Abstract
It is well recognized that peritumoral edema is vasogenic cerebral edema in malignant glioma, and vascular endothelial growth factor (VEGF) induced by phosphorylated signal transducer and activator of transcription factor 3 (pSTAT3) strongly contributes to tumor angiogenesis in glioblastoma. However, there is no study with regard to the correlation between pSTAT3 or VEGF and peritumoral edema. Such evidence may contribute to providing new targets for the management of peritumoral cerebral. In this study, newly diagnosed glioblastoma tissues from 84 patients were collected to investigate pSTAT3 and VEGF expression by immunohistochemistry, and peritumoral edema was detected by preoperative magnetic resonance imaging. We found that a significantly positive correlation emerged between VEGF and pSTAT3 expression (P = 0.000) in glioblastoma tissues, but they were not related to patient gender and age (P > 0.05); the expression of pSTAT3 and VEGF were associated with peritumoral edema extent (P = 0.005), but not with edema shape (P > 0.05). Therefore, the pSTAT3-VEGF signaling pathway, which is correlated with peritumoral edema extent, might be a regulatory mechanism in the course of peritumoral edema formation during glioblastoma tumorigenesis and progression, thereby suggesting that STAT3 inhibition might be helpful for alleviation of peritumoral cerebral edema.
Keywords: Glioblastoma, peritumoral edema, signal transducer and activator of transcription factor 3, phosphorylation, vascular endothelial growth factor
Introduction
Glioblastoma multiforme (GBM), the most frequent primary malignant glioma in adults, exhibits a poor prognosis with a median overall survival rate of 9.4 to 19.0 months despite taking radiotherapy plus temozolomide postoperatively [1]. Peritumoral edema (PTE), a main biological behavior characteristic of malignant glioma, is one of significant contributors to mortality in glioblastoma patients [2]. PTE not only influences cognitive function in patients with GBM [3], but also may serve as a predictor of poor prognosis [4-6]. Therefore, clarifying the molecular mechanism associated with the evolution of PTE is clearly necessary.
Several studies have showed that high expression of vascular endothelial growth factor (VEGF) is the pivotal molecular event of peritumoral edema in glioma [7,8]. VEGF is able to not only increase the formation of cleft and fenestra between vascular endothelial cells through down-regulating the expression of tight junction proteins, such as occludin, ZO-1, etc. [9], but also promote the vesiculo-vacuolar organelles formation within endothelium [8,10], all of which may contribute to the increase of vascular permeability, thereby leading to PTE.
Signal transducer and activator of transcription factor 3 (STAT3), which is aberrantly phosphorylated at tyrosine (Tyr) residue 705 in human glioblastoma [11,12], is associated with a variety of biological behavior of glioma cells. Phosphorylated STAT3 (pSTAT3) not only involves in glioma cell proliferation, anti-apoptosis and immunosuppression, but also is a pivotal driver of glioma cell migration and invasion [13-17]. Moreover, pSTAT3, a molecular hub for signal transduction pathways in glioma [18], is able to regulate the activity of VEGF promoter and induce VEGF transcription [19,20], thereby playing an important role in glioblastoma neo-angiogenesis [20,21]. And it is well recognized that PTE is vasogenic cerebral edema in malignant glioma. Thus, we speculate that pSTAT3-VEGF signaling pathway might be a significant molecular mechanism contributing to the formation of PTE in GBM. Currently there is no relative study on this subject. Such evidence might contribute to providing new targets for the management of PTE. In the present study, the expression of pSTAT3 and VEGF in the specimens of GBM were detected using immunohistochemistry technique, we focused our analysis on the relationship of pSTAT3-VEGF signaling pathway with peritumoral edema in GBM patients.
Materials and methods
Study samples
In this retrospective study, 84 patients with newly diagnosed GBM were selected who had undergone surgical resection in the Department of Neurosurgery, the First Affiliated Hospital of Fujian Medical University from March 2007 to October 2013. The inclusion criteria were as follows: (1) newly diagnosed glioblastoma, the pathologic diagnosis was reaffirmed according to the principles of WHO classification of tumors of the central nervous system [22]; (2) preoperative magnetic resonance imaging (MRI) data were available for the assessment of PTE; (3) tumor resection specimens were available for immunohistochemical assay. This study was approved by the Ethics Committee of Fujian Medical University and conformed to the principles of the Helsinki convention. Informed consent was provided by each patient.
Peritumoral edema
Preoperative MRI data, including T1-w, T2-w, and contrast-enhanced T1-w sequences, were obtained from each patient. All MRI scans were analyzed by two investigators who blinded to the pathological diagnosis and the results of pSTAT3 or VEGF staining. A region of very bright T2-w signal and low T1-w signal without enhancement around the tumor was determined as PTE. The extent and shape of PTE were determined on axial T2-w MRI sequences. Sagittal or coronal images were applied for edema determination when edema extent was larger in the sagittal or coronal direction than in the axial direction [23]. Edema extent was evaluated according to the maximum distance between the outer edge of edema and the nearest point of tumor margin and graded according to a previous study [24]: edema extending ≤ 2 cm from the tumor margin was determined as grade 1 (Figure 1A and 1E), and edema extending more than 2 cm beyond the tumor margin was determined as grade 2 (Figure 1B and 1F). Edema shape was classified as follows [25]: roundish (Figure 1C and 1G): the shape of edema is not radial and similar to round; irregular (Figure 1D and 1H): edema shape tends to be irregular, such as radial shape or finger-like shape.
Figure 1.
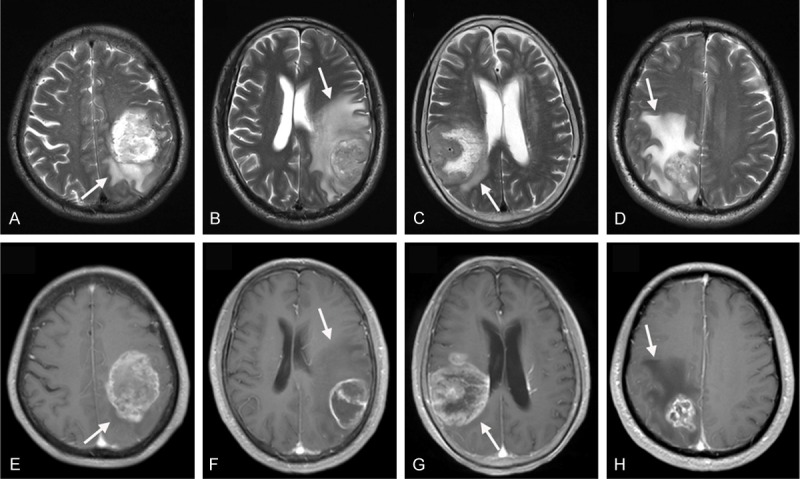
T2-w (A-D) and contrast-enhanced T1-w (E-H) MR images showing peritumoral edema in patients with GBM. (A, E) Show tumors with edema grade 1 (edema extending ≤ 2 cm from the tumor margin); (B, F) Show edema grade 2 (edema extending > 2 cm from the tumor margin); (C, G) Show the roundish shape of edema (shape is not radial and similar to round); (D, H) Show the irregular shape of edema (radial shape or finger-like shape). Arrows mark the edema around tumors.
Immunohistochemistry
Tumor tissues, which were available from patients during surgical resection, were fixed in formaldehyde and embedded by paraffin for immunohistochemistry. Briefly, Paraffin-embeded tissues were cut into 4-μm-thick slices. These pathological sections were dewaxed by treatment in xylene and rehydrated in a graded ethanol series. Then, the antigen retrieval was performed in ethylenediamine tetraacetic acid (pH 9.0) for 20 min in a boiling water bath. The endogenous peroxidase became inactivated after incubation with methanol containing 3% hydrogen peroxide for 10 minutes. The specimens were then exposed for 1 hour at room temperature to the following antibodies, respectively: against phosphorylated(p)-STAT3 (Tyr705, D3A7, rabbit monoclonal antibody, Cell Signaling Technology, USA) diluted 1:100; anti-VEGF (MAB-0234, mouse anti-human VEGF monoclonal antibody, Maixin-Bio, China). Next, according to the manufacturer’s instruction, the sections were incubated with polyperoxidase-anti-rabbit/mouse IgG (ZSGB-BIO, China) for 30 min. Peroxidase activity was developed using diaminobenzidine detection kit (ZSGB-BIO, China). Sections were counterstained with hematoxylin. According to the manufacturer’s instruction, human lung carcinoma tissue and human breast carcinoma tissue were performed as positive control for pSTAT3 and VEGF, respectively. Phosphate buffer solution (0.01 M, pH 7.2) acted as primary antibody was served as negative control.
Assessment of outcomes
Positive expression of VEGF was determined when brown staining appeared in the cytoplasm of tumor cells. Positive pSTAT3 was considered when brown staining appeared in the nucleus. In each case, the proportion of VEGF positive tumor cells was determined by assessing the randomized five visual fields under high power lens; the percentage of pSTAT3 positive tumor cells was calculated through evaluating the high power field with a highest density of stained tumor cells [12]. For statistical analysis, according to a previous study [26] the median of the proportion of positive expression was served as the cut-off point for data grouping: the positive expression rates which were less than the median were defined as low expression, and the positive rates which were greater than or equal to the median was defined as high expression. Two neuropathologists (X.F.W., Y.P.C.) blinded to patient clinical data analyzed each section independently.
Statistical methods
SPSS 19.0 statistical software was applied to analyze the data. Association of p-STAT3 with VEGF expression and correlations between p-STAT3 or VEGF expression and either patient gender or age were tested using non-parametric tests (Spearman’s rank correlation coefficient and Mann-Whitney U test, as appropriate). Chi-square test was adopted to analyze the relationships between PTE and p-STAT3, VEGF expression, patient gender or age. Statistical significance was determined as the level of P value < 0.05 (two-tailed).
Results
Clinical data and expression of pSTAT3, VEGF
Out of pSTAT3, VEGF Out of 84 cases, 33 patients (39.3%) were female and 51 (60.7%) were male, sex ratio was 1.54:1; 43 patients (51.2%) were < 55 years and 42 (48.8%) were ≥ 55 years, the median age at diagnosis was 54 years. The expression of pSTAT3 (Figure 2A and 2B) and VEGF (Figure 2D and 2E) in neoplastic cells were highly variable in GBM specimens. The range of VEGF-positive expression was 12% to 95%, with a median of 65%; pSTAT3 expression ranged from 0 to 78%, with a median of 20%. In addition to neoplastic cells, immunoreactivity was also observed in some of the neovascular endothelial cells (Figure 2C and 2F). In some specimens, VEGF-positive neurons were found as well.
Figure 2.
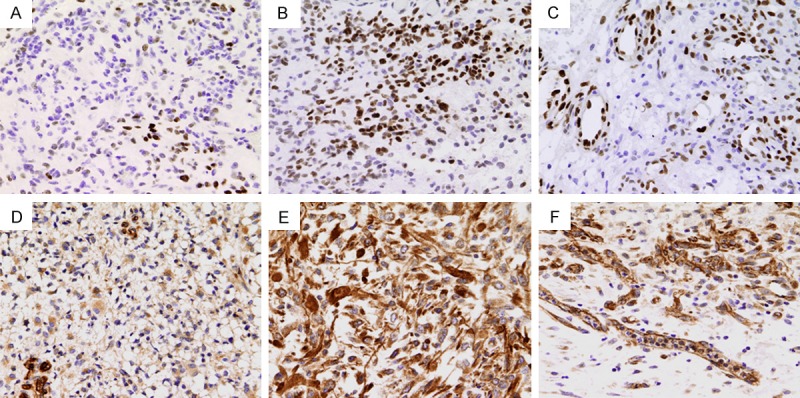
Immunohistochemical staining showing the expression of pSTAT3 (A-C) and VEGF (D-F) in GBM tissues. (A, B) Show low and high pSTAT3 expression, respectively; (D, E) Show low and high VEGF expression, respectively. Immunostaining is also seen in neovascular endothelial cells. (C, F) Show pSTAT3 and VEGF expression, respectively. Original magnification: × 400.
Expression of pSTAT3 and VEGF had positive correlation in glioblastoma tissues
It was concluded that a significant positive correlation emerged between VEGF expression and pSTAT3 expression in glioblastoma (Spearman, r = 0.590, P = 0.000), the expression level of VEGF and pSTAT3 were not related to patient gender (Mann-Whitney U test, P > 0.05), as well as age (Spearman, P > 0.05) (Table 1).
Table 1.
Correlations of pSTAT3, VEGF expression with patient gender or age
| Variables | n | pSTAT3 expression | VEGF expression | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Median (%) | Rang (%) | P value | Median (%) | Rang (%) | P value | ||
| Total | 84 | 20 | 0-78 | 65 | 12-95 | ||
| Gender | |||||||
| Male | 51 | 20 | 0-78 | 0.246 | 66 | 12-95 | 0.132 |
| Female | 33 | 17 | 0-64 | 48 | 16-94 | ||
| Age | 84 | 54 | 18-79 | 0.903 | 54 | 18-79 | 0.582 |
Correlations of VEGF, pSTAT3 expression with peritumoral edema
Among the group of the low expression of VEGF, edema extent tended to be grade 1 (32/45), whereas in 39 high expressed VEGF cases, 23 cases of edema extent exhibited grade 2. Thus, it could be seen that a positive correlation existed between VEGF expression and PTE extent in glioblastoma (Chi-square test, P = 0.005). A similar result was observed for pSTAT3 protein (Chi-square test, P = 0.017). Among 43 cases of the low expression of pSTAT3, only 13 cases of edema extent showed grade 2, and when pSTAT3 protein was highly expressed, edema extent tended to be grade 2 (23/41). Additionally, no significant difference was observed between edema extent and patient gender or age (Chi-square test, P > 0.05). When the groups between the roundish shape and irregular shape of edema were compared, however, we found that edema shape did not correlate with either VEGF or p-STAT3 expression, as well as patient gender or age (Table 2).
Table 2.
Relationships between PTE and VEGF expression, pSTAT3 expression, gender or age
| Group | n | Edema extent | Edema shape | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Grade 1 | Grade 2 | P value | Roundish | Irregular | P value | ||
| VEGF | |||||||
| Low expression | 45 | 32 | 13 | 0.005 | 11 | 34 | 0.668 |
| High expression | 39 | 16 | 23 | 8 | 31 | ||
| Pstat3 | |||||||
| Low expression | 43 | 30 | 13 | 0.017 | 10 | 33 | 0.886 |
| High expression | 41 | 18 | 23 | 9 | 32 | ||
| Gender | |||||||
| Male | 51 | 31 | 20 | 0.402 | 13 | 38 | 0.434 |
| Female | 33 | 17 | 16 | 6 | 27 | ||
| Age | |||||||
| < 55 years | 43 | 26 | 17 | 0.529 | 9 | 34 | 0.705 |
| ≥ 55 years | 41 | 22 | 19 | 10 | 31 | ||
Discussion
Peritumoral edema, a frequently encountered phenomenon, affects the clinical outcome in patients with glioblastoma [6]. Only after a better understanding of the molecular mechanism could an apparent improvement in the clinical management of PTE be achieved. Here, we demonstrate that pSTAT3 and VEGF expression have positive correlation in GBM, and PTE extent shown by MRI is positively associated with the expression of pSTAT3 and VEGF. These observations suggest that the two molecules - pSTAT3-VEGF signaling pathway, may play a relevant role in the course of peritumoral edema formation during GBM tumorigenesis.
The statistical results show that the expression of pSTAT3 is closely associated with VEGF expression, which is in accordance with a recent research [26]. Indeed, constitutively activated STAT3 in GBM is an upstream signaling molecule for VEGF expression, which contributes to tumor angiogenesis. By binding to STAT3-binding element (SBE) in the promoter of target genes, pSTAT3 regulates the transcription of a wide range of genes involved in angiogenesis, mainly including the potent pro-angiogenesis factor - VEGF [27]. A previous study using chromatin immunoprecipitation assay has showed that activated STAT3 directly binds to the VEGF promoter in vivo [28], leading to the upregulation of VEGF expression and tumor angiogenesis. Moreover, several reports reveal that for a maximum transcriptional level of the VEGF, the simultaneous binding of both pSTAT3 and other transcription factors (such as HIF-1) to the VEGF promoter is required [29,30], indicating that pSTAT3 could enhance the transcription level of VEGF in the condition of hypoxia. Additionally, in the context of a SBE-free promoter, the transcription of VEGF could be triggered via the interaction of pSTAT3 with Sp1 in GBM [19], thereby validating pSTAT3 as an important mediator of VEGF expression. Furthermore, STAT3 knockdown in malignant glioma cells results in the downregulation of VEGF transcription [31], further providing evidence that aberrant pSTAT3 may directly contribute to VEGF overexpression.
Generally, PTE related to the blood-brain barrier disturbance and the extent of neovascularization is considered to be vasogenic cerebral edema in malignant glioma. It is well recognized that neovascularization is a major pathological hallmark of GBM. Compared to normal blood-brain barrier, there exist neovascular endothelial cells dysfunction and the disruptions of tight junction proteins induced by VEGF in malignant glioma [8-10], all of which contribute to the increase of vascular permeability, thereby leading to the formation of PTE. A recent study demonstrates that a positive correlation between pSTAT3 or VEGF expression with microvascular density [26]. Moreover, it is via STAT3 signaling that VEGF induced by pSTAT3 facilitate endothelial cell survival, proliferation, migration, as well as microvascular tube formation in the course of tumor angiogenesis [27]. In the present study, we demonstrate that PTE extent is positively associated with pSTAT3 and VEGF expression. These findings suggest that pSTAT3-VEGF signaling pathway which contributes to the neovascularization of malignant glioma plays an important role in the evolution of peritumoral edema and might be a regulatory mechanism in the formation of peritumoral edema during GBM tumorigenesis.
It has previously been thought that PTE would be conducive to the spread of glioma cells, thus promoting tumor invasion [32]. Nevertheless, anti-VEGF antibody treatment of glioblastoma might lead to a wide invasion of tumor cell, although it could alleviate cerebral edema in clinical practice [33,34]. This phenomenon prompts us that PTE which is mainly mediated by VEGF might be an accompanying phenomenon of tumor invasion, and VEGF might be as a downstream molecular event of invasion-related signaling pathways regulated by glioma cells. Indeed, VEGF is a downstream signaling molecule of pSTAT3, which might be used as a significant molecule reflecting the invasion of glioma cells. Several studies have showed that pSTAT3 is not only able to up-regulate the expression of pro-invasive molecules to degrade the extracellular matrix, such as metalloproteases-2 and metalloproteases-9 [13,35], but also could modulate actomyosin activity within glioma cell leading to promotion of tumor cell motility [36], thereby enhancing the migration capacity of glioma cell and promoting tumor invasion [13,17]. In our study, we demonstrate that pSTAT3-VEGF signaling molecules are related with peritumoral edema. Therefore, we speculate that pSTAT3-VEGF signaling pathway might be a significant molecular mechanism of glioma cell invasion accompanied by the formation of peritumoral edema, and the use of STAT3 inhibitors might suppress tumor angiogenesis and invasion, as well as alleviate peritumoral cerebral edema. To understand the exact relationships among pSTAT3-VEGF pathway, PTE and tumor invasion, the nature of pSTAT3-VEGF signaling pathway shall be investigated in further studies.
Nevertheless, some studies have revealed no significant correlation in glioma between VEGF expression and PTE [37,38]. The different result is probably due to the different cases studied, the quantitative criterion of PTE, the detection method of VEGF, and the way groups were formed. Another possible explanation for the discrepancy might be that there exist other factors causing PTE, in addition to VEGF-independent pathway. Additionally, we should acknowledge that there are many limitations in our retrospective study. Thus, random-designed and prospective researches are preferable in the future study in order to avoid bias.
In conclusion, this is the first study to confirm that PTE extent in GBM is positively associated with the expression of pSTAT3 and VEGF, and to validate the positive correlation between pSTAT3 and VEGF expression in these patients. Our study provides evidence that the pSTAT3-VEGF signaling pathway may serve as a regulatory mechanism of PTE in glioblastoma tumorigenesis and progression, indicating that STAT3 inhibition might be helpful for alleviation of peritumoral cerebral edema.
Acknowledgements
This work was supported by a grant from the National Natural Science Foundation of China (No. 30973083) and Key Clinical Special Discipline Construction Program of Fujian, People’s Republic of China.
Disclosure of conflict of interest
None.
References
- 1.Yang LJ, Zhou CF, Lin ZX. Temozolomide and radiotherapy for newly diagnosed glioblastoma multiforme: a systematic review. Cancer Invest. 2014;32:31–36. doi: 10.3109/07357907.2013.861474. [DOI] [PubMed] [Google Scholar]
- 2.Hildebrand J, Amoura Z, Baumert B, Behin A, Capelle L, Chinot O, Delattre JY, Graus F, Guerrero D, Kardamakis , Perilongo G, Raffin-Sanson ML, Twijnstra A. Management of peritumoral edema. EANO-Guideline-Discussion peritumoral edema. 2003:1–9. [Google Scholar]
- 3.Talacchi A, Santini B, Savazzi S, Gerosa M. Cognitive effects of tumour and surgical treatment in glioma patients. J Neurooncol. 2011;103:541–549. doi: 10.1007/s11060-010-0417-0. [DOI] [PubMed] [Google Scholar]
- 4.Hammoud MA, Sawaya R, Shi W, Thall PF, Leeds NE. Prognostic significance of preoperative MRI scans in glioblastoma multiforme. J Neurooncol. 1996;27:65–73. doi: 10.1007/BF00146086. [DOI] [PubMed] [Google Scholar]
- 5.Pope WB, Sayre J, Perlina A, Villablanca JP, Mischel PS, Cloughesy TF. MR imaging correlates of survival in patients with high-grade gliomas. AJNR Am J Neuroradiol. 2005;26:2466–2474. [PMC free article] [PubMed] [Google Scholar]
- 6.Schoenegger K, Oberndorfer S, Wuschitz B, Struhal W, Hainfellner J, Prayer D, Heinzl H, Lahrmann H, Marosi C, Grisold W. Peritumoral edema on MRI at initial diagnosis: an independent prognostic factor for glioblastoma? Eur J Neurol. 2009;16:874–878. doi: 10.1111/j.1468-1331.2009.02613.x. [DOI] [PubMed] [Google Scholar]
- 7.Lin ZX. Glioma-related edema: new insight into molecular mechanisms and their clinical implications. Chin J Cancer. 2013;32:49–52. doi: 10.5732/cjc.012.10242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin ZX, Yang LJ, Huang Q, Lin JH, Ren J, Chen ZB, Zhou LY, Zhang PF, Fu J. Inhibition of tumor-induced edema by antisense VEGF is mediated by suppressive vesiculo-vacuolar organelles (VVO) formation. Cancer Sci. 2008;99:2540–2546. doi: 10.1111/j.1349-7006.2008.00974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Papadopoulos MC, Saadoun S, Davies DC, Bell BA. Emerging molecular mechanisms of brain tumour oedema. Br J Neurosurg. 2001;15:101–108. doi: 10.1080/02688690120036775. [DOI] [PubMed] [Google Scholar]
- 10.Yang L, Lin Z, Huang Q, Lin J, Chen Z, Zhou L, Zhang P. Effect of vascular endothelial growth factor on remodeling of C6 glioma tissue in vivo. J Neurooncol. 2011;103:33–41. doi: 10.1007/s11060-010-0356-9. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Chen L, Bao Z, Li S, You G, Yan W, Shi Z, Liu Y, Yang P, Zhang W, Han L, Kang C, Jiang T. Inhibition of STAT3 reverses alkylator resistance through modulation of the AKT and beta-catenin signaling pathways. Oncol Rep. 2011;26:1173–1180. doi: 10.3892/or.2011.1396. [DOI] [PubMed] [Google Scholar]
- 12.Birner P, Toumangelova-Uzeir K, Natchev S, Guentchev M. STAT3 tyrosine phosphorylation influences survival in glioblastoma. J Neurooncol. 2010;100:339–343. doi: 10.1007/s11060-010-0195-8. [DOI] [PubMed] [Google Scholar]
- 13.Senft C, Priester M, Polacin M, Schroder K, Seifert V, Kogel D, Weissenberger J. Inhibition of the JAK-2/STAT3 signaling pathway impedes the migratory and invasive potential of human glioblastoma cells. J Neurooncol. 2011;101:393–403. doi: 10.1007/s11060-010-0273-y. [DOI] [PubMed] [Google Scholar]
- 14.Kohanbash G, Okada H. MicroRNAs and STAT interplay. Semin Cancer Biol. 2012;22:70–75. doi: 10.1016/j.semcancer.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kiu H, Nicholson SE. Biology and significance of the JAK/STAT signalling pathways. Growth Factors. 2012;30:88–106. doi: 10.3109/08977194.2012.660936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol. 2007;7:41–51. doi: 10.1038/nri1995. [DOI] [PubMed] [Google Scholar]
- 17.Ou Y, Ma L, Dong L, Ma L, Zhao Z, Ma L, Zhou W, Fan J, Wu C, Yu C, Zhan Q, Song Y. Migfilin protein promotes migration and invasion in human glioma through epidermal growth factor receptor-mediated phospholipase C-gamma and STAT3 protein signaling pathways. J Biol Chem. 2012;287:32394–32405. doi: 10.1074/jbc.M112.393900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brantley EC, Benveniste EN. Signal transducer and activator of transcription-3: a molecular hub for signaling pathways in gliomas. Mol Cancer Res. 2008;6:675–684. doi: 10.1158/1541-7786.MCR-07-2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loeffler S, Fayard B, Weis J, Weissenberger J. Interleukin-6 induces transcriptional activation of vascular endothelial growth factor (VEGF) in astrocytes in vivo and regulates VEGF promoter activity in glioblastoma cells via direct interaction between STAT3 and Sp1. Int J Cancer. 2005;115:202–213. doi: 10.1002/ijc.20871. [DOI] [PubMed] [Google Scholar]
- 20.Kang SH, Yu MO, Park KJ, Chi SG, Park DH, Chung YG. Activated STAT3 regulates hypoxia-induced angiogenesis and cell migration in human glioblastoma. Neurosurgery. 2010;67:1386–1395. doi: 10.1227/NEU.0b013e3181f1c0cd. discussion 1395. [DOI] [PubMed] [Google Scholar]
- 21.Xu Y, Li X, Zhang S, Shen D, Li H, Wu Y, Qiu Y, Ji Y, Chen F. Targeting Stat3 suppresses growth of U251 cell-derived tumours in nude mice. J Clin Neurosci. 2012;19:443–446. doi: 10.1016/j.jocn.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 22.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seidel C, Dorner N, Osswald M, Wick A, Platten M, Bendszus M, Wick W. Does age matter? - A MRI study on peritumoral edema in newly diagnosed primary glioblastoma. BMC Cancer. 2011;11:127. doi: 10.1186/1471-2407-11-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carlson MR, Pope WB, Horvath S, Braunstein JG, Nghiemphu P, Tso CL, Mellinghoff I, Lai A, Liau LM, Mischel PS, Dong J, Nelson SF, Cloughesy TF. Relationship between survival and edema in malignant gliomas: role of vascular endothelial growth factor and neuronal pentraxin 2. Clin Cancer Res. 2007;13:2592–2598. doi: 10.1158/1078-0432.CCR-06-2772. [DOI] [PubMed] [Google Scholar]
- 25.Hartmann M, Jansen O, Egelhof T, Forsting M, Albert FK, Sartor K. [Effect of brain edema on the recurrence pattern of malignant gliomas] . Radiologe. 1998;38:948–953. doi: 10.1007/s001170050447. [DOI] [PubMed] [Google Scholar]
- 26.Piperi C, Samaras V, Levidou G, Kavantzas N, Boviatsis E, Petraki K, Grivas A, Barbatis C, Varsos V, Patsouris E, Korkolopoulou P. Prognostic significance of IL-8-STAT-3 pathway in astrocytomas: correlation with IL-6, VEGF and microvessel morphometry. Cytokine. 2011;55:387–395. doi: 10.1016/j.cyto.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 27.Chen Z, Han ZC. STAT3: a critical transcription activator in angiogenesis. Med Res Rev. 2008;28:185–200. doi: 10.1002/med.20101. [DOI] [PubMed] [Google Scholar]
- 28.Niu G, Wright KL, Huang M, Song L, Haura E, Turkson J, Zhang S, Wang T, Sinibaldi D, Coppola D, Heller R, Ellis LM, Karras J, Bromberg J, Pardoll D, Jove R, Yu H. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene. 2002;21:2000–2008. doi: 10.1038/sj.onc.1205260. [DOI] [PubMed] [Google Scholar]
- 29.Jung JE, Lee HG, Cho IH, Chung DH, Yoon SH, Yang YM, Lee JW, Choi S, Park JW, Ye SK, Chung MH. STAT3 is a potential modulator of HIF-1-mediated VEGF expression in human renal carcinoma cells. FASEB J. 2005;19:1296–1298. doi: 10.1096/fj.04-3099fje. [DOI] [PubMed] [Google Scholar]
- 30.Gray MJ, Zhang J, Ellis LM, Semenza GL, Evans DB, Watowich SS, Gallick GE. HIF-1alpha, STAT3, CBP/p300 and Ref-1/APE are components of a transcriptional complex that regulates Src-dependent hypoxia-induced expression of VEGF in pancreatic and prostate carcinomas. Oncogene. 2005;24:3110–3120. doi: 10.1038/sj.onc.1208513. [DOI] [PubMed] [Google Scholar]
- 31.Priester M, Copanaki E, Vafaizadeh V, Hensel S, Bernreuther C, Glatzel M, Seifert V, Groner B, Kogel D, Weissenberger J. STAT3 silencing inhibits glioma single cell infiltration and tumor growth. Neuro Oncol. 2013;15:840–852. doi: 10.1093/neuonc/not025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohnishi T, Sher PB, Posner JB, Shapiro WR. Capillary permeability factor secreted by malignant brain tumor. Role in peritumoral brain edema and possible mechanism for anti-edema effect of glucocorticoids. J Neurosurg. 1990;72:245–251. doi: 10.3171/jns.1990.72.2.0245. [DOI] [PubMed] [Google Scholar]
- 33.Kamoun WS, Ley CD, Farrar CT, Duyverman AM, Lahdenranta J, Lacorre DA, Batchelor TT, di Tomaso E, Duda DG, Munn LL, Fukumura D, Sorensen AG, Jain RK. Edema control by cediranib, a vascular endothelial growth factor receptor-targeted kinase inhibitor, prolongs survival despite persistent brain tumor growth in mice. J. Clin. Oncol. 2009;27:2542–2552. doi: 10.1200/JCO.2008.19.9356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rubenstein JL, Kim J, Ozawa T, Zhang M, Westphal M, Deen DF, Shuman MA. Anti-VEGF antibody treatment of glioblastoma prolongs survival but results in increased vascular cooption. Neoplasia. 2000;2:306–314. doi: 10.1038/sj.neo.7900102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen F, Xu Y, Luo Y, Zheng D, Song Y, Yu K, Li H, Zhang L, Zhong W, Ji Y. Down-regulation of Stat3 decreases invasion activity and induces apoptosis of human glioma cells. J Mol Neurosci. 2010;40:353–359. doi: 10.1007/s12031-009-9323-3. [DOI] [PubMed] [Google Scholar]
- 36.Agudelo-Garcia PA, De Jesus JK, Williams SP, Nowicki MO, Chiocca EA, Liyanarachchi S, Li PK, Lannutti JJ, Johnson JK, Lawler SE, Viapiano MS. Glioma cell migration on three-dimensional nanofiber scaffolds is regulated by substrate topography and abolished by inhibition of STAT3 signaling. Neoplasia. 2011;13:831–840. doi: 10.1593/neo.11612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vaquero J, Zurita M, Morales C, Cincu R, Oya S. Expression of vascular permeability factor in glioblastoma specimens: correlation with tumor vascular endothelial surface and peritumoral edema. J Neurooncol. 2000;49:49–55. doi: 10.1023/a:1006453428508. [DOI] [PubMed] [Google Scholar]
- 38.Dickinson PJ, Sturges BK, Higgins RJ, Roberts BN, Leutenegger CM, Bollen AW, LeCouteur RA. Vascular endothelial growth factor mRNA expression and peritumoral edema in canine primary central nervous system tumors. Vet Pathol. 2008;45:131–139. doi: 10.1354/vp.45-2-131. [DOI] [PubMed] [Google Scholar]


