Abstract
Objective: As a definite diagnosis of prostate cancer, puncture biopsy of the prostate is invasive method. The aim of this study was to evaluate the value of OPSAD (the ratio of PSA to the outer gland volume of prostate) as a non-invasive screening and diagnosis method for prostate cancer in a select population. Methods: The diagnosis data of 490 subjects undergoing ultrasound-guided biopsy of the prostate were retrospectively analyzed. This included 133 patients with prostate cancer, and 357 patients with benign prostate hyperplasia (BPH). Results: The OPSAD was significantly greater in patients with prostate cancer (1.87 ± 1.26 ng/ml2) than those with BPH (0.44 ± 0.21 ng/ml2) (P < 0.05). Receiver operating characteristic (ROC) curve analysis revealed that the performance of OPSAD as a diagnostic tool is superior to PSA and PSAD for the diagnosis of prostate cancer. In the different groups divided according to the Gleason score of prostate cancer, OPSAD is elevated with the rise of the Gleason score. Conclusion: OPSAD may be used as a new indicator for the diagnosis and prognosis of prostate cancer, and it can reduce the use of unnecessary puncture biopsy of the prostate.
Keywords: Prostate cancer, prostate specific antigen, benign prostate hyperplasia, prostate specific antigen density, outer gland of the prostate, diagnosis
Introduction
Prostate cancer is a disease which mainly affects middle to aged men. It is the most common malignancy among European and American men, and it is the second leading cause of death due to a cancer, behind only lung cancer [1]. China has a relatively low incidence of prostate cancer. However, with the rise in life expectancy, changes in lifestyle and application of new diagnostic techniques, a gradually increasing incidence is noted in China [2]. To confirm the diagnosis of prostate cancer, puncture biopsy of the prostate is the usual choice but this is invasive and has several complications [3]. In clinical practice, many subjects undergo puncture biopsy of the prostate after having tested with an elevation of the serum prostate-specific antigen (PSA) level. However, the considerable overlap in PSA levels among the men with benign prostate hyperplasia (BPH) usually leads to unnecessary puncture biopsy of the prostate. It is indicated that PSA density (PSAD) and the ratio of free prostate specific antigen (FPSA) to total prostate specific antigen (TPSA) can help in reducing the numbers of unnecessary and the negative prostate biopsies, but the sensitivity and specificity remain unsatisfactory [4]. It is reported that the inner gland (transition zone) of the prostate is the exclusive site of BPH origin, while almost all carcinomas arise from the outer gland (including peripheral and central zones) [5]. Transrectal ultrasound is found to clearly display the zonal anatomy of the prostate and precisely measure the prostate volume. It is therefore hypothesized that OPSAD (the ratio of PSA to the volume of the outer gland of prostate) could be used as an indicator for diagnosis of prostate cancer, to enhance the rate of the positive prostate biopsy and reduce the use of unnecessary puncture biopsy of the prostate.
Subjects and methods
Subjects
In the present study, the medical records of the subjects undergoing the puncture biopsy of the prostate in our hospital during the period from January 2010 to December 2013 were retrospectively analyzed, so as to evaluate the value of OPSAD for diagnosis of prostate cancer and screening of the subjects with puncture biopsy of the prostate. The study received institutional review board approval.
During the study period, 490 subjects underwent ultrasound-guided puncture biopsy of the prostate in our hospital due to elevation of the serum PSA level, or suspected nodes revealed by the digital rectal examination or ultrasonography.
Determination of PSA
The TPSA was determined using luminescent-labeled immunometric assays following the manufacturer’s instructions (Sorin Group; Modena, MO, Italy).
Puncture biopsy of the prostate
The transverse diameter, anteroposterior diameter and supero-inferior diameter of the prostate gland on the whole and the inner glandular structure of the prostate were measured using the ATL 5000 transrectal ultrasound system equipped with an end-fire probe with frequency of 510-910 MHz (Advanced Technology Laboratories, Inc.; Bothell, WA, USA). The biopsy specimens of the prostate were sampled for pathological examinations. The prostate volume and the volumes of the inner and outer glands of the prostate were calculated using the following formulae.
Prostate volume (PV, ml) = 0.52 × supero-inferiordiameter × transverse diameter × anteroposterior diameter, the volume of the inner gland of the prostate (IPV, ml) = 0.52 × supero-inferior diameter × transverse diameter × anteroposterior diameter of the inner gland of the prostate [6], and the volume of the outer gland of the prostate (OPV, ml) = the prostate volume (PV)- the volume of the inner gland of the prostate (IPV).
PSAD was estimated using the following formula: PSAD (ng/ml2) = PSA/prostate volume. OPSAD was estimated using the following formula: OPSAD (ng/ml2) = PSA/the outer gland volume of prostate.
Statistical analyses
All statistical analyses were performed using the statistical software SPSS version 19.0 (SPSS Inc.; Chicago, IL, USA). Comparison of the differences of the means of age, PSA level, prostate volume, PSAD, volume of the inner gland of the prostate, OPSAD were tested for statistical significance with an independent samples t-test. A P-value < 0.05 was considered statistically significant. The sensitivity and specificity of the OPSAD, PSAD and PSA in diagnosis of prostate cancer were expressed using ROC curves and the cut-off value was simultaneously identified using the ROC curve.
Results
The subjects had a median age of 69.9 years (range from 54-86 years), a mean serum PSA level of 18.1 ng/ml (range, 3.14-100 ng/ml), a mean prostate volume of 49 ml (17-109 ml), and a mean volume of the outer glands of the prostate of 23 ml (7-67 ml). The pathology of the prostate biopsy revealed prostate cancer in 133 cases and BPH in 357 cases.
The demographic and basic characteristics of the patients with BPH and prostate cancer are shown in Table 1. There was no significant difference in age between the patients with BPH and with prostate cancer (P > 0.05). The PSA, PSAD and OPSAD were significantly higher in the patients with prostate cancer than those in the patients with BPH (P < 0.05), while PV and OPV of the patients with prostate cancer was significantly smaller than that of the patients with BPH (P < 0.05) (Figure 1).
Table 1.
Demographic and basic characteristics of patients with BPH and prostate cancer
| Characteristic | BPH | PCA | P value |
|---|---|---|---|
| No. of cases | 357 | 133 | |
| Age (mean ± SD, years) | 69.8 ± 7.6 | 70.4 ± 7.0 | > 0.05 |
| PSA (mean ± SD, ng/ml) | 11.8 ± 7.9 | 37.8 ± 35.6 | < 0.05 |
| PV (mean ± SD, ml) | 52 ± 17 | 39 ± 10 | < 0.05 |
| PSAD (mean ± SD, ng/ml2) | 0.21 ± 0.11 | 0.93 ± 0.82 | < 0.05 |
| OPV (mean ± SD, ml) | 25 ± 9 | 17 ± 7 | < 0.05 |
| OPSAD (mean ± SD, ng/ml2) | 0.44 ± 0.21 | 1.87 ± 1.26 | < 0.05 |
BPH, benign prostate hyperplasia; PCA, prostate cancer; PSA, prostate-specific antigen; PV, prostate volume; PSAD, prostate-specific antigen density; OPV, the outer gland volume of prostate; OPSAD, the ratio of prostate specific antigen to the outer gland volume of prostrate.
Figure 1.
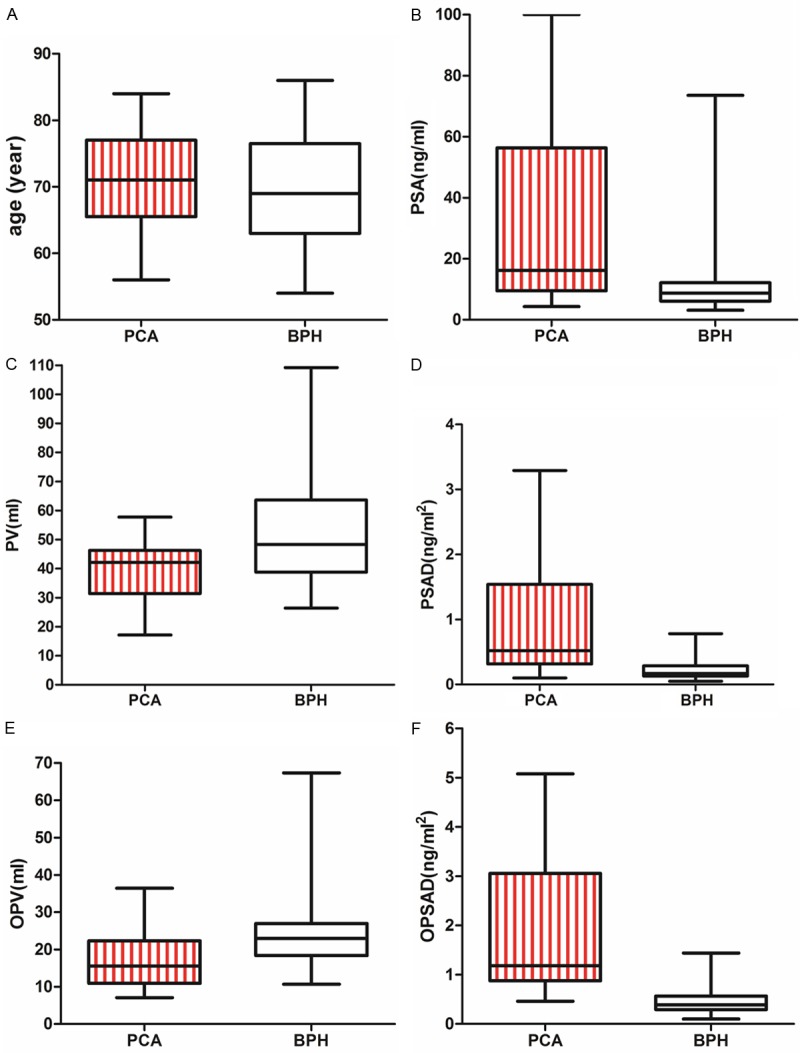
A. The age difference of patients with PCA and BPH is no statistical significance (t = 0.8437, P > 0.05). B. The PSA in patients with PCA (37.8 ± 35.6 ng/ml) is higher than that in patients with BPH (11.8 ± 7.9 ng/ml) (t = 13.53, P < 0.05); C. The PV of patients with PCA (39 ± 10 ml) is smaller than that of patients with BPH (52 ± 17 ml (t = 8.073, P < 0.05)); D. The PSAD in patients with PCA (0.93 ± 0.82 ng/ml2) is higher than that in patients with BPH (0.21 ± 0.11 ng/ml2) (t = 16.29, P < 0.05); E. The OPV of patients with PCA (17 ± 7 ml) is smaller than that of patients with BPH (25 ± 9 ml) (t = 8.293, P < 0.05); F. The OPSAD in patients with PCA (1.87 ± 1.26 ng/ml2) is higher than that in patients with BPH (0.44 ± 0.21 ng/ml2) (t = 20.65, P < 0.05); *BPH, benign prostate hyperplasia; PCA, prostate cancer; OPSAD, the ratio of prostate specific antigen to the outer gland volume of prostrate.
ROC curve analysis revealed that the cut-off value of OPSAD was 0.69 ng/ml2, when the sensitivity was 94.7% and the specificity was 88.2%. The ROC curves also compared the sensitivity and specificity of PSA, PSAD and OPSAD for diagnosis of prostate cancer (Figure 2). ROC curve analyses showed that the area under the ROC curve regarding the OPSAD was 0.917, which was higher than those regarding PSA (0.772) and PSAD (0.862). This suggests that the OPSAD had the highest accuracy in predicting prostate cancer.
Figure 2.
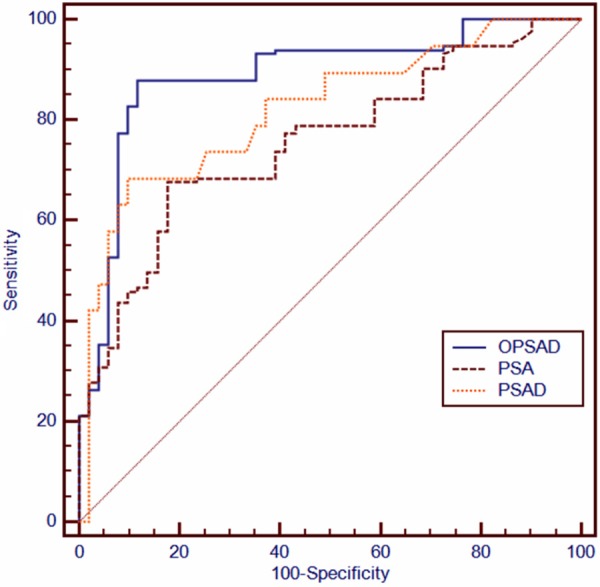
Comparation of the sensitivity and specificity of PSA, PSAD, and OPSAD for diagnosis of prostate cancer by ROC curves; the AUC of PSA was 0.772, the AUC of PSAD was 0.862, the AUC of OPSAD was 0.967 (P < 0.05). ROC, receiver operating characteristic; AUC, area under curve; PSA, prostate specific antigen; PSAD, prostate specific antigen density; OPSAD, the ratio of prostate specific antigen to the outer gland volume of prostrate.
Our findings showed that the OPSAD was related to the Gleason score of the prostate cancer (Figure 3). With the rise of the Gleason score, the OPSAD was elevated (Gleason score 6 was 0.92 ± 0.24 ng/ml2; Gleason score 7 was 2.37 ± 1.90 ng/ml2; Gleason score 8 was 3.41 ± 0.35 ng/ml2), and the difference among the different Gleason score groups was significant by One-way analysis (P < 0.05).
Figure 3.
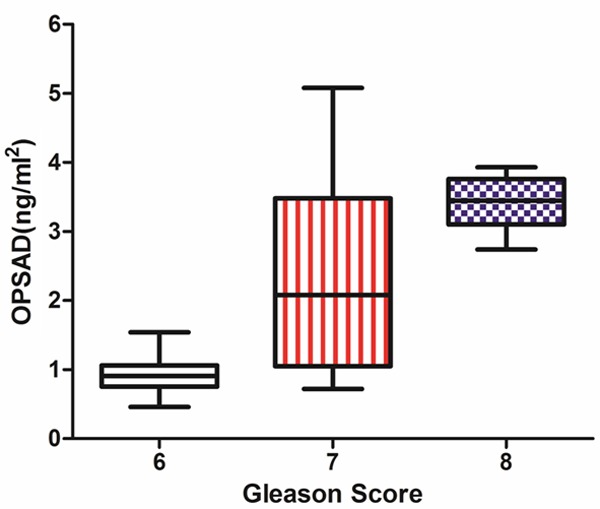
OPSAD of PCA is elevated with the rise of Gleason score. Gleason score 6 was 0.92 ± 0.24 ng/ml2; Gleason score 7 was 2.37 ± 1.90 ng/ml2; Gleason score 8 was 3.41 ± 0.35 ng/ml2. The ANOVA test showed that the difference of OPSAD among the groups with Gleason score 6, 7 and 8 (F = 57.53, P < 0.05). *PCA, prostate cancer; OPSAD, the ratio of prostate specific antigen to the outer gland volume of prostrate.
Discussion
With the rise in aging population and improvement in diagnostic techniques, the incidence of prostate cancer shows a remarkably increase among Chinese men. As a tool for definite diagnosis of prostate cancer, the puncture biopsy of prostate can improve the detection of prostate cancer through enhancing the punctures of the prostate [7].
The puncture biopsy of the prostate is the most accurate method for screening prostate cancer; however, it is invasive and may cause complications like hematospermia [8] and infection [9,10]. Development of the approach to reduce the unnecessary puncture biopsy of the prostate has been given a high priority. It has been recently shown that PSA-based screening enhances the detection of prostate cancer but is associated with a high risk of over diagnosis and additional treatment [11]. However, it is considered that the FPSA level cannot be used as the only indicator for the prostate biopsy [12]. The FPSA/TPSA ratio is reported to facilitate the improvement of the detection of prostate cancer and the reduction of unnecessary puncture biopsy of the prostate [13]. In addition, the PSA velocity (PSAV) [14] and single nucleotide polymorphisms (SNPs) [15] are shown to improve the detection of prostate cancer. However, PSAV is considered to have some limitations in diagnosis of prostate cancer [16], and overscreening and overtreatment are challenges in diagnosis and treatment of prostate cancer in recent times [17]. Moreover, it is considered that repeated prostate biopsies are required for detection of prostate cancer in subjects with negative prostate biopsies [18]. Therefore, a search for noninvasive methods or predictors with a higher sensitivity and specificity for screening prostate cancer is required so as to reduce the unnecessary prostate biopsy and missing diagnosis of prostate cancer.
Prostate cancer is found to occur predominantly in the peripheral zone of the prostate, while benign prostate hyperplasia mainly occurs in the transition zone. It has been shown that the PSA level in the patients with prostate cancer is higher than that in the patients with benign prostate hyperplasia. Considering that PSA is affected by the prostate volume, it is considered that PSAD may enhance the diagnosis of prostate cancer [19]. Combined PSA and the outer gland volume of prostate, it is hypothesized that the OPSAD may serve as a new indicator for diagnosis of prostate cancer.
We analyzed 490 patients performed puncture prostate biopsy in our hospital from January 2010 to December 2013. It showed that OPSAD in patients with prostate cancer is higher than that in patients with benign prostate hyperplasia. It also showed that PSAD had a higher diagnostic efficacy than the PSA alone, and OPSAD to detect the prostate cancer was more sensitive and specific compared to PSA and PSAD by ROC curve analysis. So OPSAD was a super method applied in the screening of prostate cancer compared to the traditional methods of PSA and PSAD.
The data of patients with prostate cancer were further analyzed according to Gleason score. It was showed that OPSAD is elevated with the rise of Gleason score, which suggested that OPSAD may be a better indicator to predict the malignancy of the prostate cancer.
One of the major limitations of the present study is its relatively small sample size. When the PSA level was < 4 ng/ml, there were no prostate cancer patients detected. Further studies to evaluate the value of OPSAD for diagnosis of prostate cancer with normal PSA levels are needed due to the small sample size in the current study. In addition, OPSAD is proposed based on the tumors that predominantly occur in the peripheral zone of the prostate, therefore, it may have some limitations in the diagnostic efficacy of the prostate cancer that occur from the anterior zone or transition zone [20,21].
From this study it can be concluded that OPSAD may be used as a new indicator for diagnosis of prostate cancer, and it can reduce the use of unnecessary puncture biopsy of the prostate. At the same time, OPSAD is related to Gleason score, so it may be used as a new predictor for prognosis of prostate cancer. In the future, it needs further studies to assess the relationship between OPSAD and prostate cancer with normal PSA level.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Hu Y, Zhao Q, Rao J, Deng H, Yuan H, Xu B. Longitudinal trends in prostate cancer incidence, mortality, and survival of patients from two Shanghai city districts: a retrospective population-based cohort study, 2000-2009. BMC Public Health. 2014;14:356. doi: 10.1186/1471-2458-14-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hung T, Chang HY. Long noncoding RNA in genome regulation: prospects and mechanisms. RNA Biol. 2010;7:582–585. doi: 10.4161/rna.7.5.13216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang M, Lin Y, Xu A, Uhlman M, Deng X, Lin X, Wu S, Diao P, Xie K, Tang P. Percent free prostate-specific antigen does not improve the effectiveness of prostate cancer detection in Chinese men with a prostate-specific antigen of 2.5-20.0 ng/ml: a multicenter study. Med Oncol. 2014;31:925. doi: 10.1007/s12032-014-0925-4. [DOI] [PubMed] [Google Scholar]
- 5.Lee F, Siders DB, Torp-Pedersen ST, Kirscht JL, McHugh TA, Mitchell AE. Prostate cancer: transrectal ultrasound and pathology comparison. A preliminary study of outer gland (peripheral and central zones) and inner gland (transition zone) cancer. Cancer. 1991;67:1132–1142. doi: 10.1002/1097-0142(19910215)67:4+<1132::aid-cncr2820671506>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 6.Tang P, Du W, Xie K, Deng X, Fu J, Chen H, Yang W. Transition zone PSA density improves the prostate cancer detection rate both in PSA 4.0-10.0 and 10.1-20.0 ng/ml in Chinese men. Urol Oncol. 2013;31:744–748. doi: 10.1016/j.urolonc.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 7.Ploussard G, Nicolaiew N, Marchand C, Terry S, Vacherot F, Vordos D, Allory Y, Abbou CC, Salomon L, de la Taille A. Prospective evaluation of an extended 21-core biopsy scheme as initial prostate cancer diagnostic strategy. Eur Urol. 2014;65:154–161. doi: 10.1016/j.eururo.2012.05.049. [DOI] [PubMed] [Google Scholar]
- 8.Abdelkhalek MA, Abdelshafy M, Elhelaly HA, El Nasr MK. Hemospermia after transrectal ultrasound (TRUS)-guided prostatic biopsy: a prospective study. J Egypt Soc Parasitol. 2012;42:63–70. doi: 10.12816/0006295. [DOI] [PubMed] [Google Scholar]
- 9.Gil-Vernet Sedo JM, Alvarez-Vijande Garcia R. Effect of intrarectal povidone-iodine in the incidence of infectious complications after transrectal prostatic biopsy. Arch Esp Urol. 2012;65:463–466. [PubMed] [Google Scholar]
- 10.Wagenlehner FM, van Oostrum E, Tenke P, Tandogdu Z, Cek M, Grabe M, Wullt B, Pickard R, Naber KG, Pilatz A, Weidner W, Bjerklund-Johansen TE, investigators G. Infective complications after prostate biopsy: outcome of the Global Prevalence Study of Infections in Urology (GPIU) 2010 and 2011, a prospective multinational multicentre prostate biopsy study. Eur Urol. 2013;63:521–527. doi: 10.1016/j.eururo.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto S, Kato M, Tomiyama Y, Amiya Y, Sasaki M, Shima T, Suzuki N, Murakami S, Nakatsu H, Shimazaki J. Management of men with a suspicion of prostate cancer after negative initial prostate biopsy results. Urol Int. 2014;92:258–263. doi: 10.1159/000355355. [DOI] [PubMed] [Google Scholar]
- 12.Vickers AJ, Cronin AM, Roobol MJ, Hugosson J, Jones JS, Kattan MW, Klein E, Hamdy F, Neal D, Donovan J, Parekh DJ, Ankerst D, Bartsch G, Klocker H, Horninger W, Benchikh A, Salama G, Villers A, Freedland SJ, Moreira DM, Schroder FH, Lilja H. The relationship between prostate-specific antigen and prostate cancer risk: the Prostate Biopsy Collaborative Group. Clin Cancer Res. 2010;16:4374–4381. doi: 10.1158/1078-0432.CCR-10-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agnihotri S, Mittal RD, Ahmad S, Mandhani A. Free to total serum prostate specific antigen ratio in symptomatic men does not help in differentiating benign from malignant disease of the prostate. Indian J Urol. 2014;30:28–32. doi: 10.4103/0970-1591.124202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loeb S, Metter EJ, Kan D, Roehl KA, Catalona WJ. Prostate-specific antigen velocity (PSAV) risk count improves the specificity of screening for clinically significant prostate cancer. BJU Int. 2012;109:508–513. doi: 10.1111/j.1464-410X.2011.10900.x. discussion 513-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kader AK, Sun J, Reck BH, Newcombe PJ, Kim ST, Hsu FC, D’Agostino RB Jr, Tao S, Zhang Z, Turner AR, Platek GT, Spraggs CF, Whittaker JC, Lane BR, Isaacs WB, Meyers DA, Bleecker ER, Torti FM, Trent JM, McConnell JD, Zheng SL, Condreay LD, Rittmaster RS, Xu J. Potential impact of adding genetic markers to clinical parameters in predicting prostate biopsy outcomes in men following an initial negative biopsy: findings from the REDUCE trial. Eur Urol. 2012;62:953–961. doi: 10.1016/j.eururo.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vickers AJ, Wolters T, Savage CJ, Cronin AM, O’Brien MF, Pettersson K, Roobol MJ, Aus G, Scardino PT, Hugosson J, Schroder FH, Lilja H. Prostate-specific antigen velocity for early detection of prostate cancer: result from a large, representative, population-based cohort. Eur Urol. 2009;56:753–760. doi: 10.1016/j.eururo.2009.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schaefer C, Weissbach L. Cancer screening: curative or harmful? An ethical dilemma facing the physician. Urologe A. 2011;50:1595–1599. doi: 10.1007/s00120-011-2727-z. [DOI] [PubMed] [Google Scholar]
- 18.Dall’Era MA, Albertsen PC, Bangma C, Carroll PR, Carter HB, Cooperberg MR, Freedland SJ, Klotz LH, Parker C, Soloway MS. Active surveillance for prostate cancer: a systematic review of the literature. Eur Urol. 2012;62:976–983. doi: 10.1016/j.eururo.2012.05.072. [DOI] [PubMed] [Google Scholar]
- 19.Oh JJ, Hong SK, Lee JK, Lee BK, Lee S, Kwon OS, Byun SS, Lee SE. Prostate-specific antigen vs prostate-specific antigen density as a predictor of upgrading in men diagnosed with Gleason 6 prostate cancer by contemporary multicore prostate biopsy. BJU Int. 2012;110:E494–499. doi: 10.1111/j.1464-410X.2012.11182.x. [DOI] [PubMed] [Google Scholar]
- 20.Patel V, Merrick GS, Allen ZA, Andreini H, Taubenslag W, Singh S, Butler WM, Adamovich E, Bittner N. The incidence of transition zone prostate cancer diagnosed by transperineal template-guided mapping biopsy: implications for treatment planning. Urology. 2011;77:1148–1152. doi: 10.1016/j.urology.2010.11.052. [DOI] [PubMed] [Google Scholar]
- 21.Cole E, Margel D, Greenspan M, Shayegan B, Matsumoto E, Fischer MA, Patlas M, Daya D, Pinthus JH. Is there a role for anterior zone sampling as part of saturation trans-rectal ultrasound guided prostate biopsy? BMC Urol. 2014;14:34. doi: 10.1186/1471-2490-14-34. [DOI] [PMC free article] [PubMed] [Google Scholar]


