Abstract
High expression of matrix metalloproteinase-9 (MMP-9) was found to be correlated with tumor progression and poor prognosis in a variety of carcinomas. However, few studies have investigated the role of MMP-9 in human hilar cholangiocarcinoma. In this study, a total of 58 patients with hilar cholangiocarcinoma who underwent curative resection were included in this study. The expression of MMP-9 was analyzed by immunohistochemistry using the streptavidin peroxidase complex method. The correlation of MMP-9 overexpression with clinicopathological features and survival time of patients was investigated. The results showed that MMP-9 overexpression was prominent in cancer cells and mainly localized in the cytoplasm. MMP-9 overexpression was observed in 46.5% tumors, which showed no correlation with clinicopathological parameters. Patients with high MMP-9 expression had a significantly poorer overall survival rate than those with negative or low MMP-9 expression (P = 0.038). Multivariate analysis confirmed that MMP-9 overexpression was an independent prognostic factor (P = 0.007). In conclusion, overexpression of MMP-9 is a valuable independent prognostic indicator in hilar cholangiocarcinoma.
Keywords: Cholangiocarcinoma, MMP, prognosis
Introduction
Hilar cholangiocarcinoma, also called Klatskin tumor, is known as an extremely fatal tumor due to its difficult diagnosis at early stage and notoriously high mortality [1]. Both early invasion and metastasis contribute to the devastating prognosis. Radical surgery is to date the only curative treatment either in the form of hepatic resection or liver transplantation for hilar cholangiocarcinoma [2-4]. However, only a minority of patients could receive curative surgery. Furthermore, even many patients who undergo surgery still have to face a high risk of recurrence with a varying survival to a large extent [5]. Adjuvant therapy such as chemotherapy and radiation therapy has not clearly shown a satisfactory result [6]. Therefore, a better understanding of the molecular regulation involved in hilar cholangiocarcinoma progression might help to identify patients at risk for an unfavorable outcome, and such prognostic markers could also serve in developing new treatment strategies.
During the dissemination of tumor cells, there are a series of necessary steps including adherence to extracellular matrix (ECM), a natural barrier against tumor invasion, and subsequent degradation of its components [7]. Matrix metalloproteinases (MMPs) comprise such family of zinc-dependent proteolytic enzymes that specifically degrade ECM glycoproteins [8], which play important roles not only in embryo development, morphogenesis, tissue remodeling, but also in tumor growth, metastasis and angiogenesis [9,10]. Loss of the tight control of MMPs activity in the context of tumor microenvironment seems to increase the destruction of ECM, neovascularization, tumor spread and metastasis [11]. Upregulated expression of MMPs has been reported to be associated with tumor aggressiveness and metastatic potential, and can have prognostic significance in several human malignancies [12-14].
Among the most important members of MMPs in particular, MMP-9 (gelatinase B or or type IV collagenase B) is characterized by the potency to degrade Type IV collagen, which is a major component of basement membrane, the first pivotal barrier penetrated by tumor cells when they become invasive [15]. Clinical studies have indicated that high MMP-9 expression is related to lymph node metastasis, tumor differentiation, and other clinicopathological features in a variety of carcinomas [16-18]. Moreover, elevated serum MMP-9 or immunohistochemical MMP-9 expression significantly correlates with poor disease-free and overall survival, and may be of possible prognostic value in some cancers [17-20].
However, to our knowledge limited studies have investigated associations between the MMP-9 expression and clinicopathological factors in patients with hilar cholangiocarcinoma. In this study, we retrospectively studied the expression of MMP-9 expression in hilar cholangiocarcinoma and evaluated the significance of MMP-9 expression with respect to clinicopathological factors and outcomes.
Materials and methods
Patients
A total of 70 patients with histopathological diagnosis as hilar cholangiocarcinoma who underwent curative tumor resection in Qilu Hospital of Shandong University between January 2005 and December 2011 were retrospectively reviewed in the study. Written consent to use stored specimens was obtained from each patient and the study was approved by the Ethics Committee of Qilu Hospital, Shandong University. Patients who died within 90 days of surgery and died from non-tumor reasons were excluded. As a result, 58 patients complied with the criteria were included in the present study. There were 41 males and 17 females, with a median age of 58 years ranging from 36 to 77 years. None of the patients had received adjuvant therapy, such as chemotherapy, radiotherapy prior to or after resection. They were all submitted to the same therapeutic program after resection. There was no evidence of predisposing conditions such as hepatolithiasis and primary sclerosing cholangitis in these cases. Clinical and histopathological characteristics were obtained by a retrospective review of patient records. The median follow-up was 16 months (range 5-98 months). Follow-up began on the date of surgery and ended in January 2013. Regular history and physical examinations were performed in all patients every 3 months during the first 2 years after surgery and every 6 months thereafter. Routine radiological examinations were performed when necessary.
Immunohistochemistry
Tissue samples were obtained from the Department of Pathology, Qilu Hospital of Shandong University. Representative tumor samples, which included the most invasive areas of tumor were embedded in paraffin and sectioned successively into 4 μm thick for staining. Anti-MMP-9 rabbit monoclonal antibody (Epitomics, California, USA) was used for MMP-9 immunohistochemical staining. Immunohistochemical staining was performed with the streptavidin peroxidase complex method. Briefly, slides were deparaffinized and rehydrated with xylene and graded alcohol. Then slides were immersed into citrate buffer (pH 6.0) using the microwave-induced optimal epitope retrieval protocol. Endogenous peroxidase activity was inactivated with 3% hydrogen peroxide for 30 min at room temperature, and then the slides were treated with 5% blocking serum for 30 min at 37°C to block nonspecific reac tions. After that, slides were incubated with anti-MMP-9 (dilution 1:50) antibody overnight at 4°C. After several washes, the slides were incubated with biotinylated goat anti-rabbit immunoglobulin as secondary antibody for 30 min at 37°C. Subsequently, streptavidin peroxidase complex reagents were applied and the desired staining of antibody-specific binding was achieved with 3, 3-diaminobenzidine solution. Finally, the sections were washed briefly with water and counterstained with hematoxylin for 30 s. Control sections were incubated with rabbit nonimmuno IgG.
Evaluation of MMP-9 immunostaining
The slides were routinely examined under a microscope and evaluated separately by two independent observers blinded to patients’ clinical information. Disagreements were resolved simultaneously by observers using a double-headed microscope to make a conclusive judgment. Immunohistochemical staining of MMP-9 for each sample was defined as detectable immunoreactions in cancer cells as described previously [21]. According to the immunoreactive proportion, the case was consid ered as negative expression MMP-9 (-) when no more than 10% of the cells were positive, as low expression MMP-9 (+) when more than 10% to 50% of the cells were positive, and as high expression MMP-9 (++) when more than 50% of the tumor cells showed a positive staining. In addition to cancer cells, most cases also showed MMP-9 expression in the stromal cells with a smaller proportion than that in the malignant epithelial cells. Moreover, all the cases had no significant difference of stromal MMP-9 staining from one another. Therefore, we evaluate the cancerous MMP-9 expression in the following.
Statistical analysis
Statistical analyses were performed with SPSS 18.0 software. Chi-square test and Fisher exact test were used to examine the relationship between the expression of MMP-9 and clinicopathological features. Kaplan-Meier estimates and log-rank tests were used for overall survival analyses. Cox proportional hazards regression model was used to analyze the independent prognostic factors. For all analyses, P < 0.05 was considered statistically significant.
Results
MMP-9 expression in hilar cholangiocarcinoma
MMP-9 expression was prominent in cancer cells and localized in the cytoplasm (Figure 1). In the 58 carcinoma specimens, MMP-9 expression in tumor cells was as follows: MMP-9 (-) expression was seen in 19 patients (32.8%), MMP-9 (+) expression was seen in 12 patients (20.7%), and MMP-9 (++) expression was seen in 27 patients (46.5%).
Figure 1.
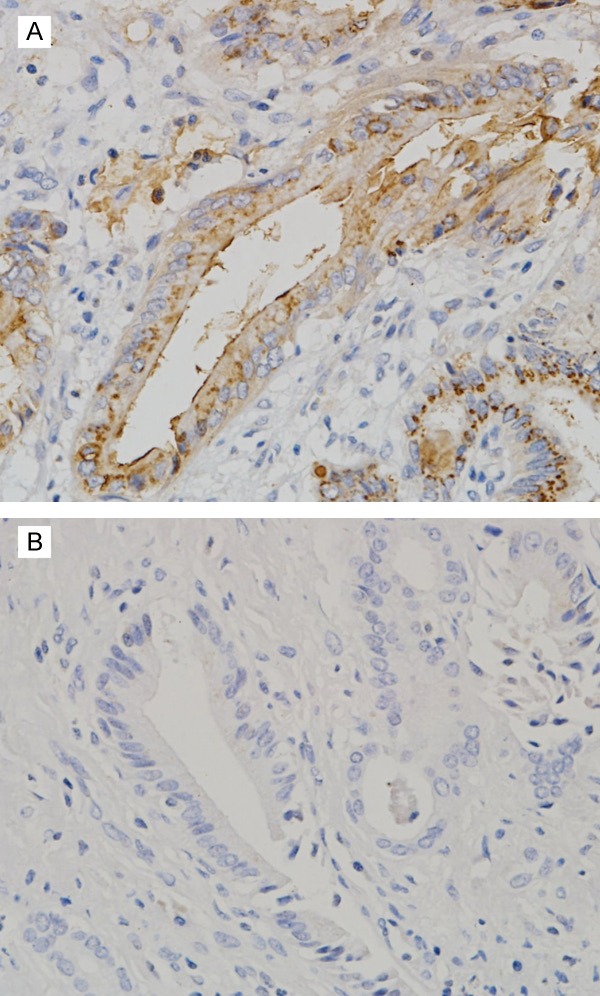
Cytoplasmic immunostaining of MMP-9 in human hilar cholangiocarcinoma (original magnification, × 400). A: Positive MMP-9 expression. B: Negative MMP-9 expression.
Lack of association between high expression of MMP-9 and clinicopathological parameters
No correlation was found between high expression of MMP-9 and the clinicopathological parameters (Table 1). In particular, we could not find any association between the high expression of MMP-9 and tumor size or lymph node metastasis (P = 0.438, P = 0.690 respectively). Additionally, there was no correlation between MMP-9 overexpression and the histological differentiation of the tumor (P = 0.201). Neither was there any correlation between patients’ age or gender and the positive immunoreaction for MMP-9 (P = 0.266, P = 0.228). Among the groups of patients with different Bismuth-Corlette classification, no association was found with MMP-9 expression (P = 0.788).
Table 1.
Relationships between the expression of MMP-9 and clinicopathological features in human hilar cholangiocarcinoma
| Clinicopathological features | n | MMP-9 | P* | |
|---|---|---|---|---|
|
|
||||
| +/- | ++ | |||
| Gender | 0.228 | |||
| Male | 41 | 24 | 17 | |
| Female | 17 | 7 | 10 | |
| Age (years) | 0.266 | |||
| < 60 | 32 | 15 | 17 | |
| ≥ 60 | 26 | 16 | 10 | |
| Differentiation | 0.201 | |||
| Well/moderately | 20 | 13 | 7 | |
| Poorly | 38 | 18 | 20 | |
| Bismuth-Corlette classification | 0.879 | |||
| Type I | 15 | 7 | 8 | |
| Type II | 6 | 3 | 3 | |
| Type IIIa | 9 | 6 | 3 | |
| Type IIIb | 10 | 6 | 4 | |
| Type IV | 18 | 9 | 9 | |
| Tumor size (cm) | 0.438 | |||
| < 3 | 15 | 9 | 6 | |
| 3-5 | 11 | 4 | 7 | |
| >5 | 32 | 18 | 14 | |
| Tumor stage | 0.648 | |||
| T1 | 12 | 7 | 5 | |
| T2 | 19 | 8 | 11 | |
| T3 | 27 | 12 | 15 | |
| Lymph node metastasis | 0.690 | |||
| No | 36 | 23 | 13 | |
| Yes | 22 | 8 | 14 | |
| TNM stage | 0.518 | |||
| I | 9 | 4 | 5 | |
| II | 15 | 9 | 6 | |
| IIIa | 6 | 3 | 3 | |
| IIIb | 7 | 5 | 2 | |
| IVa | 21 | 8 | 13 | |
X2 test.
MMP-9 was an independent prognostic factor for overall survival in hilar cholangiocarcinoma
Kaplan-Meier analysis by log-rank test indicated that patients with MMP-9 (++) expression had a significantly poorer overall survival rate than those with MMP-9 (+/-) expression (Figure 2). In addition to MMP-9 expression, univariate analysis by log-rank test also suggested that histological differentiation and lymph node metastasis were significantly associated with overall patient survival (P = 0.038, Table 2). To further determine the relationship between survival and clinicopathological features, the Cox proportional hazard regression model was performed to establish the independent prognostic factors. Multivariate analysis confirmed MMP-9 as an independent prognostic factor for hilar cholangiocarcinoma (P = 0.007, Table 3), suggesting that high expression of MMP-9 was a high-risk factor for patient survival. In addition to MMP-9, lymph node metastasis (P = 0.003) also showed independent influence on survival in hilar cholangiocarcinoma whereas histological differentiation (P = 0.990) did not.
Figure 2.
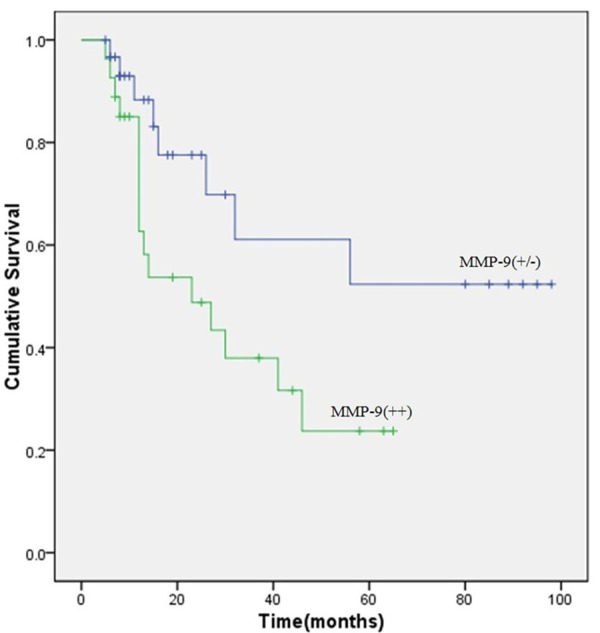
Overall survival curves of patients for hilar cholangiocarcinoma with different MMP-9 expression levels. Patients with high expression of MMP-9 have a significantly poorer survival rate than patients with negative and low MMP-9 expression (P = 0.038).
Table 2.
Univariate analysis of clinicopathological features for overall survival of 58 patients with hilar cholangiocarcinoma
| Characteristics | n | Survival rate (%) | P* |
|---|---|---|---|
| Gender | 0.434 | ||
| Male | 41 | 41.5 | |
| Female | 17 | 41.2 | |
| Age (years) | 0.209 | ||
| <60 | 32 | 43.7 | |
| ≥ 60 | 26 | 38.5 | |
| Differentiation | 0.011 | ||
| Well/moderately | 20 | 50.0 | |
| Poorly | 38 | 36.8 | |
| Bismuth-Corlette classification | 0.788 | ||
| Type I | 15 | 33.3 | |
| Type II | 6 | 50.0 | |
| Type IIIa | 9 | 55.6 | |
| Type IIIb | 10 | 40.0 | |
| Type IV | 18 | 38.9 | |
| Tumor size (cm) | 0.207 | ||
| <3 | 15 | 46.7 | |
| 3-5 | 11 | 54.5 | |
| >5 | 32 | 34.3 | |
| Tumor stage | 0.498 | ||
| T1 | 12 | 58.3 | |
| T2 | 19 | 36.8 | |
| T3 | 27 | 37.0 | |
| Lymph node metastasis | 0.029 | ||
| No | 36 | 47.2 | |
| Yes | 22 | 31.4 | |
| TNM stage | 0.614 | ||
| I | 9 | 44.4 | |
| II | 15 | 46.7 | |
| IIIa | 6 | 33.3 | |
| IIIb | 7 | 28.6 | |
| IVa | 21 | 42.9 | |
| MMP-9 expression | 0.038 | ||
| +/- | 31 | 51.6 | |
| ++ | 27 | 29.6 |
Log-rank test.
Table 3.
Multivariate analysis of clinicopathological features for overall survival of 58 patients with hilar cholangiocarcinoma
| Factors | Category | P | HR | 95% CI |
|---|---|---|---|---|
| MMP-9 expression | +/- | 0.007 | 4.302 | 1.489-12.426 |
| ++ | ||||
| Differentiation | Well/moderately | 0.990 | 0.384 | 0.207-0.715 |
| Poorly | ||||
| Lymph node metastasis | No | 0.003 | 1.008 | 0.302-3.364 |
| Yes |
HR, hazard ratio; CI, confidence interval.
Discussion
MMP-9 is well-known for its involvement in many malignant tumors. Traditionally, MMP-9 has been associated with the aggressive nature of many cancers, due to its potency to degrade type IV collagen as major components of basement membranes. Up to now, the spectrum of MMP-9 matrix substrates has significantly increased, in addition to collagen. MMP-9 has other bioactive substrates that independently modulate carcinogenesis, including the pro-transforming growth factor-β1 (TGF-β1) and the pro-tumor necrosis factor-α (TNF-α) [22,23]. The inactive form of TGF-β1 can be activated by MMP-9. Many other cytokines are also substrates for MMP-9, including CXCL8, and interleukin-1β [24,25]. Some immunohistochemical studies have demonstrated that MMP-9 can have prognostic value in predicting long-term outcome in different types of tumors [18-20]. Furthermore, some studies have also indicated that serum MMP-9 can represent a prognostic marker [17,26].
On the other hand, the role of MMP-9 in progression of neoplasias remains to be fully elucidated. In fact, novel studies have shown that it can act as a protective molecule during carcinogenesis and metastasis. For example, MMP9 expression was decreased in regional metastasis of head and neck carcinoma [27], and MMP-9 expression predicts a better overall survival in salivary gland cancer [28]. What is more, MMP-9 expression is associated with a better outcome in breast and colitis-associated carcinomas [29,30]. Scorilas et al. [31] as well demonstrated that MMP-9 over expression in breast cancer was associated with a favorable prognosis in node-negative patients. These findings suggest that MMP-9 may not be a universal cancer progression promotion factor in cancers; instead, it may have fluctuating roles [32]. It can act as either a carcinoma protector or promoter depending on the specific situation, which is related to patient characteristics.
In our study, we demonstrated that MMP-9 was overexpressed in hilar cholangiocarcinoma. Furthermore, MMP-9 overexpression showed the independent prognostic value of immunohistochemically determined for shortened survival, in addition to that of lymph node metastasis. To the best of our knowledge, limited studies have investigated immunohistochemical expression of MMP-9 in cholangiocarcinoma. Terada T et al. [33] showed increased expression of MMP-9 as well as some other MMPs and TIMPs in intrahepatic cholangiocarcinoma. Ken Shirabe et al. [21] studied 37 patients and found that up-regulated expression of MMP-9 in intrahepatic cholangiocarcinoma was a prognostic factor related to lymph node metastasis. Most of those studies just specially focused on intrahepatic cholangiocarcinoma. Li Y et al. [34] demonstrated MMP-9 expression in hilar cholangiocarcinoma with no prognosis study conducted. Kirimlioğlu H et al. [35] studied just nine Klatskin tumors with MMP-9 expression. Therefore, our study specially addresses the correlation between MMP-9 expression and prognostic analysis in surgically resected hilar cholangiocarcinoma.
Here, the overall survival was found to be strongly dependent on MMP-9 overexpression, which was in line with most MMP-9 studies in other tumor entities. In this work no association was found between the MMP-9 expression level and the traditional histopathological or clinical prognostic variables, such as tumor grade or differentiation. Similarly, a previous study in head and neck squamous cell carcinoma by Henni Ruokolainen et al. [18] either showed no correlation between MMP-9 immunohistochemical staining and the traditional clinicopathological prognostic factors. In the Cox regression analysis, the most significant prognostic factor for survival in the present study was lymph node metastasis. Meanwhile, it was observed that MMP-9 immunohistochemical staining also had prognostic value, independent of lymph node metastasis of the tumors.
Thus, assessment of MMP-9 expression level could help predict the outcome of patients with hilar cholangiocarcinoma and could also be useful for planning adjuvant therapies during follow-up. Furthermore, the specific alteration of the MMPs observed in malignant tissues and their participation in some of the major oncogenic mechanisms have fueled interest in the design and evaluation of MMP inhibitors as anticancer agents. Discovery of MMP-9 as prognostic markers indicates that disturbing the expression of MMP-9 with synthetic MMP inhibitors might be a new target for future cancer therapy. However, it is noteworthy that the dual roles of MMP-9 in tumors are highly context-dependent. The influence of MMP-9 on cancers is to some extent controversial and needs a further study.
There are some limitations in this study because the antibody does not distinguish between the pro- and active forms of MMP-9. The MMP-9 expression does not necessarily mean an active form, which can be misleading. Gelatin zymography or in situ zymography [36] are better methods to evaluate the level of gelatinase activity, which can provide more information. Other techniques like in situ hybridization can define the location and number of cells that express MMP-9 mRNA in tissue sections [37]. Further studies enrolling a larger number of patients with better methodology including serum samples will be required to confirm the results of this study. In summary, the present study suggested that MMP-9 expression, as detected by immunohistochemistry, could be used as a valuable prognostic indicator for human hilar cholangiocarcinoma after curative resection.
Acknowledgements
This work was supported by a research Grant (grant number 81272653) from the National Natural Sciences Foundation of China and research Grants (grant numbers 2012TS133) from The Independent Innovation Foundation of Shandong University.
Disclosure of conflict of interest
None.
References
- 1.Park J, Kim MH, Kim KP, Park do H, Moon SH, Song TJ, Eum J, Lee SS, Seo DW, Lee SK. Natural History and Prognostic Factors of Advanced Cholangiocarcinoma without Surgery, Chemotherapy, or Radiotherapy: A Large-Scale Observational Study. Gut Liver. 2009;3:298–305. doi: 10.5009/gnl.2009.3.4.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soares KC, Kamel I, Cosgrove DP, Herman JM, Pawlik TM. Hilar cholangiocarcinoma: diagnosis, treatment options, and management. Hepatobiliary Surg Nutr. 2014;3:18–34. doi: 10.3978/j.issn.2304-3881.2014.02.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ito F, Agni R, Rettammel RJ, Been MJ, Cho CS, Mahvi DM, Rikkers LF, Weber SM. Resection of hilar cholangiocarcinoma: concomitant liver resection decreases hepatic recurrence. Ann Surg. 2008;248:273–279. doi: 10.1097/SLA.0b013e31817f2bfd. [DOI] [PubMed] [Google Scholar]
- 4.Guglielmi A, Ruzzenente A, Bertuzzo F, Iacono C. Assessment of nodal status for perihilar cholangiocarcinoma location, number, or ratio of involved nodes. Hepatobiliary Surg Nutr. 2013;2:281–283. doi: 10.3978/j.issn.2304-3881.2013.08.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Igami T, Nishio H, Ebata T, Yokoyama Y, Sugawara G, Nimura Y, Nagino M. Surgical treatment of hilar cholangiocarcinoma in the “new era”: the Nagoya University experience. J Hepatobiliary Pancreat Sci. 2010;17:449–454. doi: 10.1007/s00534-009-0209-0. [DOI] [PubMed] [Google Scholar]
- 6.Jarnagin WR, Shoup M. Surgical management of cholangiocarcinoma. Semin Liver Dis. 2004;24:189–199. doi: 10.1055/s-2004-828895. [DOI] [PubMed] [Google Scholar]
- 7.Kleiner DE, Stetler-Stevenson WG. Matrix metalloproteinases and metastasis. Cancer Chemother Pharmacol. 1999;43(Suppl):S42–51. doi: 10.1007/s002800051097. [DOI] [PubMed] [Google Scholar]
- 8.Nagase H, Woessner JF Jr. Matrix metalloproteinases. J Biol Chem. 1999;274:21491–21494. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- 9.John A, Tuszynski G. The role of matrix metalloproteinases in tumor angiogenesis and tumor metastasis. Pathol Oncol Res. 2001;7:14–23. doi: 10.1007/BF03032599. [DOI] [PubMed] [Google Scholar]
- 10.Stetler-Stevenson WG. The role of matrix metalloproteinases in tumor invasion, metastasis, and angiogenesis. Surg Oncol Clin N Am. 2001;10:383–392. x. [PubMed] [Google Scholar]
- 11.Nelson AR, Fingleton B, Rothenberg ML, Matrisian LM. Matrix metalloproteinases: biologic activity and clinical implications. J. Clin. Oncol. 2000;18:1135–1149. doi: 10.1200/JCO.2000.18.5.1135. [DOI] [PubMed] [Google Scholar]
- 12.Miwa S, Miyagawa S, Soeda J, Kawasaki S. Matrix metalloproteinase-7 expression and biologic aggressiveness of cholangiocellular carcinoma. Cancer. 2002;94:428–434. doi: 10.1002/cncr.10235. [DOI] [PubMed] [Google Scholar]
- 13.Yamashita K, Mori M, Shiraishi T, Shibuta K, Sugimachi K. Clinical significance of matrix metalloproteinase-7 expression in esophageal carcinoma. Clin Cancer Res. 2000;6:1169–1174. [PubMed] [Google Scholar]
- 14.Yamamoto H, Itoh F, Iku S, Adachi Y, Fukushima H, Sasaki S, Mukaiya M, Hirata K, Imai K. Expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in human pancreatic adenocarcinomas: clinicopathologic and prognostic significance of matrilysin expression. J. Clin. Oncol. 2001;19:1118–1127. doi: 10.1200/JCO.2001.19.4.1118. [DOI] [PubMed] [Google Scholar]
- 15.Stetler-Stevenson WG. Type IV collagenases in tumor invasion and metastasis. Cancer Metastasis Rev. 1990;9:289–303. doi: 10.1007/BF00049520. [DOI] [PubMed] [Google Scholar]
- 16.Nagakawa Y, Aoki T, Kasuya K, Tsuchida A, Koyanagi Y. Histologic features of venous invasion, expression of vascular endothelial growth factor and matrix metalloproteinase-2 and matrix metalloproteinase-9, and the relation with liver metastasis in pancreatic cancer. Pancreas. 2002;24:169–178. doi: 10.1097/00006676-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Ranuncolo SM, Armanasco E, Cresta C, Bal De Kier Joffe E, Puricelli L. Plasma MMP-9 (92 kDa-MMP) activity is useful in the follow-up and in the assessment of prognosis in breast cancer patients. Int J Cancer. 2003;106:745–751. doi: 10.1002/ijc.11288. [DOI] [PubMed] [Google Scholar]
- 18.Ruokolainen H, Paakko P, Turpeenniemi-Hujanen T. Expression of matrix metalloproteinase-9 in head and neck squamous cell carcinoma: a potential marker for prognosis. Clin Cancer Res. 2004;10:3110–3116. doi: 10.1158/1078-0432.ccr-03-0530. [DOI] [PubMed] [Google Scholar]
- 19.Tanioka Y, Yoshida T, Yagawa T, Saiki Y, Takeo S, Harada T, Okazawa T, Yanai H, Okita K. Matrix metalloproteinase-7 and matrix metalloproteinase-9 are associated with unfavour able prognosis in superficial oesophageal cancer. Br J Cancer. 2003;89:2116–2121. doi: 10.1038/sj.bjc.6601372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sienel W, Hellers J, Morresi-Hauf A, Lichtinghagen R, Mutschler W, Jochum M, Klein C, Passlick B, Pantel K. Prognostic impact of matrix metalloproteinase-9 in operable non-small cell lung cancer. Int J Cancer. 2003;103:647–651. doi: 10.1002/ijc.10841. [DOI] [PubMed] [Google Scholar]
- 21.Shirabe K, Shimada M, Kajiyama K, Hasegawa H, Gion T, Ikeda Y, Takenaka K, Sugimachi K. Expression of matrix metalloproteinase-9 in surgically resected intrahepatic cholangiocarcinoma. Surgery. 1999;126:842–846. [PubMed] [Google Scholar]
- 22.Bjorklund M, Koivunen E. Gelatinase-mediated migration and invasion of cancer cells. Biochim Biophys Acta. 2005;1755:37–69. doi: 10.1016/j.bbcan.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 23.Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, Hanahan D. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2:737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van den Steen PE, Proost P, Wuyts A, Van Damme J, Opdenakker G. Neutrophil gelatinase B potentiates interleukin-8 tenfold by aminoterminal processing, whereas it degrades CTAP-III, PF-4, and GRO-alpha and leaves RANTES and MCP-2 intact. Blood. 2000;96:2673–2681. [PubMed] [Google Scholar]
- 25.Ito A, Mukaiyama A, Itoh Y, Nagase H, Thogersen IB, Enghild JJ, Sasaguri Y, Mori Y. Degradation of interleukin 1beta by matrix metalloproteinases. J Biol Chem. 1996;271:14657–14660. doi: 10.1074/jbc.271.25.14657. [DOI] [PubMed] [Google Scholar]
- 26.Ylisirnio S, Hoyhtya M, Turpeenniemi-Hujanen T. Serum matrix metalloproteinases-2, -9 and tissue inhibitors of metalloproteinases-1, -2 in lung cancer: TIMP-1 as a prognostic marker. Anticancer Res. 2000;20:1311–1316. [PubMed] [Google Scholar]
- 27.Stokes A, Joutsa J, Ala-Aho R, Pitchers M, Pennington CJ, Martin C, Premachandra DJ, Okada Y, Peltonen J, Grenman R, James HA, Edwards DR, Kahari VM. Expression profiles and clinical correlations of degradome components in the tumor microenvironment of head and neck squamous cell carcinoma. Clin Cancer Res. 2010;16:2022–2035. doi: 10.1158/1078-0432.CCR-09-2525. [DOI] [PubMed] [Google Scholar]
- 28.Luukkaa H, Klemi P, Leivo I, Makitie AA, Irish J, Gilbert R, Perez-Ordonez B, Hirsimaki P, Vahlberg T, Kivisaari A, Kahari VM, Grenman R. Expression of matrix metalloproteinase-1, -7, -9, -13, Ki-67, and HER-2 in epithelial-myoepithelial salivary gland cancer. Head Neck. 2010;32:1019–1027. doi: 10.1002/hed.21277. [DOI] [PubMed] [Google Scholar]
- 29.Bendrik C, Robertson J, Gauldie J, Dabrosin C. Gene transfer of matrix metalloproteinase-9 induces tumor regression of breast cancer in vivo. Cancer Res. 2008;68:3405–3412. doi: 10.1158/0008-5472.CAN-08-0295. [DOI] [PubMed] [Google Scholar]
- 30.Garg P, Sarma D, Jeppsson S, Patel NR, Gewirtz AT, Merlin D, Sitaraman SV. Matrix metalloproteinase-9 functions as a tumor suppressor in colitis-associated cancer. Cancer Res. 2010;70:792–801. doi: 10.1158/0008-5472.CAN-09-3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scorilas A, Karameris A, Arnogiannaki N, Ardavanis A, Bassilopoulos P, Trangas T, Talieri M. Overexpression of matrix-metalloproteinase-9 in human breast cancer: a potential favourable indicator in node-negative patients. Br J Cancer. 2001;84:1488–1496. doi: 10.1054/bjoc.2001.1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vilen ST, Salo T, Sorsa T, Nyberg P. Fluctuating roles of matrix metalloproteinase-9 in oral squamous cell carcinoma. ScientificWorldJournal. 2013;2013:920595. doi: 10.1155/2013/920595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Terada T, Okada Y, Nakanuma Y. Expression of immunoreactive matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases in human normal livers and primary liver tumors. Hepatology. 1996;23:1341–1344. doi: 10.1053/jhep.1996.v23.pm0008675149. [DOI] [PubMed] [Google Scholar]
- 34.Li Y, Zhang Y, Zheng Q. Expression of RECK gene and MMP-9 in hilar cholangiocarcinoma and its clinical significance. J Huazhong Univ Sci Technolog Med Sci. 2005;25:552–554. doi: 10.1007/BF02896015. [DOI] [PubMed] [Google Scholar]
- 35.Kirimlioglu H, Turkmen I, Bassullu N, Dirican A, Karadag N, Kirimlioglu V. The expression of matrix metalloproteinases in intrahepatic cholangiocarcinoma, hilar (Klatskin tumor), middle and distal extrahepatic cholangiocarcinoma, gallbladder cancer, and ampullary carcinoma: role of matrix metalloproteinases in tumor progression and prognosis. Turk J Gastroenterol. 2009;20:41–47. [PubMed] [Google Scholar]
- 36.Snoek-van Beurden PA, Von den Hoff JW. Zymographic techniques for the analysis of matrix metalloproteinases and their inhibitors. Biotechniques. 2005;38:73–83. doi: 10.2144/05381RV01. [DOI] [PubMed] [Google Scholar]
- 37.Singh RD, Haridas N, Patel JB, Shah FD, Shukla SN, Shah PM, Patel PS. Matrix metalloproteinases and their inhibitors: correlation with invasion and metastasis in oral cancer. Indian J Clin Biochem. 2010;25:250–259. doi: 10.1007/s12291-010-0060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


