Abstract
Introduction: MicroRNAs (miRNAs) are noncoding RNAs that regulate multiple cellular processes during cancer progression. MiR-335 has recently been identified to be involved in tumorigenesis of several cancers such as ovarian cancer and gastric cancer. However, the regulation of miR-335 in esophageal squamous cell carcinoma (ESCC) has not been reported yet. Methods: Expression of miR-335 in tumor and their normal matched tissues was determined by quantitative real-time PCR in 67 ESCC patients and its association with overall survival of patients was analyzed by statistical analysis. Results: The expression level of miR-335 was reduced in malignant tissue samples in comparison to normal matched tissue (P < 0.05). It was also proved that miR-335 expression was associated with ESCC histological grade, lymph node metastasis, tumor stage and clinical stage (P < 0.05). In addition, the Kaplan-Meier survival curves revealed that low miR-335 expression was associated with poor prognosis in ESCC patients. Multivariate analysis showed that miR-335 expression was an independent prognostic marker of overall survival of ESCC patients. Conclusions: The study proves for the first time that miR-335 is down regulated in a majority of ESCC patients. Our results indicate that miR-335 expression is an independent prognostic factor for patients with esophageal cancer, which might be a potential valuable biomarker for ESCC.
Keywords: miR-335, esophageal squamous cell carcinoma, quantitative real-time PCR, prognosis
Introduction
Esophageal cancer (EC) is the eighth most common cancer in the world and the sixth leading cause of cancer mortality [1]. Esophageal squamous cell carcinoma (ESCC) is the most prevalent pathological type of EC, predominates in east countries, particularly in China, with a proportion of more than 90% of all EC [2]. Despite the wide application of radical esophagectomy and systemic chemoradiotherapy, the overall 5-year survival rate of patients for ESCC is approximately 10-41% [3]. To date, extensive molecular biology studies of ESCC have identified a mass of dysregulated molecular events involved in esophageal carcinogenesis, which cover a wide range of genes with diverse functions. However, the reliable biomarkers for high-risk population screening, for clinical diagnosis and prognosis are still lacking. Therefore, it is imperative to identify and characterize more effective biomarkers for such purposes.
MicroRNAs (miRNAs) are short, and are noncoding RNAs of roughly 22-nucleotides in length, repress protein translation through binding to target mRNAs and play an important role in carcinogenesis [4]. Accumulating evidence has shown that miRNAs can act either as oncogenes or as tumor suppressors in ESCC [5,6], and measurement of miRNA expression in malignancies may have diagnostic and prognostic implications [7]. Their very small size in principle makes them less prone to degradation processes, unlike messenger RNAs, which were previously proposed as molecular markers. miR-335, which is the predicted homologue of a miRNA cloned from rat neuronal tissue and later verified in human, is transcribed from the genomic region on chromosome 7q32.2 [8]. It has been demonstrated to function as an oncogenic or a tumor suppressor miRNA in various human malignancies. For example, Yan indicated that miR-335 was upregulated in gastric cancer, and a high frequency of recurrence and poor survival was observed in gastric cancer cases with high levels of miR-335 [9]. In contrast, Xu et al. found that miR-335 was dramatically downregulated in gastric cancer cell lines than in the normal gastric cell line GES-1. Low expression of miR-335 was significantly associated with lymph-node metastasis, poor pT stage, poor pN stage and invasion of lymphatic vessels [10]. Lynch reported that miR-335 suppresses neuroblastoma cell invasiveness by direct targeting of multiple genes from the non-canonical TGF-β signaling pathway [11]. Cao showed that miR-335 was downregulated in the recurrence group of epithelial ovarian cancer [12].
However, to our knowledge, the clinicopathologic or prognostic significance of miR-335 expression in ESCC has not been studied systematically. In the present study, we determined the differential expression of miR-335 in ESCC from 67 patients. We found that miR-335 was an independent prognostic indicator in ESCC, and the reduction of miR-335 may be a selective pressure within neoplastic tissue. We have identified miR-335 as having the potential to predict the risk of ESCC.
Materials and methods
Patients and specimens
A total of 67 ESCC tumor tissues and matched adjacent normal esophageal tissues were obtained from patients at Henan Provincial Chest Hospital between the years 2006 and 2008. The tissue specimens were immediately frozen in liquid nitrogen after surgery and stored at -80°C until the extraction of total RNA. The clinical stage of all ESCC patients was classified or reclassified according to the seventh edition of the American Joint Committee on Cancer staging system. All patients recruited to this study did not receive any pre-operative treatments. This study was approved by The Human Ethics Committee of Henan Provincial Chest Hospital and, all patients signed an informed consent form. Clinical data of all the patients were collected from hospitalization and subsequent records. Detailed information is listed in Table 1. All patients were followed up until September 2012 with a median observation time of 48 months.
Table 1.
Association of miR-335 with clinicopathological characteristics of ESCC patients
| Parameters | Group | Total | MiR-335 expression | P value | |
|---|---|---|---|---|---|
|
| |||||
| Low | High | ||||
| Gender | Male | 54 | 39 | 15 | 0.731 |
| Female | 13 | 10 | 3 | ||
| Age (years) | < 60 | 40 | 30 | 10 | 0.675 |
| ≥ 60 | 27 | 19 | 8 | ||
| Tumor size (cm) | < 4 cm | 46 | 34 | 12 | 0.831 |
| ≥ 4 cm | 21 | 15 | 6 | ||
| Histological grade | G1 | 28 | 15 | 13 | 0.003 |
| G2+G3 | 39 | 33 | 5 | ||
| Tumor stage | T1-T2 | 20 | 10 | 10 | 0.005 |
| T3-T4 | 47 | 39 | 8 | ||
| Lymph nodes metastasis | Absence | 39 | 23 | 16 | 0.002 |
| Presence | 28 | 26 | 2 | ||
| Clinical stage | I-II | 41 | 24 | 17 | 0.001 |
| III-IV | 26 | 25 | 1 | ||
| Tumor location | Upper | 6 | 5 | 1 | 0.555 |
| Middle | 44 | 32 | 12 | ||
| Lower | 17 | 12 | 5 | ||
Quantitative real-time PCR
Total RNA was purified from all the 67 ESCC and matching adjacent normal specimens by the manufacturer using Trizol reagent (Invitrogen, CA, USA). Only those total RNA samples with OD A260/A280 ratio close to value of 2.0, which indicates that the RNA is pure, were subsequently analyzed. The miR-335 and RNU44 internal control specific cDNA were synthesized from total RNA using gene-specific primers according to the TaqMan microRNA assays protocol (Applied Biosystems, CA, USA). The reverse transcription products were then amplified and detected by real-time PCR using Taqman MicroRNA Assay (Applied Biosystems) specific for miR-335. Each sample was examined in triplicate and the raw data were presented as the relative quantification of miR-335 expression evaluated by the comparative cycle threshold (CT) method, normalized with respect to RNU44. Mean normalized miR-335 expression ± standard deviation (SD) was calculated from triplicate analysis. Real-time PCR was performed using an ABI 7900 system (Applied Biosystems) and comparative 2-ΔΔCt analysis was performed using SDS 2.2.2 software (Applied Biosystems).
Statistical analysis
All computations were carried out using the SPSS software version 17.0 for Windows (IBM Corporation, NY, USA). Data were expressed as mean ± SD. Paired Student’s t-test was conducted to compare miR-335 expression in paired clinical samples. The association between miR-335 expression and clinicopathologic characteristics of ESCC patients was assessed by Mann-Whitney U and Kruskal-Wallis tests. Survival curves were obtained by using the Kaplan-Meier method and compared by using the log-rank test. Multivariate survival analysis was performed using the Cox proportional hazards model. The factors selected from univariate analysis, based on a P < 0.05, were entered into the Cox proportional hazards model. P < 0.05 were considered statistically significant.
Results
Reduced expression of miR-335 in human ESCC
The expression of miR-335 was detected in 67 human ESCC and matched adjacent normal tissues by quantitative real-time PCR. After normalization to RNU44, the expression level of miR-335 in ESCC tissues (1.47 ± 0.39) was significantly lower than that in adjacent normal specimens (3.56 ± 1.17, P < 0.001). These results indicated that miR-335 might play a tumor suppressor role in ESCC. ESCC patients who expressed miR-335 at levels less than the cutoff value were assigned to the low expression group (mean expression value 0.96, n = 49), and those with expression above than the cutoff value were assigned to the high expression group (mean expression value 1.94, n = 18).
Relationship between miR-335 expression and ESCC patients’ clinicopathologic variables
In our ESCC cohort, the relationship between the expression of miR-335 and patient clinical characteristics was shown in Table 1. Low expression of miR-335 was found to significantly correlate with higher histological grade (P = 0.003), lymph node metastasis (P = 0.002), tumor stage (P = 0.005) and clinical stage (P = 0.001). However, miR-335 expression was not significantly related to gender, age, tumor size and tumor location (P > 0.05).
Relationship between clinicopathologic features, miR-335 expression, and ESCC patients’ survival: univariate survival analysis
In univariate survival analyses, cumulative survival curves were calculated according to the Kaplan-Meier method. Differences in survival times were assessed using the log-rank test. First, to confirm the representativeness of the ESCC in our study, we analyzed established prognostic predictors of patient survival. Kaplan-Meier analysis demonstrated a significant impact of well-known clinical pathological prognostic parameters, such as histological grade, lymph node metastasis, tumor stage and clinical stage on patient survival (P < 0.05, Table 2). Assessment of survival in ESCC patients revealed that lower expression of miR-335 was correlated with adverse survival of ESCC patients (P < 0.001, Table 2; Figure 1).
Table 2.
Prognostic factors in Cox proportional hazards model
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Risk ratio | 95% CI | P | Risk ratio | 95% CI | P | |
| Gender | 1.173 | 0.619-1.587 | 0.594 | |||
| Male vs. Female | ||||||
| Age (years) | 1.439 | 0.517-1.894 | 0.264 | |||
| < 60 vs. ≥ 60 | ||||||
| Tumor size | 2.642 | 1.784-3.921 | 0.149 | |||
| < 4 cm vs. ≥ 4 cm | ||||||
| Histological grade | 5.264 | 3.976-12.043 | < 0.001 | 2.781 | 1.867-4.638 | 0.006 |
| G1 vs. G2-3 | ||||||
| Tumor stage | 3.796 | 2.117-6.484 | 0.013 | 2.966 | 1.937-6.714 | 0.021 |
| T1-2 vs. T3-4 | ||||||
| Lymph node | 5.108 | 2.974-8.265 | 0.015 | 3.681 | 2.171-4.472 | 0.031 |
| Absence vs. Presence | ||||||
| Clinical stage | 4.369 | 1.744-9.015 | 0.007 | 3.979 | 1.114-7.528 | 0.014 |
| I-II vs. III-IV | ||||||
| miR-335 | 5.718 | 2.219-9.448 | < 0.001 | 5.011 | 2.074-8.526 | 0.008 |
| high vs. low | ||||||
Abbreviations: CI, confidence interval; miR, microRNA.
Figure 1.
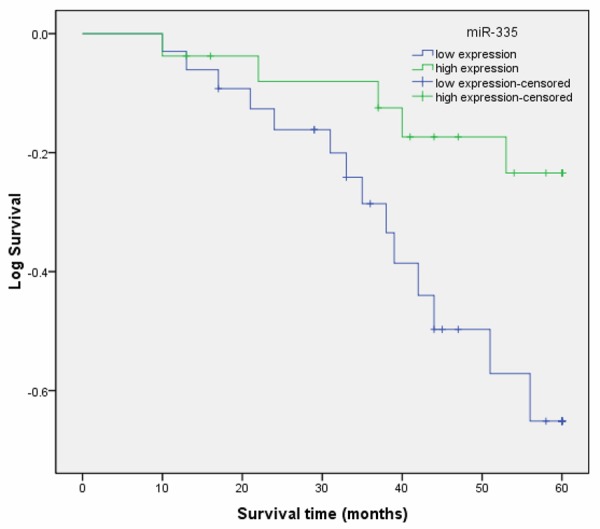
Kaplan-Meier postoperative survival curve for patterns of patients with ESCC and miR-335 expression.
Independent prognostic factors for ESCC: multivariate Cox regression analysis
Since variables observed to have a prognostic influence by univariate analysis may covariate, the expression of miR-335 and those clinicalopathological parameters that were significant in univariate analysis (histological grade, lymph node metastasis, tumor stage and clinical stage) were further examined in multivariate analysis. The results showed that the expression of miR-335 was an independent prognostic factor for overall patient survival (relative risk: 5.011, CI: 2.074-8.526, P = 0.008, Table 2). With regard to other parameters, histological grade, lymph node metastasis, tumor stage and clinical stage were also shown to be an independent prognostic factor for overall survival (P < 0.05, Table 2).
Discussion
Esophageal cancer (EC) is one of the most common malignant diseases with a poor prognosis. Although several clinicopathologic features have been the standard for determining the clinical outcome of ESCC patients, this classification scheme is probably an imprecise predictor of the prognosis of an individual patient [13]. Therefore, it is necessary to identify novel and effective biologic markers which are associated with advanced tumor progression for the early diagnosis and the discovery of a therapeutic target.
Recent studies showed more than 1,900 human miRNAs have been identified, which are estimated to regulate over 60% of genes in mammals [14]. Due to their great importance in the regulation of gene expression, it has been widely accepted that miRNAs are involved in multiple cellular functions such as differentiation, proliferation and apoptosis, thus, have been implemented in diverse physiological and pathological processes ranging from developmentto cancer [15,16]. However, the role of miRNAs in ESCC is yet to be fully elucidated. Among human miRNAs, miR-335 as an evolutionarily conserved miRNA has been demonstrated to have a close correlation with cancer development. But the expression patterns and function of miR-335 are different in different cancers. Dohi et al. indicated that the expression levels of miR-335 were significantly lower in primary hepatocellular carcinoma, compared to their non-tumor tissue counterparts, and the expression levels of miR-335 were significantly lower in hepatocellular carcinoma with distant metastasis compared to those without distant metastasis [17]. Cao found that miR-335 suppressed the invasiveness of ovarian cancer cell lines by targeting Bcl-w and the Bcl-w effector, matrix metallopeptidase 2, and showed that miR-335 was downregulated in primary ovarian cancers in comparison with normal ovarian epithelium, and an even sharper reduction in miR-335 expression was revealed in omental metastases [12]. Wang found that miR-335 acts as tumor suppressor by targeting the ROCK1 gene and inhibiting osteosarcoma cells migration and invasion [18]. In contrast, Yan found that high frequency recurrence and poor survival were observed in gastric cancer cases with high level of miR-335 and they evaluated that miR-335 were involved in regulating target genes in several oncogenic signal-pathways, such as p53, MAPK, TGF-β, Wnt, ERbB, mTOR, Toll-like receptor and focal adhesion [10]. Jiang’s results indicated that miR-335 expression was increased in human gliomas and was associated with advanced tumor progression. And miR-335 expression was demonstrated for the first time to be an independent marker for predicting the clinical outcome of patients with gliomas [19]. Shi found that miR-335 is a typical microRNA overexpressed in meningiomas in humans and play an essential role in the proliferation of meningioma cells by directly targeting the Rb1 signaling pathway [20].
However there have been no reports on the clinical relevance of miR-335 to ESCC, in the present study, we sought to determine whether there was any difference in miR-335 expression between ESCC and normal tissue samples which had not been studied previously. This study showed that miR-335 were down-regulated in the ESCC, and explored available evidence of close correlation of miR-335 expression and the total patients’ survival during a 5-year follow-up survey.
To directly address the potential roles for miR-335 in the occurrence and development of ESCC, an elaborate experiment was conducted and a rigorous analysis was performed of human miR-335 expression on ESCC samples. Our results revealed that the miR-335 expression in ESCC specimens was remarkably lower than that in normal esophageal tissues (P < 0.05). In the present study, we found the expression level of miR-335 was significantly associated with histological grade (P = 0.003), lymph node metastasis (P = 0.002), tumor stage (P = 0.005) and clinical stage (P = 0.001). It is suggested that miR-335 may play important roles in esophageal cancer carcinogenesis and progression and may affect tumor invasion and metastasis. We further imagined that miR-335 may affect the activation of cellular signal transduction pathway, cell division cycle and tumor angiogenesis to influence biological behavior of tumor, and this had just been unraveled in the latest relevant researches. Recent study showed that miR-335 regulates a set of genes, which are SOX4, RUNX2, Rb1, PTPRN2, BRCA1, MERTK and SP1, the collective expression of which in a large cohort of human tumors is associated with the risk of aggressive tumor progression [21].
Ultimately, A total of 67 patients histologically proven esophageal cancer with follow-up information were conducted a systematically analysis to confirm the relationship of miR-335 and outcome of patient initially. Our finding demonstrated that patients with lower expression of miR-335 in tumor tissue had a worse overall survival than patients with higher expression (P < 0.05, respectively), providing evidence that reduced expression of miR-335 in esophageal cancer might facilitate an increased malignant and worse prognostic phenotype. It is noteworthy that by multivariate Cox analysis combining expression of miR-335 with other parameters, miR-335 was found as an independent prognostic factor (P = 0.008) for patient survival. The aberrant expression of miR-335 linked to a poor prognosis of patients has never been investigated in esophageal cancer before.
In conclusion, our data indicated that miR-335 expression was decreased in human ESCC and was associated with advanced tumor progression. Furthermore, miR-335 expression was demonstrated for the first time to be an independent marker for predicting the clinical outcome of patients with ESCC. Apparently, a further understanding of the molecular mechanism by miR-335 in human ESCC would help in the discovery of novel targeted agents and might also lead to the development of new approaches for effective therapy of human ESCC.
Disclosure of conflict of interest
None.
References
- 1.Wheeler JB, Reed CE. Epidemiology of esophageal cancer. Surg Clin North Am. 2012;92:1077–1087. doi: 10.1016/j.suc.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 2.Umar SB, Fleischer DE. Esophageal cancer: epidemiology, pathogenesis and prevention. Nat Clin Pract Gastroenterol Hepatol. 2008;5:517–526. doi: 10.1038/ncpgasthep1223. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 4.Yates LA, Norbury CJ, Gilbert RJ. The long and short of microRNA. Cell. 2013;153:516–519. doi: 10.1016/j.cell.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 5.Ni Y, Meng L, Wang L, Dong W, Shen H, Wang G, Liu Q, Du J. MicroRNA-143 functions as a tumor suppressor in human esophageal squamous cell carcinoma. Gene. 2013;517:197–204. doi: 10.1016/j.gene.2012.12.031. [DOI] [PubMed] [Google Scholar]
- 6.Zhang J, Cheng C, Yuan X, He JT, Pan QH, Sun FY. microRNA-155 acts as an oncogene by targeting the tumor protein 53-induced nuclear protein 1 in esophageal squamous cell carcinoma. Int J Clin Exp Pathol. 2014;7:602–610. [PMC free article] [PubMed] [Google Scholar]
- 7.Larne O, Martens-Uzunova E, Hagman Z, Edsjö A, Lippolis G, den Berg MS, Bjartell A, Jenster G, Ceder Y. miQ--a novel microRNA based diagnostic and prognostic tool for prostate cancer. Int J Cancer. 2013;132:2867–2875. doi: 10.1002/ijc.27973. [DOI] [PubMed] [Google Scholar]
- 8.Zu Y, Ban J, Xia Z, Wang J, Cai Y, Ping W, Sun W. Genetic variation in a miR-335 binding site in BIRC5 alters susceptibility to lung cancer in Chinese Han populations. Biochem Biophys Res Commun. 2013;430:529–534. doi: 10.1016/j.bbrc.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Yan Z, Xiong Y, Xu W, Gao J, Cheng Y, Wang Z, Chen F, Zheng G. Identification of hsa-miR-335 as a prognostic signature in gastric cancer. PLoS One. 2012;7:e40037. doi: 10.1371/journal.pone.0040037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu Y, Zhao F, Wang Z, Song Y, Luo Y, Zhang X, Jiang L, Sun Z, Miao Z, Xu H. MicroRNA-335 acts as a metastasis suppressor in gastric cancer by targeting Bcl-w and specificity protein 1. Oncogene. 2012;31:1398–1407. doi: 10.1038/onc.2011.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lynch J, Fay J, Meehan M, Bryan K, Watters KM, Murphy DM, Stallings RL. MiRNA-335 suppresses neuroblastoma cell invasiveness by direct targeting of multiple genes from the non-canonical TGF-β signalling pathway. Carcinogenesis. 2012;33:976–985. doi: 10.1093/carcin/bgs114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao J, Cai J, Huang D, Han Q, Chen Y, Yang Q, Yang C, Kuang Y, Li D, Wang Z. miR-335 Represents an Independent Prognostic Marker in Epithelial Ovarian Cancer. Am J Clin Pathol. 2014;141:437–42. doi: 10.1309/AJCPLYTZGB54ISZC. [DOI] [PubMed] [Google Scholar]
- 13.Zhang T, Wang Q, Zhao D, Cui Y, Cao B, Guo L, Lu SH. The oncogenetic role of microRNA-31 as a potential biomarker in oesophageal squamous cell carcinoma. Clin Sci. 2011;121:437–447. doi: 10.1042/CS20110207. [DOI] [PubMed] [Google Scholar]
- 14.Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12:861–874. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- 15.Jansson MD, Lund AH. MicroRNA and cancer. Mol Oncol. 2012;6:590–610. doi: 10.1016/j.molonc.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kasinski AL, Slack FJ. Epigenetics and genetics. MicroRNAs en route to the clinic: progress in validating and targeting microRNAs for cancer therapy. Nat Rev Cancer. 2011;11:849–864. doi: 10.1038/nrc3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dohi O, Yasui K, Gen Y, Takada H, Endo M, Tsuji K, Konishi C, Yamada N, Mitsuyoshi H, Yagi N, Naito Y, Tanaka S, Arii S, Yoshikawa T. Epigenetic silencing of miR-335 and its host gene MEST in hepatocellular carcinoma. Int J Oncol. 2013;42:411–418. doi: 10.3892/ijo.2012.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Zhao W, Fu Q. miR-335 suppresses migration and invasion by targeting ROCK1 in osteosarcoma cells. Mol Cell Biochem. 2013;384:105–111. doi: 10.1007/s11010-013-1786-4. [DOI] [PubMed] [Google Scholar]
- 19.Jiang J, Sun X, Wang W, Jin X, Bo X, Li Z, Bian A, Jiu J, Wang X, Liu D, Hui X, Wang Y, Wang A, Ding L. Tumor microRNA-335 expression is associated with poor prognosis in human glioma. Med Oncol. 2012;29:3472–3477. doi: 10.1007/s12032-012-0259-z. [DOI] [PubMed] [Google Scholar]
- 20.Shi L, Jiang D, Sun G, Wan Y, Zhang S, Zeng Y, Pan T, Wang Z. miR-335 promotes cell proliferation by directly targeting Rb1 in meningiomas. J Neurooncol. 2012;110:155–162. doi: 10.1007/s11060-012-0951-z. [DOI] [PubMed] [Google Scholar]
- 21.Tomé M, López-Romero P, Albo C, Sepúlveda JC, Fernández-Gutiérrez B, Dopazo A, Bernad A, González MA. miR-335 orchestrates cell proliferation, migration and differentiation in human mesenchymal stem cells. Cell Death Differ. 2011;18:985–995. doi: 10.1038/cdd.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]


