Abstract
Intraspecific assisted migration (ISAM) through seed transfer during artificial forest regeneration has been suggested as an adaptation strategy to enhance forest resilience and productivity under future climate. In this study, we assessed the risks and benefits of ISAM in white spruce based on long-term and multilocation, rangewide provenance test data. Our results indicate that the adaptive capacity and growth potential of white spruce varied considerably among 245 range-wide provenances sampled across North America; however, the results revealed that local populations could be outperformed by nonlocal ones. Provenances originating from south-central Ontario and southwestern Québec, Canada, close to the southern edge of the species' natural distribution, demonstrated superior growth in more northerly environments compared with local populations and performed much better than populations from western Canada and Alaska, United States. During the 19–28 years between planting and measurement, the southern provenances have not been more susceptible to freezing damage compared with local populations, indicating they have the potential to be used now for the reforestation of more northerly planting sites; based on changing temperature, these seed sources potentially could maintain or increase white spruce productivity at or above historical levels at northern sites. A universal response function (URF), which uses climatic variables to predict provenance performance across field trials, indicated a relatively weak relationship between provenance performance and the climate at provenance origin. Consequently, the URF from this study did not provide information useful to ISAM. The ecological and economic importance of conserving white spruce genetic resources in south-central Ontario and southwestern Québec for use in ISAM is discussed.
Keywords: Assisted migration, genetic conservation, genetic gain, geographic genetic variation, local adaptation, universal response function
Introduction
Climate change is projected to alter the growing conditions of forest trees considerably (Rahmstorf and Ganopolski 1999; IPCC 2007; McKenney et al. 2007). The likely scenarios of future climate for the boreal regions of North America include higher temperature, longer growing season, and more frequent climate extremes such as prolonged drought (McKenney et al. 2007; IPCC 2012). As long-lived organisms, boreal forest tree species may face considerable challenges in adapting to changes in climate that are expected to be more rapid than those that occurred in the past (Savolainen et al. 2007; Johnston et al. 2009).
A potential consequence of climate change for forest ecosystems is the disruption of evolutionary equilibrium between local forest populations and their surrounding environments. Having evolved under local selection pressures, especially those imposed by the colder climate of the postglacial and preindustrial era for thousands of years, forest populations have become more or less adapted to their local climatic conditions (Savolainen et al. 2007; Aitken et al. 2008). Local adaptation of plant and forest tree species across the landscape has resulted in population differentiation of morphological and physiological traits (Skrøppa 1982; Blum 1988; Rehfeldt 1989; Sork et al. 1993; Li et al. 1997; Wu and Ying 2004) that may be detected by functional gene markers (Namroud et al. 2008; Fournier-Level et al. 2011). Knowledge and observations about the evolutionary process of local adaptation have established the principle that “local seed is best,” which is often explicitly or implicitly assumed in delineating seed zones for tree species to guide artificial forest regeneration (Campbell 1979; Rehfeldt 1989; Parker 1992). Unfortunately, a rapidly changing climate and the adaptation “lags” of forest tree populations resulting from their long lifespan and limited gene flow distance (Aitken 2000; Young et al. 2000) may have disrupted the rate of synchronization. Results from provenance trials of tree species have suggested that: (1) at least for quantitative traits such as growth rate, local climatic conditions are becoming suboptimal for some forest populations; and (2) the potential for enhanced land productivity resulting from a warmer climate in boreal forest regions will not be fully realized by local tree populations (Schmidtling 1994; Carter 1996; Morgenstern et al. 2006; Wang et al. 2006; Rweyyongeza et al. 2011).
Intraspecific assisted migration (ISAM) (Leech et al. 2011), a forest management practice that guides tree seed transfer within a species' natural range during artificial forest regeneration, can be an effective strategy to enhance forest adaptation to climate change, as well as to increase forest productivity (Johnston et al. 2009). Abundant information suggests that forest species with widespread natural distribution have large natural genetic variation in adaptation and growth among geographical natural populations, or provenances, which have evolved in synchronization with local climate (Morgenstern and Mullin 1990; Matyas and Yeatman 1992; Li et al. 1997; Morgenstern and Copis 1999). Understanding provenances' adaptation and growth potential in their native growing environments could improve our understanding of the stability and resilience of present forests, while also indicating their vulnerability to climate change. Matching provenances to their optimal growing environments through remapping their geographic deployment zones (Wang et al. 2006; Ying and Yanchuk 2006; Thomson and Parker 2008) could potentially improve the stability and resilience of newly regenerated forests, at least temporarily, depending on the rate of climate change. By enabling the full expression of tree growth potential, and hence higher forest productivity (Johnston et al. 2009), ISAM may also result in greater rates of carbon sequestration and ecological resilience in boreal forests (Leech et al. 2011).
Long-term rangewide provenance trials are a valuable source of information for understanding vulnerability to impacts from climate change and the development of ISAM strategies (Matyas 1994; Carter 1996; Wang et al. 2006). Data from rangewide provenance tests reveal landscape-level intraspecific genetic variability and its spatial trends. Additionally, multiple field trial locations under varying climatic conditions, particularly long-term data from older trials, can enhance data reliability in revealing the both vulnerability and adaptive capacity (such as to frost and drought) of individual provenances and their interactions with climate of existing forests and of planting sites using ISAM. The use of such information for ISAM has been demonstrated using provenance response and transfer functions (Rehfeldt et al. 1999; Wang et al. 2006, 2010; Hamann et al. 2011). Because of the high value of information from provenance test data in guiding assisted migration, trials involving wide range provenances (from California to the Yukon) of 18 commercially important species were recently established in multiple locations along a broad geographic region and climatic gradient (O'Neill et al. 2013).
White spruce (Picea glauca [Moench] Voss) is one of the few boreal conifer species with a transcontinental natural distribution in North America (Nienstaedt and Zasada 1990). In Ontario, Canada, white spruce is a major component of natural forests and provides many products of economic value to the province (Ontario Ministry of Natural Resources [OMNR], 2006). During the late 1970s and early 1980s, the Canadian Forest Service, in cooperation with OMNR, established the 410-series of white spruce rangewide provenance tests, which included 16 field trial locations within Ontario (Morgenstern and Copis 1999). Early results from this series were previously reported for individual trials or provenances (Morgenstern and Copis 1999; Cherry and Parker 2003; Lesser et al. 2004; Morgenstern et al. 2006). In this study, we examine large-scale spatial patterns of genetic variation in adaptation and growth indicators of white spruce provenances across all 16 trials. We anticipate the usefulness of such information in: (1) assisting the development of an ISAM strategy to adapt white spruce to climate change within and outside Ontario and (2) assessing vulnerability of existing forests to climate change, and (3) contributing to policies for conserving genetic resources of this species.
Materials and Methods
Provenance samples & field trial establishment
Provenance seed was sampled from the natural range of white spruce across North America between 1972 and 1976; and 245 provenances were included in the 410-series of experiments across Ontario sites. The objectives of the 410-series were to assess genetic variation across the entire range of white spruce and to examine within-region genetic variation for selected areas, including Ontario (Morgenstern and Copis 1999) (Fig. 1A). Detailed information about the individual provenances was published by Morgenstern and Copis (1999).
Figure 1.
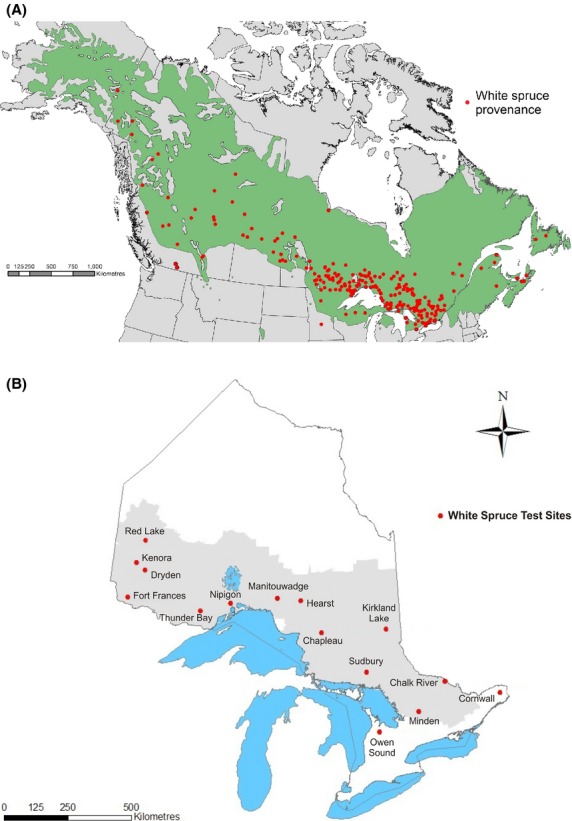
Locations of (A) white spruce provenance origin and (B) field trial sites in Ontario used in the white spruce 410-series rangewide test. Green shading in map (A) indicates the natural range of white spruce distribution and gray shading in map (B) indicates the managed forest area in Ontario.
Between 1978 and 1985, 16 field trials in the 410-series were established within Ontario. Although additional field trials of this series were established in other eastern Canadian provinces, data from these locations were not available for inclusion in our study. Field trial sites in Ontario varied in soil depth and texture and occurred along a climatic gradient (Fig. 1B) that encompassed the provincial range of white spruce growing environments. Summary information, including climate norms (1971–2000) (obtained using the SEEDWHERE software of McKenney et al. 1999) and experimental design for the Ontario trial locations, is presented in Table 1. All trial areas were mechanically site prepared prior to planting. The age of planting stock varied from 1 to 3 years among trials and postplanting (1–2 year) tree survival rate was uniformly high (≥94%; Table 1). The experimental design for each field trial was a randomized complete block design with multiple-tree plots. These were replicated 5–8 times and the number of trees per provenance plot within an experimental block varied from 4 to 10 among the trials (Table 1). No field trials contained all 245 provenances. Rather, each field trial tested a subset of 48–86 provenances. While many of the common provenances overlapped between pairwise trials (Table 2), 105 of the 245 provenances were tested in fewer than four Ontario field trials and only two provenances were tested across all 16 trials.
Table 1.
Location details, composition, and initial survival for the 410-series white spruce provenance trials in Ontario
| Trial location | Lat. (°N) | Long. (°W) | Elev. (m) | MAT (°C) | MAP (mm) | GSL (day) | Tree age | No. of provenances | No. of replications | Trees/plot | Post-planting survival (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cornwall | 45°22′ | 74°44′ | 24 | 5.57 | 1011 | 205 | 23 | 64 | 6 | 8 | 98.0 |
| Chalk River | 45°59′ | 77°26′ | 170 | 4.62 | 846 | 193 | 20 | 71 | 8 | 4 | – |
| Chalk River | 45°59′ | 77°26′ | 170 | 4.62 | 846 | 193 | 20 | 71 | 8 | 4 | – |
| Minden | 45°00′ | 78°53′ | 376 | 4.78 | 1090 | 193 | 25 | 80 | 5 | 10 | 99.0 |
| Kirkland Lake | 48°00′ | 80°20′ | 308 | 1.73 | 839 | 172 | 19 | 80 | 5 | 10 | 97.0 |
| Owen Sound | 44°24′ | 80°55′ | 200 | 6.29 | 1058 | 211 | 22 | 64 | 6 | 8 | 98.0 |
| Sudbury | 46°31′ | 81°24′ | 350 | 3.72 | 878 | 185 | 24 | 86 | 5 | 9 | 97.0 |
| Chapleau | 47°59′ | 83°42′ | 451 | 1.13 | 888 | 165 | 25 | 80 | 5 | 10 | 97.0 |
| Hearst | 49°04′ | 84°53′ | 320 | 0.39 | 789 | 161 | 21 | 85 | 5 | 9 | 94.0 |
| Manitouwadge | 49°12′ | 86°01′ | 300 | 0.92 | 803 | 165 | 26 | 80 | 6 | 10 | 96.0 |
| Nipigon | 48°58′ | 88°32′ | 190 | 1.38 | 724 | 165 | 26 | 80 | 6 | 8 | 98.0 |
| Thunder Bay | 48°38′ | 90°09′ | 475 | 1.56 | 753 | 161 | 26 | 78 | 6 | 5 | 98.0 |
| Dryden | 49°54′ | 93°20′ | 410 | 1.88 | 681 | 176 | 26 | 78 | 5 | 10 | 99.0 |
| Red Lake | 50°56′ | 93°29′ | 370 | 1.19 | 651 | 173 | 21 | 80 | 5 | 9 | 98.0 |
| Kenora | 50°08′ | 93°50′ | 410 | 1.86 | 657 | 177 | 28 | 48 | 5 | 5 | 99.0 |
| Fort Frances | 48°45′ | 93°58′ | 340 | 2.85 | 686 | 181 | 28 | 65 | 5 | 5 | 99.0 |
Lat., long., MAT, MAP, and GSL are latitude, longitude, mean annual temperature, mean annual precipitation, and growing season length, respectively. Two trials were conducted in Chalk River.
Table 2.
Numbers of common provenances of white spruce represented between paired field trial locations
| No. of common provenances | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trial location | Total no. of provenances | Trial no. | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 |
| Cornwall | 64 | 1 | 30 | 29 | 46 | 43 | 28 | 34 | 42 | 25 | 32 | 25 | 37 | 25 | 25 | 14 | 17 |
| Chalk River G1 | 71 | 2 | 70 | 31 | 27 | 22 | 46 | 30 | 53 | 26 | 31 | 24 | 29 | 21 | 9 | 13 | |
| Chalk River G2 | 71 | 3 | 31 | 28 | 21 | 45 | 29 | 54 | 25 | 31 | 23 | 28 | 21 | 9 | 13 | ||
| Minden | 80 | 4 | 30 | 39 | 43 | 61 | 26 | 38 | 27 | 43 | 27 | 28 | 17 | 21 | |||
| Owen Sound | 67 | 5 | 24 | 28 | 28 | 37 | 25 | 28 | 27 | 26 | 25 | 15 | 18 | ||||
| Kirkland Lake | 80 | 6 | 31 | 50 | 30 | 44 | 44 | 36 | 34 | 24 | 14 | 18 | |||||
| Sudbury | 86 | 7 | 47 | 53 | 29 | 29 | 28 | 26 | 25 | 14 | 19 | ||||||
| Chapleau | 80 | 8 | 29 | 40 | 31 | 41 | 28 | 27 | 15 | 20 | |||||||
| Hearst | 85 | 9 | 29 | 38 | 25 | 34 | 28 | 10 | 16 | ||||||||
| Manitouwadge | 80 | 10 | 60 | 68 | 52 | 39 | 25 | 29 | |||||||||
| Nipigon | 80 | 11 | 52 | 55 | 32 | 17 | 22 | ||||||||||
| Thunder Bay | 78 | 12 | 52 | 39 | 26 | 32 | |||||||||||
| Dryden | 78 | 13 | 42 | 24 | 32 | ||||||||||||
| Red Lake | 80 | 14 | 40 | 55 | |||||||||||||
| Kenora | 48 | 15 | 48 | ||||||||||||||
| Fort Frances | 65 | 16 | |||||||||||||||
Two trials were conducted in Chalk River.
Trials were assessed in the summer of 2001 at tree ages from 19 to 28 years and measurements included individual tree height, diameter at breast height (DBH), and tree health status (mortality and mechanical damage). Individual tree stem volume was subsequently estimated using a taper equation (Popovich 1972) developed using data from white spruce plantations grown in Québec, Canada. This equation was independently validated and found to produce unbiased results with high accuracy for white spruce grown in plantations in the upper Great Lakes region of the United States (Rauscher and Harding 1993).
Predicting provenance effects
The experimental data were largely balanced within each of the individual trials. However, because individual trials included only a subset of provenances, the data became highly imbalanced when the 16 trials were pooled to perform a combined analysis, a procedure necessary to evaluate the relative performance of all 245 provenances. In addition to the differing subsets of provenances, data imbalances resulted from differences among field trials in: (1) age of planting stock (1–3 years), (2) tree age at measurement (19–28 years), (3) number of replications per trial (5–8), and (4) number of trees per plot (4–10 trees). Additionally, varying climate, soil conditions, competing vegetation, and damage by insects and frost across trial locations (Morgenstern and Copis 1999) resulted in considerable differences in mean tree sizes among field trials. As such, the marginal means of provenance performance across trials were biased and poor indicators of the intrinsic growth potential of the provenances.
Data standardization is effective in removing scale effects caused by tree size differences in tree genetic evaluation (White and Hodge 1989), thereby satisfying the underlying homogeneous variance assumptions often associated with analytical linear models (Lynch and Walsh 1998; White et al. 2007). In tree genetic data analysis, data standardization is recommended because it does not alter analytical outcomes in either estimating genetic parameters (such as heritability and genetic correlations) or genotype ranking (White et al. 2007). In this study, prior to statistical analysis, data were standardized to transform observations of individual trials with the same mean and variance (which were taken from the summary statistics of pooled data). Because larger phenotypic variance is often associated with larger tree size (White and Hodge 1989), the means and variances were consequently compressed after data standardization for trials of above average size and expanded for trials of below average size.
To evaluate the performance of all 245 rangewide provenances, which were directly or indirectly connected through overlapping common provenances across the 16 field trials (Table 2), a mixed linear model (eq. 1) was used in which the effects of provenances and their interactions with trial locations were treated as random, while trial location and blocks within trials were treated as fixed. In matrix notation, the analytical model was:
| (1) |
where y is the vector of observations of individual trees; X is the incidence matrix of fixed effects included in vector β (e.g., trial locations and blocks within a trial); Z is incidence matrix of random effects in vector u, which comprises provenance and the interaction between provenance and trial location; and e is the vector of residuals. Henderson's mixed linear model equation (Henderson 1984) has provided the theoretical basis for simultaneously yielding best linear unbiased estimation (BLUE) of fixed effects and best linear unbiased prediction (BLUP) of random provenance effects (Searle et al. 1992; Lynch and Walsh 1998; White et al. 2007) for mixed models like equation 1. In this study, this analytical approach permits the utilization of the genetic variance/covariance matrix of provenances across field trials to improve evaluation of provenance effects. The SAS Proc Mixed procedure (SAS Institute 2011) was used to implement the BLUP analysis with standardized data for continuous traits (e.g., tree height, DBH & volume) and the Proc Glimmix procedure was used for binary trait (e.g., survival), respectively.
Modeling geographic patterns and provenance response
The BLUPs of provenance effects were modeled with the geographic coordinates of provenance origins (i.e., latitudinal & longitudinal coordinates) to reveal large-scale spatial patterns of variation in growth and adaptation, which may have differentiated under the influence of local climate in the past. Polynomial curves with the appropriate orders were used to approximate the distribution patterns of data points.
Because most provenances were tested in less than one-third of the field trials, and each field trial included only a subset of the 245 provenances (Table 2), it is less informative to examine the response of individual provenances to climatic conditions of the trial sites. Instead, the growth response by regional provenance groups was examined, with each group representing a geographic area from which a relatively large number of provenances (n ≥ 63) were sampled. Specifically, provenances were assigned to southern (lat. <47°N, n = 84), central (47°N ≥ lat. <50°N, n = 94), and northern (lat. ≥ 50°N, n = 63) regional groups (four provenances were not assigned because of missing geographic coordinates). Although the boundaries of these regional provenance groups were determined arbitrarily, efforts were made to ensure that provenances within a regional group exhibited similar survival and growth patterns. Our exploratory analysis indicated that this classification of regional provenance groups resulted in a clearer delineation of the response patterns to climatic conditions of the trial sites than a classification from a multivariate regression tree analysis (Hamann et al. 2011).
Analysis of regional groups at each of the 16 trial locations provided a simple but insightful indication of the response of white spruce to climatic conditions. For this analysis, the relative growth of provenance groups (expressed as ratio of provenance group mean to the overall trial mean) was plotted against some commonly used thermal indicators of planting site climate, that is, mean annual temperature (MAT), growing season length (GSL), and latitude.
Developing a universal response function
Wang et al. (2010) proposed a multiple regression approach to use climatic variables to predict population performance across test sites, and named the predictive equation a universal response function (URF). Conceptually, climate variables from the site of provenance origin can be superior to geocoordinates in predicting provenance response to climatic conditions at planting sites, because climate at provenance origin could contribute an evolutionary force to cause genetic differentiation and local adaptation (Aitken et al. 2008). Analytically, the URF approach uses climatic variables at provenance origin and those at planting site as independent prediction variables (as well as their quadratic effects and interactions), with population performance (such as mean tree height growth) as the dependent variable (Wang et al. 2010). Using stepwise selection in a multiple regression analysis, the URF may be able to identify influential climatic variables explaining provenance growth variation across planting sites. In this study, we followed Wang et al. (2010) in developing a URF for white spruce based on the 410-series provenance tests data. Climatic variables of both provenance origins and planting sites were generated using SEEDWHERE software (McKenney et al. 1999). Specific climatic variables at provenance origin we included in the analysis included mean annual temperature (MAT_p)(°C), January minimum temperature (JMT_p)(°C), February minimum temperature (FMT_p)(°C), growing season length (GSL_p)(days), annual precipitation (AP_p)(mm), and precipitation of the driest period (PDP_p)(mm). Climatic variables at planting sites were mean annual temperature (MAT_s)(°C), mean annual minimum temperature (MAMT_s)(°C), growing season length (GSL_s)(days) and annual precipitation (AP_s)(mm). The quadratic effects and interactions between the above variables were generated as the product of a climatic variable with itself or with another variable. In total, 43 independent variables were used in model building.
Provenance mean height growth at individual field trials was used as the dependent variable. Because field trials differed in age at measurement (Table 1), an adjustment was made to approximate provenance mean height growth at a standard age of 25 years across field trials. This was estimated by using Goelz and Burk's (1992) base-age invariant site index model, which was parameterized for white spruce by Payandeh and Wang (1995) as:
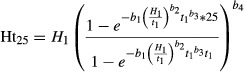 |
(2) |
where Ht25 is the estimated provenance height at age 25, H1 is provenance height measured at the age t1, e is the base of natural logarithm and b1–b4 are model parameter estimates with the values of 5.7336, 0.8236, −1.880 and 2.7703, respectively. The SAS Proc Reg procedure with the stepwise option (SAS Institute 2011) was applied for variable selection. The P values for independent variables to enter and stay in the model were set at 0.15 and 0.05, respectively.
Results
Provenance survival
Initial survival was uniformly high (≥94%) across the 16 field trials, but then declined. At the time of measurement in 2001, survival was <70% at five trial sites (i.e., Kirkland Lake, Hearst, Manitouwadge, Thunder Bay, and Dryden), remained above 80% for 10 sites, with the remaining site (Sudbury) at 72% (Table 3). While individual trees were not assessed to define specific causes of mortality (e.g., pests, frost/cold damage, competition), survival rates were weakly correlated with mean annual temperature (MAT) (P < 0.07) and significantly affected by growing season length (GSL) (P < 0.03; Fig. 2), with shorter GSL and lower MAT associated with lower survival rates.
Table 3.
Survival and mean tree height, diameter at breast height (DBH) and stem volume at time of measurement for 16 white spruce provenance trials in Ontario
| Height (m) | DBH (cm) | Volume (m3) | |||||
|---|---|---|---|---|---|---|---|
| Trial location | Survival (%) | Mean | SD | Mean | SD | Mean | SD |
| Cornwall | 85.7 | 6.90 | 1.85 | 11.51 | 3.87 | 0.064 | 0.067 |
| Chalk River G1 | 92.7 | 5.95 | 1.32 | 10.26 | 2.98 | 0.035 | 0.032 |
| Chalk River G2 | 91.2 | 5.57 | 1.31 | 9.45 | 2.79 | 0.026 | 0.025 |
| Minden | 80.0 | 6.08 | 1.88 | 7.88 | 2.94 | 0.021 | 0.027 |
| Kirkland Lake | 67.3 | 2.89 | 1.18 | 3.51 | 2.15 | 0.001 | 0.002 |
| Owen Sound | 87.0 | 3.46 | 1.00 | 4.67 | 1.65 | 0.003 | 0.006 |
| Sudbury | 72.3 | 5.04 | 1.62 | 6.70 | 2.79 | 0.012 | 0.016 |
| Chapleau | 82.2 | 6.88 | 1.63 | 8.92 | 2.66 | 0.028 | 0.027 |
| Hearst | 64.7 | 5.66 | 1.41 | 9.36 | 3.28 | 0.028 | 0.028 |
| Manitouwadge | 64.6 | 3.39 | 1.45 | 5.12 | 3.19 | 0.007 | 0.014 |
| Nipigon | 84.6 | 4.42 | 1.19 | 6.72 | 2.06 | 0.008 | 0.009 |
| Thunder Bay | 55.8 | 7.20 | 1.54 | 9.93 | 3.00 | 0.040 | 0.038 |
| Dryden | 67.6 | 2.88 | 1.01 | 3.76 | 1.68 | 0.001 | 0.003 |
| Red Lake | 90.4 | 4.32 | 1.14 | 7.51 | 2.61 | 0.011 | 0.012 |
| Kenora | 92.7 | 6.53 | 1.43 | 9.10 | 2.40 | 0.026 | 0.022 |
| Fort Frances | 88.4 | 4.28 | 1.40 | 6.08 | 2.59 | 0.008 | 0.011 |
SD is the standard deviation of measurements within a trial. Two trials were conducted in Chalk River.
Figure 2.
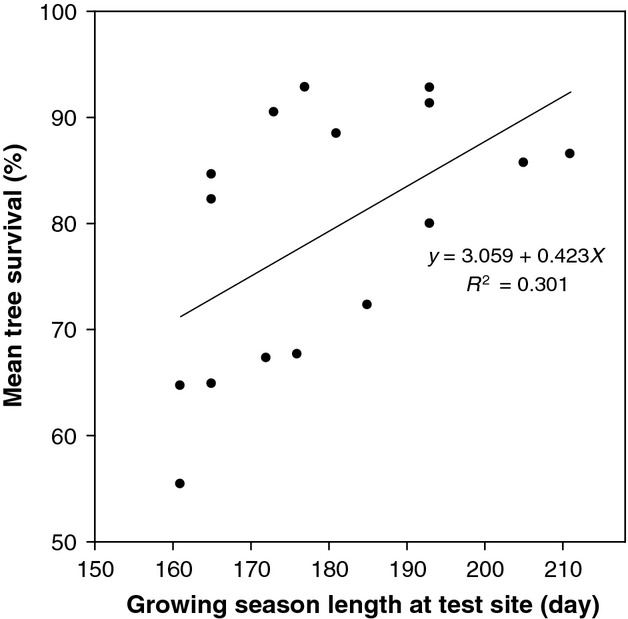
The relationship of provenance survival with growing season length. The linear regression equation and coefficient of determination (R2) are presented.
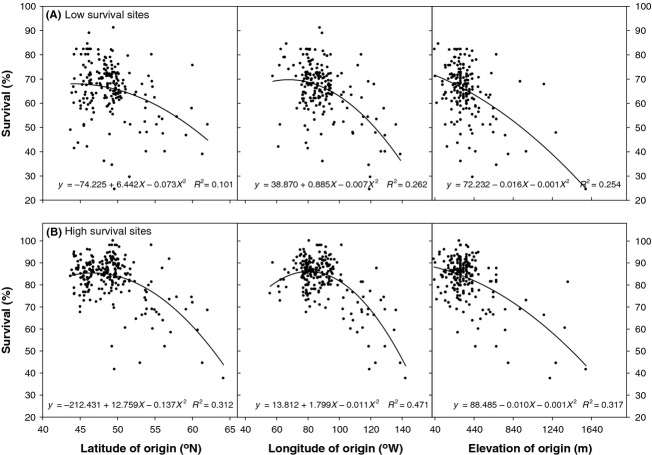
The relationship of survival rate with latitude, longitude, and elevation of provenance origin was examined to elucidate survival differences among provenances (Fig. 3). Across the five test sites with low survival, which involved 196 provenances, the latitudinal, longitudinal, and elevational trends were delineated by statistically significant quadratic polynomial curves (Fig. 3A). Provenances from southern Ontario and southwestern Québec (i.e., lat. <47°N) survived as well as local provenances originating close to the trial locations (i.e., lat. 47°–50°N; long. 85°–94°W) (P > 0.63), and significantly better than provenances from western Canada and Alaska (i.e., lat. > 50°N) (P < 0.004). For the 11 trials with higher survival rates (72–93%), the 241 provenances planted showed patterns of survival (Fig. 3B) similar to the lower survival trials (Fig. 3A), although latitudinal and longitudinal polynomial curves explained a greater portion of the variation in survival among provenances (Fig. 3A and B). These quadratic polynomial curves approximated the survival data better than linear lines, with 5–44% increase in R2 of model fitting although the improvement was not always statistically significant. Combined, these results indicated that across the field trials, provenances from southern Ontario and southwestern Québec demonstrated similar adaptability to those from northern Ontario and eastern Canada and significantly higher adaptability than those from western Canada.
Figure 3.
Relationship of provenance survival with latitude, longitude, and elevation of provenance origin for trial locations with survival of (A) (<70%; n = 5) and (B) (>72%; n = 11). Quadratic polynomial models and coefficients of determination (R2) are presented.
When field trials were grouped into relatively harsh (i.e., MAT < 3°C; 10 trials) and relatively mild (i.e., MAT ≥ 3°C; 6 trials) thermal environments, survival rates of provenances from south-central Ontario and southwestern Québec (i.e., 44°–47°N; 74°–85°W) were again similar to those from northern Ontario and eastern Canada (i.e., 47°–50°N) in the harsh environment; both had significantly better survival (P < 0.004) than provenances from western Canada and Alaska. In the milder environment, white spruce provenances from south-central Ontario survived similar to those from northern Ontario and eastern Canada (87 vs. 85%; P < 0.10); and again survived significantly better than provenances from western Canada and Alaska (85–87 vs. 82%; P < 0.0001).
Provenance growth potential
Tree growth varied considerably across the 16 trial sites, reflecting differences in climate, soil, tree age, etc. (Table 3). After removing inter-trial tree size variation using standardized data, the BLUPs of provenance effects on tree height, DBH, and stem volume varied from 2.70 to 6.08 m (mean 4.9 m), 4.1 to 9.8 cm (mean 7.7 cm), and 0.001 to 0.037 m3 (mean 0.017 m3), respectively, among the 245 rangewide provenances tested. Similar to survival, height, DBH, and stem volume for the rangewide provenances were related to their geographic origins, which were slightly better approximated by quadratic or cubic polynomial curves (Fig. 4) than linear lines, with up to 15% increase in R-square in model fitting (although the improvement may not be statistically significant). Provenances from south-central Ontario and southwestern Québec exhibited the highest growth rates, followed by provenances from northern Ontario and eastern Canada. Provenances from western Canada and Alaska exhibited substantially lower growth rates than those from other regions (Fig. 4; Table 4).
Figure 4.
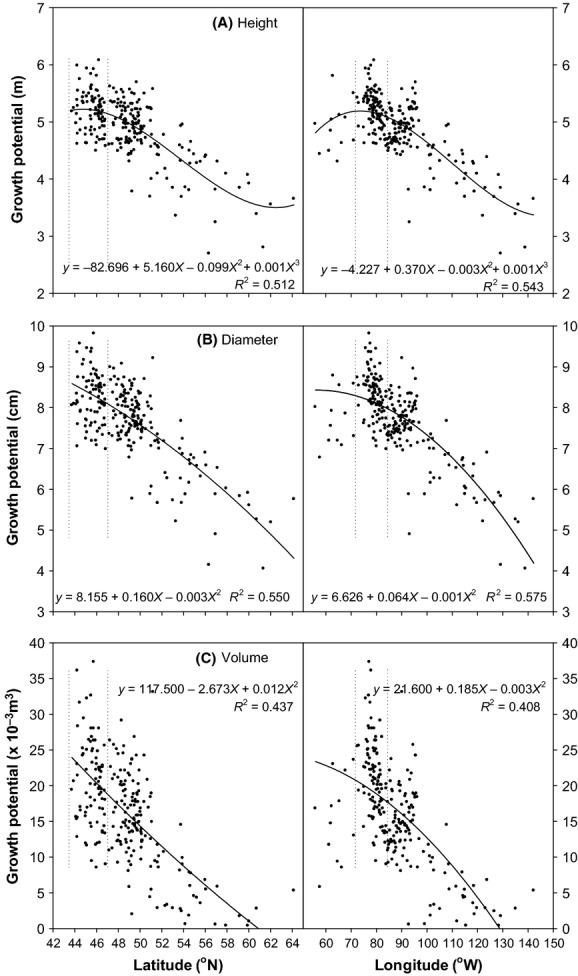
Relationship of (A) tree height, (B) stem diameter, and (C) stem volume growth of provenances with latitude and longitude of provenance origin. The area between the two vertical lines represents the geographic region of provenances from south-central Ontario and southwestern Québec associated with superior tree growth. Polynomial models and coefficients of determination (R2) are presented.
Table 4.
Mean tree height, diameter, and stem volume of three regional provenance groups of white spruce trees measured in 2001
| Regional group (No. of provenances) | Height (m) | DBH (cm) | Volume (m3) |
|---|---|---|---|
| Southern (84) | 5.19a | 8.25a | 0.020a |
| Central (94) | 5.02b | 7.95b | 0.017b |
| Northern (63) | 4.41c | 6.78c | 0.010c |
DBH, diameter at breast height.
The three regional provenance groups are southern (lat. < 47°N), central (47°N ≥ lat. <50°N), and northern (lat. ≥ 50°N). Mean values with different superscript letters differ significantly (P < 0.001) according to Tukey's multiple comparison test.
Response of provenance groups to trial site climate
The three regional provenance groups exhibited contrasting, distinct trends in response to MAT of field trial locations (Fig. 5). In general, the southern group of provenances exhibited the highest relative growth at the warmest sites. This relative growth superiority decreased gradually as MAT decreased across trial locations. When planted further north, where MAT was close to 1°C, the growth of southern provenances was similar to that of local provenances. Growth performance of provenances in the central group, mostly from northern Ontario and eastern Canada, showed the opposite trend. Their growth was comparable with the southern group at northern sites but became increasingly inferior at warmer trial locations. Provenances in the northern group, generally associated with higher latitude and altitude of origin, showed a parallel growth response to that of the central group, but with the intercept of the response line shifted substantially downward (Fig. 5), indicating much lower growth potential. Provenance group-by-trial location interaction was evident, which mainly reflected the relative differences among provenance group means because their rankings remained unchanged.
Figure 5.
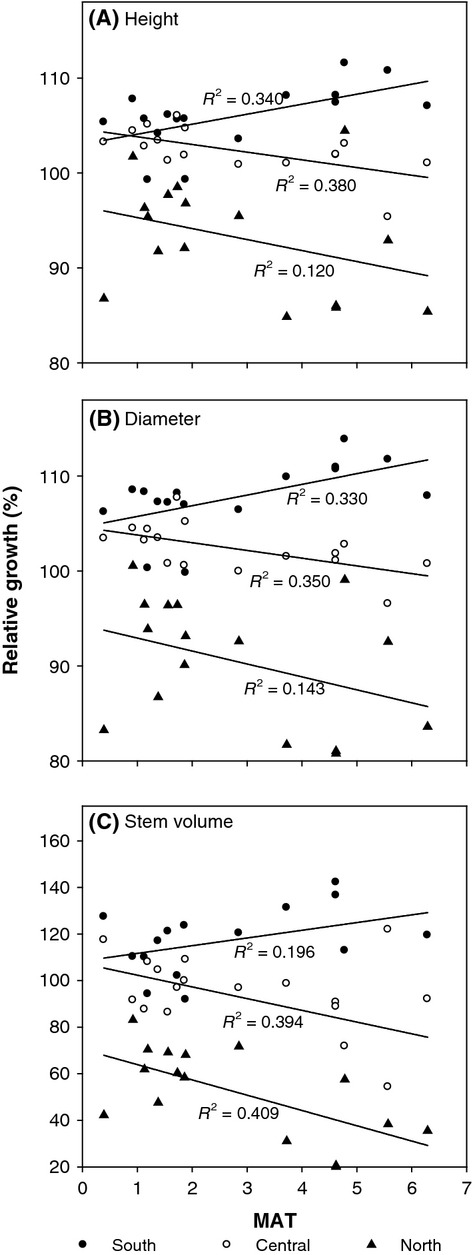
Relative (A) height, (B) diameter, and (C) stem volume growth of three regional provenance groups to trial means as related to the mean annual temperature (MAT) of the field trial locations. The three regional provenance groups are southern (lat. < 47°N, n = 84), central (47°N ≥ lat. <50°N, n = 94) and northern (lat. ≥ 50°N, n = 63), where n is the number of provenances included. Coefficients of determination (R2) for linear regression equations are presented.
Other thermal indicators of field trial sites, such as GSL and latitude, were highly correlated with MAT, and the relationship trends of growth responses of provenance groups with these indicators were similar (data not shown).
The universal response function (URF)
Among the 43 candidate climatic variables used to predict provenance mean height growth across field trials, eight were selected into the URF (cumulative R2 = 0.60) (Table 5), with six of them representing the climatic conditions of field trial locations and two representing interactions between climatic variables at trial locations and at provenance origins. While Mallow's Cp statistic suggested that provenance growth difference could be further explained by adding independent variables, there was no other climatic variables that could stay in the model at the significance level of P < 0.05. The six climatic variables at trial locations were mean annual temperature (MAT_s), mean annual minimum temperature (MAMT_s), annual precipitation (AP_s) and their quadratic effects (i.e., MAT_s2, MAMT_s2 and AP_s2). Predictive climatic variables at provenance origins were mean annual temperature (MAT_p) and February minimum temperature (FMT_p), which interacted with climatic variables at trial locations (i.e., MAT_p × AP_s and FMT_p × GSL_s). In the established URF, no single climatic variable was predominantly predictive of provenance performance as shown by the partial R2s; however, selected climatic variables for trial locations have jointly explained the majority of the growth variation across field trials, with a cumulative partial R2 of 0.539 (Table 5). The interaction between annual mean temperature at provenance origin and annual precipitation at field trial site (MAT_p × AP_s) indicated that a combination of higher MAT_p and greater AP_s could have a positive effect on provenance growth, while the interpretation for the interaction between February mean minimum temperature at provenance origin and the growing season length at field trial site (FMT_p × GSL_s) was not straightforward. Nevertheless, these two interaction terms in the URF accounted for only 0.063 cumulative partial R2.
Table 5.
Multiple regression analysis of provenance mean tree height growth across field test against climatic variables at test locations and provenance origins
| Independent variable | Variable domain | Parameter estimate | Partial R2 | Model R2 | C(p) | F | P |
|---|---|---|---|---|---|---|---|
| Intercept | −44.27 | ||||||
| MAT_s2 (°C) | 0.15–39.56 | 2.41233 | 0.0740 | 0.0740 | 1616.91 | 93.95 | <0.0001 |
| MAMT_s (°C) | −5.96 to 1.51 | −10.22872 | 0.0690 | 0.1431 | 1410.91 | 94.58 | <0.0001 |
| MAMT_s2 | 0.12–35.52 | −1.20823 | 0.1492 | 0.2922 | 963.475 | 247.22 | <0.0001 |
| MAT_s (°C) | 0.39–6.29 | −9.92411 | 0.1127 | 0.4049 | 626.001 | 221.90 | <0.0001 |
| AP_s2 | 423801–1188100 | −0.0000545 | 0.0606 | 0.4655 | 445.404 | 132.78 | <0.0001 |
| AP_s (mm) | 651–1090 | 0.08333 | 0.0737 | 0.5392 | 225.465 | 187.02 | <0.0001 |
| MAT_p × AP_s | −3222 to 7706 | 0.00074057 | 0.0257 | 0.5649 | 149.943 | 69.13 | <0.0001 |
| FMT_p × GSL_s | −5389 to −1360 | −0.00146 | 0.0376 | 0.6025 | 38.6002 | 110.54 | <0.0001 |
MAT, MAMT, AP, FMT, GSL, are mean annual temperature, mean annual minimum temperature, annual precipitation, February minimum temperature and growing season length, respectively, with _p or _s indicating climatic variable at provenance origins or trial site location. C(p) is Mallow's Cp statistic.
Discussion
The 410-series rangewide provenance test represented a large-scale historical effort in Canada to understand the intraspecific genetic variability of white spruce growth and adaptation (Morgenstern and Copis 1999). This study is the first to compare adaptation and performance for rangewide provenances across 16 field trials established in Ontario.
Provenance adaptation
The adaptability of white spruce provenances to different growing environments was mainly indicated by survival. Unfortunately, the causes of tree mortality were not assessed in this long-term provenance test. Field trials with lowest survival were located in northern Ontario where winters are coldest. The statistically significant association between mean survival rate and GSL at a trial location suggests that thermal regime and low temperature are factors influencing survival in northern Ontario.
Southern provenance survived equally well as local provenances did at northern planting sites, while provenances whose origins were north of the northern planting sites had significantly poorer survival. This result is contrary to the general view that tree species populations from milder environments will suffer at sites north of their origin because they are less cold hardy than those originating from more northern, colder environments (Matyas and Yeatman 1992). However, white spruce, as a boreal tree species, can tolerate very low winter temperatures (<−40°C) through the mechanisms of “deep undercooling” or “extra organ freezing” (Sakai 1979, 1983; Bannister and Neuner 2001) when trees are dormant. Thus, minimum winter temperature in northern Ontario is unlikely to cause mortality of white spruce. Southern Ontario white spruce provenances also survived and grew well in central Alberta where winter climatic conditions were cold as or colder than those of the northern Ontario 410-series trial locations (Rweyyongeza et al. 2011). Therefore, as shown by our results, almost all the white spruce provenances can survive the historical winter climate in northern Ontario while in winter dormancy.
In contrast to minimum winter temperature, relatively mild freezing in spring and fall may damage or kill young white spruce. Fall frost damage is relatively infrequent and less consequential to white spruce (Nienstaedt and Zasada 1990). Spring frost is more frequent and more damaging to white spruce than to other spruces, such as black (Picea mariana [Mill.] BSP) and red spruce (Picea rubens Sarg.), as white spruce requires lower thermal sums to initiate bud flush (e.g., O'Reilly and Parker 1982; Blum 1988). Spring frost damage to white spruce can be particularly high when warm spring temperatures induce earlier than normal dehardening and bud burst (Clements et al. 1972; Man et al. 2009). However, conifer species of more southerly origins often require higher thermal sums to flush buds than those from northerly populations (Ekberg et al. 1994; Partanen and Beuker 1999; Søgaard et al. 2008). Southern white spruce provenances were found to delay bud flushing for several days (Blum 1988) and were therefore cold hardier than more local northern provenances under the same thermal regime in the spring (Simpson 1994).
Thus, at northern Ontario trial locations, the southern provenances tested in our study may have suffered less mortality from low temperatures than the northern provenances due to avoidance of spring frost by slightly delayed bud break (Blum 1988; Simpson 1994).
Provenance growth
The relationship of predicted height, DBH, and volume growth with latitude of provenance origin indicated a decreasing trend of growth potential as latitude at origin increased (Fig. 4). Along a longitudinal gradient, provenance performance increased slightly as provenance origins moved from eastern Canada to Ontario (i.e., from 55° to 72°W), peaked in Québec and southern Ontario (between 72° and 85°W), and then decreased markedly further west. These latitudinal and longitudinal trends are consistent because latitude and longitude are highly autocorrelated in the range of white spruce distribution (Fig. 1A) (Nienstaedt and Zasada 1990). That is, latitude of provenance origins decreased from eastern Canada to southwestern Québec and southern Ontario and then increased toward to the west coast (Fig. 1). Both the latitudinal and longitudinal patterns identified southwestern Québec and southern Ontario as the source of provenances with the best overall performance. These patterns of growth variation of white spruce provenances are consistent with results from other studies in Canada and the United States (Khalil 1985; Nienstaedt and Zasada 1990; Cherry and Parker 2003).
Considerable differences in growth were evident among white spruce provenances tested at sites across Ontario. The best performing provenances exhibited nearly double the growth in height and DBH of the worst growing provenances. The superior growth of provenances from south-central Ontario and southwestern Québec is consistent with results for several trial locations scattered across the range of white spruce (Nienstaedt 1969; Khalil 1985; Li et al. 1993, 1997; Rweyyongeza et al. 2011). These populations typically outperform local provenances at test sites located at large distances from their geographic origin. For example, Rweyyongeza et al. (2011) reported that at the age of 24 years, white spruce provenances from southern Ontario and Québec had the highest growth rate in central Alberta, compared with local provenances and those from western and eastern Canada. Our results suggest that seed sources of white spruce native to northern Ontario and Quebec will grow relatively more slowly, compared with more southerly provenances, as temperatures warm. Provenances native to western Canada and Alaska may be even more severely affected by climate warming within their home range, with relative volume growth decreasing by as much as 50% for a mean annual temperature increase by 5°C, compared with southerly provenances. We cannot project how white spruce in south-central Ontario and southwestern Québec will respond to climate warming, as the trial lacks test sites at locations that are warmer than those in this part of the species' range. These results confirm recommendations that their use for planting may increase forest productivity and carbon sequestration, and indicate that these populations represent a promising candidate genetic base for ISAM of white spruce through its northern range.
Predicting provenance performance with URF
Based on the derived URF, white spruce provenance mean height growth across field trials was partially predicted by a combination of climatic variables describing field trial locations and provenance origins. The predictability (based on R2) of this study's URF was 0.60, compared to 0.81 found by Wang et al. (2010). Several factors could have contributed to the poorer quality of the URF in the present study, including: (1) potentially high variability in soil fertility among trial sites that affected mean tree height growth independent of climate. This is suggested by field trials which are spatially close and have similar climatic conditions differing considerably in overall mean tree height growth (for example, Owen Sound vs. Minden, and Dryden vs. Kenora), (Tables 1 and 3); and (2) age differences among trials at time of measurement (Table 3). Although adjustment was made to account for age differences, we cannot rule out the possibility that height adjustments of provenances not present at all sites was imprecise or insufficient. In addition, the highly predictability of the URF in the lodgepole pine (Pinus contorta) study (Wang et al. 2010) could have benefited from uniform trial age and soil conditions among that study's field trials.
We interpret the finding that the independent prediction variables selected into the URF represented mostly climate at field trial locations to mean that URF primarily indicates phenotypic plasticity of white spruce to climate. The absence of a strong relationship between provenance performance and climate at provenance origin indicates that climatic selection pressures on white spruce were not important in causing provenance genetic differentiation. The URF from this study thus seemed to have limited utility in guiding ISAM.
Implications for climate change impacts and an intraspecific assisted migration strategy
The results of this study suggest that climate change will cause white spruce populations in northern parts of the species' range to grow relatively slowly, compared with southerly provenances. If the goal of implementing an ISAM strategy is to reduce climate maladaptation, this study indicates there is relatively low risk of mortality of southern white spruce populations planted in colder northern Ontario locations. The higher survival rates of southern provenances at northern sites after 19–28 years suggests that thermal conditions in northern regions (i.e., 47° ≤ lat. < 50°N) were within their cold tolerance capacity. While recognizing the potential for increased disturbance by fire, insects, and disease in boreal forests as a result of a warming climate, a benefit may be higher growth and productivity of some boreal species and populations (Huang et al. 2010). If climate change does not dramatically increase moisture stress as predicted for some boreal regions of North America (IPCC 2012), growth rates may increase for some tree species due to longer growing seasons and higher photosynthetic rates under warmer temperatures (Pastor and Post 1988; Goldblum and Rigg 2005). Growth in northern regions may be further increased by regenerating southern provenances of white spruce that showed improved growth in northern Ontario (Fig. 5). For example, at an average site in northern Ontario with MAT of 1.5°C, over the past 30–40 years provenances from the southern group (moved northward by about 3° latitude) would have produced about 13.5% more mean stem volume than the local populations (i.e., central group) (Fig. 5). An estimated additional 8% increase in stem volume would have occurred under a 1°C increase in MAT. Indeed, it appeared as though the growth of northern white spruce populations is becoming, if not the case already, suboptimal to a warming climate.
Conserving genetic resources of white spruce in south-central Ontario and southwestern Québec is of high ecological and economic importance for adapting this species to climate change. The gene pool of white spruce populations in this region seems to produce the most productive genetic material to enhance climate change adaptation through ISAM in Ontario and other regions where white spruce is grown. Very importantly, it appears that such relocations could take place now, since climate conditions during the 20–30 years as these trials were established did not result in freezing damage to southern populations planted at northern sites.
Genetic and physiological mechanisms linked to superior growth and adaptability in diverse climatic habitats are largely unknown and information (such as genetic diversity and allele richness) is lacking from direct comparisons between the southern and northern populations of white spruce. Pollen and macrofossil data and recent analyses of chloroplast DNA suggest that postglacial expansion of white spruce from two refugia may have resulted in genetically distinct white spruce populations across eastern Canada and the United States (Ritchie and MacDonald 1986; Anderson et al. 2006; de Lafontaine et al. 2010). White spruce is believed to have spread from an Appalachian (Pennsylvania) refugium into New England, the Altantic Provinces, and Québec, while the Mississippian (Kansas, Iowa, Missouri, Illinois) refugium migrated to colonize the Great Lakes basin, northern Ontario, and westerly into central Canada (Ritchie and MacDonald 1986; de Lafontaine et al. 2010). We propose that the founder populations of white spruce in southern Ontario and southwestern Québec may have originated from migration from both glacial refugia, resulting in a broader genetic base to harbor beneficial alleles associated with superior tree growth and climatic adaptability. The genetic base of white spruce outside this region could have been narrowed by either the selection forces of local adaptation (Aitken et al. 2008) or the founder populations may have arisen from only one of these glacial refugia (Ritchie and MacDonald 1986). If confirmed, this may help explain why white spruce populations in southern Ontario and southwestern Québec are more productive than populations from other regions of the species' natural range. Emerging genomic tools such as high-density functional gene array (Namroud et al. 2008) may eventually provide insight into this phenomenon.
Acknowledgments
We thank pioneer forest geneticists of the Canadian Forest Service (CFS) and former staff of the Ontario Ministry of Natural Resources (OMNR) for establishing the research trials; Dale Simpson and Peter Copis (CFS), Denny Irving (OMNR), Mark Lesser and Joel Symonds (Lakehead University) for assistance in trial measurement; Lisa Buse (OMNR) for editorial assistance and three anonymous reviewers and Dr. Peter Manning, Associate Editor of Ecology & Evolution, for constructive comments on an earlier version of this manuscript. Funding for this study was provided by the Government of Ontario's Interministerial Climate Change Committee under the Project CC-145.
Conflict of Interest
None declared.
References
- Aitken SN. Conserving adaptive variation in forest ecosystems. J. Sust. For. 2000;10:1–12. [Google Scholar]
- Aitken SN, Yeaman S, Holliday JA, Wang T, Curtis-McLane S. Adaptation, migration or extirpation: climate change outcomes for tree populations. Evol. Appl. 2008;1:95–111. doi: 10.1111/j.1752-4571.2007.00013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson LL, Hu FS, Nelson DM, Petit RJ, Paige KN. Ice-age endurance: DNA evidence of a white spruce refugium in Alaska. Proc. Natl Acad. Sci. USA. 2006;103:12447–12450. doi: 10.1073/pnas.0605310103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannister P. Frost resistance and the distribution of conifers. In: Bigras F, Colombo SJ, Neuner G, editors. Conifer cold hardiness. Dordrecht, The Netherlands: Kluwer Academic Publishers; 2001. pp. 3–22. Chapter 1. [Google Scholar]
- Blum BM. Variation in the phenology of bud flushing in white and red spruce. Can. J. For. Res. 1988;18:315–319. [Google Scholar]
- Campbell RK. Genecology of Douglas-fir in a watershed in the Oregon Cascades. Ecology. 1979;60:1036–1050. [Google Scholar]
- Carter KK. Provenance tests as indicators of growth response to climate change in 10 north temperate tree species. Can. J. For. Res. 1996;26:1089–1095. [Google Scholar]
- Cherry M, Parker WH. Sault Ste. Marie, ON: Ontario Ministry of Natural Resources, Applied Research and Development; 2003. Utilization of genetically improved stock to increase carbon sequestration. Forest Research Report No. 160. 15 pp. [Google Scholar]
- Clements JR, Fraser JW, Yeatman CW. Frost damage to white spruce buds. Can. J. For. Res. 1972;2:62–63. [Google Scholar]
- Ekberg I, Eriksson G, Namkoong G, Nilsson C. Genetic correlation for growth rhythm and growth capacity at age 3-8 years in provenance hybrids of Picea abies. Scand. J. For. 1994;9:25–33. [Google Scholar]
- Fournier-Level A, Korte A, Cooper MD, Nordborg M, Schmitt J, Wilczek AM. A map of local adaptation in Arabidopsis thaliana. Science. 2011;334:87–89. doi: 10.1126/science.1209271. [DOI] [PubMed] [Google Scholar]
- Goelz JCG, Burk TE. Development of a well-behaved site index equation: jack pine in north central Ontario. Can. J. For. Res. 1992;22:776–784. [Google Scholar]
- Goldblum D, Rigg LS. Tree growth response to climate change at the deciduous–boreal forest ecotone, Ontario, Canada. Can. J. For. Res. 2005;35:2709–2718. [Google Scholar]
- Hamann A, Gylander T, Chen P-Y. Developing seed zones and transfer guidelines with multivariate regression trees. Tree Genet. Genom. 2011;7:399–408. [Google Scholar]
- Henderson CR. Applications of linear models in animal breeding. Guelph, ON: University of Guelph; 1984. p. 462. [Google Scholar]
- Huang J, Tardif JC, Bergeron WY. Radial growth response of four dominant boreal tree species to climate along a latitudinal gradient in the eastern Canadian boreal forest. Glob. Change Biol. 2010;16:711–731. doi: 10.1111/j.1365-2486.2009.01990.x. [Google Scholar]
- Intergovernmental Panel on Climate Change (IPCC) 2007. Climate Change 2007: The Physical Science Basis. Summary for Policy Makers. Available at http://www.ipcc.ch/ (accessed 13 November 2007)
- IPCC. Summary for policymakers. A Special Report of Working Groups I and II of the Intergovernmental Panel on Climate Change. In: Field CB, Barros V, Stocker TF, Qin D, Dokken DJ, Ebi KL, Mastrandrea MD, Mach KJ, Plattner G-K, Allen SK, Tignor M, Midgley PM, editors. Managing the risks of extreme events and disasters to advance climate change adaptation. Cambridge, U.K., and New York, NY: Cambridge Univ. Press; 2012. pp. 1–19. [Google Scholar]
- Johnston M, Campagna M, Gray P, Kope H, Ogden A, O'Neill GA, et al. Library Archive Canadian Catalogue, Publication; 2009. Vulnerability of Canada's tree species to climate change and management options for adaptation: an overview for policy makers and practitioners. ISBN 978-1-100-13845-9, Cat. no.: Fo4-28/2009E-PDF (Electronic) 40p. [Google Scholar]
- Khalil MAK. Genetic variation in eastern white spruce (Picea glauca (Moench) Voss) populations. Can. J. For. Res. 1985;15:444–452. [Google Scholar]
- de Lafontaine G, Turgeon J, Payette S. Phylogeography of white spruce (Picea glauca) in eastern North America reveals contrasting ecological trajectories. J. Biogeogr. 2010;37:741–751. [Google Scholar]
- Leech SM, Almuedo PL, O'Neill G. Assisted migration: adapting forest management to a changing climate. BC J. Ecosyst. Manage. 2011;12:18–34. [Google Scholar]
- Lesser MR, Cherry M, Parker WH. Investigation of limestone ecotypes of white spruce based on a provenance test series. Can. J. For. Res. 2004;34:1119–1127. [Google Scholar]
- Li P, Beaulieu J, Corriveau A, Bousquet J. Genetic variation in juvenile growth and phenology in a white spruce provenance-progeny test. Silvae Genet. 1993;42:52–60. [Google Scholar]
- Li P, Beaulieu J, Bousquet J. Genetic structure and patterns of genetic variation among populations in eastern white spruce (Picea glauca. Can. J. For. Res. 1997;27:189–198. [Google Scholar]
- Lynch M, Walsh B. Genetics and analysis of quantitative traits. Sunderland, MA: Sinauer Associates Inc., Publishers; 1998. p. 980. [Google Scholar]
- Man R, Kayahara GJ, Dang Q, Rice JA. A case of severe frost damage prior to budbreak in young conifers in Northeastern Ontario: consequence of climate change? Forest. Chron. 2009;85:453–462. [Google Scholar]
- Matyas C. Modelling climate change effects with provenance data. Tree Physiol. 1994;14:797–804. doi: 10.1093/treephys/14.7-8-9.797. [DOI] [PubMed] [Google Scholar]
- Matyas C, Yeatman CW. Effect of geographic transfer on growth and survival of jack pine (Pinus banksiana Lamb.) provenances. Silvae Genet. 1992;41:370–376. [Google Scholar]
- McKenney DW, Mackey BG, Joyce D. SEEDWHERE: a computer tool to support seed transfer and ecological restoration decisions. Environ. Model. Softw. 1999;14:589–595. [Google Scholar]
- McKenney DW, Pedlar JH, Lawrence K, Campbell K, Hutchinson MF. Potential impacts of climate change on the distribution of North American trees. Bioscience. 2007;57:939–948. [Google Scholar]
- Morgenstern EK, Copis P. Ottawa, ON: Canadian Forest Service Science; 1999. Best white spruce provenances in Ontario. Information Report, ST-X-16, 34 pp. [Google Scholar]
- Morgenstern EK, Mullin TJ. Growth and survival of black spruce in the range wide provenance study. Can. J. For. Res. 1990;20:130–143. [Google Scholar]
- Morgenstern EK, D'Eon S, Penner M. White spruce growth to age 44 in a provenance test at the Petawawa Research Forest. Forest. Chron. 2006;82:572–578. [Google Scholar]
- Namroud MC, Beaulieu J, Juge N, Laroche J, Bousquet J. Scanning the genome for gene single nucleotide polymorphisms involved in adaptive population differentiation in white spruce. Mol. Ecol. 2008;17:3599–3613. doi: 10.1111/j.1365-294X.2008.03840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nienstaedt H. St. Anne de Bellevue, QC: Macdonald Coll; 1969. White spruce and seed source variation and adaptation to 14 planting sites in northeastern United States and Canada; pp. 183–194. Proc. 11th Meet. Comm. For. Tree Breed. Canada. [Google Scholar]
- Nienstaedt H. Picea glauca (Moench) Voss. In: Burns RM, Honkala BH, Zasada JC, editors. Silvics of North America. Vol. 1 Conifers. USDA Forest Service: Washington, DC; 1990. pp. 204–226. USDA Forest Service Agriculture Handbook. 654. [Google Scholar]
- O'Neill G, Berger V, Carlson M, Ukrainetz N. 2013. Assisted migration Bulletin. B.C. Min. For., Lands and Nat. Resour. Ops., Tree Improve. Br. http://www.for.gov.bc.ca/hre/forgen/interior/AMAT.htm.
- Ontario Ministry of Natural Resources (OMNR) Toronto, ON: OMNR; 2006. State of the forest report 2006; p. 153. [Google Scholar]
- O'Reilly C, Parker WH. Vegetative phenology in a clonal seed orchard of Picea glauca and Picea mariana in northwestern Ontario. Can. J. For. Res. 1982;12:408–413. [Google Scholar]
- Parker WH. Focal point seed zones: site-specific seed zone delineation using geographic information systems. Can. J. For. Res. 1992;22:267–271. [Google Scholar]
- Partanen J, Beuker E. Effects of photoperiod and thermal time on growth rhythm of Pinus sylvestris seedlings. Scand. J. For. 1999;14:487–497. [Google Scholar]
- Pastor J, Post WM. Response of northern forests to CO2-induced climate change. Nature. 1988;334:55–58. [Google Scholar]
- Payandeh B, Wang Y. Preliminary site index equations for three planted species in northern Ontario. Northern J. Appl. For. 1995;12:57–63. [Google Scholar]
- Popovich S. 1972. Volume tables for plantation white spruce Grand ‘Mere, Quebec. Can. For. Serv. Laurentian For. Res. Cent. Inf. Rep. Z-X-29. 17 pp.
- Rahmstorf S, Ganopolski A. Long-term global warming scenarios computed with an efficient coupled climate model. Clim. Change. 1999;43:353–367. [Google Scholar]
- Rauscher MH, Harding BR. Testing the accuracy of white spruce total-tree volume equations. Northern J. Appl. For. 1993;10:112–116. [Google Scholar]
- Rehfeldt GE. Ecological adaptations in Douglas-fir (Pseudotsuga menziesii var glauca) – a synthesis. For. Ecol. Manage. 1989;28:203–215. [Google Scholar]
- Rehfeldt GE, Ying CC, Spittlehouse DL, Hamilton DA. Genetic responses to climate in Pinus contorta: niche breadth, climate change, and reforestation. Ecol. Monogr. 1999;69:375–407. [Google Scholar]
- Ritchie JC, MacDonald GM. The patterns of postglacial spread of white spruce. J. Biogeogr. 1986;13:527–540. [Google Scholar]
- Rweyyongeza DM, Barnhardt LK, Hansen C. 2011. Patterns of optimal growth for white spruce provenances in Alberta. Alberta Sustai. Resour. Develop. Centre, Smokey Lake, Alberta. Pub. No: Ref. T/255. 37 pp.
- Sakai A. Freezing avoidance mechanism of primordial shoots of conifer buds. Plant Cell Physiol. 1979;20:1381–1390. [Google Scholar]
- Sakai A. Comparative study on freezing resistance of conifers with special reference to cold adaptation and its evolutive aspects. Can. J. Bot. 1983;61:2323–2332. [Google Scholar]
- SAS Institute. Cary, NC: SAS Inc; 2011. SAS/STAT 9.2. User's guide. [Google Scholar]
- Savolainen O, Pyhajarvi T, Knurr T. Gene flow and local adaptation in trees. Annu. Rev. Ecol. Evol. Syst. 2007;38:595–619. [Google Scholar]
- Schmidtling RC. Use of provenance tests to predict response to climate change: loblolly pine and Norway spruce. Tree Physiol. 1994;14:805–817. doi: 10.1093/treephys/14.7-8-9.805. [DOI] [PubMed] [Google Scholar]
- Searle SR, Casella G, McCulloch CE. Variance components. New York, NY: John Wiley & Sons, Inc; 1992. p. 501. [Google Scholar]
- Simpson DG. Seasonal and geographic origin effects on cold hardiness of white spruce buds, foliage and stems. Can. J. For. Res. 1994;24:1066–1070. [Google Scholar]
- Skrøppa T. Genetic variation in growth rhythm characteristics within and between natural populations of Norway spruce. A preliminary report. Silva Fennica. 1982;16:160–167. [Google Scholar]
- Søgaard G, Johnsen Ø, Nilsen J, Junttila O. Climate control of bud burst in young seedlings of nine provenances of Norway spruce. Tree Physiol. 2008;28:311–320. doi: 10.1093/treephys/28.2.311. [DOI] [PubMed] [Google Scholar]
- Sork VL, Stowe KA, Hochwender C. Evidence for local adaptation in closely adjacent subpopulations of northern red oak (Quercus rubra L.) expressed as resistance to leaf herbivores. Am. Nat. 1993;142:928–936. doi: 10.1086/285581. [DOI] [PubMed] [Google Scholar]
- Thomson AM, Parker WH. Boreal forest provenance tests used to predict optimal growth and response to climate change. 1. Jack pine. Can. J. For. Res. 2008;38:157–170. [Google Scholar]
- Wang T, Hamann A, Yanchuk A, O'Neill GA, Aitken SN. Use of response functions in selecting lodgepole pine populations for future climates. Glob. Change Biol. 2006;12:2404–2416. [Google Scholar]
- Wang T, O'Neill GA, Aitken SN. Integrating environmental and genetic effects to predict responses of tree populations to climate. Ecol. Appl. 2010;20:153–163. doi: 10.1890/08-2257.1. [DOI] [PubMed] [Google Scholar]
- White TL, Hodge GR. Predicting breeding values with applications in forest tree improvement. Dordrecht, NL: Kluwer Academic Publisher; 1989. p. 367. [Google Scholar]
- White TL, Adams WT, Neale DB. Forest genetics. CAB International, Wallingford, UK: CABI Publishing; 2007. p. 682. [Google Scholar]
- Wu HX, Ying CC. Geographic pattern of local optimality in natural populations of lodgepole pine. For. Ecol. Manage. 2004;194:177–198. [Google Scholar]
- Ying CC, Yanchuk AD. The development of British Columbia's tree seed transfer guidelines: purpose, concept, methodology, and implementation. For. Ecol. Manage. 2006;227:1–13. [Google Scholar]
- Young A, Boshier D, Boyle T. Forest conservation genetics: principles and practices. Collingwood, Vic., Australia: CSIRO Publishing; 2000. p. 352. [Google Scholar]