Abstract
The study was to investigate the effects of oxygen concentration at different levels for culturing pre-compaction embryos on human embryo development competence. A total of 1254 oocytes from 92 patients treated with conventional in vitro fertilization (IVF) were harvested in this study. Oocytes were randomly assigned to the atmospheric (~20%) or low (~5%) oxygen concentration groups on the retrieval day (day 0). Groups were compared with respect to fertilization rates, embryo development, and reproductive outcome. We failed to detect a significant difference on fertilization rate between two groups. However, the low oxygen group yielded more optimal embryos on day 3 when compared with the atmospheric group (72.4% vs. 64.2%). The low oxygen group had a significantly higher blastocyst formation rate than the atmospheric oxygen group (64.5% vs. 52.9%). It is seemly that the optimal blastocyst and frozen blastocyst rates was higher in the low oxygen group, but the data did not reach a statistical significance. Although the use of low oxygen will not affect the clinical outcome in the fresh cleavage-transfer cycles, but it will result in more favorable clinical outcomes in the subsequent warming blastocyst-transfer cycles, with statistically significantly higher clinical pregnancy rate (CPR) and implantation rate (IR) compared with atmospheric oxygen. In conclusion, a low oxygen concentration may significantly improve the developmental potential of pre-compaction embryos, thus resulting in a positive effect on subsequent blastocyst cultivation and optimizing the treatment cycle.
Keywords: Oxygen concentration, atmospheric oxygen, embryo development, sibling oocytes
Introduction
Although remarkable progress has been made since the establishment of human assisted reproductive technology (ART), a major challenge facing in vitro fertilization (IVF) clinics around the world is to maintain acceptable pregnancy rates. In addition to precise clinical protocols, Embryo culture conditions, such as oxygen concentration, pH, and temperature, are thought to influence the developmental competence of embryos [1].
Based on animal models, it has been demonstrated that embryos in vivo are exposed to an oxygen concentration of 2%-8% [2]. Embryos encounter a decreasing oxygen concentration gradient during the transit between the fallopian tube and uterine cavity [3]. Thus, embryo cultivation that mimics particular in vivo reproduction conditions, such as oxygen concentration, should be more beneficial for developing embryos with reproductive competence. However, in traditional IVF, embryos are exposed to a gas phase of atmospheric (~20%) oxygen, a protocol derived from earlier somatic tissue techniques. It was not until the middle of the 1990s, with the advances in blastocyst cultivation technology, as well as the increased risk of multiple pregnancies, low oxygen concentration has become the focus of attention [4].
The pre- and post-compaction stage embryos differed significantly in many respects. Pre-compaction embryos have limited mechanisms for maintaining metabolic homeostasis, and are therefore extremely sensitive to conditions that induce oxidative stress. Post-compaction embryos have complex systems and their regulation ability could be enhanced [5]. Numerous studies with animals conducted over 30 years or more have demonstrated that low oxygen concentration has more superiority for blastocyst cultivation than atmospheric oxygen at the post-compaction stage [6-8]. It remains unclear whether or not a low oxygen concentration is beneficial or needed for embryos at the pre-compaction stage.
As early as 1999, Dumoulin et al. compared the effect of oxygen on human cleavage stage embryos development and found no differences in clinical outcomes [9]. Recently, a preliminary study in a mouse model demonstrated that oxygen had detrimental effect on embryo development with the greatest effect during the cleavage stages, reflecting a greater sensitivity of embryos to oxygen toxicity prior to compaction [10]. It remains to be determined whether or not the effect of the oxygen concentration is stage-specific.
Although human embryo development shares many features with other species, there are still some notable differences. There are also individual differences between different populations. Considering these deficiencies, a sibling-oocyte model was established to minimize potential variations between different species and people that may obscure the impact of different oxygen concentrations on human embryonic development.
Thus, the primary end-point of this prospective randomized trial on sibling oocytes was to evaluate the effect of atmospheric (~20%) and low (~5%) oxygen concentration in the gas mixture for culturing pre-compaction embryos on human embryo development competence to determine the optimal oxygen concentration for human embryo culture.
Materials and methods
Subjects and study design
This prospective randomized trial was approved by the Institutional Review Board of Tongji Hospital in Wuhan, China. During a period of 6 months, 92 patients with conventional IVF treatment at the Reproductive medicine center of Tongji Hospital, Huazhong University of Science and technology, Wuhan, China, from April 2013 to October 2013, were recruited in this study. All women were younger than 35 years and for whom 8-20 oocytes were retrieved. They had normal basal endocrine examinations and morphologically normal oocytes. None had a history of hydrosalpinx. Male partners had normal semen quality according to World Health Organization (WHO) criteria. A total of 1254 oocytes from 92 couples undergoing conventional IVF treatment were randomly assigned to the atmospheric (~20%) or low (~5%) oxygen concentration groups on the retrieval day (day 0). Our study was conducted by two experienced embryologists who were responsible for the randomization, morphological assessment and selection of the embryos, in order to avoid subject differences during the experiments.
Randomized methods
All patients used double lumen oocyte harvesting needles for follicles puncture. Each follicle was individually punctured and each oocyte was cultured in one droplet. The first oocyte of the patient was assigned to the atmospheric (~20%) or low (~5%) oxygen concentration groups, and then the second oocyte of the patient was assigned to the low (~5%) or atmospheric (~20%) oxygen concentration groups. So the cycle repeated, all oocytes of the patients in turn divided into atmospheric (~20%) and low (~5%) oxygen concentration groups.
Ovarian stimulation and sperm preparation
The gonadotropin-releasing hormone (GnRH) agonist (Decapeptyl, Ferring, Kiel; Germany; Diphereline Ipsen, Paris, France) was used from midluteal phase of the preceding cycle in a standard long ovarian stimulation protocol. Ovarian stimulation was carried out with 150-300IU recombinant follicle stimulation hormone (FSH) (Gonal-F, Serono, Aubonne, Switzerland) from 3-6 days after menstrual cycle onset until oocyte retrieval. We would adjust the dosage of FSH according to the vaginal ultrasound combined with serum hormone level. A single 10,000IU dose of Human chorionic gonadotropin (HCG) was administratedwhen the leading follicle reached a mean diameter of 17-18 mm, after 34-36 h, oocytes were retrieved though ultrasound-guided transvaginal puncture.
All semen samples were collected following masturbation after 3-7 days of sexual abstinence and were allowed to liquefy for at least 30 min at 37°C before the swim-up purification processing. Sperm concentration, volume, motility and morphology were analyzed according to the World Health Organization (WHO) criteria. The oocytes were incubated with 50,000-100,000 forward motile sperm 4-6 h after the oocytes retrieved.
Culture procedures
In conventional IVF cycles, the oocytes were incubated with motile sperm 2-3 hours after retrieved. The corona cells were removed to assess fertilization. Oocytes from each patients were randomly divided into the atmospheric (~20%) or low (~5%) oxygen concentration cultivation groups on the retrieval day (day 0).Two models of incubators were used for embryo cultivation. For culture at atmospheric (~20%) oxygen concentration, a Labotect C200 incubator (Germany) was used. For culture at low (~5%) oxygen concentration, a K-Minc 1000 incubator (Australia) was used. The doors were infrequently opened so that the conditions within the incubator remained constant for as long as possible. The gas concentrations were measured each day before the incubator doors were opened using an IR sensor (Labotect InControl, 1050, Germany). Oocytes and embryos of both groups were cultures individually in drops of G1 medium (Vitrolife, Gotheburg, Sweden) (until day 3) under sterile mineral oil. The embryologist was blinded to the comaparitive assessment of the embryos correct. Usually the two best-quality embryos were selected for transfer on day 3, with surplus embryos being extensively cultured to the blastocyst stage for possible cryopreservation. The morphological assessment of blastocyst quality were conducted on day 5 or 6 according to Gardner’score system [11].
Assessment of observation indicators
Fertilization was confirmed by the observation of two pronuclei (PN) 16-18 h after conventional IVF. An optimal embryo on day 3 was which with at least six blastomeres, a degree of fragmentation less than 10% and equal sized blastomeres. According to Gardner’ scoring system, a optimal blastocyst was defined that with a blastocoel fully filling out the embryo, a tightly packed inner cell mass and a trophectoderm with many cells forming a cohesive epithelium on day 5 or day 6. Embryos reaching the blastocyst stage (minimum expansion: early blastocyst; inner cell mass and trophectoderm layer: score A or B) were cryopreserved on day 5 or day 6. A rising HCG concentration indicated the establishment of pregnancy. Clinical pregnancy was defined as the presence of a gestational sac with fetal heartbeat on untrasound screening. Implantation rate was defined as the number of gestational sacs observed, divided by the number of embryos transferred.
Statistics analysis
Continuous data were presented as mean ± SD and as proportions if they were ordinal. The data were analyzed by chi-squared test with SPSS 10.0 (SPSS , Chicago, 1 L), and a P value < 0.05 was considered statistically significant.
Results
A total of 1254 oocytes from 92 conventional IVF patients were randomly assigned to the atmospheric (~20%) or low (~5%) oxygen concentration groups on the retrieval day (day 0), in which 626 (49.9%) oocytes of atmospheric and 628 (51%) of low oxygen concentration groups. Demographic data of patients were shown in Table 1. The mean age of the patient was 29.63 ± 3.62 year, and the mean retrieved oocyte number was 13.99 ± 3.72. The 92 patients consisted of 67 cases of tubal infertility, 4 of endometriosis, 7 of polycystic ovary syndrome (PCOS), 10 of mild male factor infertility and 4 of unexplained infertility.
Table 1.
Demographic data of patients included in the study is shown
| Patients included (n) | 92 |
|---|---|
| Female age, mean ± SD (year) | 29.63 ± 3.623 |
| Infertility diagnosis, n (%) | |
| Tubal factor | 67 (72.8) |
| Endometriosis | 4 (4.3) |
| Polycystic ovary syndrome (PCOS) | 7 (7.6) |
| Unexplained | 4 (4.3) |
| Male factor | 10 (10.9) |
| No. of oocytes retrieved per patient, mean ± SD | 13.99 ± 3.719 |
No significant difference was found in the normal fertilization rate (59.6% vs. 58.0%) and abnormal fertilization rate (14.53% vs. 15.60%) of the two different oxygen concentration on day 1 (Table 2). No significant difference was observed in the number of blastomeres on day 2 (Table 3). However, embryos of low oxygen group on day 3 had higher percentage of 8 blastomeres (39.84% vs. 32.44%, P= 0.037) and lower percentage of less than 5 blastomeres (18.51% vs. 26.01%, P= 0.013) compared to atmospheric oxygen group. In addition, high quality embryo rate in low oxygen group was significantly higher than atmospheric oxygen group (72.4% vs. 64.2%, P= 0.018) (Table 4). When the surplus embryos were cultivated on day 5 or 6, we found that the low oxygen group had a significantly higher blastocyst formation rate than the atmospheric oxygen group (64.5% vs. 52.9%, P= 0.009). Moreover, the optimal blastocyst (40.3% vs. 37.4%) and frozen blastocyst rates (61.7% vs. 56.1%) had a favorable trend, although no statistically significant differences exited in the low oxygen group compared with the atmospheric group (Figure 1).
Table 2.
Comparison of fertilization characteristics between different oxygen concentration groups
| 20% O2 | 5% O2 | P-value | |
|---|---|---|---|
| Oocyte obtained per cycle (n) | 626 | 628 | NS |
| Metaphase II oocytes, n (%) | 568 (90.7) | 569 (90.6) | NS |
| Normal fertilization (2PN), n (%) | 373 (59.6) | 364 (58.0) | NS |
| Polyspermy rates (> 2PN), n (%) | 91 (14.53) | 98 (15.60) | NS |
NS = not statistically significant.
Table 3.
Comparison of human embryo development competence on the second day (day 2) after fertilization between different oxygen concentration groups
| 20% O2 | 5% O2 | P-value | |
|---|---|---|---|
| ≤ 2 cell | 33 (8.85) | 40 (10.99) | NS |
| 3 cell | 39 (10.46) | 25 (6.87) | NS |
| 4 cell | 225 (60.32) | 227 (62.36) | NS |
| ≥ 5 cell | 76 (20.38) | 71 (19.51) | NS |
NS = not statistically significant.
Table 4.
Comparison of human embryo development competence on the third day (day 3) after fertilization between different oxygen concentration groups
| 20% O2 | 5% O2 | P-value | |
|---|---|---|---|
| ≤ 5 cell | 97 (26.01) | 67 (18.41) | 0.013* |
| 6 cell | 53 (14.21) | 43 (11.81) | NS |
| 7 cell | 59 (15.82) | 59 (16.21) | NS |
| 8 cell | 121 (32.44) | 145 (39.84) | 0.037* |
| 9 cell | 25 (6.7) | 24 (6.59) | NS |
| ≥ 10 cell | 18 (4.83) | 26 (7.41) | NS |
| Optimal day 3 embryoa | 239 (64.2) | 263 (72.4) | 0.018* |
NS = not statistically significant;
P< 0.05 as compared with 20% oxygen concentration group;
Optimal day 3 embryo: 6-cell and above, < 10% fragmentation, equal sized blastomeres.
Figure 1.
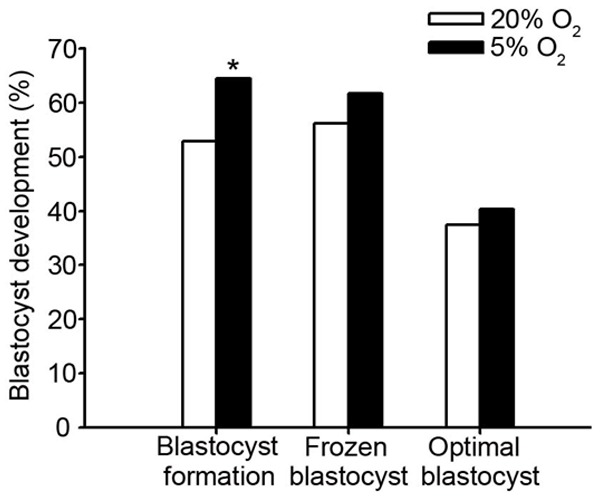
Comparison of human blastocyst development competence between different oxygen concentration groups. * = Statistically significant from the control (P < 0.05).
The clinical outcomes of fresh and warming cycles after transfer of embryos chosen only derived from 20% O2 or only from 5% O2 group were presents in Table 5. No significant differences were found between the two groups with respect to their fresh cycle characteristics such as clinical pregnancy rate (CPR) and implantation rate (IR). However, in the subsequent warming blastocyst-transfer (BT) cycles of our study, the improved CPR and IR were seen with the low oxygen group.
Table 5.
Clinical outcomes of fresh and warming cycles after transfer of embryos chosen only derived from 20% O2 or only from 5% O2 group
| Only from 20% O2 | Only from 5% O2 | P-value | |
|---|---|---|---|
| Fresh cycles: clinical outcome | - | ||
| No. of ET | 30 | 43 | - |
| Transferred cleavage embryos | 60 | 86 | |
| Clinical pregnancy rate per ET | 15/30 (50.0) | 19/43 (54.2) | NS |
| Implantation rate | 20/60 (33.3) | 24/86 (27.9) | NS |
| Warming cycles: clinical outcome | |||
| No. of ET | 19 | 22 | - |
| Transferred blastocysts | 37 | 40 | - |
| Clinical pregnancy rate per ET | 5/19 (26.3) | 14/22 (63.6) | 0.017a |
| Implantation rate | 9/37 (24.3) | 23/40 (57.5) | 0.003a |
Data are expressed as absolute and percentage frequency. ET: Embryo transfer.
P < 0.05 as compared with 20% oxygen concentration group.
Discussion
The present study confirms that a low oxygen concentration may signifi cantly improve the developmental potential of cleavage stage embryos, thus resulting in a positive effect on subsequent blastocyst cultivation. The use of low oxygen will not affect the clinical outcome in the fresh cleavage-transfer cycles, but it will result in more favorable clinical outcomes in the subsequent warming blastocyst-transfer cycles, with statistically significantly higher CPR and IR compared with atmospheric oxygen, indicating that the detrimental effect of atmospheric oxygen on human pre-compaction embryos are cumulative and would manifest at the later stages of preimplantation development. It is reasonable for IVF clinics to use low oxygen concentration from embryo cul ture beginning, and it will optimize the patients’ treatment outcome in a long-term perspective.
Suboptimal in vitro culture conditions, specifically exposure to high oxygen concentrations have been associated with increased reactive oxygen species (ROS) generation and compromised developmental ability. A low oxygen concentration (~5%) had a favorable effect on embryo quality by reducing the ROS levels produced in high oxygen cultures. The ROS may block or retard early embryonic development by affecting key cellular organelles required for rapid cell division [3,12]. Methods such as antioxidants, lower oxygen concentration could be effective to regulate intra-and extra-embryonic environments, ensuring minimal oxidative damage to embryos [13].
Wale et al. clearly showed that the zygote stage is most sensitive to oxygen toxicity. Exposure of mouse embryos to atmospheric oxygen results in irreversible damage, such as a delay in cleavage divisions [10]. Bedaiwy reported that high ROS levels on day 1 in the culture media are associated with a low fertilization rate, low cleavage rate, and high embryonic fragmentation during ICSI cycles [14]. Our prospective non-blinded trial involving sibling oocytes showed no significant difference in the fertilization and cleavage rates in the first two cleavage divisions. This finding can be explained by the potential antioxidant activity of the cumulus cell mass and normal spermatozoa present inconventional IVF cycles. Additionally, the Vitrolife G-Series™ media with its built-in VitROShield help protect embryos during culture and handling in the laboratory. VitROShield is the combination of hyaluronan, gentamicin and the antioxidant lipoate in the culture system and protects from pH induced stress, free oxygen radicals, infections and cryo damage. Antioxidant activity neutralizes the ROS and negates the effect on fertilization and cleavage rates in the first two cleavage divisions.
During preimplantation period, several important events occur, including 1. first cleavage cleavage division; 2. the activation of embryonic genome; 3. compaction of the morula; 4) blastocyst formation [15]. As development proceeds, maternally-inherited molecules decay, accompanied by gradual activation of the embryonic genome, which is responsible for the comprehensive regulation of embryonic development. Activation of the human embryonicgenome is reportedly initiated at the 4-8 cell stage [16], and high transcriptional activity in the embryo is detected from the 8-cell stage, or even later at the 16-cell stage [5]. Thus, this could be a possible explanation that the detrimental effects of atmospheric oxygen on embryo development were observed obviously on day 3 or blastocyst stage. Our results may coincide with the findings of Rinaudo et al. [17], who showed certain beneficial effects for faster embryo development and increased cell number if cultured at a low oxygen concentration. Dumoulin et al. [9] compared the effect of oxygen on human cleavage stage embryos development and found no differences in morphometric parameters. However, their conclusions were drawn from different populations and the parameters comparisons were not evaluated in a specific patient cycle.
Culturing embryos in low oxygen concentration improves embryo utilization rate and increases the chance of pregnancy. Kovacic et al. [8] reported low oxygen is recommended for poor responder patients with more optimal embryos to select (62.5%. in 5 %O2 vs. 44.7% in 20% O2). In the present study, low oxygen cultivation had more high quality cleavage-embryos and blastocysts available for cryopreservation, providing patients with more opportunities for achieving pregnancy. de los Santos et al. [18] in an ovum donation cycles found that reduced oxygen tension improved embryo quality but not clinical pregnancy rates in a cleavage-transfer program. Our observation may be similar to Maria’s. However, in the subsequent warming blastocyst-transfer (BT) cycles of our study, we noted that the low oxygen group resulted in more favorable clinical outcomes. Supernumerary optimal embryos, these are cryopreserved and transferred in subsequent cycles can improve the overall pregnancy rates. Meintjes et al. [19] also observed that human embryos cultured in 5% oxygen consistently yield better clinical results in a fresh blastocyst-transfer (BT) program. In china, it is uncommon to perform fresh BT cycles for each patient and all available fresh blastocysts would be cryopreserved for transfer in a subsequent cycle. No matter what protocol adopt, low oxygen cultivation indeed optimize patients’ treatment outcomes to some extent.
The blastocysts derived from high oxygen cultivation may be accompanied by compromised development ability, which may be related to the fact that alterations derived from the maternal genome during the pre-compaction stage at different oxygen concentrations may have a far-reaching influence on the embryonic genome, resulting in differences in subsequent embryo health and developmental potential. Lonergan et al. [20] pointed out that changes in transcript abundance at the blastocyst stage in the bovine embryos were in many cases a consequence of perturbed transcription earlier in development, reflecting the sensitivity of embryos to suboptimal culture conditions at the cleavage stage in vitro.
Oxygen at a concentration of 20% has been shown to give rise to the epigenetics modifications. Embryos cultured in a high oxygen concentration had fewer cells, perturbed cell signaling and abnormal energy metabolism, indicating a cultured environment-induced differential expression pattern [21]. Rinaudo and Schultz [17] compared global patterns of gene expression in preimplantation mouse embryos derived from culture in 20% oxygen with that of 5% oxygen using the Affymetrix MOE430 chip. The gene expression profile of embryos cultured in 5% oxygen more closely approximated that of embryos that developed in vivo when compared with those that developed in 20% oxygen concentration. Gardner [22] reported that nineteen genes were upregulated in the 20% O2 group, while twelve genes were downregulated compared to the 5% O2 group. Elamaran [23] used a quantitative RT-PCR assay to analyze the effects of O2 concentration (5% vs. 20%) on the relative abundance of gene transcripts in buffalo embryos produced in vitro and noted an increased expression of anti-apoptotic genes BCL-2 and MCL-1 and a decrease in the expression of pro-apoptotic genes BAX and BID in pre-implantation stage if culturing under 5% oxygen concentration.
Currently the value of low oxygen concentrations in human IVF laboratories is a continuing question and the effect of low oxygen concentration on human embryo development competence are controversial. In some cases, the benefits reflected in embryo quality and clinical outcome [24], in others improvements were found exclusively for pregnancy or implantation outcomes, but embryo quality was not affected [19], and in others, improvements in embryo quality [18,25]. These inconsistencies are mostly due to inter-laboratory differences, but also in intra-laboratory variation, including culture media composition, stimulation regimes and laboratory protocols.
This is the first report involving the impact of oxygen concentration on human pre-compaction embryos using a prospective randomized sibling-oocyte study model in a Chinese population. A low oxygen concentration significantly improves the developmental potential of pre-compaction stage embryos, resulting in a positive effect on subsequent blastocyst cultivation. Low oxygen cultivation had more embryos available for cryopreservation, providing patients with more opportunities for achieving pregnancy in subsequent cycles, and thus optimizing the treatment outcomes. However, as a prospective study, the sample size of this study was relatively small, and further studies are needed.
Disclosure of conflict of interest
None.
References
- 1.Gardner DK, Lane M, Stevens J, Schoolcraft WB. Noninvasive assessment of human embryo nutrient consumption as a measure of developmental potential. Fertil Steril. 2001;76:1175–1180. doi: 10.1016/s0015-0282(01)02888-6. [DOI] [PubMed] [Google Scholar]
- 2.Catt JW, Henman M. Toxic effects of oxygen on human embryo development. Hum Reprod. 2000;15(Suppl 2):199–206. doi: 10.1093/humrep/15.suppl_2.199. [DOI] [PubMed] [Google Scholar]
- 3.Kovacic B. Culture systems: low-oxygen culture. Methods Mol Biol. 2012;912:249–272. doi: 10.1007/978-1-61779-971-6_15. [DOI] [PubMed] [Google Scholar]
- 4.Higdon HL 3rd, Blackhurst DW, Boone WR. Incubator management in an assisted reproductive technology laboratory. Fertil Steril. 2008;89:703–710. doi: 10.1016/j.fertnstert.2007.03.040. [DOI] [PubMed] [Google Scholar]
- 5.Lane M, Gardner DK. Embryo culture medium: which is the best? Best Pract Res Clin Obstet Gynaecol. 2007;21:83–100. doi: 10.1016/j.bpobgyn.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Umaoka Y, Noda Y, Narimoto K, Mori T. Effects of oxygen toxicity on early development of mouse embryos. Mol Reprod Dev. 1992;31:28–33. doi: 10.1002/mrd.1080310106. [DOI] [PubMed] [Google Scholar]
- 7.Booth PJ, Holm P, Callesen H. The effect of oxygen tension on porcine embryonic development is dependent on embryo type. Theriogenology. 2005;63:2040–2052. doi: 10.1016/j.theriogenology.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Kovacic B, Sajko MC, Vlaisavljevic V. A prospective, randomized trial on the effect of atmospheric versus reduced oxygen concentration on the outcome of intracytoplasmic sperm injection cycles. Fertil Steril. 2010;94:511–519. doi: 10.1016/j.fertnstert.2009.03.077. [DOI] [PubMed] [Google Scholar]
- 9.Dumoulin JC, Meijers CJ, Bras M, Coonen E, Geraedts JP, Evers JL. Effect of oxygen concentration on human in-vitro fertilization and embryo culture. Hum Reprod. 1999;14:465–469. doi: 10.1093/humrep/14.2.465. [DOI] [PubMed] [Google Scholar]
- 10.Wale PL, Gardner DK. Time-lapse analysis of mouse embryo development in oxygen gradients. Reprod Biomed Online. 2010;21:402–410. doi: 10.1016/j.rbmo.2010.04.028. [DOI] [PubMed] [Google Scholar]
- 11.Gardner DK, Lane M, Stevens J, Schlenker T, Schoolcraft WB. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril. 2000;73:1155–1158. doi: 10.1016/s0015-0282(00)00518-5. [DOI] [PubMed] [Google Scholar]
- 12.Bavister B. Oxygen concentration and preimplantation development. Reprod Biomed Online. 2004;9:484–486. doi: 10.1016/s1472-6483(10)61630-6. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi M. Oxidative stress and redox regulation on in vitro development of mammalian embryos. J Reprod Dev. 2012;58:1–9. doi: 10.1262/jrd.11-138n. [DOI] [PubMed] [Google Scholar]
- 14.Bedaiwy MA, Falcone T, Mohamed MS, Aleem AA, Sharma RK, Worley SE, Thornton J, Agarwal A. Differential growth of human embryos in vitro: role of reactive oxygen species. Fertil Steril. 2004;82:593–600. doi: 10.1016/j.fertnstert.2004.02.121. [DOI] [PubMed] [Google Scholar]
- 15.Lonergan P, Fair T, Corcoran D, Evans AC. Effect of culture environment on gene expression and developmental characteristics in IVF-derived embryos. Theriogenology. 2006;65:137–152. doi: 10.1016/j.theriogenology.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 16.Galan A, Montaner D, Poo ME, Valbuena D, Ruiz V, Aguilar C, Dopazo J, Simon C. Functional genomics of 5- to 8-cell stage human embryos by blastomere single-cell cDNA analysis. PLoS One. 2010;5:e13615. doi: 10.1371/journal.pone.0013615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rinaudo PF, Giritharan G, Talbi S, Dobson AT, Schultz RM. Effects of oxygen tension on gene expression in preimplantation mouse embryos. Fertil Steril. 2006;86:1252–1265. 1265.e1251–1236. doi: 10.1016/j.fertnstert.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 18.de los Santos MJ, Gamiz P, Albert C, Galan A, Viloria T, Perez S, Romero JL, Remohi J. Reduced oxygen tension improves embryo quality but not clinical pregnancy rates: a randomized clinical study into ovum donation cycles. Fertil Steril. 2013;100:402–407. doi: 10.1016/j.fertnstert.2013.03.044. [DOI] [PubMed] [Google Scholar]
- 19.Meintjes M, Chantilis SJ, Douglas JD, Rodriguez AJ, Guerami AR, Bookout DM, Barnett BD, Madden JD. A controlled randomized trial evaluating the effect of lowered incubator oxygen tension on live births in a predominantly blastocyst transfer program. Hum Reprod. 2009;24:300–307. doi: 10.1093/humrep/den368. [DOI] [PubMed] [Google Scholar]
- 20.Lonergan P, Rizos D, Gutierrez-Adan A, Moreira PM, Pintado B, de la Fuente J, Boland MP. Temporal divergence in the pattern of messen ger RNA expression in bovine embryos cultured from the zygote to blastocyst stage in vitro or in vivo. Biol Reprod. 2003;69:1424–1431. doi: 10.1095/biolreprod.103.018168. [DOI] [PubMed] [Google Scholar]
- 21.Wale PL, Gardner DK. Oxygen regulates amino acid turnover and carbohydrate uptake during the preimplantation period of mouse embryo development. Biol Reprod. 2012;87:24, 21–28. doi: 10.1095/biolreprod.112.100552. [DOI] [PubMed] [Google Scholar]
- 22.Gardner DK, Lane M. Ex vivo early embryo development and effects on gene expression and imprinting. Reprod Fertil Dev. 2005;17:361–370. doi: 10.1071/rd04103. [DOI] [PubMed] [Google Scholar]
- 23.Elamaran G, Singh KP, Singh MK, Singla SK, Chauhan MS, Manik RS, Palta P. Oxygen concentration and cysteamine supplementation during in vitro production of buffalo (Bubalus bubalis) embryos affect mRNA expression of BCL-2, BCL-XL, MCL-1, BAX and BID. Reprod Domest Anim. 2012;47:1027–1036. doi: 10.1111/j.1439-0531.2012.02009.x. [DOI] [PubMed] [Google Scholar]
- 24.Waldenstrom U, Engstrom AB, Hellberg D, Nilsson S. Low-oxygen compared with high-oxygen atmosphere in blastocyst culture, a prospective randomized study. Fertil Steril. 2009;91:2461–2465. doi: 10.1016/j.fertnstert.2008.03.051. [DOI] [PubMed] [Google Scholar]
- 25.Ciray HN, Aksoy T, Yaramanci K, Karayaka I, Bahceci M. In vitro culture under physiologic oxygen concentration improves blastocyst yield and quality: a prospective randomized survey on sibling oocytes. Fertil Steril. 2009;91:1459–1461. doi: 10.1016/j.fertnstert.2008.07.1707. [DOI] [PubMed] [Google Scholar]


