Abstract
Background: Angiotensin I-converting enzyme (ACE) gene plays an important role in the pathogenesis of cancers. The association between ACE insertion/deletion (I/D) polymorphism and the risk of various cancers has been studied. However, the results of these studies remain conflicting. Therefore, we performed a meta-analysis to evaluate the association between ACE I/D polymorphism and the risk of cancers. Methods: PubMed, Embase, ScienceDirect, Springer, CNKI, Wanfang, Weipu, CBM databases and Google Scholar were searched for case-control studies on ACE I/D polymorphism and the risk of cancers, published up to Dec 31, 2013. Odds ratios (ORs) with 95% confidence intervals (CIs) were used to assess the strength of the association between ACE I/D polymorphism and cancer risk. Results: Thirty-five published studies with 5007 cases and 8173 controls were included. Overall, there were no significant association between ACE I/D polymorphism and the risk of cancers (II vs. ID+DD OR = 1.05, 95% CI = 0.89-1.23, I vs. D OR = 1.00, 95% CI = 0.89-1.13). However, when stratified by ethnicity, we found a significant association between this polymorphism and cancer risk in Caucasians (II vs. ID+DD: OR = 1.43, 95% CI = 1.02-2.00, I vs. D: OR = 1.23, 95% CI 1.01-1.49). Conclusion: ACE I/D polymorphism is associated with the cancer risk in Caucasians.
Keywords: ACE I/D, single nucleotide polymorphism, cancer risk, meta-analysis
Introduction
Angiotensin I-converting enzyme (ACE) is a zinc metallopeptidase which converts angiotensin I to angiotensin II. ACE is one of the key enzymes in human renin-angiotensin system (RAS) [1]. It plays an important role in the modulation of vascular homeostasis, inflammation and angiogenesis [2-4]. ACE is expressed in many tissues and systems including lung, vasculature, kidney, heart, and testes [5]. Emerging evidence has shown that the expression of ACE is up-regulated in several types of cancers [6-9]. Moreover, ACE inhibitors are currently considered being used as novel antineoplastic therapies [6,10].
The ACE gene is located on human’s chromosome 17q23 that consists of 26 exons and 25 introns [11]. The ACE insertion/deletion (I/D) polymorphism of 287bp Alu repeat sequence in intron 16 (rs4646994) has been reported [11]. Although the I/D polymorphism is not located in the coding region of the ACE gene, subjects with ACE D allele exhibits a higher plasma ACE level and activity [12]. The I/D polymorphism account for 20% to 50% of the variance in ACE expression or activity in blood and tissues among individuals [13].
Up to now, a number of studies were conducted to evaluate the association between ACE I/D polymorphism and risk of different types of cancers in diverse populations. However, the results from the published studies remain conflicting rather than conclusive. Therefore, we performed a meta-analysis on all eligible case-control studies to clarify the association between ACE I/D polymorphism and cancer risk.
Methods
Literature search
We conducted the literature search by using the PubMed, Embase, ScienceDirect, Springer, CNKI, Wanfang, Weipu, CBM databases and Google Scholar for relevant articles published (update to Dec 31, 2013) with the following search terms: “ACE” or “angiotensin I converting” and “polymorphism” or “insertion/deletion” and “cancer” or “carcinoma” or “tumor”. In addition, the studies were identified by manual search of the reference lists of reviews and retrieved studies. The inclusion criteria were: (1) the study evaluated the association between ACE polymorphism and cancer risk in human; (2) a case-control study; (3) genotype distributions in both cases and controls were available for estimating an odds ratio with 95% confidence interval (CI) and P value, (4) genotype distributions of controls must be consistent with Hardy-Weinberg equilibrium (HWE). Main exclusion criteria of studies were as follows: (1) case reports, reviews, letters and editorial articles; (2) only case population; (3) duplicate of previous publication; and (4) the distribution of genotypes among controls are consistent with HWE.
Data extraction
Two investigators (Zhang and Cheng) extracted the data from all eligible studies independently. We checked all potentially relevant studies and reached a consensus on all items. From each study, the following information was extracted: first author’s name, year of publication, country of origin, ethnicity, definition of case, source of control selection and the genotype frequencies in cases and controls.
Statistical analysis
For each case-control study, we first examined whether the genotype distributions in control group were consistent with Hardy-Weinberg equilibrium by Pearson’s X2 test. Heterogeneity was evaluated by the X2 based Q statistic and was considered statistical significant at P value < 0.10. I 2 value was also used to measure the percentage of variability in studies that due to heterogeneity rather than chance. When the effects were assumed to be homogenous, fixed-effects model was used (the Mantel-Haenszel method); otherwise, it was more appropriate to use random-effects model (DerSimonian and Laird method) [14-16]. The strength of associations between ACE I/D polymorphism and cancer risk were measured by ORs with 95% CIs. The pooled ORs were evaluated for the homozygote comparison (II vs. DD), heterozygote comparison (ID vs. DD), dominant model (II+ID vs. DD) recessive model (II vs. ID+DD), and haploid model (I vs. D) comparison. The funnel plots as well as Begg’s tests and Egger’s test were used to investigate publication bias [17]. Sensitivity analysis was performed to assess the stability of the results by sequentially excluding each study [18]. All statistical analyses were performed by using the Revman 5.2 software (Cochrane Library Software, Oxford, UK) and STATA11.0 (STATA Corporation, College Station, TX, USA).
Results
Studies characteristics
Overall, 35 publications [13,19-52] including 5007 cases and 8137 controls were available for this meta-analysis based on the inclusion and exclusion criteria (Figure 1). The main characteristics of these studies are summarized in Table 1. There were 22 studies of Asian populations, 13 studies of Caucasians population. Of the 35 studies, 7 articles were population-based and 28 articles were hospital-based. The diagnosis of most of the cases was based on pathology. Healthy subjects matched for age and sex were used as controls. Polymerase chain reaction (PCR) was performed for genotyping.
Figure 1.
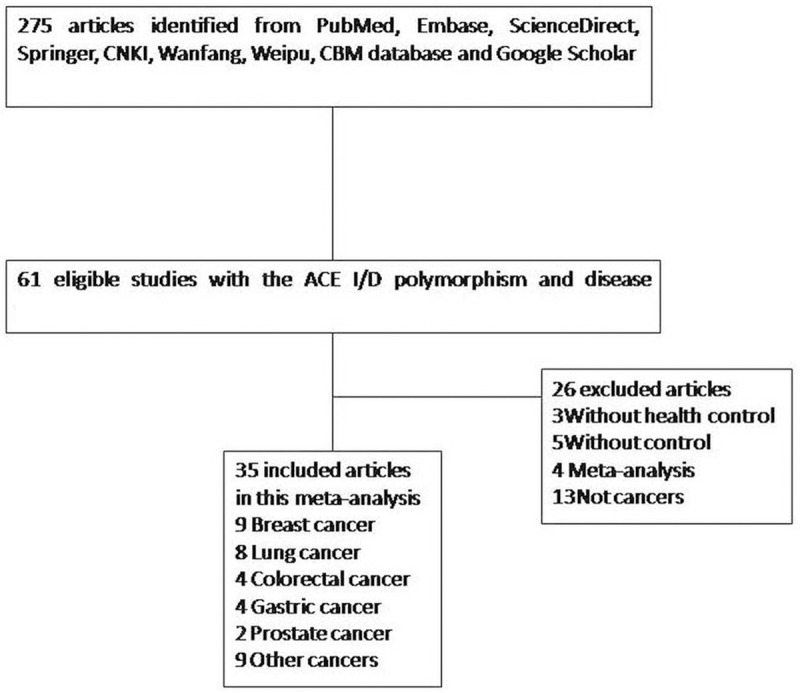
Flow diagram of study selection.
Table 1.
Distribution of ACE genotype and allele among cancer patients and controls
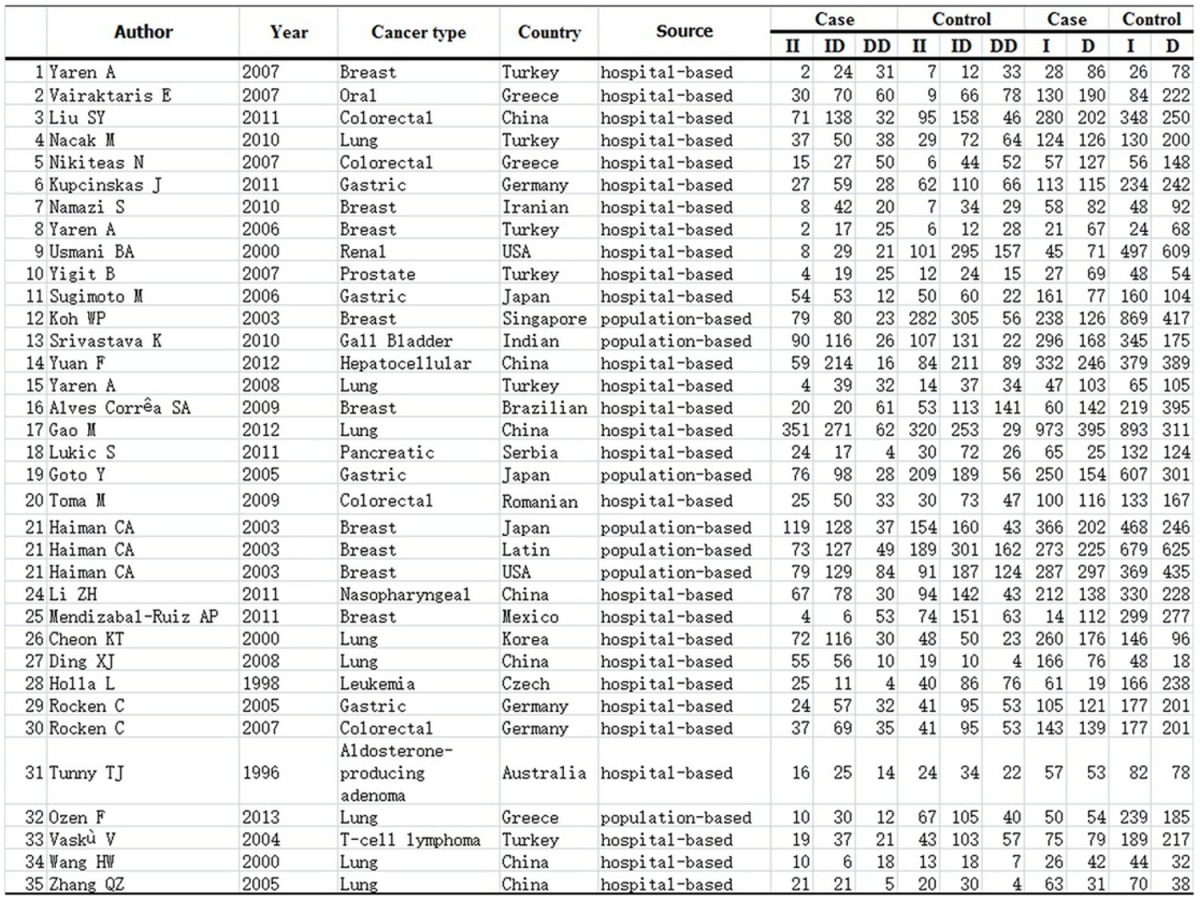
|
Meta-analysis
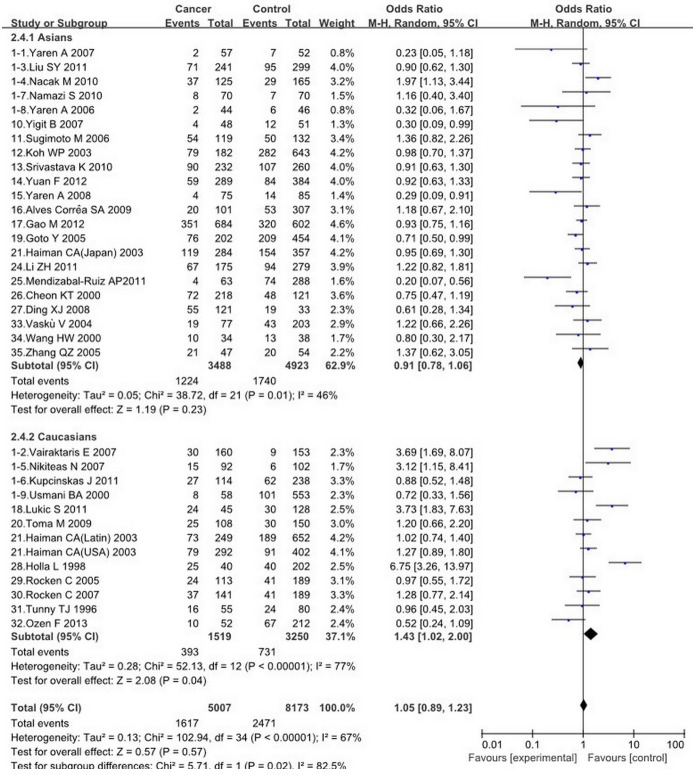
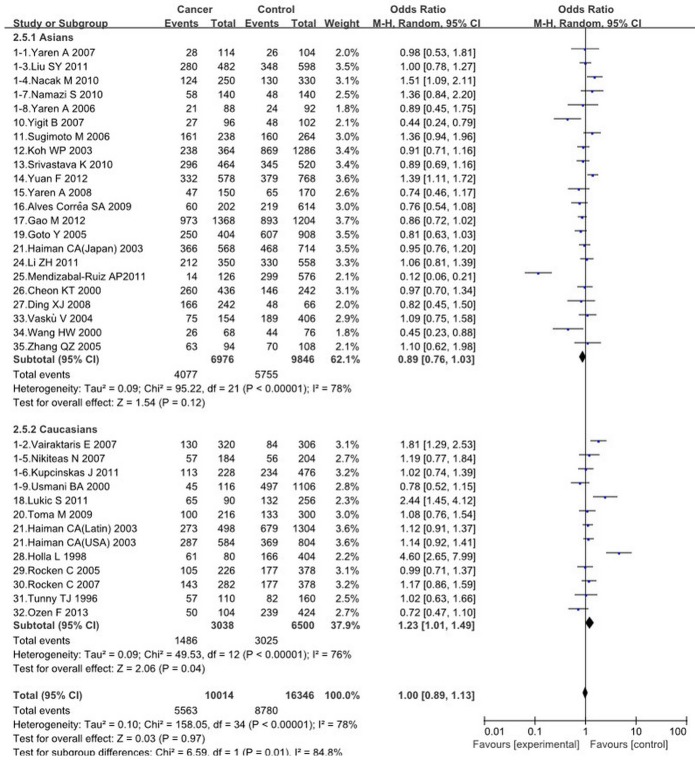
A summary of the meta-analysis results of the association between ACE I/D polymorphism and cancer risk is shown, there are no significant association was found between ACE I/D polymorphism and the risk of cancers (II vs. DD OR = 0.97, 95% = CI 0.76-1.24, ID vs. DD OR = 0.98, 95% CI = 0.79-1.21, II+ID vs. DD OR = 0.99, 95% CI = 0.80-1.23, II vs. ID+DD OR = 1.05, 95% CI = 0.89-1.23, I vs. D OR = 1.00, 95% CI = 0.89-1.13). However, in the subgroup analyses by ethnicity, there was a significant association between this ACE I/D polymorphism and cancer risk in Caucasians (II vs. ID+DD: OR = 1.43, 95% CI = 1.02-2.00, I vs. D: OR = 1.23, 95% CI 1.01-1.49) (Figures 2, 4). In the subgroup analyses by cancer types, no significant association was found under different genetic models.
Figure 2.
The association between ACE I/D polymorphism and cancer risk in subgroup analysis by ethnicity (II VS. ID+DD).
Figure 4.
The association between ACE I/D polymorphism and cancer risk in subgroup analysis by ethnicity (I VS. D).
Test of heterogeneity
For the overall analysis, the Q-statistic was significant and I 2 showed stable variation under the comparisons (II vs. DD: P < 0.00001, I 2 = 74%; ID vs. DD P < 0.00001, I 2 = 76%; II+ID vs. DD P < 0.00001, I 2 = 79%; II vs. ID+DD P < 0.00001, I 2 = 67%; I vs. D P < 0.00001, I 2 = 78%). In the subgroup analyses of ethnicity, the I 2 showed inconsistent with the former, the I 2 of II vs. ID+DD are 46% and 77%. While there is no notable difference of I 2 in I vs. D, the former is 78%, the latter are 78% and 76 % (Figures 2, 4).
Sensitivity analysis
The influence of a single study on the overall meta-analysis estimate was investigated by excluding each study at a time. The omission of any study made no significant difference. This is indicating that the results of our meta-analysis were statistically reliable.
Publication bias
Begg’s funnel plot and Egger’s test were performed to assess the publication bias of the literatures. Egger’s test did not show any evidence of publication bias (t = 0.16, P = 0.871 for II vs. ID+DD and t = -0.78, P = 0.440 for I vs. D, respectively) (Figures 3, 5).
Figure 3.
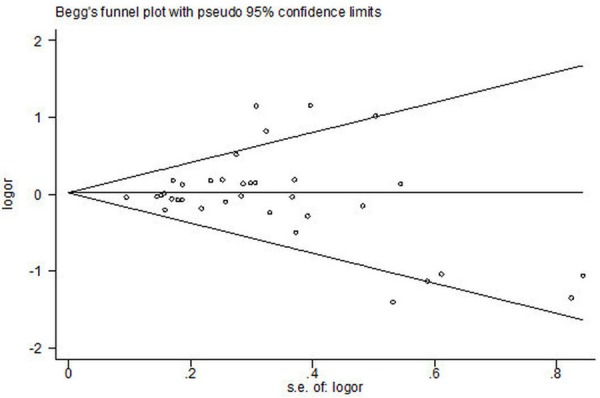
Begg’s funnel plot for publication bias in selection of studies on ACE I/D polymorphism (II VS. ID + DD; bias = 0.871).
Figure 5.
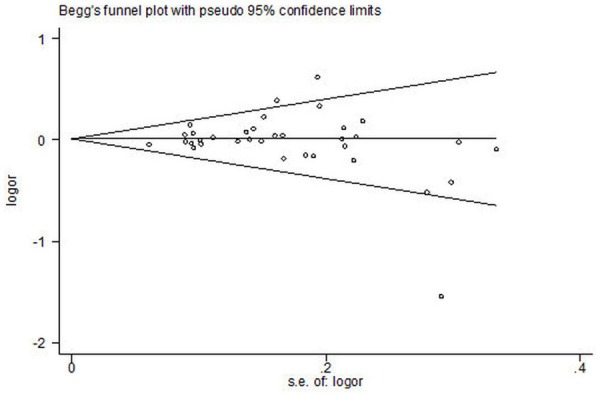
Begg’s funnel plot for publication bias in selection of studies on ACE I/D polymorphism (I VS. D; bias = 0.440).
Discussion
Human ACE is the key enzyme in the renin-angiotensin system, which works in the regulation of blood pressure, the number of red blood cell, cardiovascular homeostasis and serum electrolytes. In recent years there were more evidences indicating that ACE was associated with the pathogenesis of cancer, even it was the trigger events at least in some group of patients with cancer. It may influence tumor cell adhesion, proliferation, migration, angiogenesis and metastatic behaviors [53]. Some studies showed that the ACE inhibitor could lower the breast cancer risk [10]. But in some meta-analyses, show that there were no significant association between the ACE I/D polymorphisms and breast cancer risk. In different cancer studies, have the inconsistent and conflict result. On the other hand, there are some studies about the risk of ACE gene polymorphism with variety of cancers, for example, in prostate cancer, it has been reported that the ACE gene polymorphism is associated with clinical outcome parameters [54]. It is noteworthy that RAS inhibitors caused reductions in growth and angiogenesis in tumor cell lines [55,56]. Also it have been demonstrated that the ACE, besides angiotensin II production, is the inactivation of bradykinin [57]. That is known to established role in tumor formation through its ability to stimulate growth and increase vascular permeability [57]. In these sex hormone-related neoplasias cancers, oestrogens increase hepatic synthesis of the renin substrate angiotensinogen, which is converted to angiotensin I, the substrate of ACE [58].
In short, the ACE gene plays an important role in the pathogenesis of cancers. Up to now, a number of original studies have been carried out to investigate whether ACE I/D polymorphism confer individual’s susceptibility to cancer. However, the results from the published studies were conflicting. We conducted an up-dated meta-analysis including with 5007 cases and 8173 controls from 35 case-control studies to evaluate the association between ACE I/D polymorphism and the cancer risk.
There are no significant association between ACE I/D polymorphism and cancer risks under any genetic model in the total population. However, in the subgroup analyses by ethnicity, we found that the ACE I/D polymorphism were associated with increased cancers risk in Caucasians. There was an aggregated OR of 1.43 (95% CI = 1.02-2.00) for increased cancer susceptibility under recessive comparison. This indicates that the ACE I/D polymorphism may contribute to pathogenesis of cancers in Caucasians. Even though the D genotype has been reported that associated coronary heart disease and hypertension. No associations were found between this polymorphism and the cancers risk in Asians, which was consistent with previous reports [13,28,30,31].
One of the unique of meta-analysis is heterogeneity. The heterogeneity was found in almost all comparisons in our meta-analysis. To get more full and accurate detail of the precious date, we used the random-effect models. The results are stable with the sensitivity analysis which did not change the results of the meta-analysis. Meanwhile, there are no publication bias for the risk of cancer in the ACE I/D polymorphism studies.
There were some limitations of our meta-analysis. First, the control subjects were not uniformly defined because of some study only including unitary gender and some reproductive system cancer such as prostatic cancer. Second, in several studies, the larger tumor sizes and lymph node metastases were significantly associated with the DD genotype. Third, all the included studies were from European, Asian and Latino populations, further studies are necessary to contain more findings for other ethnic populations. Fourth, cancer is a multi-factorial disease. Due to lack of original data, we could not evaluate the potential interactions of gene-gene and gene-environment.
In conclusion, the I allele of ACE I/D genotype may confer the risk of cancer in Caucasians, but not in Asian. More studies would be of great value to explore the interaction between the ACE I/D polymorphism and cancer risk.
Acknowledgements
This study was supported by National Natural Science Foundation of China Grant 81170022.
Disclosure of conflict of interest
None.
References
- 1.Stroth U, Unger T. The renin angiotensin system and its receptors. J Cardiovasc Pharmacol. 1999:S41–43. doi: 10.1097/00005344-199900001-00005. [DOI] [PubMed] [Google Scholar]
- 2.Murphey LJ, Gainer JV, Vaughan DE, Brown NJ. Angiotensin-converting enzyme insertion/deletion polymorphism modulates the human in vivo metabolism of bradykinin. Circulation. 2000;102:829–832. doi: 10.1161/01.cir.102.8.829. [DOI] [PubMed] [Google Scholar]
- 3.Deshayes F, Nahmias C. Angiotensin receptors: a new role in cancer? Trends Endocrinol Metab. 2005;16:293–299. doi: 10.1016/j.tem.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 4.Egami K, Murohara T, Shimada T, Sasaki K, Shintani S, Sugaya T, Ishii M, Akagi T, Ikeda H, Matsuishi T, Imaizumi T. Role of host angiotensin II type 1 receptor in angiogenesis and growth. J Clin Invest. 2003;112:67–75. doi: 10.1172/JCI16645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaufman KM, Kelly J, Gray-McGuire C, Asundi N, Yu H, Reid J, Baird T, Hutchings D, Bruner G, Scofield RH, Moser K, Harley JB. Linkage insertion/deletion polymorphism and systemic lupus erythematosus. Mol Cell Endocrinol. 2001;177:81–85. doi: 10.1016/s0303-7207(01)00424-5. [DOI] [PubMed] [Google Scholar]
- 6.Abali H, Gullu IH, Engin H, Haznedaroğlu IC, Erman M, Tekuzman G. Old antihypertensives as novel antineoplastics: angiotensin-I-converting enzyme inhibitors and angiotensin II type 1 receptor antagonists. Med Hypotheses. 2002;59:344–348. doi: 10.1016/s0306-9877(02)00185-8. [DOI] [PubMed] [Google Scholar]
- 7.Yoshiji H, Kuriyama S, Fukui H. Angiotensin- I-converting enzyme inhibitors may be an alternative anti-angiogenic strategy in the treatment of liver fibrosis and hepatocellular carcinoma. Possible role of vascular endothelial growth factor. Tumour Biol. 2002;23:348–356. doi: 10.1159/000069792. [DOI] [PubMed] [Google Scholar]
- 8.Bauvois B. Transmembrane proteases in cell growth and invasion: new contributors to angiogenesis? Oncogene. 2004;23:317–329. doi: 10.1038/sj.onc.1207124. [DOI] [PubMed] [Google Scholar]
- 9.Carl-McGrath S, Lendeckel U, Ebert M, Wolter AB, Roessner A, Röcken C. The ectopeptidases CD10, CD13, CD26, and CD143 are upregulated in gastric cancer. Int J Oncol. 2004;25:1223–1232. [PubMed] [Google Scholar]
- 10.Lever AF, Hole DJ, Gillis CR, McCallum IR, McInnes GT, MacKinnon PL, Meredith PA, Murray LS, Reid JL, Robertson JW. Do inhibitors of angiotensin-I-converting enzyme protect against risk of cancer? Lancet. 1998;352:179–184. doi: 10.1016/S0140-6736(98)03228-0. [DOI] [PubMed] [Google Scholar]
- 11.Rigat B, Hubert C, Alhenc-Gelas F, Cambien F, Corvol P, Soubrier F. An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J Clin Invest. 1990;86:1343–1346. doi: 10.1172/JCI114844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Winkelmann BR, Nauck M, Klein B, Russ AP, Böhm BO, Siekmeier R, Ihnken K, Verho M, Gross W, März W. Deletion polymorphism of the angiotensin I-converting enzyme gene is associated with increased plasma angiotensin-converting enzyme activity but not with increased risk for myocardial infarction and coronary artery disease. Ann Intern Med. 1996;125:19–25. doi: 10.7326/0003-4819-125-1-199607010-00004. [DOI] [PubMed] [Google Scholar]
- 13.Rocken C, Lendeckel U, Dierkes J, Westphal S, Carl-McGrath S, Peters B, Kruger S, Malfertheiner P, Roessner A, Ebert MP. The number of lymph node metastases in gastric cancer correlates with the angiotensin I-converting enzyme gene insertion/deletion polymorphism. Clin Cancer Res. 2005;11:2526–2530. doi: 10.1158/1078-0432.CCR-04-1922. [DOI] [PubMed] [Google Scholar]
- 14.Diggle Peter J, Heagerty P, Liang KY, Zeger Scott L, editors. Analysis of Longitudinal Data. 2nd ed. Oxford University Press; 2002. pp. 169–171. [Google Scholar]
- 15.Hoboken JWS, Fitzmaurice GM, Laird NM, Ware JH, editors. Applied Longitudinal Analysis. Hoboken: John Wiley & Sons; 2004. pp. 326–328. [Google Scholar]
- 16.Laird NM, Ware JH. Random-Effects Models for Longitudinal Data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 17.Munafo MR, Clark TG, Flint J. Assessing publication bias in genetic association studies: evidence from a recent meta-analysis. Psychiatry Res. 2004;129:39–44. doi: 10.1016/j.psychres.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 18.Zhang YG, Huang J, Zhang J, Li XB, He C, Xiao YL, Tian C, Wan H, Zhao YL, Tsewang YG, Fan H. RANTES gene polymorphisms and asthma risk: a meta-analysis. Arch Med Res. 2010;41:50–58. doi: 10.1016/j.arcmed.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 19.Yaren A, Turgut S, Kursunluoglu R, Oztop I, Turgut G, Degirmencioglu S, Kelten C, Erdem E. Insertion/Deletion polymorphism of the angiotensin I-converting enzyme gene in patients with breast cancer and effects on prognostic factors. J Investig Med. 2007;55:255–261. doi: 10.2310/6650.2007.00006. [DOI] [PubMed] [Google Scholar]
- 20.Namazi S, Monabati A, Ardeshir-Rouhani-Fard S, Azarpira N. Association of angiotensin I converting enzyme (Insertion/Deletion) and angiotensin II type 1 receptor (A1166C) polymorphisms with breast cancer prognostic factors in Iranian population. Mol Carcinog. 2010;49:1022–1030. doi: 10.1002/mc.20685. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez-Zuloeta Ladd AM, Vasquez AA, Sayed-Tabatabaei FA, Coebergh JW, Hofman A, Njajou O, Stricker B, van Duijn C. Angiotensin-converting enzyme gene insertion/deletion polymorphism and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2005;14:2143–2146. doi: 10.1158/1055-9965.EPI-05-0045. [DOI] [PubMed] [Google Scholar]
- 22.Vairaktaris E, Yapijakis C, Tsigris C, Vassiliou S, Derka S, Nkenke E, Spyridonidou S, Vylliotis A, Vorris E, Ragos V, Neukam FW, Patsouris E. Association of angiotensin-converting enzyme gene insertion/deletion polymorphism with increased risk for oral cancer. Acta Oncol. 2007;6:1097–1102. doi: 10.1080/02841860701373579. [DOI] [PubMed] [Google Scholar]
- 23.Liu SY, Sima X, Wang CH, Gao M. The association between ACE polymorphism and risk of colorectal cancer in a Chinese population. Clin Biochem. 2011;44:1223–1226. doi: 10.1016/j.clinbiochem.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 24.Sugimoto M, Furuta T, Shirai N, Ikuma M, Sugimura H, Hishida A. Influences of chymase and angiotensin I-converting enzyme gene polymorphisms on gastric cancer risks in Japan. Cancer Epidemiol Biomarkers Prev. 2006;15:1929–1934. doi: 10.1158/1055-9965.EPI-06-0339. [DOI] [PubMed] [Google Scholar]
- 25.van der Knaap R, Siemes C, Coebergh JW, van Duijn CM, Hofman A, Stricker BH. Renin-angiotensin system inhibitors, angiotensin I-converting enzyme gene insertion/deletion polymorphism, and cancer: the Rotterdam study. Cancer. 2008;112:748–757. doi: 10.1002/cncr.23215. [DOI] [PubMed] [Google Scholar]
- 26.Nacak M, Nacak I, Sanli M, Ozkur M, Pektaş M, Aynacioğlu AS. Association of angiotensin converting enzyme gene insertion/deletion polymorphism with lung cancer in Turkey. Cancer Genet Cytogenet. 2010;198:22–26. doi: 10.1016/j.cancergencyto.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 27.Nikiteas N, Tsigris C, Chatzitheofylaktou A, Yannopoulos A. No Association with risk for colorectal cancer of the insertion/deletion polymorphism which affects levels of angiotensin-converting enzyme. In Vivo. 2007;21:1065–1068. [PubMed] [Google Scholar]
- 28.Kupcinskas J, Wex T, Bornschein J, Selgrad M, Leja M, Juozaityte E, Kiudelis G, Jonaitis L, Malfertheiner P. Lack of association between gene polymorphismsof angiotensin converting enzyme, Nod-like receptor 1, Toll-like receptor 4, FAS/FASL and the presence of helicobacter pylori-induced premalignant gastric lesions and gastric cancer in Caucasians. BMC Med Genet. 2011;12:112. doi: 10.1186/1471-2350-12-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yaren A, Turgut S, Kursunluoglu R, Oztop I, Turgut G, Kelten C, Erdem E. Association between the polymorphism of the angiotensin-converting enzyme gene and tumor size of breast cancer in premenopausal patients. Tohoku J Exp Med. 2006;210:109–116. doi: 10.1620/tjem.210.109. [DOI] [PubMed] [Google Scholar]
- 30.Usmani BA, Janeczko M, Shen R, Mazumdar M, Papandreou CN, Nanus DM. Analysis of the insertion/deletion polymorphism of the human angiotensin converting enzyme (ACE) gene in patients with renal cancer. Br J Cancer. 2000;82:550–552. doi: 10.1054/bjoc.1999.0962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yigit B, Bozkurt N, Narter F, Yilmaz H, Yucebas E, Isbir T. Effects of ACE I/D polymorphism on prostate cancer risk, tumor grade and metastatis. Anticancer Res. 2007;27:933–936. [PubMed] [Google Scholar]
- 32.Koh WP, Yuan JM, Sun CL, van den Berg D, Seow A, Lee HP, Yu MC. Angiotensin I-converting enzyme (ACE) gene polymorphism and breast cancer risk among Chinese women in Singapore. Cancer Res. 2003;63:573–578. [PubMed] [Google Scholar]
- 33.Srivastava K, Srivastava A, Mittal B. Angiotensin I-converting enzyme insertion/deletion polymorphism and increased risk of gall bladder cancer in women. DNA Cell Biol. 2010;29:417–422. doi: 10.1089/dna.2010.1033. [DOI] [PubMed] [Google Scholar]
- 34.Yuan F, Zhang LS, Li HY, Liao M, Lv M, Zhang C. Influence of Angiotensin I-converting enzyme gene polymorphism on hepatocellular carcinoma risk in China. DNA Cell Biol. 2013;32:268–273. doi: 10.1089/dna.2012.1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yaren A, Oztop I, Turgut S, Turgut G, Degirmencioglu S, Demirpence M. Angiotensin-converting enzyme gene polymorphism is associated with anemia in non small-cell lung cancer. Exp Biol Med (Maywood) 2008;233:32–37. doi: 10.3181/0705-RM-141. [DOI] [PubMed] [Google Scholar]
- 36.Alves Corrêa SA, Ribeiro de Noronha SM, Nogueira-de-Souza NC, Valleta de Carvalho C, Massad Costa AM, Juvenal Linhares J, Vieira Gomes MT, Guerreiro da Silva ID. Association between the angiotensin-converting enzyme (insertion/deletion) and angiotensin II type 1receptor (A1166C) polymorphisms and breast cancer among Brazilian women. J Renin Angiotensin Aldosterone Syst. 2009;10:51–58. doi: 10.1177/1470320309102317. [DOI] [PubMed] [Google Scholar]
- 37.Gao M, Wang Y, Shi Y, Liu D, Liang Y, Yu Y, Zhaobin J, Zhu L, Jin S. The relationship between three well-characterized polymorphisms of the angiotensin converting enzyme gene and lung cancer risk: a case-control study and a meta-analysis. J Renin Angiotensin Aldosterone Syst. 2012;13:455–460. doi: 10.1177/1470320312443912. [DOI] [PubMed] [Google Scholar]
- 38.Lukic S, Nikolic A, Alempijevic T, Popovic D, Sokic Milutinovic A, Ugljesic M, Knezevic S, Milicic B, Dinic D, Radojkovic D. Angiotensin-converting enzyme gene insertion/deletion polymorphism in patients with chronic pancreatitis and pancreatic cancer. Dig Surg. 2011;28:258–262. doi: 10.1159/000328666. [DOI] [PubMed] [Google Scholar]
- 39.Goto Y, Ando T, Nishio K, Ishida Y, Kawai S, Goto H, Hamajima N. The ACE gene polymorphism is associated with the incidence of gastric cancer among H. pylori seropositive subjects with atrophic gastritis. Asian Pac J Cancer Prev. 2005;6:464–467. [PubMed] [Google Scholar]
- 40.Toma M, Cimponeriu D, Apostol P, Stavarachi M, Cojocaru M, Belusică L, Crăciun AM, Radu I, Gavrilă L. Lack of association between ACE ID polymorphism and colorectal cancer in Romanian patients. Chirurgia (Bucur) 2009;104:553–556. [PubMed] [Google Scholar]
- 41.Haiman CA, Henderson SO, Bretsky P, Kolonel LN, Henderson BE. Genetic variation in angiotensin I-converting enzyme (ACE) and breast cancer risk: the multiethnic cohort. Cancer Res. 2003;63:6984–6987. [PubMed] [Google Scholar]
- 42.Li ZH, Pan XM, Han BW, Han HB, Zhang Z, Gao LB. No association between ACE polymorphism and risk of nasopharyngeal carcinoma. J Renin Angiotensin Aldosterone Syst. 2012;13:210–215. doi: 10.1177/1470320311426168. [DOI] [PubMed] [Google Scholar]
- 43.Mendizábal-Ruiz AP, Morales J, Castro Martinez X, Gutierrez Rubio SA, Valdez L, Vásquez-Camacho JG, Sanchez Corona J, Moran Moguel MC. RAS polymorphisms in cancerous and benign breast tissue. J Renin Angiotensin Aldosterone Syst. 2011;12:85–92. doi: 10.1177/1470320310383735. [DOI] [PubMed] [Google Scholar]
- 44.Cheon KT, Choi KH, Lee HB, Park SK, Rhee YK, Lee YC. Gene polymorphisms of endothelial nitric oxide synthase and angiotensin-converting enzyme in patients with lung cancer. Lung. 2000;178:351–360. doi: 10.1007/s004080000039. [DOI] [PubMed] [Google Scholar]
- 45.Ding XJ. Analysis of the relationship between the polymorphism of angiotensin-converting enzyme gene and lung cancer (Chinese) 2008 doi: 10.3779/j.issn.1009-3419.2005.03.11. [DOI] [PubMed] [Google Scholar]
- 46.Holla L, Vasku A, Soucek M, Krahulcova E, Hajek D, Znojil V. Angiotensin-converting enzyme gene polymorphism and blood groups of the ABO system in Leukaemias compared with controls and hypertensives. Scripta Medica (Brno) 1998;71:471–477. [Google Scholar]
- 47.Röcken C, Neumann K, Carl-McGrath S, Lage H, Ebert MP, Dierkes J, Jacobi CA, Kalmuk S, Neuhaus P, Neumann U. The gene polymorphism of the angiotensin I-converting enzyme correlates with tumor size and patient survival in colorectal cancer patients. Neoplasia. 2007;9:716–722. doi: 10.1593/neo.07418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tunny TJ, Xu L, Richardson KA, Stowasser M, Gartside M, Gordon RD. Insertion/deletion polymorphism of the angiotensin-converting enzyme gene and loss of the insertion allele in aldosterone-producing adenoma. J Hum Hypertens. 1996;10:827–830. [PubMed] [Google Scholar]
- 49.Ozen F, Polat F, Arslan S, Ozdemir O. Combined germline variations of thrombophilic genes promote genesis of lung cancer. Asian Pac J Cancer Prev. 2013;14:5449–5454. doi: 10.7314/apjcp.2013.14.9.5449. [DOI] [PubMed] [Google Scholar]
- 50.Vaskù V, Vaskù JA, Goldbergová MP, Vaskù A. Association of variants in angiotensin-converting enzyme and endothelin-1 genes with phototherapy in cutaneous T-cell lymphoma. Acta Dermatovenerol Alp Panonica Adriat. 2004;13:111–116. 118. [PubMed] [Google Scholar]
- 51.Wang HW, Nie ZS, Duan YZ, Liu DD, Han ZH, Zhang XH, Hua B. Association between polymorphism in ACE gene with lung cancer and COPD patients (Chinese) 2000 [Google Scholar]
- 52.Zhang QZ, Liu XM, Zhang ZG, Wang LH. Analysis of the relationship between polymorphism of angiotensin-converting enzyme gene and lung cancer. Zhongguo Fei Ai Za Zhi. 2005;8:211–214. doi: 10.3779/j.issn.1009-3419.2005.03.11. [DOI] [PubMed] [Google Scholar]
- 53.Zhang Y, He J, Deng Y, Zhang J, Li X, Xiang Z, Huang H, Tian C, Huang J, Fan H. The insertion/deletion (I/D) polymorphism in the Angiotensin-converting enzyme gene and cancer risk: a meta-analysis. BMC Med Genet. 2011;12:159. doi: 10.1186/1471-2350-12-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mendoza J, Martinez J, Hernandez C, Perez-Montiel D, Castro C, Fabián-Morales E, Santibáñez M, González-Barrios R, Díaz-Chávez J, Andonegui MA, Reynoso N, Oñate LF, Jiménez MA, Núñez M, Dyer R, Herrera LA. Linkage of angiotensin I-converting enzyme gene insertion/deletion polymorphism to the progression of human prostate cancer. J Pathol. 2004;202:330–335. doi: 10.1002/path.1529. [DOI] [PubMed] [Google Scholar]
- 55.Uemura H, Ishiguro H, Nakaigawa N, Nagashima Y, Miyoshi Y, Fujinami K, Sakaguchi A, Kubota Y. Angiotensin II receptor blocker shows antiproliferative activity in prostate cancer cells: a possibility of tyrosine kinase inhibitor of growth factor. Mol Cancer Ther. 2003;2:1139–1147. [PubMed] [Google Scholar]
- 56.Fujita M, Hayashi I, Yamashina S, Fukamizu A, Itoman M, Majima M. Angiotensin type 1a receptor signaling-dependent induction of vascular endothelial growth factor in stroma is relevant to tumor-associated angiogenesis and tumor growth. Carcinogenesis. 2005;26:271–279. doi: 10.1093/carcin/bgh324. [DOI] [PubMed] [Google Scholar]
- 57.Wu J, Akaike T, Hayashida K, Miyamoto Y, Nakagawa T, Miyakawa K, Müller-Esterl W, Maeda H. Identification of bradykinin receptors in clinical cancer specimens and murine tumor tissues. Int J Cancer. 2002;98:29–35. doi: 10.1002/ijc.10142. [DOI] [PubMed] [Google Scholar]
- 58.Harrap SB, Davidson HR, Connor JM, Soubrier F, Corvol P, Fraser R, Foy CJ, Watt GC. The angiotensin I converting enzyme gene and predisposition to high blood pressure. Hypertension. 1993;21:455–460. doi: 10.1161/01.hyp.21.4.455. [DOI] [PubMed] [Google Scholar]