Abstract
Objective: This study is to explore the relationship between the chronic hepatitis B virus (HBV) infection and the expressions of toll-like receptor 2/4 (TLR2/4) in peripheral blood dendritic cells (DCs), to find out the immunological significance of TLR2/4 in HBV progression. Methods: Patients had been divided into the HBV, HBV-related liver cirrhosis (HBV-LC), and HBV-related hepatocellular carcinoma (HBV-HCC) groups. Healthy individuals served as normal controls (NC). Flow cytometry was used to determine the percentage of DCs in peripheral blood, and the expression of TLR2/4 in DCs as well as the expression of HBeAg. Real-time quantitative PCR was performed to measure the content of HBV-DNA. Results: The percentages of DCs in peripheral blood exhibited a slightly decreasing trend, without statistical significances, along with the disease severity in HBV patients (9.40 ± 2.05%, 7.11 ± 3.82%, 6.51 ± 4.38% and 6.00 ± 4.73% for the groups of NC, HBV, HBV-LC, and HBV-HCC, respectively). The expression of TLR2 was significantly increased in the disease progression, with the TLR2 expression rates of 2.60 ± 1.70%, 2.67 ± 2.89%, 3.53 ± 3.41% and 5.11 ± 4.93 for NC, HBV, HBV-LC, HBV-HCC, respectively. Similar results were found for TLR4 (expression rates: 45.34 ± 4.46%, 53.94 ± 5.21%, 65.16 ± 5.92% and 75.54 ± 6.12%), which was positively correlated with TLR2. Furthermore, the HBeAg level was increased, while the amount of HBV-DNA exhibited a declining trend, along with the disease severity. Correlation analysis revealed that the expression of HBeAg was positively correlated with TLR2. Conclusions: The elevated expressions of TLR2/4 on DC cell surfaces in peripheral blood may synergistically promote the disease progression of chronic HBV infection.
Keywords: Hepatitis B virus, chronic infection, dendritic cells, toll-like receptors 2/4
Introduction
Chronic hepatitis B virus (HBV) infection could end up with differential clinical outcomes, mainly depending on the interaction between the viral replication and the body immune response. In recent years, the role of the innate immunity in anti-virus processes has attracted increasing attention. Toll-like receptors (TLRs), recently discovered pattern recognition receptors (PRRs), can recognize the single-/double-stranded RNA/DNAs of the viruses and produce proinflammatory cytokines to activate innate immune cells. Meanwhile, TLRs can also activate dendritic cells (DCs), strengthen the antigen presentation, and start T-cell immune responses [1,2].
DCs are the strongest antigen-presenting cells (APCs) in vivo. DC cell number is usually low, accounting for no more than 5% of the peripheral blood mononuclear cells. DCs could activate the resting T cells to initiate immune responses. TLRs are abundantly expressed receptors on DC cell surfaces, especially those peripheral blood monocyte-derived DCs. TLR2 and TLR4 (TLR2/4) are first found TLRs with wide ranges of anti-virus capabilities, which identify HBV antigens and participate in immune responses during the HBV infection, including virus removal and immune tolerance [3-5].
In this study, the expressions of TLR2/4 in the peripheral blood-derived DCs at different clinical stages during HBV infection were detected by flow cytometry. The immunological significance of TLR2/4 expressions in DCs in chronic HBV infection and the outcomes were preliminarily discussed.
Materials and methods
Patients
Patients with chronic hepatitis B infection admitted to the First Affiliated Hospital of Xinjiang Medical University from September, 2011 to October, 2012 were enrolled in this study.
The inclusion criteria were as follows: patients who were clearly diagnosed as viral hepatitis according to the history, clinical manifestations, biochemical tests, and immunological and imaging examinations, as defined by the guideline of prevention and treatment for chronic hepatitis B jointly formulated by the Chinese Society of Hepatology, CMA and the Society of Infectious Diseases, CMA in 2005 [6]. The patients did not undergo antiretroviral therapy in the recent six months. Exclusion criteria: patients with other viral hepatitis, alcoholic hepatitis, autoimmune hepatitis, and schistosomiasis liver disease.
These patients were divided into the following groups: the chronic hepatitis B virus infection (HBV) group (16 males and 19 females; average age of 37.33 ± 15.05 years, ranged from 9 to 68 years), the HBV-related liver cirrhosis (HBV-LC) group (21 males and 13 females; average age of 42.96 ± 11.70 years, ranged from 21 to 65 years), and the HBV-related hepatocellular carcinoma (HBV-HCC) group (17 males and 14 females; average age of 46.38 ± 11.93 years, ranged from 25 to 70 years). In addition, 30 healthy individuals from the hospital medical center were included to serve as normal controls (NC) (14 males and 16 females; average age of 31.18 ± 8.83 years, ranged from 25 to 55 years), whose HBV/HCV/HIV viral markers were all negative, with no fatty liver, cirrhosis, or heart, brain, kidney, and other organ diseases. All subjects were informed and signed the informed consent forms before blood sampling.
Instruments and reagents
Flow cytometer (LSR II) and K3-EDTA anticoagulant tubes were purchased from Becton, Dickinson and Company (Franklin Lakes, NJ, USA). RBC lysing solution, lineage2-FITC-labeled monoclonal antibody, anti-human MHC II PerCP-cy5-labeled monoclonal antibody, anti-human TLR2 PE-cy7-labeled monoclonal antibody, and anti-human TLR4 PE-labeled monoclonal antibody were all purchased from eBiosciences (San Diego, CA, USA) and Gibco (Grand Island, NY, USA).
Immunofluorescence and flow cytometry
EDTA-K3 anticoagulated whole blood was collected from fasting patients in the morning. FITC-labeled mouse anti-human Lineage2 antibody (20 μl), PE-labeled mouse anti-human TLR4 antibody (20 μl), PerCP-cy5 labeled mouse anti-human MHC II monoclonal antibody (20 μl), and PE-cy7 labeled mouse anti-human TLR2 antibody (20 μl), or FITC-labeled mouse anti-human CD3 antibody (20 μl), PE-labeled mouse anti-human CD8 antibody (20 μl), and PerCP-labeled mouse anti-human CD4 monoclonal antibody (20 μl) were added to 100 μl anticoagulated blood, respectively. An isotype control antibody (20 μl) was added to 100 μl anticoagulated blood to serve as controls. Samples were incubated in the dark at room temperature for 30 min. After that, 500 μl red blood cell lysing solution was added. The samples were washed with PBS twice, and the supernatant was discarded after centrifugation. 100 μl FACS buffer was then used to resuspend the cells and they were detected by the flow cytometry. According to the procedures, the detected cells were first gated with Lineage2-MHCII + analysis, and DC cells were selected. Then the TLR2/TLR4 expressions on DC cell surfaces were detected.
Hepatitis B detection
For the measurement of HBeAg, ARCHITECT i2000 system was used, and the chemiluminescence was detected. HBeAg values > 1 PEIU/ml was considered as positive. For the HBV-DNA quantitative detection, ABIPrism7000 fluorescence quantitative PCR assay was performed. The normal range was ≤ 1000 copies/ml, and the detection limit was 100 copies/ml.
Statistical analysis
The SPSS 17.0 statistical software was applied for statistical analysis. Data were expressed as mean ± standard deviation (SD). Normal distribution was compared using ANOVA. LSD-t test was used for pairwise comparisons between groups. Spearman correlation analysis was applied for linear correlation analysis. P < 0.05 was considered statistically significant.
Results
Percentage of DCs in peripheral blood is slightly diminished in HBV patients
DC cells are the most potent antigen-presenting cells, playing an important role in the initiation of T-cell responses. In order to find out whether the peripheral blood monocyte-derived DCs from HBV patients were influenced, flow cytometry was performed to detect the amounts of mononuclear cells and DCs in peripheral blood from these patients. A representative result was shown in Figure 1. Our results indicated that the percentage of mononuclear cells remained relatively stable among all these groups, while a slightly decreasing trend in DC cell number was observed in the disease progression (Table 1). The percentage of DCs in peripheral blood from the normal control (NC) group, chronic hepatitis B virus infection (HBV) group, HBV-related liver cirrhosis (HBV-LC) group, and HBV-related hepatocellular carcinoma (HBV-HCC) group were 9.40 ± 2.05%, 7.11 ± 3.82%, 6.51 ± 4.38%, and 6.00 ± 4.73%, respectively. Despite the decreasing trend in DC amount in disease pathogenesis, there were no statistically significant differences between these groups (Table 1, P > 0.05). These results suggest that the percentage of DCs in peripheral blood is slightly diminished along with the disease severity in HBV patients.
Figure 1.
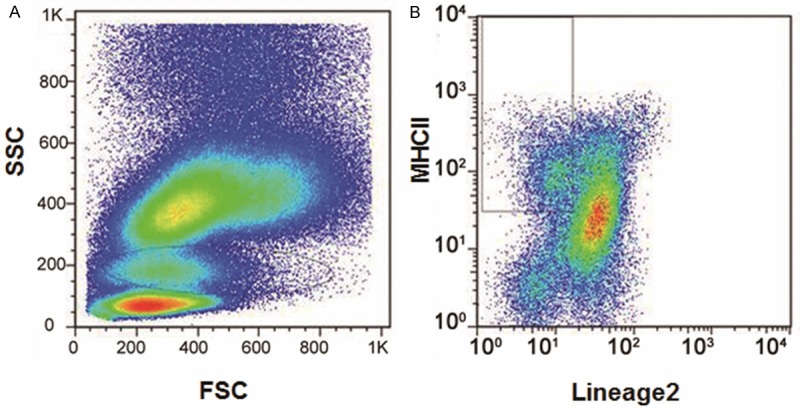
Representative pictures showing the detection of DC cells in peripheral blood from HBV patients. A. The peripheral cells could be divided into the lymphocyte group, the mononuclear cell group, and the white blood cell group, according to the FSC and SSC cytometric values. B. DC cells were selected with MHCII as surface recognition receptors.
Table 1.
Frequencies of mononuclear cells and DC cells in peripheral blood in normal control (NC) group and hepatitis B virus infection (HBV) groups
| Groups | Patient numbers | Mononuclear cells (%) | DC cells (%) |
|---|---|---|---|
| Normal control (NC) | 30 | 8.14 ± 2.24 | 9.4 ± 2.05 |
| Chronic hepatitis B virus infection (HBV) | 35 | 8.40 ± 2.73 | 7.11 ± 3.82 |
| HBV-related liver cirrhosis (HBV-LC) | 34 | 8.52 ± 3.13 | 6.51 ± 4.38 |
| HBV-related hepatocellular carcinoma (HBV-HCC) | 31 | 8.75 ± 3.49 | 6.00 ± 4.73 |
Expressions of TLR2 and TLR4 in DCs are elevated in HBV patients
TLRs are abundantly expressed receptors in DCs, which may participate in the signaling pathway involved in liver diseases. To further find out the role of TLR2/4 in HBV infection, their expression in DCs was assessed in HBV patients with flow cytometry (Figure 2A). As shown in Table 2, the proportion of TLR2-positive DCs in the NC group, HBV group, HBV-LC group, and HBV-HCC group were 2.60 ± 1.70%, 2.67 ± 2.89%, 3.53 ± 3.41%, and 5.11 ± 4.93%, respectively. TLR2 expression in DCs was increased gradually as disease progressed, and there were statistical significances between these groups (Table 2, P < 0.05). On the other hand, the proportion of TLR4-positive DCs in the NC group, HBV group, HBV-LC group, and HBV-HCC group were 45.34 ± 24.46%, 53.94 ± 16.21%, 65.16 ± 12.92%, and 75.54 ± 11.12%, respectively. The expression of TLR4 in DCs was increased gradually in disease pathogenesis. No statistical significance was observed between the HBV group and the NC group, while there were statistically significant differences between the remaining groups (Table 2, P < 0.05). Furthermore, the correlation analysis indicated that the expressions of TLR2 and TLR4 were positively correlated with each other (Figure 2B) (r = 0.379, P = 0.02). These results suggest that the expressions of TLR2/4 were positively correlated and significantly increased in the disease progression, which might contribute to the disease pathogenesis.
Figure 2.
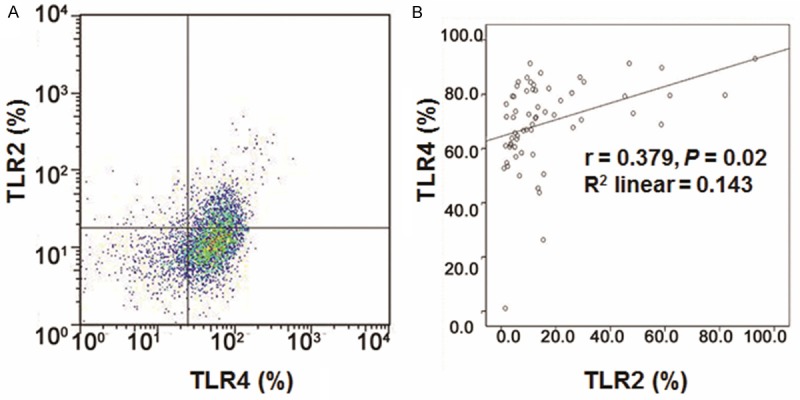
Expression of TLR2 and TLR4 in DCs in HBV patients. A. Flow cytometry was applied to detect the expressions of TLR2 and TLR4 in DCs. Cells in Q1, Q4 were considered as negative for TLR2 and TLR4 respectively, while Q2 and Q3 were considered as positive for TLR2 and TLR4, respectively. B. TLR2 and TLR4 were positively correlated (r = 0.379, P = 0.02).
Table 2.
Expression of TLR2 and TLR4 in DCs in NC group and HBV groups
| Groups | Patient numbers | DC cells (%) | TLR2 (%) | TLR4 (%) |
|---|---|---|---|---|
| NC | 30 | 9.4 ± 2.05 | 2.60 ± 1.70 | 45.34 ± 24.46 |
| HBV | 35 | 7.11 ± 3.82 | 2.67 ± 2.89a | 53.94 ± 16.21 |
| HBV-LC | 34 | 6.51 ± 4.38 | 3.53 ± 3.41a,b | 65.16 ± 12.92a,b |
| HBV-HCC | 31 | 6.00 ± 4.73 | 5.11 ± 4.93a,b,c | 75.54 ± 11.12a,b,c |
| F value | 8.86 | 11.34 | 12.75 | |
| P value | 0.60 | 0.001 | 0.001 |
Note: Compared with the NC group;
P < 0.05.
Compared with the HBV group;
P < 0.05.
Compared with the HBV-LC group;
P < 0.05.
TLR2 expression is positively correlated with HBeAg in HBV patients
To further investigate the mechanism(s) through which TLR2/4 elevation might be related to the pathogenesis of HBV infection, the expression level of HBeAg and the amount of HBV-DNA in HBV patients were detected with chemiluminescence and real-time quantitative PCR, respectively, and correlation analyses were also performed. HBeAg and HBV-DNA are both clinical indicators for HBV infection. Our results indicated that HBeAg levels were increased, while the amount of HBV-DNA exhibited a declining trend, along with the disease severity (Table 3). Furthermore, the correlation analyses revealed that the expression of HBeAg was positively correlated to TLR2 (Figure 3A) (r = 0.486, P = 0.0001) and HBV-DNA (r = 0.372, P = 0.002), respectively, while there was no obvious correlation between HBeAg and TLR4 (Figure 3B) (r = 0.048, P = 0.072). The HBV-DNA content had no correlation to either TLR2 (r = 0.080, P = 0.537) or TLR4 (r = 0.074, P = 0.567) (Figure 3C, 3D).
Table 3.
Correlations between TLR2, TLR4, HBeAg, and HBV-DNA in DCs
| n | TLR2 | TLR4 | HBeAg | HBV-DNA | |
|---|---|---|---|---|---|
| TLR2 | 30 | - | 0.379a | 0.486b | -0.080 |
| TLR4 | 35 | 0.379 | - | 0.048 | -0.074 |
| HBeAg | 34 | 0.486b | 0.048 | - | 0.372c |
| HBV-DNA | 31 | -0.080 | -0.074 | 0.372c | - |
Note: TLR2 vs. TLR4;
P < 0.05.
HBeAg vs. TLR2;
P < 0.05.
HBeAg vs. HBV-DNA;
P < 0.05.
Figure 3.
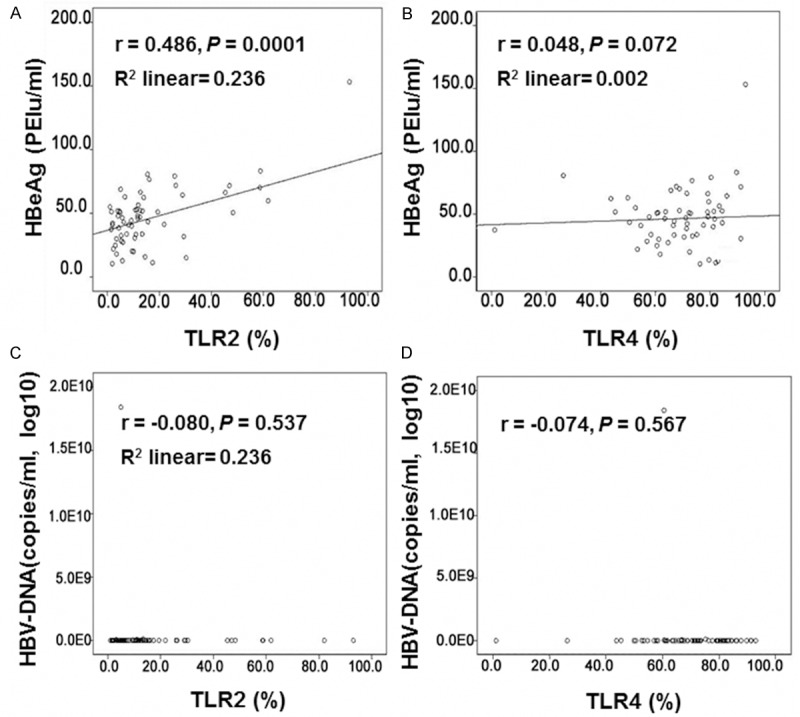
Correlation analyses between HBeAg and TLR2/4, HBV-DNA. (A, B) The expression of HBeAg was positively correlated to TLR2 (A) (r = 0.486, P = 0.0001) and HBV-DNA (r = 0.372, P = 0.002), respectively, while there was no obvious correlation between HBeAg and TLR4 (B) (r = 0.048, P = 0.072). (C, D) The HBV-DNA content had no correlation to either TLR2 (C) (r = -0.080, P = 0.537) or TLR4 (D) (r = -0.074, P = 0.567).
Discussion
In recent years, studies showed that hepatitis B virus and hepatitis B surface antigens can enter the DC cells and cause damages, leading to decline of DC cell numbers and function impairment [7,8]. Because DC cells have antiviral activity during the HBV infection, in the case of decreased DC cell numbers and function damage, the virus cannot be promptly removed, which in turn worsens the condition. There were also reports suggesting that the DC cell numbers and the cellular function in the peripheral blood of patients with chronic hepatitis B infection were declined, compared with normal controls [9,10]. Our results indicated that the DC cell numbers in peripheral blood of the patients in the NC group, HBV group, HBV-LC group, and HBV-HCC group were decreased, along with the disease severity, i.e., DC cells were diminished in disease pathogenesis. It was considered that the long-term HBV infection would decrease DC cell numbers and cause cellular dysfunctions, so declined antiviral capacity of DC cells might lead to a positive feed-back loop to induce long-lasting viral infection and further damages to these cells.
TLR2 and TLR4 are the earliest found toll-like receptors, which possess specific features as multiple surface receptors and play extensive roles in cell functions. Current studies are mainly using immunohistochemistry, ELISA, and other methods, to detect TLR expressions in peripheral blood and/or mononuclear cells, and compare the differences between HBV groups with different severities. These studies suggested that the peripheral blood levels of TLR2 and TLR4 in HBV groups were up-regulated in comparison with the normal control group [11-15]. In the present study, flow cytometry was used to detect the TLR2 and TLR4 expressions in the peripheral blood from HBV patients, and their expression levels followed an increasing tendency from the normal control group, HBV group, HBV-LC group, to HBV-HCC group, indicating that TLR2 and TLR4 might participate in the pathogenesis of HBV infection, probably through altering the innate immune responses during infection. In contrast, Chen et al. indicated that TLR2 and TLR4 were down-regulated to affect the innate immune responses during the HBV infection [16]. Such contradictions may be because of the different experimental methods, reagents, and stages of the patient’s condition.
Our results suggested that the expressions of TLR2 and TLR4 were positively correlated, indicating that they may exert a synergistic effect during the infection. HBeAg, a protein product of the viral replication, stimulates the immune system as a viral immunogen, causing inflammatory responses. In our results, HBeAg expression level was positively correlated with the expression of TLR2, which indicated that TLR2 might be closely related to hepatitis B-specific immunity. No obvious correlation was observed between TLR4 and HBeAg, which was considered to be due to the different ways TLR2 and TLR4 participate in signaling pathways. TLR2 exerts effects only through MyD88-dependent signaling pathways, while TLR4 can work through either MyD88-dependent or -independent signaling pathways. Whether the independent signaling pathway negatively regulates TLR4 and causes immune tolerance, resulting in non-response of TLR4 to HBeAg, needs to be further studied. There were differences between TLR2 and TLR4 expression patterns in DC cells. The abilities of DC to uptake and process antigens are highly associated with its maturity. Immature DC cells mainly recognize antigens, but do not present antigens, causing immune tolerance. On the other hand, mature DC cells are able to present antigens to activate immune responses. However, when the immune response is too vigorous, it will lead to liver cell damage. We hypothesize that if TLR4 were mainly expressed in immature DC cells, HBeAg might not be recognized and immune tolerance would occur. If TLR2 were mainly expressed in mature DC cells, the immune activation would be enhanced. Of course, the expression patterns of TLR2 and TLR4 on the surfaces of mature and immature DC cells need further investigation.
The positive correlation between TLR2 and HBeAg, together with the non-obvious correlation between TLR2 and HBV-DNA, indicated that the viral replication did not directly affect the expression of TLR2, which would be affected indirectly by the viral proteins. HBV-DNA is a direct marker of HBV replication in vivo. As all specimens used herein did not undergo antiretroviral treatment, the immune status in response of HBV would be reflected. Wang et al. reported that there was no significant correlation between the TLR4 expression and the serum HBV-DNA level in HBV patients [17]. In our study, HBV-DNA exhibited no obvious correlation with either TLR2 or TLR4, indicating that the up-regulated expressions of TLR2 and TLR4 were irrelevant to the viral replication, and their expressions were not induced by the viral RNAs. However, in contrast to our these results, Wang et al. from another group found that TLR2 was negatively correlated to HBV-DNA contents, which was considered due to the differences in detection methods, model cells, or whether or not the enrolled patients were under antiviral therapy.
In conclusion, this study showed that the high-level expressions of TLR2 and TLR4 on DC cell surfaces might synergistically promote the disease progression of chronic HBV infection, which provided theoretical basis for future researches on the mechanism of viral hepatitis and immune responses. The relationship between TLR2/4 receptors and chronic HBV infection has not yet reached a unified conclusion, and the viral antigen components regulating DC surface TLR2 and TLR4 during chronic HBV infection and related mechanism(s) still need in-depth studies.
Acknowledgements
This work was supported by the seed fund from The Institut Pasteur of Shanghai, Chinese Academy of Sciences and The Department of Infectious Diseases and Immunology, The First Affiliated Hospital of Xinjiang Medical University (No. GRMY-2011-03).
Disclosure of conflict of interest
None.
References
- 1.Li N, Chen MQ, Qian ZP, Zhu MQ, Li Q, Zheng JM, Wang XY, Shi GF. Correlation of the expression of toll-like receptors in monocyte-derived dendritic cells with prognosis of chronic severe hepatitis B. J Dig Dis. 2011;12:117–24. doi: 10.1111/j.1751-2980.2011.00486.x. [DOI] [PubMed] [Google Scholar]
- 2.Rong Y, Song H, You S, Zhu B, Zang H, Zhao Y, Li Y, Wan Z, Liu H, Zhang A, Xiao L, Xin S. Association of Toll-like receptor 3 polymorphisms with chronic hepatitis B and hepatitis B-related acute-on-chronic liver failure. Inflammation. 2013;36:413–8. doi: 10.1007/s10753-012-9560-4. [DOI] [PubMed] [Google Scholar]
- 3.Zhou H. Recognition of HBV antigens and HBV-DNA by dendritic cells. Hepatobiliary Pancreat Dis. 2010;9:584–92. [PubMed] [Google Scholar]
- 4.Busca A, Kumar A. Innate immune responses in hepatitis B virus (HBV) infection. Virol J. 2014;11:22. doi: 10.1186/1743-422X-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang L, Zou ZQ, Liu CX, Liu XZ. Immunotherapeutic interventions in chronic Hepatitis B virus infection: A review. J Immunol Methods. 2014;407:1–8. doi: 10.1016/j.jim.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Chinese Society of Hepatology and Chinese Society of Infectious Diseases; Chinese Medical Association. The guideline of prevention and treatment for chronic hepatitis B. Zhonghua Gan Zang Bing Za Zhi. 2005;13:881–91. [PubMed] [Google Scholar]
- 7.Untergasser A, Zedler U, Langenkamp A, Hösel M, Quasdorff M, Esser K, Dienes HP, Tappertzhofen B, Kolanus W, Protzer U. Dendritic cells take up viral antigens but do not support the early steps of hepatitis B virus infection. Hepatology. 2006;43:539–47. doi: 10.1002/hep.21048. [DOI] [PubMed] [Google Scholar]
- 8.Moffat JM, Cheong WS, Villadangos JA, Mintern JD, Netter HJ. Hepatitis B virus-like particles access major histocompatibility class I and II antigen presentation pathways in primary dendritic cells. Vaccine. 2013;31:2310–6. doi: 10.1016/j.vaccine.2013.02.042. [DOI] [PubMed] [Google Scholar]
- 9.Shi B, Ren G, Hu Y, Wang S, Zhang Z, Yuan Z. HBsAg Inhibits IFN-α Production in Plasmacytoid Dendritic Cells through TNF-α and IL-10 Induction in Monocytes. PLoS One. 2012;7:44900. doi: 10.1371/journal.pone.0044900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koumbi LJ, Papadopoulos NG, Anastassiadou V, Machaira M, Kafetzis DA, Papaevangelou V. Dendritic Cells in Uninfected Infants Born to Hepatitis B Virus-Positive Mothers. Clin Vaccine Immunol. 2010;17:1079–85. doi: 10.1128/CVI.00074-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, Lian JQ, Huang CX, Wang JP, Wei X, Nan XP, Yu HT, Jiang LL, Wang XQ, Zhuang Y, Li XH, Li Y, Wang PZ, Robek MD, Bai XF. Overexpression of Toll-like receptor 2/4 on monocytes modulates the activities of CD4+CD25+ regulatory T cells in chronic hepatitis B virus infection. Virology. 2010;397:34–42. doi: 10.1016/j.virol.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Wei XQ, Wen ZF, Zheng FP, Yao JL. Changes in toll-like receptor 2 and 4 in peripheral blood mononuclear cells in patients with chronic hepatitis B. Zhonghua Gan Zang Bing Za Zhi. 2007;15:354–7. [PubMed] [Google Scholar]
- 13.Zhuang Y, Xie Q, Yan CG, Wang H, Cai W, Lin LY, An BY, Liu YY, Zhou XQ, Yu H, Guo Q. Expression of Toll-like receptor 2,4 in peripheral Mood mononuclear cells from patients with hepatitis B virus related cirrhosis. Chinese Journal of Infectious Diseases. 2009;27:133–7. [Google Scholar]
- 14.Wang XQ, Zhang Y, Bai XF, Huang CX, Lian JQ. Detection of circulating Toll-like receptor 2 and 4 and CD4 + CD25 + regulatory T cells in patients with HBV-related liver cirrhosis. Chinese Journal of Microbiology and Immunology. 2009;29:411–5. [Google Scholar]
- 15.Soares JB, Pimentel-Nunes P, Afonso L, Rolanda C, Lopes P, Roncon-Albuquerque R Jr, Gonçalves N, Boal-Carvalho I, Pardal F, Lopes S, Macedo G, Lara-Santos L, Henrique R, Moreira-Dias L, Gonçalves R, Dinis-Ribeiro M, Leite-Moreira AF. Increased hepatic expression of TLR2 and TLR4 in the hepatic inflammation-fibrosis-carcinoma sequence. Innate Immun. 2012;18:700–8. doi: 10.1177/1753425912436762. [DOI] [PubMed] [Google Scholar]
- 16.Chen Z, Cheng Y, Xu Y, Liao J, Zhang X, Hu Y, Zhang Q, Wang J, Zhang Z, Shen F, Yuan Z. Expression profiles and function of Toll-like receptors 2 and 4 in peripheral blood mononuclear cells of chronic hepatitis B patients. Clin Immunol. 2008;128:400–8. doi: 10.1016/j.clim.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 17.Wang H, Du WJ, Qin LY, Liu LW, Chen SJ. Study on the expression of Toll-like receptor 4 in peripheral blood mononuclear cell of patients with chronic hepatitis B virus infection. Chinese Journal of Infectious Diseases. 2007;25:298–302. [Google Scholar]


