Abstract
Background: Human herpesvirus 8 (HHV8)-positive plasmablastic lymphoma is a disease which correlates with acquired immunodeficiency syndrome (AIDS). Little is known about the pathogenesis of the disease due to its rarity. We report an autopsy case about AIDS related HHV-8-positive plasmablastic lymphoma and presents an examination about HHV8 related proteins for the disease by using immunohistochemical techniques. Case presentation: Two kinds of tumors complicated the male AIDS patient: one was HHV-8-positive plasmablastic lymphoma and the other was Kaposi’s sarcoma (KS). Immunohistochemically, the lymphoma cells were positive for HHV8-associated lytic early proteins as well as HHV8 latency-associated nuclear antigen 1 (LANA-1), and, on the other hand, the lymphoma cells were negative for lytic immediately early proteins. KS was positive for only LANA-1. Conclusion: These findings indicate that the lymphoma cells acquired an ability to proliferate without de novo HHV8 replication. Moreover, the onset mechanisms of HHV-8-positive plasmablastic lymphoma may be different from those of KS.
Keywords: HHV-8-associated plasmablastic lymphoma, large B-cell lymphoma arising in HHV8-associated multicentric Castleman’s disease, HHV-8 latency-associated nuclear antigen 1, open reading frame 59, viral interleukin-6
Introduction
Infection with human herpesvirus 8 (HHV-8), also known as Kaposi’s sarcoma-associated herpesvirus, can lead to the development of Kaposi’s sarcoma (KS), primary effusion lymphoma (PEL), or multicentric Castleman’s disease (MCD). KS frequently occurs in HIV-infected men who have sex with men, whereas the occurrence of PEL and MCD in HIV-1 infected individuals is relatively rare [1]. MCD is a lymphoproliferative disorder of unknown etiology that presents with systematic lymphadenopathy and splenomegaly. Typical laboratory abnormalities observed in MCD include anemia, thrombocytosis or thrombocytopenia, elevated inflammatory markers, and high levels of interleukin 6 (IL-6) expression. HHV-8 is commonly associated with MCD in patients with acquired immunodeficiency syndrome (AIDS) [2]. Although MCD is recognized as a non-neoplastic disease, HHV-8-positive plasmablasts localize in the mantle zone of B-cell follicles [3]. Monoclonal expansions of the HHV-8-positive plasmablasts are referred to as microlymphomas. The extranodal and/or massive expansion by HHV-8-positive plasmablasts is defined as HHV-8-positive plasmablastic lymphoma and is classified by the World Health Organization as “large B-cell lymphoma (LBL) arising in HHV-8-associated multicentric Castleman’s disease” [4]. HHV-8-positive plasmablastic lymphoma is distinguished from plasmablastic lymphoma which usually occurs in oral cavity of AIDS patients and is not associated with HHV-8 infection.
The HHV-8 genome encodes more than 80 viral genes/proteins. Like other types of herpes viruses, HHV-8 genes are classified as latent genes or lytic genes, which include immediate-early, early, and late genes [5]. Latency-associated nuclear antigen 1 (LANA-1) is always expressed in HHV-8-infected cells. Among the lytic viral proteins, the replication and transcription activator protein (RTA) encoded by the open reading frame 50 genes (ORF50) of HHV-8 serves a critical role in switching from latency to the lytic phase, and the K8 protein is a transactivator of other lytic proteins. Early proteins such as ORF59 and viral interleukin 6 (vIL-6) are expressed after the immediate-early proteins [6,7]. Immunohistochemical studies have demonstrated that HHV-8-encoded lytic proteins such as RTA, K8, ORF59, and vIL-6 are expressed in plasmablasts in MCD lesions, while KS cells predominantly express LANA-1, but rarely express lytic proteins [8]. Differences in expression patterns of HHV-8-encoded proteins suggest they serve different roles in KS and MCD. However, the expression profiles of HHV-8-encoded proteins are unknown in LBL arising in HHV-8 MCD or HHV-8-positive plasmablastic lymphoma.
Herein, we report expression patterns of HHV-8-encoded proteins in lymphoma cells from an autopsy case with AIDS-related HHV-8-positive plasmablastic lymphoma, as determined by immunohistochemical techniques.
Clinical summary
A 46-year-old homosexual man presented with an exanthema in his thigh. Pathological examination revealed that the patient had KS, prompting us to determine his HIV/AIDS status. Following diagnosis with AIDS, antiretroviral therapy was initiated, as was anticancer therapy including liposomal doxorubicin for treating KS. Two years after being diagnosed with AIDS, he was admitted to our hospital with high fever and splenomegaly. Laboratory analyses revealed an elevated C-reactive protein level (11.3 mg/dL), thrombocytopenia (4.4 × 104 platelets/mL), and increased HHV-8 copy number in the peripheral blood (2.9 × 104 HHV-8 copies/106 white blood cells). Although no histological examination was performed, MCD was strongly suspected, and treatment with liposomal doxorubicin and valganciclovir was initiated. Although the HHV-8 copy number in peripheral blood decreased markedly, he developed progressive dyspnea and chylothorax and died 3 years following the diagnosis of AIDS.
Pathological findings
The autopsy revealed tumors of 1 cm in diameter in both the ureters. Histologically, the tumor was characterized by the infiltration of plasmablast-like large cells with irregularly shaped nuclei and abundant cytoplasm (Figure 1A). Immunohistochemical staining showed that the tumor cells were positive for CD45 (leukocyte common antigen) and CD138, but negative for CD3, CD4, CD8, CD20, and CD79a. Moreover, almost all tumor cells were positive for HHV-8 LANA-1 (Figure 1B), cytoplasmic-IgM, and the immunoglobulin lambda light chain. The Epstein-Barr virus was not detected in the tumor cells by in situ hybridization. On the basis of these findings, the tumor was diagnosed as an HHV-8-positive plasmablastic lymphoma [4]. Immunohistochemistry performed using antibodies specific for HHV-8-encoded proteins [9] revealed that 3-10% of the lymphoma cells were positive for two early proteins of HHV-8, namely, ORF59 (Figure 1C) and vIL-6 (Figure 1D), whereas the RTA and K8 proteins were not expressed in the lymphoma cells. In addition to the ureters, lymphoma cells were observed in the lung, heart, bone marrow, urinary bladder, mesenterium, and skin. In the lung, there were many lymphoma cell embolisms in pleural lymphatic vessels; these were suspected to cause severe chylothorax and dyspnea (Figure 1E).
Figure 1.
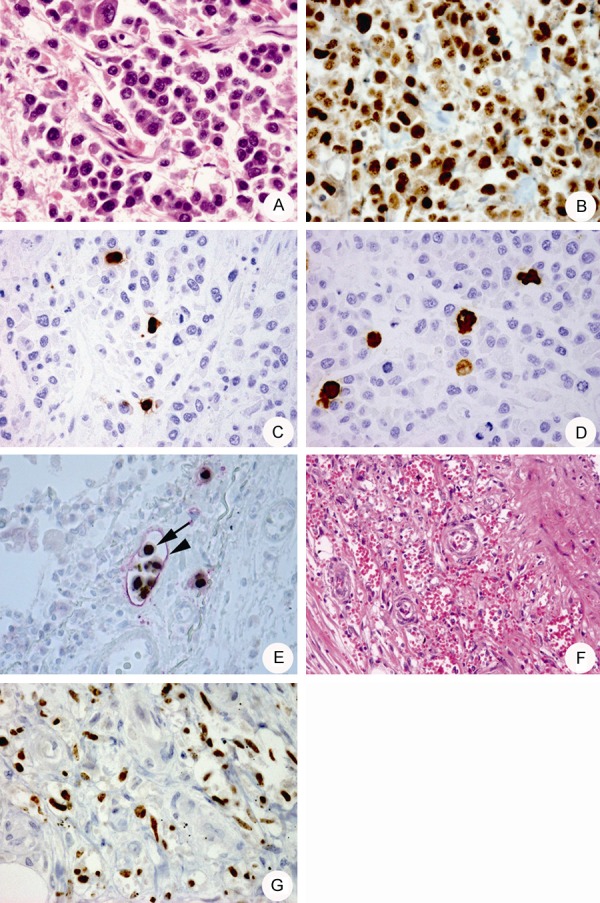
(A) Microscopic morphology of a ureter tumor, following hematoxylin and eosin (HE) staining. 400× magnification. (B) HHV-8 LANA-1 detection in tumor cells with punctate staining. 400× magnification. (C, D) A small number of ORF59 (C) and vIL-6 (D) positive tumor cells are observed. 400× magnification. (E) Histology of a lymphoma cell embolism in the pleural lymphatic vessels with double immunohistostaining using antibodies against HHV-8 LANA-1 (arrow) or D2-40 (arrowhead). 400× magnification. (F) Histology of the Kaposi’s sarcoma (KS) in the ureter (HE stain). 100× magnification. (G) Detection of HHV-8 LANA-1 expression in KS cells. 400× magnification.
KS lesions remained in the thigh and were also observed in the ureters adjacent to the lymphoma lesion (Figure 1F). Although LANA-1 was detected in the KS cells (Figure 1G), the other HHV-8-associated proteins, including RTA, ORF59, vIL-6, and K8 were not detected. The spleen showed marked atrophy and no MCD lesions were observed in the spleen or lymph nodes at the time of the autopsy.
Discussion
In the present study, we demonstrated expression patterns of HHV-8-encoded proteins in a case of HHV-8-positive plasmablastic lymphoma. In this case, the lymphoma cells partially expressed ORF59 and vIL-6, but not RTA or K8. To our knowledge, this is the first report describing the detailed expression patterns of HHV-8 proteins in HHV-8-positive plasmablastic lymphoma. RTA and K8 expression are necessary and sufficient for viral replication in HHV-8-infected cells [10], suggesting that the proliferation of lymphoma cells occurred without de novo HHV-8 replication. This possibility is supported by the observation that the growth and infiltration of lymphoma cells had progressed, while the HHV-8 copy number in peripheral blood markedly decreased.
In this patient, MCD lesions could not be proved histologically during his lifetime or at the time of autopsy. A high copy number of HHV-8 in the peripheral blood is a useful marker for MCD activity, and liposomal doxorubicin used to treat KS not only reduces HHV-8 copy numbers but is also effective in treating MCD [8,11,12]. The occurrence of HHV-8-positive plasmablastic lymphomas and a high copy number of HHV-8 in the peripheral blood strongly suggest that MCD lesions were present before the onset of HHV-8-positive plasmablastic lymphoma. Unlike KS, HHV-8-infected cells in mantle zones of follicles in MCD lesions express lytic proteins, indicating that de novo replication of HHV-8 is associated with the pathogenesis of MCD [9]. Clinical studies have showed that anti-herpes virus treatment with valganciclovir is effective for improving MCD [12]. However, the present case demonstrated that HHV-8 replication did not occur in HHV-8-positive plasmablastic lymphoma, although the lymphoma is thought to arise from HHV-8-positive plasmablasts in MCD lesions. Thus, this case suggests that HHV-8-positive plasmablastic lymphoma cells underwent additional transformations from HHV-8-positive plasmablasts, resulting in increased proliferation without de novo HHV-8 replication.
Consequently, this case suggests that HHV-8-positive plasmablastic lymphoma cells show different HHV-8 protein expression patterns compared to HHV-8-positive plasmablasts in MCD lesions. High copy numbers of HHV-8 in peripheral blood samples can be a diagnostic marker of MCD, but not for HHV-8-positive plasmablastic lymphoma. Reduction in HHV-8 copies in peripheral blood may reflect improvement in MCD; however, attention should be given to the onset of HHV-8-positive plasmablastic lymphoma after the reduction in HHV-8 copies in the peripheral blood of patients with MCD. Because the prognosis of HHV-8-associated lymphomas is very poor, the accumulation of clinical data and the elucidation of mechanisms underlying the development of lymphomas are expected to greatly benefit the establishment of effective treatment.
Acknowledgements
The authors are grateful to Kouji Fujita for performing the immunohistochemical stains.
Disclosure of conflict of interest
None.
References
- 1.Ota Y, Hishima T, Mochizuki M, Kodama Y, Moritani S, Oyaizu N, Mine S, Ajisawa A, Tanuma J, Uehira T, Hagiwara S, Yajima K, Koizumi Y, Shirasaka T, Kojima Y, Nagai H, Yokomaku Y, Shiozawa Y, Koibuchi T, Iwamoto A, Oka S, Hasegawa H, Okada S, Katano H. Classification of AIDS-related lymphoma cases between 1987 and 2012 in Japan based on the WHO classification of lymphomas, fourth edition. Cancer Med. 2014;3:143–153. doi: 10.1002/cam4.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soulier J, Grollet L, Oksenhendler E, Cacoub P, Cazals-Hatem D, Babinet P, d’Agay MF, Clauvel JP, Raphael M, Degos L, et al. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman’s disease. Blood. 1995;86:1276–1280. [PubMed] [Google Scholar]
- 3.Du MQ, Liu H, Diss TC, Ye H, Hamoudi RA, Dupin N, Meignin V, Oksenhendler E, Boshoff C, Isaacson PG. Kaposi sarcoma-associated herpesvirus infects monotypic (IgM lambda) but polyclonal naive B cells in Castleman disease and associated lymphoproliferative disorders. Blood. 2001;97:2130–2136. doi: 10.1182/blood.v97.7.2130. [DOI] [PubMed] [Google Scholar]
- 4.Isaacson PG, Campo E, Harris NL. WHO classification of tumours of haematopoietic and lymphoid tissues. World Health Organization classification of tumours. 4th edition. International Agency for Research on Cancer; 2008. Large B-cell lymphoma arising in HHV-8 associated multicentric Catleman disease; pp. 258–259. [Google Scholar]
- 5.Zhu FX, Cusano T, Yuan Y. Identification of the immediate-early transcripts of Kaposi’s sarcoma-associated herpesvirus. J Virol. 1999;73:5556–5567. doi: 10.1128/jvi.73.7.5556-5567.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore PS, Boshoff C, Weiss RA, Chang Y. Molecular mimicry of human cytokine and cytokine response pathway genes by KSHV. Science. 1996;274:1739–1744. doi: 10.1126/science.274.5293.1739. [DOI] [PubMed] [Google Scholar]
- 7.Xiao Y, Chen J, Liao Q, Wu Y, Peng C, Chen X. Lytic infection of Kaposi’s sarcoma-associated herpesvirus induces DNA double-strand breaks and impairs non-homologous end joining. J Gen Virol. 2013;94:1870–1875. doi: 10.1099/vir.0.053033-0. [DOI] [PubMed] [Google Scholar]
- 8.Abe Y, Matsubara D, Gatanaga H, Oka S, Kimura S, Sasao Y, Saitoh K, Fujii T, Sato Y, Sata T, Katano H. Distinct expression of Kaposi’s sarcoma-associated herpesvirus-encoded proteins in Kaposi’s sarcoma and multicentric Castleman’s disease. Pathol Int. 2006;56:617–624. doi: 10.1111/j.1440-1827.2006.02017.x. [DOI] [PubMed] [Google Scholar]
- 9.Katano H, Sato Y, Kurata T, Mori S, Sata T. Expression and localization of human herpesvirus 8-encoded proteins in primary effusion lymphoma, Kaposi’s sarcoma, and multicentric Castleman’s disease. Virology. 2000;269:335–344. doi: 10.1006/viro.2000.0196. [DOI] [PubMed] [Google Scholar]
- 10.West JT, Wood C. The role of Kaposi’s sarcoma-associated herpesvirus/human herpesvirus-8 regulator of transcription activation (RTA) in control of gene expression. Oncogene. 2003;22:5150–5163. doi: 10.1038/sj.onc.1206555. [DOI] [PubMed] [Google Scholar]
- 11.Bollen J, Polstra A, Van Der Kuyl A, Weel J, Noorduyn L, Van Oers M, Cornelissen M. Multicentric Castleman’s disease and Kaposi’s sarcoma in a cyclosporin treated, HIV-1 negative patient: case report. BMC Blood Disord. 2003;3:3. doi: 10.1186/1471-2326-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casper C, Krantz EM, Corey L, Kuntz SR, Wang J, Selke S, Hamilton S, Huang ML, Wald A. Valganciclovir for suppression of human herpesvirus-8 replication: a randomized, double-blind, placebo-controlled, crossover trial. J Infect Dis. 2008;198:23–30. doi: 10.1086/588820. [DOI] [PMC free article] [PubMed] [Google Scholar]


