Abstract
Objective: The aim of the study was to assess the role of contrast-enhanced ultrasound (CEUS) in treatment response evaluation after percutaneous bipolar radiofrequency ablation (BRFA) for liver tumors. Methods: From May 2012 to May 2014, 39 patients with 73 tumors were treated by BRFA. One month after the treatment, CEUS and CEMRI/CECT were conducted to evaluate the treatment response. The results of CEUS were compared with CEMRI/CECT. Results: Of the 73 tumors ablated, eight (11.0%) were found to have residual viable tumor tissue and 65 (89.0%) were successfully ablated based on CEMRI/CECT within 1-month after ablation. CEUS detected seven of the eight residual tumors and 63 of 65 completely ablated tumors. The sensitivity, specificity, positive predictive value, negative predictive value and accuracy of CEUS were 87.5% (7/8), 96.9% (63/65), 77.8% (7/9), 98.4% (63/64) and 95.9% (70/73), respectively. The complete ablation (CR) rates for the tumors ≤3.0 cm, 3.1-5.0 cm, and >5.0 cm were 96.6% (58/60), 63.6% (7/11), and 0% (0/2), respectively (P<0.001). CR rates were 94.7% (36/38) for primary liver tumors and 82.9% (29/35) for metastatic liver tumors (P=0.212), and were 97.4% (38/39) for the tumors with curative treatment intention and 79.4% (27/34) for those with palliative treatment intention (P=0.037). Major complication was not encountered in this series. Conclusions: BRFA is an effective technique of percutaneous ablation for liver tumors and CEUS can be used to assess its therapeutic effect accurately.
Keywords: Contrast-enhanced ultrasound, contrast-enhanced MRI, contrast-enhanced CT, liver tumors, bipolar radiofrequency ablation
Introduction
Radiofrequency ablation (RFA) has been widely used for the treatment of liver tumors and its efficacy and safety has already been accepted as one of optimal methods [1-5]. Especially for small hepatocellular carcinoma, RFA has a comparable treatment outcome with surgical resection [6]. The tumor response is an important prognostic factor for patients after RFA. Contrast-enhanced magnetic resonance imaging (CEMRI) and contrast-enhanced computed tomography (CECT) were routinely regarded as the reference standard in the evaluation of tumor response. Contrast-enhanced ultrasound (CEUS) had also been proven to be useful in evaluating the posttreatment efficacy of RFA [7-10].
Bipolar radiofrequency ablation (BRFA) is a novel technique that has more advantages than conventionally used monopolar RFA (MRFA) system. It has been confirmed that BRFA had better applicability and fewer side effects than MRFA system [5,11,12]. Moreover, results of ex vivo experimental studies have shown that BRFA can achieve larger ablation volumes than MRFA system [13]. However, the actual ablation size in patients with liver cancer is not available and few studies have assessed the therapeutic effect of BRFA using CEUS and the ablation volume. The purpose of our study was to test the usefulness of CEUS in the posttreatment evaluation in comparison with CEMRI/CECT.
Materials and methods
Patients
Between May 2012 and May 2014, 39 consecutive patients with 73 liver tumors were referred to our institution for ultrasound (US)-guided percutaneous BRFA therapy. The inclusion criteria were: (1) single tumor no greater than 7cm in diameter; (2) multiple tumors no more than 5; (3) absence of portal venous thrombosis or extrahepatic metastases; (4) liver cirrhosis classified as Child-Pugh class A or B; and (5) prothrombin time ratio greater than 50% and platelet count greater than 60 000/mm3 (60 × 109/L). Exclusion criteria were advanced liver disease (i.e. Child-Pugh class C), any contraindication for percutaneous ablation (severe ascites, platelet count below 50 000/mm3 or 50 × 109/L, or prothrombin activity <50%). In addition, the patients who were pregnant and had serious heart problems were also excluded. Written informed consent from all the patients was obtained before the study.
Table 1 summarized the data of the baseline characteristics of the 39 patients with 73 tumors treated in the study (19 patients had one tumor, 10 had two, six had three, four had four). The patients consisted of 29 men and 10 women and the mean age of them was (59.9 ± 6.7) years. 23 patients had primary liver cancers and 16 patients had metastatic liver cancers (11 from the colon-rectum cancer, three from the duodenum cancer, and three from breast cancer).
Table 1.
Baseline characteristics of study patients
| Characteristics | Number of patients |
|---|---|
| M/F ratio | 29/10 |
| Age (yrs) | 59.9 ± 6.7 |
| HbsAg (+):HbsAg (-) | 17:22 |
| Cirrhosis (+):Cirrhosis (-) | 13:26 |
| α-fetoprotein level | |
| ≤20 ng/ml | 20 |
| >20 ng/ml | 19 |
| Liver cirrhosis | |
| Child A | 39 |
| Child B | 0 |
| Number of tumors | |
| Single | 19 |
| Two/Three/Four | 10/6/4 |
| Size of tumors | |
| <3 cm | 60 |
| 3-5 cm | 11 |
| >5 cm | 2 |
| Source of lesions | |
| Primary | 23 |
| Metastasis | 16 |
| Treatment intention | |
| Curative treatment | 25 |
| Palliative treatment | 14 |
Equipments and methods
A BRFA system (Celon AG Medical Instruments, Teltow, Germany) was used for all the ablation procedures in this study. The electrodes were operated by a power control unit working at 470 kHz and providing a maximum output power of 250 W (CelonLabPower; Celon AG). It is designed as a bipolar unit that does not require the use of grounding pads. The conducting part of the applicators is 20, 30 or 40 mm length, including both the insulator and the tip. In bipolar mode, the high-frequency current flows between the two electrodes at the tip of the bipolar electrode and then heats up the tissue surrounding the electrodes. An internal liquid circulation of the applicator enables increase of the coagulation efficiency and avoids burn of abdominal wall. The delivery rate of the internal liquid circulation is set to 30 ml/min using saline solution at room temperature. The liquid flow is driven by a triple peristaltic pump, which is part of the system. The unit controls up to three bipolar electrodes where the actual number could be individually adapted to the clinical situation between one and three. With one connected bipolar electrode, the unit is in bipolar operating mode, in which the device provides an acoustic output of the coagulation status. If the resistance increases beyond a specific limit value power (700 Ω), the energy delivery will stop automatically. A 15 or 20-cm-long, 15.5-gauge bipolar radiofrequency electrode with a radial array of 2-4 cm was used. Radiofrequency energy was delivered at 20-250 W until tissue impedance increased enough to prevent flow of current.
Patients with supine decubitus position were given a systemic anesthesia by administration of 0.1-0.15 mg/kg midazolam, 8-30 μg/kg sufentanil citrate, 0.1-0.4 mg/kg cisatracurium besilate via peripheral vein. A LogiQ E9 US machine (GE Medical Systems, Milwaukee, WI, USA) with a 1-5 MHz curvilinear transducer was used for guidance of RFA. On the basis of location of the targeted tumors, US was performed to scan the liver and to select an optimal puncture path. Then the RFA electrode was introduced into the target tumor through an intercostal or a subcostal approach under US guidance. For tumors smaller than 2 cm, one bipolar electrode (T30 or T40) or two T30 electrodes were usually applied. For tumors sized between 2 cm and 3 cm, two bipolar electrodes (T30 or T40) were parallelly inserted into the tumor with an inter-electrode distance of 1.5 to 2.0 cm. For tumors sized between 3 cm and 5 cm, three T40 electrodes were usually used in triangle with a 3.0 cm maximal distance from each other. If the tumors exceed 5 cm, three T40 electrodes were simultaneously applied in such a way that the triangular 3.0 cm equilateral conformation was achieved. Hyperechoic gas appeared during the ablation procedure and finally covered the tumor completely. When the BRFA system showed that the target energy was achieved or the impedance was over 250 Ω, the operation was stopped.
Treatment response evaluation
Within 1 month after the treatment, CEUS was performed to evaluate the treatment response. The CEUS examination was performed by two skilled radiologists who had more than 5 years’ experience in CEUS and were unaware of clinical and other imaging information of the patients. Sonovue (Bracco, Milan, Italy) was used as contrast agent and was administrated as a bolus injection at a dose of 1.5 ml through the antecubital vein, then followed by a flush of 5 ml normal saline. Contrast-specific mode was used and the mechanic index was set to be less than 0.2 to avoid disruption of microbubbles. Lesions were observed continuously for 6 min to register the enhancement in the arterial (10-30 s), portal (31-120 s), and late phases (121-360 s).
Reference standard
CEMRI/CECT within 1 month were used as the reference standard. Magnetic resonance imaging was performed with a 3.0-Tesla whole-body magnetic resonance imager (Verio3.0T, Siemens Medical Systems, Berlin, German). A dynamic breath-hold gadolinium-enhanced, three-dimensional gradient echo T1-weighted pulse sequence was performed with imaging in the arterial, portal venous, and delay phases. The CT images were obtained using a spiral scanner (Light Speed VCT, GE Medical Systems, Milwaukee, WI, USA) before and after injection of intravenous nonionic contrast in the hepatic arterial, portal venous and late phases of enhancement.
Image analysis
Complete response (CR) was defined as the absence of enhancement within the tumor, which reflects complete tissue necrosis. Residual or incomplete response (ICR) was defined as the persistence of contrast enhancement within the tumor area after treatment.
Statistical analysis
Quantitative data were expressed as mean ± standard deviation. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and diagnostic accuracy of CEUS for detection of residual tumor after ablation were calculated. The qualitative data were compared with chi-square statistics. If there were cells that had less than 5 observations, Fisher’s exact probability test was used. All P values were derived from two-tailed tests, and a level of less than 0.05 was accepted as statistically significant. Statistical analysis software (version 16.0; SPSS, Chicago, III, USA) was used for the analysis.
Results
According to the results of CEMRI or CECT within 1 month, CR (Figure 1) was obtained in 65/73 (89.0%) nodules while in the remaining eight nodules (11.0%) the presence of residual contrast uptake qualified them as ICR (Figure 2). Table 2 summarized the CR rates on the basis of treatment intention, type of the tumor and tumor size. Comparison between the 1-month CEMRI/CECT and CEUS studies was shown in Table 3.
Figure 1.
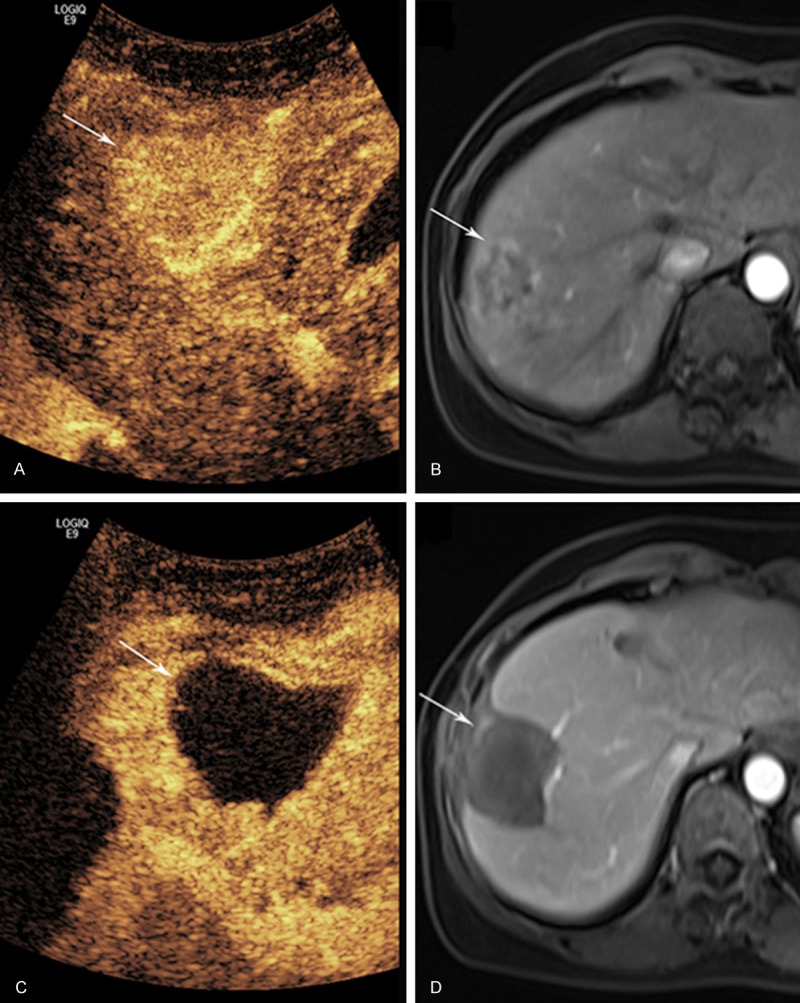
A 50-year old woman with liver metastasis cancer resulted from breast with complete response (CR) to bipolar radiofrequency ablation (BRFA). A. Arterial-phase contrast-enhanced ultrasound (CEUS) before BRFA shows a hyperenhanced tumor (arrow); B. Contrast-enhanced magnetic resonance imaging (CEMRI) shows peripheral enhancement of the tumor in the arterial phase (arrow) before BRFA; C. CEUS after BRFA: the tumor shows non-enhancement (arrow) in the arterial phase; D. CEMRI also shows non-enhancement (arrow) in the arterial phase.
Figure 2.
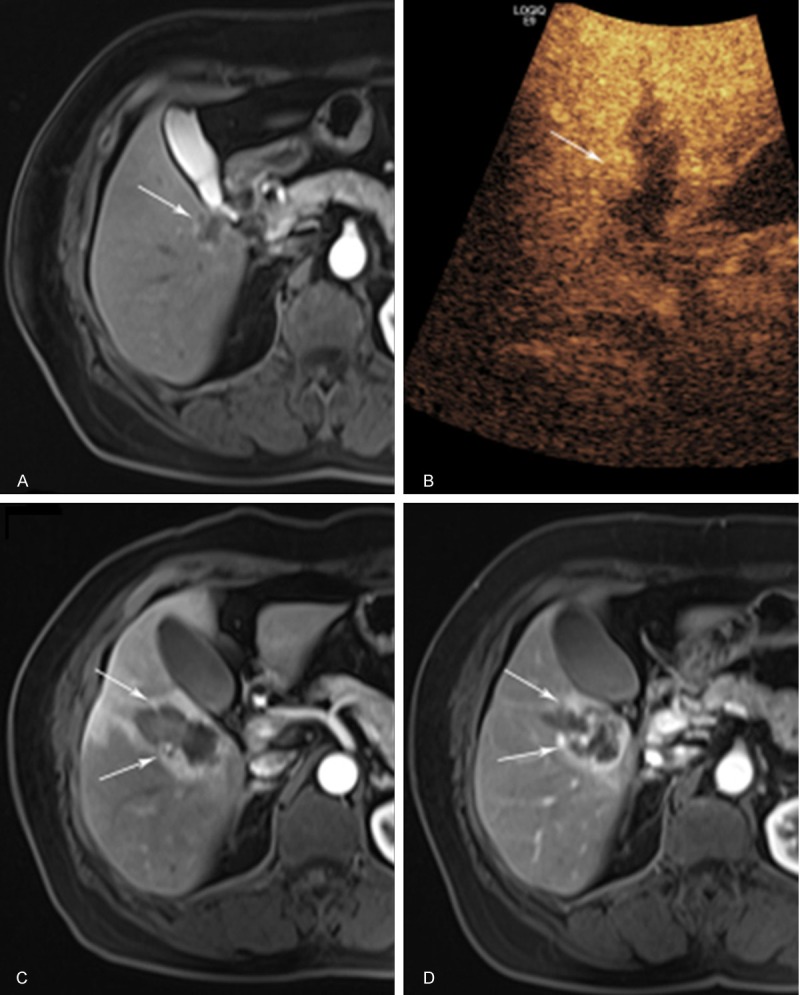
A 66-year old female with liver metastasis cancer resulted from breast with incomplete response (ICR) to BRFA. A. CEMRI shows peripheral enhancement (arrow) of the tumor in the arterial phase before BRFA; B. One month after BRFA, CEUS arterial phase at 18s after contrast administration shows a nodular enhancement (arrow) at the lateral margin of the ablated area; C. One week after BRFA, CEMRI arterial phase shows a nodular enhancement (arrow); D. Two months after BRFA, CEMRI arterial phase shows peripheral enhancement (arrow).
Table 2.
Complete response rate in terms of treatment intention, type of the tumor and tumor size
| Features | Complete response rate | P value |
|---|---|---|
| Treatment intention | 0.037 | |
| Curative treatment | 97.4% (38/39) | |
| Palliative treatment | 79.4% (27/34) | |
| Type of the tumor | 0.212 | |
| Primary liver cancer | 94.7% (36/38) | |
| Metastatic liver cancer | 82.9% (29/35) | |
| Size | <0.001 | |
| ≤3.0 cm | 96.7% (58/60) | |
| 3.1-5.0 cm | 63.6% (7/11) | |
| >5.0 cm | 0% (0/2) |
Table 3.
The usefulness of CEUS in evaluating the treatment response in comparison with CEMRI/CECT
| CEMRI or CECT within one month | CEUS within one month |
|---|---|
| CR (n=65) | 63 |
| ICR (n=8) | 7 |
| Sensitivity (%) | 87.5% (7/8) |
| Specificity (%) | 96.9% (63/65) |
| PPV (%) | 77.8% (7/9) |
| NPV (%) | 98.4% (63/64) |
| DA (%) | 95.9% (70/73) |
CR, complete response; ICR, incomplete response; PPV, positive predictive value; NPV, Negative predictive value; DA, diagnostic accuracy.
No treatment-related death appeared. Some minor complications (pain, most of the patients; fever ≥38.5°C, two patients; asymptomatic pleural effusion, 15 patients) were observed after the treatment.
CEUS identified seven of eight positive tumors with a sensitivity of 87.5 % (7/8); all tumors had concurrent MRI. Among the 65 successfully ablated tumors, CEUS showed CR in 63 with a specificity of 97.0% (63/65); 52 tumors had concurrent MRI and 11 had concurrent CT.
The overall accuracy of CEUS was 95.9% (70/73), as shown in Table 2. One false negative result was obtained by 1-month CEUS in a tumor located in segment 6. This tumor was a metastatic liver carcinoma from the colon and sized 3.2 cm in diameter. The two false positive tumors were located in segment 8 that were obscured by the pulmonary air.
In addition, Table 4 showed the tumor size before BRFA treatment and the size of the ablation zone after BRFA treatment in the tumors with complete response. For 8 tumors using one T30 electrode, the maximum diameter of the ablation zone after RFA 1-month was 2.6 ± 1.0 cm (range, 1.4-4.2 cm) whereas the minimum diameter was 2.1 ± 0.7 cm (range, 1.1-3.1 cm), according to the results of CEUS. For 6 tumors using one T40 electrode, the maximum diameter after RFA 1-month were 3.4 ± 0.8 cm (range, 2.3-4.1 cm) whereas the minimum diameter was 2.7 ± 0.9 cm (range, 1.7-3.8 cm). Regarding 29 tumors using two T30 electrodes, the maximum diameter and the minimum diameter were 3.3 ± 0.8 cm (range, 2.0-5.0 cm) and 2.4 ± 0.7 cm (range, 1.4-3.9 cm) respectively. For 8 tumors using two T40 electrodes, the maximum diameter and the minimum diameter were 4.5 ± 0.6 cm (range, 3.7-5.2 cm) and 2.8 ± 0.8 cm (range, 1.7-4.0 cm) respectively. As to 6 tumors using three T40 electrodes, the maximum diameter and the minimum diameter were 5.1 ± 0.5 cm (range, 4.3-6.0 cm) and 3.6 ± 1.0 cm (range, 2.0-5.0 cm) respectively.
Table 4.
Tumor size before BRFA and size of the ablation zone after BRFA (all tumors with complete response)
| Tumor size | Mean ± standard deviation (range) | |
|---|---|---|
| ≤3 cm | Tumor Size before BRFA | |
| maximum diameter (cm) | 1.8 ± 0.5 (0.8-2.8) | |
| minimum diameter (cm) | 1.4 ± 0.4 (0.6-2.5) | |
| Ablation zone after BRFA | ||
| maximum diameter (cm) | 3.2 ± 0.8 (1.8-5.0) | |
| minimum diameter (cm) | 2.4 ± 0.6 (1.3-3.9) | |
| 3.1-5.0 cm | Tumor size before BRFA | |
| maximum diameter (cm) | 3.8 ± 0.2 (3.5-4.2) | |
| minimum diameter (cm) | 2.8 ± 0.4 (2.1-3.3) | |
| Ablation zone after BRFA | ||
| maximum diameter (cm) | 4.6 ± 1.2 (3.6-6.9) | |
| minimum diameter (cm) | 3.7 ± 1.0 (3.0-5.9) |
Discussion
RFA is a commonly used minimally invasive method for the treatment of liver cancer [14,15]. It consists of both MRFA and BRFA [15]. BRFA system has two active electrodes so it can eliminate the need for a grounding pad and the danger of skin burns, thus fewer complications will be encountered. In the present study, no major complications (death, hemorrhage, intestinal perforation, bile duct and diaphragm injury, etc.) appeared, in consistent with the previous report using BRFA [5]. In comparison with conventional MRFA procedures, in which the complication rate was about 10% and mortality was 1.4%, BRFA looks like a safer modality [16]. The safety of BRFA can be ascribed to the following factors. The first is that the configuration of the BRFA electrode is straight and no multiple-prong design is applied. Therefore, the unexpected damage to adjacent critical structures, like colon, gallbladder, bile duct, heart, and so on, by the extended prongs is avoided. The second is that for large tumors, fewer insertions are needed because by using a combination of multiple BRFA electrodes insertion a larger coagulation volume can be achieved. In addition, the ablation time also can be greatly decreased thus the complication associated with long ablation time such as bleeding can be minimized. Finally, no need of the electrical pads also reduces the risk of skin burn.
In our study, the CR rate was 89.0%. The CR rate for the tumors with a curative treatment intention (94.7%) was remarkably higher than for those with a palliative treatment intention (82.9%), thus the treatment intention was associated the effectiveness of BRFA. For the patients with curative treatment intention, the purpose of the treatment is to eradicate the tumor and to avoid local tumor recurrence after treatment, thus great efforts should be taken to ablate the tumor completely even if the tumors are in difficult locations. On the other hand, the purpose of the treatment for those with palliative intention is to reduce the tumor burden and to alleviate the patient’s symptom. Those patients always had large tumors or tumors adjacent to critical structures, and always had a worse liver function. To treat such patients, careful consideration should be taken to avoid possible complications, whereas the local treatment efficacy is the secondary purpose. On the other hand, the CR rates were 94.7% and 82.9% for primary and metastatic liver cancer respectively. It showed that the therapeutic effect of BRFA for primary liver cancer was slightly better than that for metastatic liver cancer, although the difference was not statistically significant. The primary liver cancer such as hepatocellular carcinoma (HCC) always has a capsule so that the tumor is well-defined whereas metastatic liver cancer is always ill-defined and infiltrated, therefore, more efforts is needed for the ablation of metastatic liver cancer. A so-called “oven effect” is also happened in HCC whereas not in metastatic liver cancer. It is said that the “oven effect” could result in more homogeneous heat distribution within the tumor, which in turn avoids residual tumor. This phenomenon also indicates that for metastatic liver cancer an extensive ablation is mandatory if the treatment purpose is curative. Moreover, the CR rate in tumor ≤3.0 cm (96.7%) was prominently higher than that in tumor 3.1-5.0 cm (63.6%) and >5.0 cm (0%). These results illustrated that tumor size is also a key factor for complete response.
In the present study, using three T40 electrodes insertion simultaneously, the maximum size of the ablation volume was up to 6.8 × 6.0 × 5.8 cm. The output power used was 120 W and the distance among the electrodes was about 3 cm. This result was similar to previous study using BRFA [13], which also indicated that simultaneous application of multiple electrodes could create large coagulation volume with fewer insertions and less time as compared with conventional MRFA method.
The evaluation of therapeutic effect is very important for the management of patients. CECT or CEMRI usually acts as the reference standard for the post-treatment evaluation. Our study used CEUS to assess the treatment response and compared it with CEMRI/CECT one month after RFA. CEUS has already been used for assessing the treatment response with high sensitivity, specificity and accuracy [9,17-19]. In the current study, CEUS showed sensitivity of 87.5%, specificity of 96.9% and accuracy of 95.9%. CEUS has similar ability as compared with CEMRI/CECT. In previous studies, CEUS within 1-month after RFA had the sensitivity of 86.9%-97.0%, specificity of 96.6%-100%, and accuracy of 92%-95.1% [17,18,20]. Our results were consistent with those studies.
The present study had some limitations. Firstly, the case number was small so that further study with large case series was mandatory. Secondly, the number of tumors greater than 5cm was small, thus the real ability of BRFA for medium or large tumors were still unknown and should be verified in future study. Thirdly, long-term follow-up for the patients undergoing BRFA was not available thus the outcome was not available at the current stage.
In summary, BRFA is an effective technique of percutaneous ablation for liver cancers and CEUS can be used to assess its therapeutic effect accurately. However, future studies are needed to evaluate its usefulness regarding medium and large tumors, and long term outcome should also be evaluated.
Acknowledgements
This work was supported in part by Grant 20114003 and 2013SY066 from Shanghai Municipal Commission of Health and Family Planning, Grant 14441900900 from Science and Technology Commission of Shanghai Municipality, Grant 81301299, 81301229, and 81371570 from the National Natural Scientific Foundation of China, and Grant 2012045 from Shanghai Municipal Human Resources and Social Security Bureau.
Disclosure of conflict of interest
None.
References
- 1.Xu HX, Xie XY, Lu MD, Chen JW, Yin XY, Xu ZF, Liu GJ. Ultrasound-guided percutaneous thermal ablation of hepatocellular carcinoma using microwave and radiofrequency ablation. Clin Radiol. 2004;59:53–61. doi: 10.1016/j.crad.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 2.Lu MD, Xu HX, Xie XY, Yin XY, Chen JW, Kuang M, Xu ZF, Liu GJ, Zheng YL. Percutaneous microwave and radiofrequency ablation for hepatocellular carcinoma: a retrospective comparative study. J Gastroenterol. 2005;40:1054–60. doi: 10.1007/s00535-005-1671-3. [DOI] [PubMed] [Google Scholar]
- 3.Riccardo L, Laura C. Local-regional treatment of hepatocellular carcinoma. Radiology. 2012;262:43–58. doi: 10.1148/radiol.11110144. [DOI] [PubMed] [Google Scholar]
- 4.Xu HX, Wang Y, Lu MD, Liu LN. Percutaneous ultrasound-guided thermal ablation for intrahepatic cholangiocarcinoma. Br J Radiol. 2012;85:1078–84. doi: 10.1259/bjr/24563774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Osaki Y, Ikeda K, Izumi N, Yamashita S, Kumada H, Hatta S, Okita K. Clinical effectiveness of bipolar radiofrequency ablation for small liver cancers. J Gastroenterol. 2013;48:874–83. doi: 10.1007/s00535-012-0685-x. [DOI] [PubMed] [Google Scholar]
- 6.Peng ZW, Lin XJ, Zhang YJ, Liang HH, Guo RP, Shi M, Chen MS. Radiofrequency ablation versus hepatic resection for the treatment of hepatocellular carcinomas 2 cm or smaller: a retrospective comparative study. Radiology. 2012;262:1022–33. doi: 10.1148/radiol.11110817. [DOI] [PubMed] [Google Scholar]
- 7.Claudon M, Dietrich CF, Choi BI, Cosgrove DO, Kudo M, Nolsoe CP, Piscaglia F, Wilson SR, Barr RG, Chamma MC, Chaubal NG, Chen MH, Clevert DA, Correas JM, Ding H, Forsberg F, Fowlkes JB, Gibson RN, Goldberg BB, Lassau N, Leen ELS, Mattrey RF, Moriyasu F, Solbiati L, Weskott HP, Xu HX. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) in the liver-update 2012. Ultraschall Med. 2013;34:11–29. doi: 10.1055/s-0032-1325499. [DOI] [PubMed] [Google Scholar]
- 8.Toshikuni N, Shiroeda H, Ozaki K, Matsue Y, Minato T, Nomura T, Hayashi N, Arisawa T, Tsutsumi M. Advanced ultrasonography technologies to assess the effects of radiofrequency ablation on hepatocellular carcinoma. Radiol Oncol. 2013;47:224–9. doi: 10.2478/raon-2013-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu HP, Wilkins LR, Ziats NP, Haaga JR, Exner AA. Real-time Monitoring of radiofrequency ablation and postablation assessment: accuracy of contrast-enhanced us in experimental rat liver model. Radiology. 2014;270:107–16. doi: 10.1148/radiol.13121999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pompili M, Riccardi L, Covino M, Barbaro B, Di Stasi C, Orefice R, Gasbarrini G, Rapaccini GL. Contrast-enhanced gray-scale harmonic ultrasound in the efficacy assessment of ablation treatments for hepatocellular carcinoma. Liver Int. 2005;25:954–61. doi: 10.1111/j.1478-3231.2005.01135.x. [DOI] [PubMed] [Google Scholar]
- 11.Baldwin K, Katz SC, Rubin A, Somasundar P. Bipolar radiofrequency ablation of liver tumors: Technical experience and interval follow-up in 22 patients with 33 ablations. J Surg Oncol. 2012;106:905–10. doi: 10.1002/jso.23147. [DOI] [PubMed] [Google Scholar]
- 12.Meijerink MR, van den Tol P, van Tilborg AA, van Waesberghe JH, Meijer S, van Kuijk C. Radiofrequency ablation of large size liver tumours using novel plan-parallel expandable bipolar electrodes: Initial clinical experience. Eur J Radiol. 2011;77:167–71. doi: 10.1016/j.ejrad.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 13.Clasen S, Schmidt D, Boss A, Dietz K, Krober SM, Claussen CD, Pereira PL. Multipolar Radiofrequency Ablation with Internally Cooled Electrodes: Experimental Study in ex Vivo Bovine Liver with Mathematic Modeling. Radiology. 2006;238:881–90. doi: 10.1148/radiol.2382050571. [DOI] [PubMed] [Google Scholar]
- 14.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–22. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldberg SN, Grassi CJ, Cardella JF, Charboneau JW, Dodd GD, Dupuy DE, Gervais D, Gillams AR, Kane RA, Lee FT, Livraghi T, McGahan J, Phillips DA, Rhim H, Silverman SG Society of Interventional Radiology Technology Assessment Committee; International Working Group on Image-Guided Tumor Ablation. Image-guided tumor ablation: standardization of terminology and reporting criteria. Radiology. 2005;235:728–39. doi: 10.1148/radiol.2353042205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jansen MC, van Duijnhoven FH, van Hillegersberg R, Rijken A, van Coevorden F, van der Sijp J, Prevoo W, van Gulik TM. Adverse effects of radiofrequency ablation of liver tumors in the Netherlands. Br J Surg. 2005;92:1248–54. doi: 10.1002/bjs.5059. [DOI] [PubMed] [Google Scholar]
- 17.Salvaggio G, Campisi A, Lo Greco V, Cannella I, Meloni MF, Caruso G. Evaluation of posttreatment response of hepatocellular carcinoma: comparison of ultrasonography with second-generation ultrasound contrast agent and multidetector CT. Abdom Imaging. 2010;35:447–53. doi: 10.1007/s00261-009-9551-6. [DOI] [PubMed] [Google Scholar]
- 18.Vilana R, Bianchi L, Varela M, Nicolau C, Sanchez M, Ayuso C, Garcia M, Sala M, Llovet JM, Bruix J, Bru C. Is microbubble-enhanced ultrasonography sufficient for assessment of response to percutaneous treatment in patients with early hepatocellular carcinoma. Eur Radiol. 2006;16:2454–62. doi: 10.1007/s00330-006-0264-8. [DOI] [PubMed] [Google Scholar]
- 19.Meloni MF, Andreano A, Zimbaro F, Lava M, Lazzaroni S, Sironi S. Contrast enhanced ultrasound: Roles in immediate post-procedural and 24-h evaluation of the effectiveness of thermal ablation of liver tumors. J Ultrasound. 2012;15:207–14. doi: 10.1016/j.jus.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu MD, Yu XL, Li AH, Jiang TA, Chen MH, Zhao BZ, Zhou XD, Wang JR. Comparison of contrast enhanced ultrasound and contrast enhanced CT or MRI in monitoring percutaneous thermal ablation procedure in patients with hepatocellular carcinoma: A multi-center study in China. Ultrasound Med Biol. 2007;33:1736–49. doi: 10.1016/j.ultrasmedbio.2007.05.004. [DOI] [PubMed] [Google Scholar]


