Abstract
Aims: To identify the clinical significance of Wnt5A expression in the development and progression of cervical cancer. Methods: Real-time PCR was performed in 8 pairs of surgically resected cervical cancer and adjacent normal cervical tissues. Immunohistochemistry was performed to examine Wnt5A expression in 94 paraffin-embedded cervical cancer samples. Associatio ns of Wnt5A expression with clinicopathological factors and clinical survival were analyzed. Results: Wnt5A expression was overexpressed in cervical cancer tissues compared with adjacent normal cervix. Wnt5A expression tended to be positively correlated with lymph nodes metastasis (P = 0.028) and recurrence (P = 0.009). Moreover, patients with higher Wnt5A expression in cancer tissues had better overall (P = 0.004) and recurrent-free survival (P = 0.012) than those with lower Wnt5A expression. Multivariate analysis revealed that Wnt5A was an independent prognostic factor (P = 0.026) for predicting overall survival of cervical cancer patients. Conclusion: Upregulation of Wnt5A was associated with metastasis and progression of cervical cancer. The results of our study unravel the significance of Wnt/Ca2+ signaling in cervical cancer.
Keywords: Cervical cancer, Wnt5A, Wnt/Ca2+ signaling, prognosis
Introduction
Cervical cancer is a malignant tumor of the cervix, with an estimated 277,000 new cases and 266,000 deaths annually worldwide [1]. The prognosis has been improved substantially in these decades, and the 5-year survival rate of patients with early stage cervical cancer is more than 80% [2]. Currently, surgical resection and radiotherapy are both potentially curative treatment for localized tumors. Unfortunately, 30-35% of patients present with advanced tumors failed treatments [3]. Moreover, recurrent cervical cancer usually exhibits a poor response to many chemotherapeutic agents and radiotherapy [4]. Therefore, there is an urgent need to uncover the biological mechanisms contributing to development and progression of cervical cancer.
The Wnt signaling pathway involves numerous proteins that are required for basic developmental processes of cell proliferation and differentiation in many different species and organs [5]. It begins when proteins binds the extracellular N-terminal domain of a Frizzled (Fz) family receptor, which is a seven-transmembrane protein and constitute a distinct family of G-protein coupled receptor. Upon activation of the Fz receptor, a signal is sent to the phosphoprotein Dishevelled (Dsh). In canonical Wnt signaling pathway, phosphorylation of Dsh allows β-catenin to translocate into the nucleus and subsequently induce a cellular response via gene transduction alongside the TCF/LEF transcription factors [6]. Accumulating evidence suggests that Wnt signaling plays important role in carcinogenesis and tumor progression. Activation of Wnt signaling may promote cancer cell proliferation and migration [7,8], while suppression of Wnt signaling inhibits cancer stemness and tumor development [9,10]. Besides, Wnt signaling pathway has been proposed to be implicated in chemosensitivity [11] and radioresistance [12]. Moreover, persistent activation of Wnt/β-catenin signaling has been demonstrated to be an indicator of poor clinical prognosis in several cancers [13].
The Wnt signaling could be divided into two categories: the canonical and the non-canonical Wnt signaling pathway. The canonical Wnt signaling has been shown to be involved in the development and progression of cervical cancer. Li et al. revealed that activation of β-catenin and Akt pathways are required for the sustention of EMT-associated stem cell-like traits of cervical cancer cells [14]. Chung et al. reported that epigenetic silencing of SFRP genes leads to oncogenic activation of the Wnt pathway and contributes to cervical cancer progression through the EMT program [15]. Ramachandran et al. found that Wnt inhibitory factor 1 induces apoptosis and inhibits tumor growth, invasion and angiogenesis of cervical cancer [16]. However, the roles of non-canonical Wnt signaling in the development of cervical cancer remain largely unknown. The aim of this study was to determine the clinical significance of non-canonical Wnt signaling in cervical cancer.
Materials and methods
Patients and tissue specimens
A total of 94 paraffin-embedded human cervical squamous cell carcinoma tissues were obtained from the First Affiliated Hospital of Shenzhen University from January 2004 to December 2007. Demographic and clinicopathological data were collected from impatient medical records (summarized in Table 1). For the use of these clinical materials for research purposes, written informed consent from all patients and approval from The Institutional Research Ethics Committee were obtained. The median follow-up period was 46 months (range, 0.5-60 months). In addition, 8 pairs of cervical cancer tissues and the paired adjacent noncancerous cervical tissues were disected and frozen liquid nitrogen until further use.
Table 1.
Associations of Wnt5A expression with clinicopathological characteristics in cervical cancer
| Variable | Category | No. | Wnt5A | P | |
|---|---|---|---|---|---|
|
| |||||
| + | - | ||||
| Age (y) | ≤ 50 | 69 | 33 | 36 | 0.129 |
| > 50 | 25 | 8 | 17 | ||
| FIGO Stage | IB | 62 | 24 | 38 | 0.132 |
| > IB | 32 | 17 | 15 | ||
| Grade | 1/2 | 40 | 16 | 24 | 0.543 |
| 3 | 54 | 25 | 29 | ||
| LN Metastasis | No | 76 | 29 | 47 | 0.028 |
| Yes | 18 | 12 | 6 | ||
| Recurrence | No | 81 | 31 | 50 | 0.009 |
| Yes | 13 | 10 | 3 | ||
Real-time PCR
Approximate 100 mg tissues from cervical cancer and normal cervical tissues were used for RNA extraction using the Trizol Reagent (Invitrogen) according to manufacturer’s instructions. Isolated RNA was quantified and then subjected to RT-PCR using the Prime Script RT reagent Kit. Sequences of the primers are: Wnt5A forward primer: 5’-ATTCTTGGTGGTCGCTAGG-3’; reverse primer: 5’-CTGTCCTTGAGAAAGTCCTG-3’, GAPDH forward primer 5’-GAATCTACTGGCGTCTTCACC-3’, reverse primer 5’-GTCATGAGCCCTTCCACGATGC-3’.
Immunohistochemical staining
Paraffin-embedded samples were obtained from 94 patients for immunohistochemical analysis. In brief, 4 μm paraffin-embedded sections were deparaffinized and rehydrated. Endogenous peroxidase activity was blocked by 3% hydrogen peroxide for 15 min. After antigen retrieval, sections were incubated with 5% serum to avoid the non-specific binding. The sections were incubated overnight at 4°C with the primary antibody at dilutions of 1:100 for Wnt5A (Abcam, USA; 1:200). After the primary antibody was washed off, the sections were incubated with prediluted secondary antibody (Santa Cruz Biotechnology). Immunoreactivity was visualized by 3’, 3-diaminobezidine reaction and then the sections were counterstained with hematoxylin. For blank controls, the primary antibody was omitted. For negative controls, the primary antibody was replaced by nonimmune serum.
The stained slides were scored independently by two pathologists blinded to clinical data. The proportion of positive tumor cells was scored as follows: 0 (no positive tumor cells); 1 (< 10% positive tumor cells); 2 (10-50% positive tumor cells); 3 (51-80% positive tumor cells), and 4 (> 80% positive tumor cells). Staining intensity was graded according to the following criteria: 1 (weak staining = light yellow); 2 (moderate staining = yellow brown) and 3 (strong staining = brown). Staining index (SI) was calculated as the product of staining intensity score and the proportion of positive tumor cells. The cut-off value for distinguishing high and low Wnt5A expression was set as an SI of 6.
Statistical analysis
The Statistical Program for Social Sciences, version 16.0 (SPSS, Chicago, IL), was used for statistical analysis. The Chi-square test was performed to examine the associations of Wnt5A expression with clinicopathological factors. Survival curves were generated using the Kaplan-Meier method and compared using the log-rank test. All results were expressed as means ± standard deviation (S.D.), where P values less than 0.05 were considered statistically significant.
Results
Wnt5A expression in human cervical cancer tissues
In order to determine the role of Wnt/Ca2+ signaling in the development of cervical cancer, we investigated the expression profiling of Wnt5A in cervical cancer tissues. As shown in Figure 1, Wnt5A was significantly upregulated in primary cervical cancer samples compared with the paired adjacent noncancerous cervical tissues. Overall, 77.6% (73/94) of the paraffin-embedded cervical cancers samples showed positive expression of Wnt5A. The representative immunostaining of Wnt5A in cervical cancer was shown in Figure 2A-D.
Figure 1.
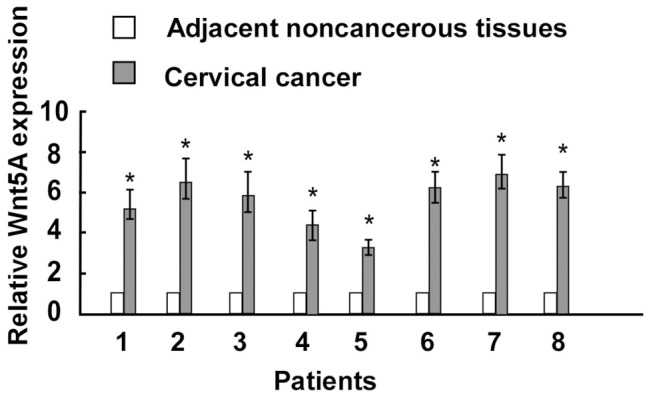
Wnt5A expression in cervical cancer specimens was detected by Real-time PCR (n = 8) compared with adjacent noncancerous tissue. asterisks, P < 0.05.
Figure 2.
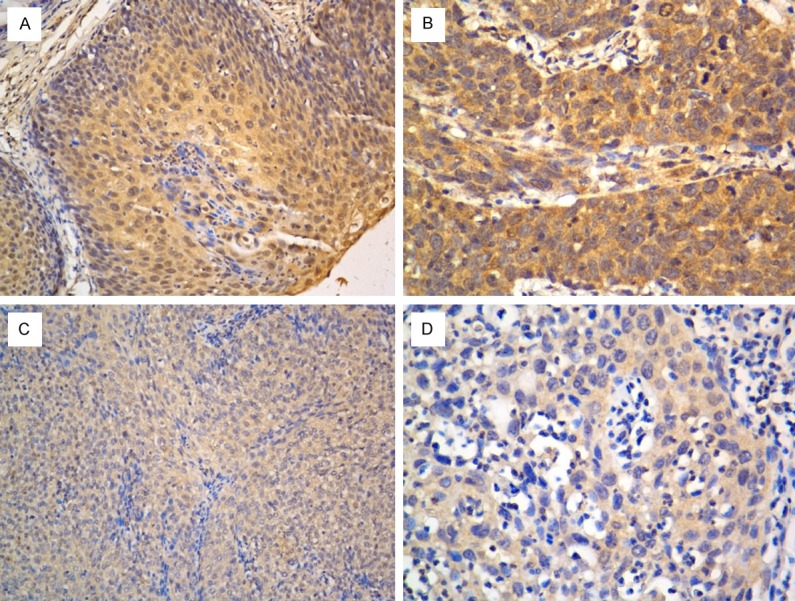
High (A, B) and low (C, D) expression of Wnt5A in cervical cancer tissues by immunohistochemistry. (A, C with 200× magnification; B, D with 400× magnification).
Associations of Wnt5A expression with clinicopathological features
The associations of Wnt5A expression with clinicopathological characteristics are summa rized in Table 1. Wnt5A expression tended to be positively correlated with lymph nodes metastasis (P = 0.028) and recurrence (P = 0.009). However, no statistic ally significant relationships were found between Wnt5A expression and age, tumor stage, or tumor grade.
Associations of Wnt5A expression with clinical survival of cervical cancer patients
The prognostic role of Wnt5A in cervical cancer was evaluated using the Kapla n-Meier survival curve analysis. As shown in Figure 3, patients with higher Wnt5A expression in cancer tissues had better overall (P = 0.004) and recurrent-free survival (P = 0.012) than those with lower Wnt5A expression. Multivariate analysis indicated that Wnt5A expression was an independent prognostic factor of patient overall survival (Table 2). Our results revealed that Wnt5A may represent a potential prognostic indicator for cervical cancer patients.
Figure 3.
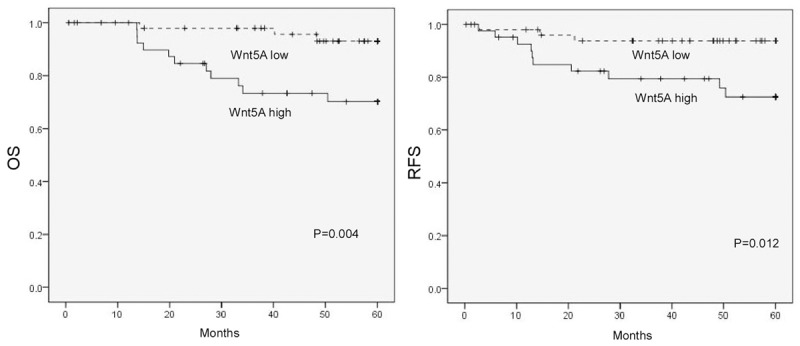
Kaplan-Meier curves of 94 cervical cancer patients with low versus high expression of Wnt5A.
Table 2.
Multivariate Cox regression analysis of OS and RFS in cervical cancer patients
| Prognostic variables | OS | RFS | ||
|---|---|---|---|---|
|
|
|
|||
| HR (95% CI) | P | HR (95% CI) | P | |
| Age (> 50 vs. ≤ 50) | 1.158 (0.248-5.409) | 0.852 | 1.614 (0.354-7.356) | 0.537 |
| FIGO Stage (> IB vs. IB) | 1.501 (0.382-5.900) | 0.561 | 1.362 (0.367-5.052) | 0.645 |
| Differentiation (Grade 3 vs. 1/2) | 1.051 (0.326-3.389) | 0.934 | 1.199 (0.374-3.839) | 0.760 |
| LN Metastasis (+ vs. -) | 3.155 (1.065-9.349) | 0.038 | 2.092 (0.656-6.679) | 0.212 |
| Wnt5A expression (+ vs. -) | 4.784 (1.208-18.941) | 0.026 | 3.507 (0.886-13.871) | 0.074 |
Discussion
In this study, we found that there is an elevation of Wnt5A expression in cervical cancer tissues in compared to normal cervical tissues by Real-time PCR. Wnt5A expression was closely correlated with lymph nodes metastasis. Moreover, statistical analysis showed that higher expression of Wnt5A was associated with poor overall and recurrent-free survival of cervical cancer. Multivariate Cox regression analysis showed that Wnt5A expression was an independent prognostic factor for patients with cervical cancer. Our study unraveled that the non-canonical Wnt signaling was activated in cervical cancer, and that non-canonical Wnt signaling may play crucial roles in the development and progression of cervical cancer.
The main non-canonical Wnt signaling pathways include the planar cell polarity (PCP) pathway, the Wnt/Ca2+ pathway. It has been demonstrated that the planar cell polarity (PCP) Wnt signaling plays important roles in embryonic development and tissue homeostasis, and the Wnt/Ca2+ pathway is implicated in cell adhesion and cell movements during gastrulation [17]. Recent studies indicated that Wnt/Ca2+ pathway was involved in the occurrence of cancer stem cell and carcinogenesis [18]. Wnt5A has been identified as a Wnt/Ca2+ signaling protein. Several recent studies have described a significant role for Wnt5A in tumor development and metastasis, contributing to invasion and migration of cancer cells [19]. However, the exact roles of non-canonical Wnt signaling pathways in cancers are less well understood.
In the present study, we found that the expression of Wnt5A was up-regulated in cervical cancer tissues in compared to adjacent noncancerous tissues, and that elevated expression of Wnt5A significantly predicted a poor overall and recurrent-free survival compared with low Wnt5A expression in cervical cancer patients. Moreover, multivariate analysis implied that Wnt5A immunoreactivity may be a useful prognostic indicator in patients with cervical cancer. These results indicate an oncogenic role of non-canonical Wnt signaling in cervical cancer, which concurs with several previous studies. Peng et al. reported that Wnt5A as a predic tor in poor clinical outcome of patients with ovarian cancer, and that it plays important roles in mediating chemosensitivity to anticancer drugs in ovarian cancer cells [20]. Da Forno et al. demonstrated that increased cytoplasmic Wnt5A was associated with melanoma progression, and strong cytoplasmic Wnt5A was an independent risk factor for reduced metastasis-free and overall survival [21]. Huang et al. shown that Wnt5A gene expression was significantly correlated with tumor proliferation and angiogenesis of non-small-cell lung cancer, and that Wnt5A status was a significant prognostic factor for non-small-cell lung cancer patients [22]. However, Syed Khaja et al., in contrast to us, described that elevated level of Wnt5A protein is associated with better clinical outcome in localized prostate cacner [23]. Ying et al. also suggested that Wnt5A could act as a tumor suppressor in colorectal cancer by antagonizing the canonical Wnt signaling [24]. This could be due to the tissue specific effect of Wnt/Ca2+ signaling in different cancers.
Metastasis remains a major cause of morbidity and mortality in cervical cancer patients. In our study, we found that overexpression is associated with lymph nodes metastasis of cervical cancer. Our result is in agreement with previous studies demo nstrating Wnt5A is capable of mediating several biological events associated with cancer cell metastasis. Kurayoshi et al. demonstrated that Wnt5A expression is correlated with gastric cancer aggressiveness through stimulating tumor cell migration and invasion [25]. Pourreyron et al. showed that Wnt5A is strongly expressed at the leading edge in non-melanoma skin cancer, indicating that Wnt5A signalling contributes to tissue invasion by non-melanoma skin cancer [26]. These findings underline the metastasis-promoting effects of Wnt/Ca2+ signaling in cancers.
Conclusion
In conclusion, our study reported an upregulation of Wnt5A in cervical cancer, and that the expression of Wnt5A is closely associated with tumor metastasis and clinical outcome of patients with cervical cancer. Our study indicates a potential role of Wnt5A in regulating E MT and metastasis in cervical cancer. However, further studies are needed to clarify the molecular mechanism of Wnt5A in cervical cancer development and progression.
Disclosure of conflict of interest
None.
References
- 1.Sahasrabuddhe VV, Parham GP, Mwanahamuntu MH, Vermund SH. Cervical cancer prevention in low- and middle-income countries: feasible, affordable, essential. Cancer Prev Res (Phila) 2012;5:11–17. doi: 10.1158/1940-6207.CAPR-11-0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Landoni F, Maneo A, Colombo A, Placa F, Milani R, Perego P, Favini G, Ferri L, Mangioni C. Randomised study of radical surgery versus radiotherapy for stage Ib-IIa cervical cancer. Lancet. 1997;350:535–540. doi: 10.1016/S0140-6736(97)02250-2. [DOI] [PubMed] [Google Scholar]
- 3.Waggoner SE. Cervical cancer. Lancet. 2003;361:2217–2225. doi: 10.1016/S0140-6736(03)13778-6. [DOI] [PubMed] [Google Scholar]
- 4.Vici P, Mariani L, Pizzuti L, Sergi D, Di Lauro L, Vizza E, Tomao F, Tomao S, Mancini E, Vincenzoni C, Barba M, Maugeri-Sacca M, Giovinazzo G, Venuti A. Emerging biological treatments for uterine cervical carcinoma. J Cancer. 2014;5:86–97. doi: 10.7150/jca.7963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lyuksyutova AI, Lu CC, Milanesio N, King LA, Guo N, Wang Y, Nathans J, Tessier-Lavigne M, Zou Y. Anterior-posterior guidance of commissural axons by Wnt-frizzled signaling. Science. 2003;302:1984–1988. doi: 10.1126/science.1089610. [DOI] [PubMed] [Google Scholar]
- 6.Komiya Y, Habas R. Wnt signal transduction pathways. Organogenesis. 2008;4:68–75. doi: 10.4161/org.4.2.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mokkapati S, Niopek K, Huang L, Cunniff KJ, Ruteshouser EC, deCaestecker M, Finegold MJ, Huff V. Beta-catenin activation in a novel liver progenitor cell type is sufficient to cause hepatocellular carcinoma and hepatoblastoma. Cancer Res. 2014;74:4515–25. doi: 10.1158/0008-5472.CAN-13-3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao C, Xiao G, Hu J. Regulation of Wnt/beta-catenin signaling by posttranslational modifications. Cell Biosci. 2014;4:13. doi: 10.1186/2045-3701-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramachandran I, Ganapathy V, Gillies E, Fonseca I, Sureban SM, Houchen CW, Reis A, Queimado L. Wnt inhibitory factor 1 suppresses cancer stemness and induces cellular senescence. Cell Death Dis. 2014;5:e1246. doi: 10.1038/cddis.2014.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu W, Li Y. Salinomycin suppresses LRP6 expression and inhibits both Wnt/beta-catenin and mTORC1 signaling in breast and prostate cancer cells. J Cell Biochem. 2014;115:1799–807. doi: 10.1002/jcb.24850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dandekar S, Romanos-Sirakis E, Pais F, Bhatla T, Jones C, Bourgeois W, Hunger SP, Raetz EA, Hermiston ML, Dasgupta R, Morrison DJ, Carroll WL. Wnt inhibition leads to improved chemosensitivity in paediatric acute lymphoblastic leukaemia. Br J Haematol. 2014 doi: 10.1111/bjh.13011. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim Y, Kim KH, Lee J, Lee YA, Kim M, Lee SJ, Park K, Yang H, Jin J, Joo KM, Nam DH. Wnt activation is implicated in glioblastoma radioresistance. Lab Invest. 2012;92:466–473. doi: 10.1038/labinvest.2011.161. [DOI] [PubMed] [Google Scholar]
- 13.Hu T, Li C. Convergence between Wnt-beta-catenin and EGFR signaling in cancer. Mol Cancer. 2010;9:236. doi: 10.1186/1476-4598-9-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li J, Zhou BP. Activation of beta-catenin and Akt pathways by Twist are critical for the maintenance of EMT associated cancer stem cell-like characters. BMC Cancer. 2011;11:49. doi: 10.1186/1471-2407-11-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chung MT, Lai HC, Sytwu HK, Yan MD, Shih YL, Chang CC, Yu MH, Liu HS, Chu DW, Lin YW. SFRP1 and SFRP2 suppress the transformation and invasion abilities of cervical cancer cells through Wnt signal pathway. Gynecol Oncol. 2009;112:646–653. doi: 10.1016/j.ygyno.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 16.Ramachandran I, Thavathiru E, Ramalingam S, Natarajan G, Mills WK, Benbrook DM, Zuna R, Lightfoot S, Reis A, Anant S, Queimado L. Wnt inhibitory factor 1 induces apoptosis and inhibits cervical cancer growth, invasion and angiogenesis in vivo. Oncogene. 2012;31:2725–2737. doi: 10.1038/onc.2011.455. [DOI] [PubMed] [Google Scholar]
- 17.Kuhl M. Non-canonical Wnt signaling in Xenopus: regulation of axis formation and gastrulation. Semin Cell Dev Biol. 2002;13:243–249. doi: 10.1016/s1084-9521(02)00050-2. [DOI] [PubMed] [Google Scholar]
- 18.Habas R, Dawid IB. Dishevelled and Wnt signaling: is the nucleus the final frontier? J Biol. 2005;4:2. doi: 10.1186/jbiol22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ren D, Minami Y, Nishita M. Critical role of Wnt5a-Ror2 signaling in motility and invasiveness of carcinoma cells following Snail-mediated epithelial-mesenchymal transition. Genes Cells. 2011;16:304–315. doi: 10.1111/j.1365-2443.2011.01487.x. [DOI] [PubMed] [Google Scholar]
- 20.Peng C, Zhang X, Yu H, Wu D, Zheng J. Wnt5a as a predictor in poor clinical outcome of patients and a mediator in chemoresistance of ovarian cancer. Int J Gynecol Cancer. 2011;21:280–288. doi: 10.1097/IGC.0b013e31820aaadb. [DOI] [PubMed] [Google Scholar]
- 21.Da Forno PD, Pringle JH, Hutchinson P, Osborn J, Huang Q, Potter L, Hancox RA, Fletcher A, Saldanha GS. WNT5A expression increases during melanoma progression and correlates with outcome. Clin Cancer Res. 2008;14:5825–5832. doi: 10.1158/1078-0432.CCR-07-5104. [DOI] [PubMed] [Google Scholar]
- 22.Huang CL, Liu D, Nakano J, Ishikawa S, Kontani K, Yokomise H, Ueno M. Wnt5a expression is associated with the tumor proliferation and the stromal vascular endothelial growth factor--an expression in non-small-cell lung cancer. J. Clin. Oncol. 2005;23:8765–8773. doi: 10.1200/JCO.2005.02.2871. [DOI] [PubMed] [Google Scholar]
- 23.Syed Khaja AS, Helczynski L, Edsjo A, Ehrnstrom R, Lindgren A, Ulmert D, Andersson T, Bjartell A. Elevated level of Wnt5a protein in localized prostate cancer tissue is associated with better outcome. PLoS One. 2011;6:e26539. doi: 10.1371/journal.pone.0026539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ying J, Li H, Yu J, Ng KM, Poon FF, Wong SC, Chan AT, Sung JJ, Tao Q. WNT5A exhibits tumor-suppressive activity through antagonizing the Wnt/beta-catenin signaling, and is frequently methylated in colorectal cancer. Clin Cancer Res. 2008;14:55–61. doi: 10.1158/1078-0432.CCR-07-1644. [DOI] [PubMed] [Google Scholar]
- 25.Kurayoshi M, Oue N, Yamamoto H, Kishida M, Inoue A, Asahara T, Yasui W, Kikuchi A. Expression of Wnt-5a is correlated with aggressiveness of gastric cancer by stimulating cell migration and invasion. Cancer Res. 2006;66:10439–10448. doi: 10.1158/0008-5472.CAN-06-2359. [DOI] [PubMed] [Google Scholar]
- 26.Pourreyron C, Reilly L, Proby C, Panteleyev A, Fleming C, McLean K, South AP, Foerster J. Wnt5a is strongly expressed at the leading edge in non-melanoma skin cancer, forming active gradients, while canonical Wnt signalling is repressed. PLoS One. 2012;7:e31827. doi: 10.1371/journal.pone.0031827. [DOI] [PMC free article] [PubMed] [Google Scholar]


