Abstract
Background: Gliosarcoma (GS) is a rare high-grade malignant tumor with poor prognosis. The survival period of GS ranges from 4 to 18.5 months. Rarely would it be over 40 months. Survival of intraventricular GS is less than 8 months. Methods: There were 2 cases of primary gliosarcoma in our hospital with long-term survival after resection, with one of pure intraventricular origin. We confirmed that our diagnosis was correct by light microscopy, GFAP immunohistochemistry and histochemistry of reticular fiber staining. Results: In the first case, a 47-year-old man with intraventricular gliosarcoma survived for 130 months after surgery. In another case, a 63-year-old woman survived for 4 years after resection. Both cases of GS exhibited biphasic glioblastoma and fibrosarcoma with necrosis. According to the review of surgical records, complete tumor resections, including extended resections were carried out in both cases. The two patients received postoperative radiation therapy and chemotherapy without any further recurrence and metastasis. Conclusions: We reported two cases of GS with long survival. The presented cases demonstrate that, in rare instances, gliosarcoma may show prolonged survival with after surgical excision combined with radiotherapy and chemotherapy.
Keywords: Primary gliosarcoma, intraventricular gliosarcoma, long survival
Introduction
Gliosarcoma (GS) is a rare high-grade malignant tumor. It is a glioblastoma (GBM) variant characterized by a biphasic glial and mesenchymal component [1]. It was first described by Stroebe et al. in 1895 [2] and increasingly recognized by Feigen et al. in 1955 [3] by detailed histological analyses. Initially, GS was considered a collision between two independent tumors. Sarcoma was believed to come from the proliferation of the vascular component [4]. In recent years, with the progression of genetic research, the occurrence of similar genetic alterations in both glial and mesenchymal components suggests a monoclonal origin [5]. The incidence of GS among patients with GS and GBM is between 1.8 and 8% [1,6]. GS is predominantly located in the cerebral hemisphere and rarely located in intraventricular areas. GS has a poor prognosis with an average of 4 months survival in untreated patients [7]. Two cases of primary GS with long-term survival, including one of an intraventricular GS reported in this essay. According to our knowledge, only a few reports on intraventricular GS (IVGS) [8-13] or long-survival GS [14-16] have been published. Immunohistochemical staining of P16, EGFR, P53, CD68, and Ki-67 was performed and the clinical and pathological features of the GS were analyzed with review of the literature.
Materials and methods
Two cases of primary GS were retrieved in Peking Union Medical College Hospital. All tissues were fixed in 10% neutral buffered formalin, routinely processed, and embedded in paraffin. Hematoxylin-eosin stained sections were reviewed independently by two experienced pathologists. The immunohistochemical staining of GFAP (polyclonal, Z0334, dilution 1:50; Dako), EGFR (monoclonal, ZA0505, dilution 1:100; ZSGB-Bio), P53 (monoclonal, code PAb 1801, prediluted; Fuzhou Maixin-Bio), P16 (monoclonal, ZM0205, prediluted; ZSGB-Bio), Ki-67 (monoclonal, ZA0502, prediluted; ZSGB-Bio), CD68 (monoclonal, ZM0060, prediluted; ZSGB-Bio) were performed in 2 cases. They were performed on 4 μm thick unstained sections cut from representative formalin-fixed paraffin-embedded blocks. For all markers, positive control and negative control were used. For GFAP and CD68, signals that appeared as tan particles in cytoplasm were positive. For EGFR, >10% tumor cell with tan granules on membrane were positive. For P53, Ki-67, tan particles in nucleus were positive. For P16, tan particles in nucleus and/or cytoplasm were positive. The sections had no tan particles in corresponding locations, so the result was negative. The histochemical staining of reticulin was also performed in 2 cases by Gordon and Sweet method, black particles in reticular fiber was positive.
Results
Case 1
A 47-year-old male patient had a two-month history of intermittent headache and a one-week history of right leg weakness. Neither nausea nor vomiting was accompanied. Neurological examination revealed positive findings in the right lower limb about hemiplegia test, chaddoch sign, cordon sign and heel-to-knee-to-shin test, with no other positive findings. Computed Tomography (CT) of the brain indicated an isointense to hyperintense lesion of 4 × 3.5 cm in size in front of the third ventricle. It was extending into both sides of ventricles with the anterior horn and body of the left lateral ventricle being occupied; Magnetic Resonance Imaging (MRI) of the brain demonstrated a hypointense lesion on T1 Weighted Image (WI) in the left ventricle (Figure 1A), an isointense to hyperintense on T2WI and hyperintense lesion on Flair WI. On gadolinium injection, the mass demonstrated heterogeneous enhancement, with the core of hypointense. On coronal imaging it appeared that the mass was located in corpus callosum with upward growth, protruding into both sides of lateral ventricle, with the left anterior horn of lateral ventricle being occupied. The mass was well-demarcated and there was obstructive hydrocephalus. The clinical diagnosis of intraventricular mass was that it was a high-grade glioma or ependymoma. In operative view, the lesion was within the left lateral ventricle and through the hole it protruded into to the right lateral ventricle and the third ventricle. The tumor was close to the lower body wall of the lateral ventricle and grew from there. The 4.3 × 4 × 4.5 cm-sized tumor seemed smoothly on the surface with adequate blood supply being resected along the surrounding gliosis belt around the tumor. Subsequently, the patient received chemotherapy (nimustine hydrochloride; 150 mg; carotid artery infusion under digital subtraction angiography) and post-operative 6MV-X ray three-dimensional conformal radiotherapy (the tumor area wild 40 Gy/20 times and shrinking the tumor area wild 16 Gy/8 times; up to a total DT 56Gy/28 times, 2 Gy/day, 5 times/week). We followed up the patient for 130 months after the operation and found no tumor recurrence or metastasis (Figure 1B).
Figure 1.
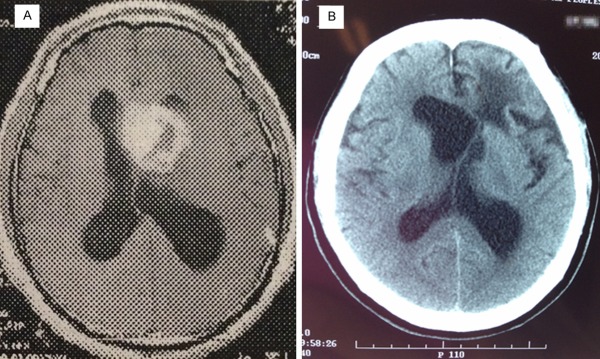
MRI scan of the tumor in Case 1 in 2003 demonstrates an irregular and uneven ring-shaped heterogeneous enhanced lesions around front left lateral ventricle, right shift of brain midline due to pressure (A: axial T-1 contrast-enhanced images). CT scan in 2014 revealed irregular structure in the anterior horn of the left lateralventricle, left shift of brain midline and a large cystic low-density areasin the anterior horn of the left lateral ventricle (B: axial plane).
Gross examination showed the tumor to be generally grey-white tissue with a total size of 5 × 4 × 2.8 cm, partial surface with capsule; the cut surface was grey-pink, solid and firm, with some areas being yellow and soft. Microscopic examination found a mix of glial and sarcomatous components (Figure 2A) with geographic necrosis (Figure 2B). The glial component was composed of large atypical cells, and some of multinucleated giant cells. Mitoses and pathological mitoses were easily found (Figure 2C). The sarcomatous element was composed of fibrosarcoma and no heterologous differentiation was found when mixed with glial components. The glial components were GFAP-positive (Figure 2D) while the mesenchymal component was GFAP-negative. The sarcomatous component was rich in reticulin (Figure 2E). P16 (Figure 2F), P53 were positive in both components, but EGFR, CD68 were negative. Ki-67 index was 20%.
Figure 2.
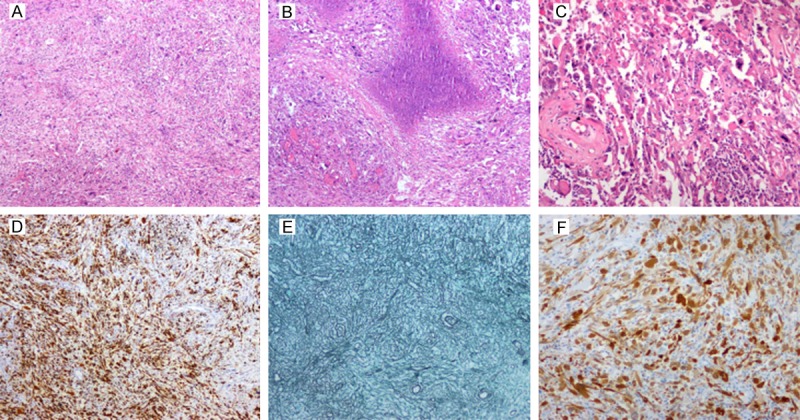
Section of the tumor in Case 1 shows a mix of fusiform glial and sarcomatous areas and the glial components are split into pieces with distinct sarcomatous cells (A: H&E, original ×40). Another area shows irregular necrosis (B: H&E, original ×40) and significant cellular atypia, mitosis and visible pathological mitosis (C: H&E, original ×150). Sections with GFAP (D: GFAP, ×150) and reticulin (E: Reticulin, ×150) show the GFAP-positive, reticulin-poor glial areas contrasting with GFAP-negative, reticulin-rich sarcomatous components. Both areas are uniformly positive for P16 (F: P16, ×150).
Case 2
A 63-year-old female patient had a 15-day history of pulsating headache in the frontal and parietal location with nausea. There was no fever, vomiting, blurred vision or significant limb weakness. Neurological examination showed no positive findings. MRI revealed a large tumor measuring 4.5 × 4 × 4 cm in the middle and rear of the right temporal lobe (Figure 3A). It was solid with cystic areas; a mass having heterogeneous enhancement and an irregular border. There was marked peritumoral edema, from the right of midline to the left. Preoperatively, a malignant glioma was suspected and the patient was scheduled for surgery. A right temporal craniotomy and decompressive craniectomy were performed. In the operation, the tumor was located in the temporal lobe, 3 cm from temporal pole, allowing sight of an approximately 4.5 cm diameter pale tumor which was completely resected; also resected was peritumoral brain tissue of about 1 cm thickness. Subsequently, she received post-operative 6MV-X ray three-dimensional conformal radiotherapy (the tumor area wild 50 Gy/25 times and shrinking the tumor area wild 10 Gy/5 times; up to a total DT 60 Gy/30 times, 2 Gy/day, 5 times/week) and chemotherapy (Temozolomide; 75 mg/m2.d, two doses). We conducted a postoperative follow-up for 48 months and found no tumor recurrence or metastasis (Figure 3B).
Figure 3.
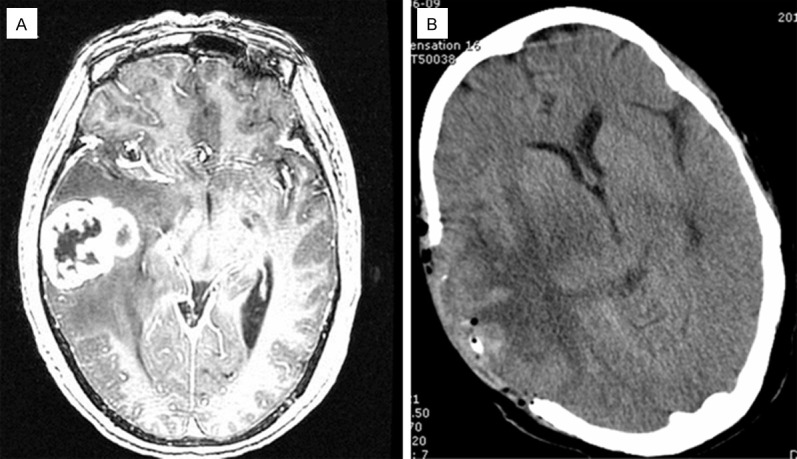
Preoperative MRI scan of the tumor in case 2 reveals a lobulated enhanced lesion in right temporal lobe, cystic area in middle and large edema around lesions (A: axial T-1 contrast-enhanced images). Postoperative CT scan displayed defections in the right temporal lobe partial skull, no residual lesions and a huge area of edema in original brain lesions (B: axial plane).
Gross examination revealed that the tumor was gray-white color with a cystic area of 1.5 cm diameter and the solid component exhibited bleeding and a soft yellow zone around the cystic area while the other area was firm. Histology revealed a distinct biphasic pattern of glial and mesenchymal components. The mesenchymal cells were spindle-shaped around thick-walled blood vessels with nuclear atypia and mitoses (Figure 4A). The glial cells had gemistocytic morphology (Figure 4B) with degeneration and necrosis; the tumor cells had nuclear atypia and more mitoses. The glial components were GFAP-positive while the mesenchymal component was GFAP-negative. P16, P53 and EGFR (Figure 4C) were also positive in both components. CD68 was negative. Ki-67 index was 50%. The sarcomatous component was rich in reticulin.
Figure 4.
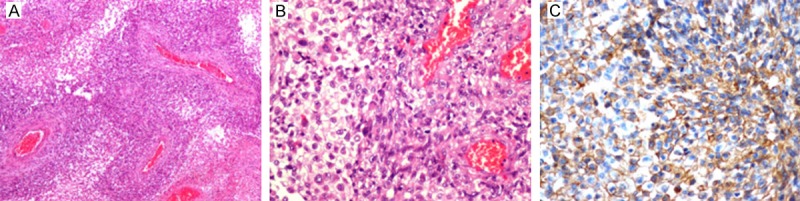
Section of the tumor in Case 2 showing spindle-shaped sarcomatous components grow around the thick-walled vessel; outside the sarcomatous cells is glial components (A: H&E, original ×40). The glial cells have gemistocytic morphology (B: H&E, original ×300). Immunohistochemical staining for EGFR demonstrates positivity in tumor cells (C: EGFR, ×300).
Discussion
Gliosarcoma (GS) is an uncommon and highly malignant tumor of the central nervous system (CNS) and is a subtype of GBM. The 2007 World Health Organization classification identified GS as a grade IV neoplasm [1]. Its clinical presentation, prognosis and therapy are similar to that of GBM [1]. In terms of age distribution, GS is common among people aged between 50 and 70 [14,17-24], with a median age of diagnosis over 60 years [19] and a slight male preponderance, a very small number of cases could occur in children [1,7,9,17-24]. The course of the disease was consistent with those of rapidly expanding intracranial tumors. The major clinical manifestations include effects of risen intracranial tension, such as headache and vomiting. There may also be some aphasia, hemiparesis, seizures and cognitive decline, depending on tumor location [9,22,25]. GS usually occurs in the cerebral hemisphere and involves the temporal, frontal, parietal and occipital lobe [1,7,25]. Rarely can GS be seen in the posterior fossa or spinal cord, while a few reports recorded a higher rate of incidence in the frontal lobe [17]. Unifocal GS is the most often formal and multifocal GS like metastasis that has also been reported. GS has a rapid progression and poor prognosis, and the mean survival time of GS has been reported from 4 to 18.5 months (Table 1) [17-26] while, if untreated, the mean survival is only four months. Winkler et al. [14] reported that the longest patient survival known following initial diagnosis has been 22 years.
Table 1.
Clinical data on gliosarcoma as reported in literature
| Author, year, ref no. | No. of patients | Age (mean or median age) in years | Sex | Survival time |
|---|---|---|---|---|
| Winkler et al., 2000, [14] | 1 | 61 | F | 264 mths |
| Salvati et al., 2005, [18] | 11 | 39-81 (52) | 9M/2F | 15.75 mths |
| Han L et al., 2008, [9] | 15 | 15-71 (44.5) | 11M/4F | 3-30 mths (mean 10.5 mths) |
| Kozak et al., 2009, [19] | 353 | 21-90 (63) | 216M/137F | 9 mths (median) |
| Han SJ et al., 2010, [25] | 219 | NM | NM | 4-11.5 mths (mean) |
| Kang et al., 2011, [20] | 12 | 25-75 (54) | 10M/2F | 13.4 mths |
| Biswas et al., 2011, [22] | 17 | 22-61 (50) | 9M/8F | 8.27 mths (median) |
| Lee et al., 2012, [21] | 26 | 11-84 (51) | 16M/10F | 12 days-36.7 mths (mean 14.4 mths) |
| Singh et al., 2012, [26] | 22 | 7-65 (37.5) | 16M/6F | 1.5-46 mths (mean 18.5 mths) |
| Walker et al., 2013, [6] | 46 | 24-92 (56) | 31M/15F | 12.5 (median) |
| Burzynski et al., 2013, [16] | 1 | 9 | M | 156 mths |
| Romero et al., 2013, [23] | 5 | 54.2 | 5M/1F | 10 mths (median) |
| Linhares et al., 2014, [15] | 1 | 51 | F | 36 mths |
| Damodaran et al., 2014, [24] | 19 | 34-86 (58.6) | 11M/8F | 9.7 (median) |
| Current study | 2 | 47.63 | M, F | 130 mths, 48 mths |
NM: not mentioned; F: female; M: male.
Common intraventricular gliomas are ependymoma and subependymoma, subependymal giant cell astrocytoma and high-grade astrocytomas. Intraventricular GBM is exceedingly rare and most often occurs in the lateral ventricle involving body and frontal horn and arises from periventricular glial elements [25]. Intraventricular gliosarcoma (IVGS) is rare too. Only seven cases have been reported in the literature (Table 2). Of these cases, one lateral IVGS arose in an ependymoma [8]; the other four cases were pure IVGS [10-13] and there were two IVGS reported in a 15 case report into MRI of primary cerebral GS [9]. In these seven cases, survival ranged from 10 days to 8 months and the mean survival was 3 months. We describe the eighth case of 130 months survival in the world literature without any further recurrence or metastasis, and this is the first case of IVGS with long survival. In addition, another case of GS located in the temporal lobe with 48 months survival without any further recurrence or metastasis has been followed up. The two long-term survival cases may make us wonder whether the patient had a highly malignant tumor. What is behind the long-term survival? The causes will be explored.
Table 2.
Clinical data on intraventricular gliosarcoma as reported in literature
| Author, year ref no. | Age/Gender | Clinical symptoms | Tumor size, cm | Location | Traetment | Follow up |
|---|---|---|---|---|---|---|
| Higashino et al., 2001, [7] | 29/M | Headache, nausea (2 mths) | 6 × 5 | The right lateral ventricle | Resection+RT+CT | 79 days (died) |
| Disseminated along in the spinal canal | ||||||
| Han L et al., 2008, [8] | 36/M | NM | 5.0 × 4 × 4 | Midline, bilateral intraventricular and trunk of corpus callosum | Resection+RT | 8 mths (alive) |
| Han L et al., 2008, [8] | 54/F | Headache (1 mth), right limb palsy (15 days) | 6.8 × 5.5 × 5.9 | Left posterior horn of lateral ventricle | Resection | 1 mth (died) |
| Govindan et al., 2008, [9] | 55/F | Severe intermittent holoacranial headache, vomit (15 days) | NM | The septum and frontal horns of lateral ventricles on both sides | Resection | 10 days (died) |
| Altered sensorium (3 days) | ||||||
| Moiyadi et al., 2010, [10] | 65/M | Progressive left-sided weakness, behavioral disturbances, headaches (3 mths) | NM | Right temporal horn and protruding into the ventricule | Resection+RT | 2 mths (alive, progression neurological deterioration) |
| Transependymal spread | ||||||
| Sarkar et al., 2012, [11] | 60/F | Headache, vomit (1.5 mths) | NM | Septal region, extending into the body and the frontal horn of the ventricle on either sides | Resection+CT | NM |
| Social disinhibition (3 week) | ||||||
| Baldawa et al., 2013, [12] | 18/F | Holocranial headache, vomit (1 mths) | 3 × 3 | Left temporal horn of left ventricle | Resection+RT | 4 mths (alive, progressive neurogicasl deterioration) |
| Diplopia (1 week) | Transependymal spread on imaging | |||||
| Current study | 47/M | Headache (2 mths), right-sided weakness (1 week) | 4.3 × 4 × 4.5 | The frontal horn of left ventricle and estending into both sides ventricle, an and trunk of corpus callosum d | Resection+RT+CT | 130 mths (alive, no recurrence, no metastases) |
RT: radiotherapy; CT: chemotherapy; NM: not mentioned.
GS reported closely related to receive radiation exposure, in particular to secondary GS. The two cases reported above had no history of radiation exposure. The genetic alterations associated with the occurrence of GS had PTEN mutation, P16 deletion and TP53 mutation and lack EGFR amplification (0% - 8%). According to the study by Reis et al., GS has a higher frequency of TP53 mutation and a lower frequency of EGFR amplification than primary GBM. On the other hand, GS has a higher frequency of PTEN mutation and P16 deletion but a lower frequency of TP53 mutation than secondary GBM. The genetic alterations in GS are intermediate-between those of primary and secondary GBM [5]. The case of IVGS by Moiyadi et al. reported P53 positive in both glial and sarcomatous components and EGFR positive only in glial component [11]. In our IVGS case, P53 and P16 were strongly positive in both components, but EGFR was negative. In the other case in our current study, P16, P53 and EGFR were all positive in both two components. In recent years, some studies related to MGMT methylation in GS have been conducted and from these there have emerged two different views. Singh et al. [26] and Kang et al. [20] found MGMT promoter methylation was often present in GS (31.25% and 58.3%), whereas the studies of Lee et al. [21] showed MGMT methylation (11.5%) are rare events in GS. On the matter of MGMT promoter methylation status on survival, Singh et al. and Kang et al. had different conclusions-Kang et al. asserted that patient survival is associated with MGMT methylation status, whereas Singh et al. believed MGMT methylation status did not correlate with survival.
On CT scan, GS can either resemble GBM with lesions, areas of necrosis and heterogeneous enhancement-or it can resemble meningiomas with hyperdense lesions having well-defined borders and homogeneous enhancement [25,27]. On MRI, the tumors showed heterogeneous contrast enhancement, well-demarcated or irregular borders and peritumoral edema. Sometimes, the tumor has cystic components with uneven and thick-walled rim-like or ring enhancement as well as intramural strip enhancement [9,25]. There has been some disagreement on tumor border issues. To be specific, Perry et al. reported that most cases of GS were infiltrating diffusely with ill-defined margins [17]. Our case 2 is a typical solid and cystic mass and has heterogeneous enhancement and irregular borders. Little is known about the features of IVGS on CT or MRI in the eight cases, we found that there had been enhancement in all cases and hypointense or isointense on T1WI, isointense to hyperintense on T2WI in 6 cases, but 2 cases were hypointense on T2WI and only 2 cases showed heterogeneous enhancement. As far as the border of the tumor is concerned, half of the cases were well-demarcated; one in four cases showed irregular borders; but a quarter of the cases were ill-demarcated and revealed trans-ependymal spread. 50% of the cases involved both sides of the ventricle with large tumor and cystic degeneration.
GS consists of biphasic glial and sarcomatous components. Meis et al. defined that a minimum of one confluent sarcomatous area must fill one medium power field [28]. Histologically, the glial component fulfills the cytological criteria of anaplastic astrocytoma, often, GBM. The sarcomatous areas commonly resemble fibrosarcoma, but may show a variety of lines of mesenchymal differentiation, such as osteogenic, chondrogenic, adipogenic, smooth and skeletal muscle [1,25]. Reticulin and GFAP helped to define the glial and mesenchymal elements; the sarcomatous areas were reticulin-rich and GFAP-negative, otherwise, the glial component would be reticulin-poor and GFAP-positive. Vimentin was positive in two components, so it was not significant in identifying them. In our two cases, the glial element was pleomorphic GBM with positive GFAP and the sarcomatous was fibrosarcoma with reticulin-rich sarcoma. In one case the sarcomatous component was around the thick-walled vessels. There were necrosis and no heterologous differentiation.
The biological features of GS closely resemble that of GBM. Yet, GS has a higher propensity (approximately 11% of cases) to metastasize extracranially than GBM via hematogenous dissemination do. The sarcomatous component seems to have a higher propensity for such dissemination, often the only component in the metastases. Han et al. reviewed 34 reports of 219 cases of GS in the literature and found that most extracranial metastases of GS are located in the lung and liver, although there are some reports of metastases on spleen, adrenal glands, kidneys, oral mucus, skin, bone marrows, skull, ribs and cervical lymph nodes. However, dissemination within the neuraxis is less common but still has been reported [25]. The intraventricular location of GS can cause trans-ependymal spread or spread throughout Cerebral Spinal Fluid (CSF) pathways, resulting in rapid dissemination within the neuraxis [13].There are two cases of IVGS with trans-ependymal spread [11,13], of which one case was with dissemination along CSF pathways [8].
Gliosarcoma has a poor prognosis. Kozak et al. analyzed a total of 353 GS and 16035 GBM patients and concluded that the prognosis for GS patients appears slightly worse than that observed for GBM patients [19]. The management of primary GS described included maximal surgery, post-operative radiotherapy and chemotherapy with nitrosourea, misonidazole, dacarbazine, mithramycin, ametophterin, thalidomide, temozolomide, irinotecan, vincritine, cisplatin and ordoxorubicn [25]. The effect of radiation (RT) or chemotherapy is not clear. Perry et al. have demonstrated improved survival in patients undergoing post-operative radiation compared to those who were kept under observation after surgery (10.6 months versus 6.25 months) [17]. Studies by Kevin et al. suggested that tumor excision, as opposed to biopsy only, and adjuvant RT may improve the outcome [19]. The radiotherapy dose used varies from 45-81Gy [25]. Although chemotherapy with temozolomide (TMZ) is now the standard of care for GBM, the precise role of chemotherapy remains uncertain for GS. In the study by Han et al., 10 patients who received TMZ and RT showed no significant difference in survival than the other 10 patients who received RT with or without other chemotherapy [29]. Salvati et al. [18] and Walker GV et al. [6] have however concluded that TMZ seems to give additional benefits. Singh et al. reported 14 cases with a mean overall survival of 18.5 months with TMZ and suggested that TMZ should be included in GS therapy [26]. Han et al. suggested that patients of GS may warrant specific treatment, separate from conventional GBM therapy [25]. IVGS has a poorer prognosis with survival from 10 days to 8 months on record and mean survival was 3 months [8-13]. In our study, the patients described in the two cases received surgical excision combined with radiotherapy and chemotherapy. We have used 56 and 60Gy in radiotherapy dose, as well as chemotherapy with nimustine hydrochloride in case 1 and TMZ in case 2. The outcome for these two patients confirms that their treatment has been useful. Surprisingly, our case of IVGS (located within the ventricle) would have had a greater potential for trans-ependymal spread or spread through CSF pathways, but there was no proof of spread. In gross examination, the tumor was coated by a membrane and therefore it was safe to conclude that it had limited its growth.
The current study has some limitations. First, the number of samples in the study was relatively small. Second, because it was a retrospective study, some data was not readily available, such as the follow-up treatment in other hospital. Third, there were no result of MGMT methylation and IDH-1 mutation in 2 cases. In this connection, we could not explain adequately the cause why the patients had long-survival and prospective studies should be performed in the future to verify our findings.
In conclusion, we reported two cases of GS with long survival. Evidently, as this report suggests, it is possible to experience long-term survival in GS and IVGS-even though the ventricle is a rare location for GS and has a poorer recorded prognosis. Furthermore, we infer that the fact that the patients mentioned in the two cases remained alive without any recurrence or metastasis for such long time may be attributable to the useful treatment of surgical excision combined with radiotherapy and chemotherapy, but we need to check MGMT methylation and IDH-1 mutation in the following study.
Acknowledgements
We thank Dr. Haibo Zhang who provided radiographic image reading guidance and also thank Dr. Yuchao Qiu for English proofreading.
Disclosure of conflict of interest
None.
References
- 1.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK. WHO classification of tumours of the central nervous system. 4th edition. Lyon: IARC; 2007. Gliosarcoma; pp. 48–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stroebe H. Ueber Entstehung und Bauder Gehirnglioma. Beitr Pathol Anat Allg Pathol. 1895;19:405–486. [Google Scholar]
- 3.Feigin IH, Gross SW. Sarcoma arising in glioblastoma of the brain. Am J Pathol. 1955;31:633–653. [PMC free article] [PubMed] [Google Scholar]
- 4.Feigin I, Allen LB, Lipkin L, Gross SW. The endothelial hyperplasia of the cerebral blood vessels with brain tumors, and its sarcomatous transformation. Cancer. 1958;11:264–277. doi: 10.1002/1097-0142(195803/04)11:2<264::aid-cncr2820110207>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 5.Reis RM, Könü-Lebleblicioglu D, Lopes JM, Kleihues P, Ohgaki H. Genetic profile of gliosarcomas. Am J Pathol. 2000;156:425–432. doi: 10.1016/S0002-9440(10)64746-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walker GV, Gilbert MR, Prabhu SS, Brown PD, McAleer MF. Temozolomide use in adult patients with gliosarcoma: an evolving clinical practice. J Neurooncol. 2013;112:83–89. doi: 10.1007/s11060-012-1029-7. [DOI] [PubMed] [Google Scholar]
- 7.Morantz RA, Feigin I, Ransohoff J. Clinical and pathological study of 24 cases of gliosarcoma. J Neurosurg. 1976;45:398–408. doi: 10.3171/jns.1976.45.4.0398. [DOI] [PubMed] [Google Scholar]
- 8.Higashino T, Inamura T, Kawashima M, Ikezaki K, Miyazono M, Yoshiura T, Iwaki T, Fukui M. A lateral ventricular gliosarcoma arising in an ependymoma. Clin Neuropathol. 2001;20:219–223. [PubMed] [Google Scholar]
- 9.Han L, Zhang X, Qiu S, Li X, Xiong W, Zhang Y, Qu H, Chang R, Chen B, Wang W, Li S. Magnetic resonance imaging of primary cerebral gliosarcoma: a report of 15 cases. Acta Radiol. 2008;l49:1058–1067. doi: 10.1080/02841850802314796. [DOI] [PubMed] [Google Scholar]
- 10.Govindan A, Bhat DI, Mahadevan A, Chakraborti S, Sampath S, Chandramouli BA, Shankar SK. An unusual case of intraventricular gliosarcoma. Clin Neuropathol. 2009;28:379–383. doi: 10.5414/npp28379. [DOI] [PubMed] [Google Scholar]
- 11.Moiyadi A, Sridhar E, Jalali R. Intraventricular gliosarcoma: unusual location of an uncommon tumor. J Neurooncol. 2010;96:291–294. doi: 10.1007/s11060-009-9952-y. [DOI] [PubMed] [Google Scholar]
- 12.Sarkar H, K S, Ghosh S. Pure intraventricular origin of gliosarcoma - a rare entity. Turk Neurosurg. 2013;23:392–394. doi: 10.5137/1019-5149.JTN.5436-12.0. [DOI] [PubMed] [Google Scholar]
- 13.Baldawa S, Kasegaonkar P, Vani S, Kelkar G. Primary intraventricular gliosarcoma. Clin Neuropathol. 2013;32:525–528. doi: 10.5414/NP300607. [DOI] [PubMed] [Google Scholar]
- 14.Winkler PA, Büttner A, Tomezzoli A, Weis S. Histologically repeatedly confirmed gliosarcoma with long survival: review of the literature and report of a case. Acta Neurochir (Wien) 2000;142:91–95. doi: 10.1007/s007010050012. [DOI] [PubMed] [Google Scholar]
- 15.Linhares P, Martinho O, Carvalho B, Castro L, Lopes JM, Vaz R, Reis RM. Analysis of a synchronous gliosarcoma and meningioma with long survival: A case report and review of the literature. Surg Neurol Int. 2013;27:151. doi: 10.4103/2152-7806.122229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burzynski SR, Janicki TJ, Burzynski GS, Marszalek A. Long-term Survival (>13 Years) in a Child with Recurrent Diffuse Pontine Gliosarcoma: A Case Report. J Pediatr Hematol Oncol. 2013 OTC; doi: 10.1097/MPH.0000000000000020. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perry JR, Ang LC, Bilbao JM, Muller PJ. Clinicopathologic features of primary and postirradiation cerebral gliosarcoma. Cancer. 1995;75:2910–2918. doi: 10.1002/1097-0142(19950615)75:12<2910::aid-cncr2820751219>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 18.Salvati M, Caroli E, Raco A, Giangaspero F, Delfini R, Ferrante L. Gliosarcomas: analysis of 11 cases do two subtypes exist? J Neurooncol. 2005;74:59–63. doi: 10.1007/s11060-004-5949-8. [DOI] [PubMed] [Google Scholar]
- 19.Kozak KR, Mahadevan A, Moody JS. Adult gliosarcoma: epidemiology, natural history, and factors associated with outcome. Neuro Oncol. 2009;11:183–191. doi: 10.1215/15228517-2008-076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang SH, Park KJ, Kim CY, Yu MO, Park CK, Park SH, Chung YG. O6-methylguanine DNA methyltransferase status determined by promoter methylation and immunohistochemistry in gliosarcoma and their clinical implications. J Neurooncol. 2011;101:477–486. doi: 10.1007/s11060-010-0267-9. [DOI] [PubMed] [Google Scholar]
- 21.Lee D, Kang SY, Suh YL, Jeong JY, Lee JI, Nam DH. Clinicopathologic and genomic features of gliosarcomas. J Neurooncol. 2012;107:643–650. doi: 10.1007/s11060-011-0790-3. [DOI] [PubMed] [Google Scholar]
- 22.Biswas A, Kumar N, Kumar P, Vasishta RK, Gupta K, Sharma SC, Patel F, Mathuriya SN. Primary gliosarcoma--clinical experience from a regional cancer centre in north India. Br J Neurosurg. 2011;25:723–729. doi: 10.3109/02688697.2011.570881. [DOI] [PubMed] [Google Scholar]
- 23.Romero-Rojas AE, Diaz-Perez JA, Ariza-Serrano LM, Amaro D, Lozano-Castillo A. Primary gliosarcoma of the brain: radiologic and histopathologic features. Neuroradiol J. 2013;26:639–648. doi: 10.1177/197140091302600606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Damodaran O, van Heerden J, Nowak AK, Bynevelt M, McDonald K, Marsh J, Lee G. Clinical management and survival outcomes of gliosarcomas in the era of multimodality therapy. J Clin Neurosci. 2014;21:478–481. doi: 10.1016/j.jocn.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 25.Han SJ, Yang I, Tihan T, Prados MD, Parsa AT. Primary gliosarcoma: key clinical and pathologic distinctions from glioblastoma with implications as a unique oncologic entity. J Neurooncol. 2010;96:313–320. doi: 10.1007/s11060-009-9973-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh G, Mallick S, Sharma V, Joshi N, Purkait S, Jha P, Sharma MC, Suri V, Julka PK, Mahapatra AK, Singh M, Kale SS, Sarkar C. A study of clinico-pathological parameters and O6-methylguanine DNA methyltransferase (MGMT) promoter methylation status in the prognostication of gliosarcoma. Neuropathology. 2012;32:534–542. doi: 10.1111/j.1440-1789.2012.01297.x. [DOI] [PubMed] [Google Scholar]
- 27.Jelinek J, Smirniotopoulos JG, Parisi JE, Kanzer M. Lateral ventricular neoplasms of the brain: differential diagnosis based on clinical, CT, and MRI findings. AJR Am J Neuroradiol. 1990;155:365–72. doi: 10.2214/ajr.155.2.2115270. [DOI] [PubMed] [Google Scholar]
- 28.Meis JM, Martz KL, Nelson JS. Mixed glioblastoma multiforme and sarcoma. A clinicopathologic study of 26 radiation therapy oncology group cases. Cancer. 1991;67:2342–2349. doi: 10.1002/1097-0142(19910501)67:9<2342::aid-cncr2820670922>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 29.Han SJ, Yang I, Ahn BJ, Otero JJ, Tihan T, McDermott MW, Berger MS, Prados MD, Parsa AT. Clinical characteristics and outcomes for a modern series of primary gliosarcoma patients. Cancer. 2010;116:1358–1366. doi: 10.1002/cncr.24857. [DOI] [PubMed] [Google Scholar]


