15680096113139990041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cox TR, Erler JT. Remodeling and homeostasis of the extracellular matrix: implications for fibrotic diseases and cancer. Dis Model Mech. 2011;4:165–178. doi: 10.1242/dmm.004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol. 2012;196:395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, Yamauchi M, Gasser DL, Weaver VM. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barker HE, Cox TR, Erler JT. The rationale for targeting the LOX family in cancer. Nat Rev Cancer. 2012;12:540–552. doi: 10.1038/nrc3319. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez C, Rodriguez-Sinovas A, Martinez-Gonzalez J. Lysyl oxidase as a potential therapeutic target. Drug News Perspect. 2008;21:218–224. doi: 10.1358/dnp.2008.21.4.1213351. [DOI] [PubMed] [Google Scholar]
- 10.Maki JM. Lysyl oxidases in mammalian development and certain pathological conditions. Histol Histopathol. 2009;24:651–660. doi: 10.14670/HH-24.651. [DOI] [PubMed] [Google Scholar]
- 11.Payne SL, Hendrix MJ, Kirschmann DA. Paradoxical roles for lysyl oxidases in cancer-a prospect. J Cell Biochem. 2007;101:1338–1354. doi: 10.1002/jcb.21371. [DOI] [PubMed] [Google Scholar]
- 12.Baker AM, Cox TR, Bird D, Lang G, Murray GI, Sun XF, Southall SM, Wilson JR, Erler JT. The role of lysyl oxidase in SRC-dependent proliferation and metastasis of colorectal cancer. J Natl Cancer Inst. 2011;103:407–424. doi: 10.1093/jnci/djq569. [DOI] [PubMed] [Google Scholar]
- 13.Gao Y, Xiao Q, Ma H, Li L, Liu J, Feng Y, Fang Z, Wu J, Han X, Zhang J, Sun Y, Wu G, Padera R, Chen H, Wong KK, Ge G, Ji H. LKB1 inhibits lung cancer progression through lysyl oxidase and extracellular matrix remodeling. Proc Natl Acad Sci U S A. 2010;107:18892–18897. doi: 10.1073/pnas.1004952107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erler JT, Bennewith KL, Cox TR, Lang G, Bird D, Koong A, Le QT, Giaccia AJ. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell. 2009;15:35–44. doi: 10.1016/j.ccr.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ping W, Jiang WY, Chen WS, Sun W, Fu XN. Expression and significance of hypoxia inducible factor-1alpha and lysyl oxidase in non-small cell lung cancer. Asian Pac J Cancer Prev. 2013;14:3613–3618. doi: 10.7314/apjcp.2013.14.6.3613. [DOI] [PubMed] [Google Scholar]
- 17.Giatromanolaki A, Koukourakis MI, Sivridis E, Turley H, Talks K, Pezzella F, Gatter KC, Harris AL. Relation of hypoxia inducible factor 1 alpha and 2 alpha in operable non-small cell lung cancer to angiogenic/molecular profile of tumours and survival. Br J Cancer. 2001;85:881–890. doi: 10.1054/bjoc.2001.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swinson DE, Jones JL, Cox G, Richardson D, Harris AL, O’Byrne KJ. Hypoxia-inducible factor-1 alpha in non small cell lung cancer: relation to growth factor, protease and apoptosis pathways. Int J Cancer. 2004;111:43–50. doi: 10.1002/ijc.20052. [DOI] [PubMed] [Google Scholar]
- 19.Wong CC, Zhang H, Gilkes DM, Chen J, Wei H, Chaturvedi P, Hubbi ME, Semenza GL. Inhibitors of hypoxia-inducible factor 1 block breast cancer metastatic niche formation and lung metastasis. J Mol Med (Berl) 2012;90:803–815. doi: 10.1007/s00109-011-0855-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ji F, Wang Y, Qiu L, Li S, Zhu J, Liang Z, Wan Y, Di W. Hypoxia inducible factor 1alpha-mediated LOX expression correlates with migration and invasion in epithelial ovarian cancer. Int J Oncol. 2013;42:1578–1588. doi: 10.3892/ijo.2013.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Q, Jin XS, Yang ZY, Wei M, Zhu XC, Wang P, Liu BY, Gu QL. Upregulated expression of LOX is a novel independent prognostic marker of worse outcome in gastric cancer patients after curative surgery. Oncol Lett. 2013;5:896–902. doi: 10.3892/ol.2012.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Albinger-Hegyi A, Stoeckli SJ, Schmid S, Storz M, Iotzova G, Probst-Hensch NM, Rehrauer H, Tinguely M, Moch H, Hegyi I. Lysyl oxidase expression is an independent marker of prognosis and a predictor of lymph node metastasis in oral and oropharyngeal squamous cell carcinoma (OSCC) Int J Cancer. 2010;126:2653–2662. doi: 10.1002/ijc.24948. [DOI] [PubMed] [Google Scholar]
- 23.Wilgus ML, Borczuk AC, Stoopler M, Ginsburg M, Gorenstein L, Sonett JR, Powell CA. Lysyl oxidase: a lung adenocarcinoma biomarker of invasion and survival. Cancer. 2011;117:2186–2191. doi: 10.1002/cncr.25768. [DOI] [PubMed] [Google Scholar]
- 24.Sakai M, Kato H, Sano A, Tanaka N, Inose T, Kimura H, Sohda M, Nakajima M, Kuwano H. Expression of lysyl oxidase is correlated with lymph node metastasis and poor prognosis in esophageal squamous cell carcinoma. Ann Surg Oncol. 2009;16:2494–2501. doi: 10.1245/s10434-009-0559-5. [DOI] [PubMed] [Google Scholar]
- 25.Giampuzzi M, Botti G, Cilli M, Gusmano R, Borel A, Sommer P, Di Donato A. Down-regulation of lysyl oxidase-induced tumorigenic transformation in NRK-49F cells characterized by constitutive activation of ras proto-oncogene. J Biol Chem. 2001;276:29226–29232. doi: 10.1074/jbc.M101695200. [DOI] [PubMed] [Google Scholar]
- 26.Bouez C, Reynaud C, Noblesse E, Thepot A, Gleyzal C, Kanitakis J, Perrier E, Damour O, Sommer P. The lysyl oxidase LOX is absent in basal and squamous cell carcinomas and its knockdown induces an invading phenotype in a skin equivalent model. Clin Cancer Res. 2006;12:1463–1469. doi: 10.1158/1078-0432.CCR-05-1456. [DOI] [PubMed] [Google Scholar]
- 27.Kagan HM, Li W. Lysyl oxidase: properties, specificity, and biological roles inside and outside of the cell. J Cell Biochem. 2003;88:660–672. doi: 10.1002/jcb.10413. [DOI] [PubMed] [Google Scholar]
- 28.Palamakumbura AH, Jeay S, Guo Y, Pischon N, Sommer P, Sonenshein GE, Trackman PC. The propeptide domain of lysyl oxidase induces phenotypic reversion of ras-transformed cells. J Biol Chem. 2004;279:40593–40600. doi: 10.1074/jbc.M406639200. [DOI] [PubMed] [Google Scholar]
- 29.Wu M, Min C, Wang X, Yu Z, Kirsch KH, Trackman PC, Sonenshein GE. Repression of BCL2 by the tumor suppressor activity of the lysyl oxidase propeptide inhibits transformed phenotype of lung and pancreatic cancer cells. Cancer Res. 2007;67:6278–6285. doi: 10.1158/0008-5472.CAN-07-0776. [DOI] [PubMed] [Google Scholar]
- 30.Yang X, Li S, Li W, Chen J, Xiao X, Wang Y, Yan G, Chen L. Inactivation of lysyl oxidase by beta-aminopropionitrile inhibits hypoxia-induced invasion and migration of cervical cancer cells. Oncol Rep. 2013;29:541–548. doi: 10.3892/or.2012.2146. [DOI] [PubMed] [Google Scholar]


