Abstract
The adequate selection of indicator groups of biodiversity is an important aspect of the systematic conservation planning. However, these assessments differ in the spatial scales, in the methods used and in the groups considered to accomplish this task, which generally produces contradictory results. The quantification of the spatial congruence between species richness and complementarity among different taxonomic groups is a fundamental step to identify potential indicator groups. Using a constructive approach, the main purposes of this study were to evaluate the performance and efficiency of eight potential indicator groups representing amphibian diversity in the Brazilian Atlantic Forest. Data on the geographic range of amphibian species that occur in the Brazilian Atlantic Forest were overlapped to the full geographic extent of the biome, which was divided into a regular equal-area grid. Optimization routines based on the concept of complementarily were applied to verify the performance of each indicator group selected in relation to the representativeness of the amphibians in the Brazilian Atlantic Forest as a whole, which were solved by the algorithm “simulated annealing,” through the use of the software MARXAN. Some indicator groups were substantially more effective than others in regard to the representation of the taxonomic groups assessed, which was confirmed by the high significance of the data (F = 312.76; P < 0.01). Leiuperidae was considered as the best indicator group among the families analyzed, as it showed a good performance, representing 71% of amphibian species in the Brazilian Atlantic Forest (i.e., 290 species), which may be associated with the diffuse geographic distribution of their species. In this sense, this study promotes understanding of how the diversity standards of amphibians can be informative for systematic conservation planning on a regional scale.
Keywords: Amphibians, Atlantic Forest, biodiversity indicators, representativeness, surrogates, systematic conservation planning
Introduction
Increased rates of habitat loss and human occupation are creating demands for more adequate strategies to maximize efforts for biodiversity conservation (Diniz-Filho et al. 2008). One of the conservation strategies mostly used to preserve threatened species is the establishment of protected areas (Lawler and White 2008). The selection of sites for the protection of biological communities and the maintenance of ecosystem processes, within the context of systematic conservation planning (see Margules and Pressey 2000), is an extremely efficient tool to preserve species and habitats (Clemens et al. 1999; Myers et al. 2000; Kati et al. 2004; Rodrigues and Brooks 2007; Loucks et al. 2008). However, the resources available for the creation of protected areas are limited (Loucks et al. 2008). Therefore, it is no surprise that the inclusion of the economic costs into conservation planning can result in more feasible conservation strategies on the ground (Naidoo et al. 2006).
A central issue in systematic conservation planning is the identification of targets to be conserved (Margules and Pressey 2000; Groves et al. 2002; Cowling and Pressey 2003; Sarkar 2004). Protected area networks are often selected to protect species of distinct taxonomic groups, communities of high biological relevance, or combinations of different abiotic conditions favorable to local ecosystems, with the assumption that such sites will also protect a wider range of biodiversity (Lawler and White 2008). Therefore, conservation planners should count on surrogates, or indicator groups, to represent the largest possible part of local biodiversity in reserve selection (Kremen 1992; Raven and Wilson 1992; Flather et al. 1997). The validity of this hypothesis depends on how well the chosen indicator group represents a wider array of biodiversity (Lawler and White 2008). In this way, the adequate selection of indicator groups is fundamental for the consistency of successful systematic conservation planning (Margules and Pressey 2000; Margules and Sarkar 2007).
Most conservation plans are based on the biodiversity surrogates (e.g., Loiselle et al. 2003; Stoms et al. 2005; Margules and Sarkar 2007; Rodrigues and Brooks 2007). These surrogates are generally based on the species, such as keystone species, umbrella species, or flagship species (Andelman and Fagan 2000; Mace et al. 2007; Grantham et al. 2010). Additionally, these surrogates may also be based on other parameters, such as vegetation structure, soil coverage, and environmental gradients (Faith and Walker 1996a,b1996b; Sarkar et al. 2005; Trakhtenbrot and Kadmon 2005), even though it is known that surrogates based on the species are more efficient than those based on environmental proxies (Rodrigues and Brooks 2007).
Quantifying the spatial congruence between species richness and complementarity among different taxonomic groups is a fundamental step to identify potential indicator groups (Howard et al. 1998; van Jaarsveld et al. 1998; Pinto et al. 2008). However, these evaluations differ in spatial scale, in the methods used and in the groups that are tested, which generally produces contradictory results (e.g., Schmit et al. 2005; Bani et al. 2006; Lamoreux et al. 2006; Chiarucci et al. 2007; Rodrigues and Brooks 2007; Grantham et al. 2010; Lewandowski et al. 2010). In spite of the importance and usefulness of systematic investigations about the consistency of indicator groups to guide conservation actions and decision-making processes, only a few studies have explicitly evaluated this aspect (e.g., Araújo et al. 2001; Manne and Williams 2003; Bani et al. 2006; Lawler and White 2008; Trindade-Filho and Loyola 2011).
There is a trend in the scientific literature in relation to studies on organisms that indicate habitat quality (Lima 2001). In this sense, amphibians have been identified as potential biological indicators due to their naked skin and their use of aquatic and terrestrial habitats, which makes them extremely vulnerable to environmental disturbances (Blaustein and Wake 1995; Tocher et al. 1997; Cosson et al. 1999; Kwet and Di-Bernardo 2002; DeGarady and Halbrook 2006; Lebboroni et al. 2006). However, these previous studies did not clearly evaluate which characteristics might make amphibians a good indicator group across different taxa (Sewell and Griffiths 2009). This suggests that some taxa previously highlighted as good indicators could have appeared so simply because they harbored many species, instead of really exhibiting good indicator qualities (Larsen et al. 2009). In order to use a straightforward approach to improve this concept, the main purpose of this study was to assess the performance of amphibian families as potential indicator groups to represent overall amphibian diversity in the Brazilian Atlantic Forest.
Materials and Methods
Study area
The Brazilian Atlantic Forest was chosen as our case study because it is one of the 34 global biodiversity hotspots for conservation priorities (Mittermeier et al. 2004), having high rate of habitat loss (Teixeira et al. 2009), which is one of the main factors that driving amphibians to extinction (Stuart et al. 2004; Becker et al. 2007). This biome originally covered approximately 150 million hectares, but it is now reduced to only 11.4–16.0% of its pristine cover (Ribeiro et al. 2009). The majority of the forest remnants cover less than 100 hectares (Ranta et al. 1998) and are isolated from each other, representing forests at early and middle succession stages (Viana et al. 1997; Metzger 2000; Metzger et al. 2009). The remaining large fragments are located in hilly terrain, hindering human occupation (Silva et al. 2007). Yet, the ranges of different altitudinal and latitudinal gradients where these remnants are found have favored a high biodiversity as compared to other biomes in Brazil (Ribeiro et al. 2009).
The Atlantic Forest is the leader biome in amphibian diversity in Brazil, comprising about 400 species (i.e., about 50% of all amphibian species within Brazil, Haddad et al. 2008). This high species richness is explained by the high diversity of habitats and microhabitats, which favor endemisms (Haddad 1998).
Data
Data on the geographic range of Atlantic Forest amphibian species were obtained from the IUCN Red List of Threatened Species database (IUCN 2012). The software ArcGIS 9.3 (ESRI 2008) was used to overlap the species ranges to the full geographic extent of the biome, which was divided into a regular equal-area grid containing cells with spatial resolution of 0.5° (i.e., about 50 km2), providing a network of 436 cells. The total land area covered by this grid was based on the atlas of the remaining Atlantic Forest (SOS Mata Atlântica and Instituto Nacional de Pesquisas Espaciais 2008).
Presence–absence data matrices were designed for 408 amphibian species occurring in the Brazilian Atlantic Forest in such a way that a given species was considered as present when its area of occurrence included any section of the grid system.
Species were divided into eight potential indicator groups, which were based on the different taxonomic groups represented by the families Brachycephalidae, Bufonidae, Cycloramphidae, Hylidae, Hylodidae, Leiuperidae, Leptodactylidae, and Microhylidae. Amphibian families with less than 20 species were excluded from the analyses because of their small sample size. These families included the Allophrynidae, Aromobatidae, Caeciliidae, Centrolenidae, Ceratophryidae, Craugastoridae, Dendrobatidae, Eleutherodactylidae, Hemiphractidae, Pipidae, Ranidae, Plethodontidae, Rhinatrematidae, and Strabomantidae. The taxonomy adopted for the families followed the classification proposed by Blackburn and Wake (2011).
Analyses
In order to evaluate the performance of indicator groups (amphibian families), the smallest set of grid cells needed to represent all species of each indicator group was selected to solve a problem known as “minimum set coverage” (Underhill 1994). Then, the species representation was maximized with the lowest possible number of cells (Church et al. 1996; Andelman et al. 1999; Cabeza and Moilanen 2001). Thus, a set of eight cells was chosen as the lowest number of cells needed to represent all species among the potential indicator groups assessed.
After that, the 20 best sets of solutions to maximize the representation of each indicator group within eight cells were selected, solving the problem known as “maximal representation problem” (Church et al. 1996). The best spatial solutions to represent the maximum number of species in each group were encountered, with the condition that these solutions do not exceed a set of eight cells in the grid system. This was necessary to evaluate the effectiveness of the selected indicator groups (i.e., the percentage of diversity represented), so they could be compared without biases related to the number of cells contained in each group (see Lawler and White 2008).
Optimization routines based on the concept of complementarity (Vane-Wright et al. 1991; Howard et al. 1998; Cabeza and Moilanen 2001) were then used to verify the performance of each indicator group in regard to the representativeness of overall amphibian species. This concept assumes a nonoverlapping representation of natural features (Cabeza and Moilanen 2001), providing a measure of the contribution of an area to the full complement of biodiversity features assessed (Margules and Sarkar 2007), which implies that the conservation benefits that follow from a particular conservation action at a site depend on the regional context of the site and conservation actions taken elsewhere (Moilanen 2008). Optimization problems were solved by the algorithm “simulated annealing” (Kirkpatrick et al. 1983; Possingham et al. 2000), which was run 10,000 times for each group, using the software MARXAN, version 2.43 (Ball et al. 2009). This is a nonsequential algorithm that looks for optimal solutions (minimum number of cells) by comparing entire sets of areas. Initially, the algorithm selects a random network of cells and, at each iteration (in this case, 10,000 iterations), it randomly changes the system by adding, deleting, and/or switching cells (Possingham et al. 2000) and thus compares the changes resulting in a cost equation (Kelley et al. 2002). The increased acceptable cost decreases at each iteration (Andelman et al. 1999). Therefore, at each step, the new solution is compared with the former solution and the best one is maintained (Kirkpatrick et al. 1983; Possingham et al. 2000).
The average conservation percentage of target species represented a measure of the performance of each indicator group selected. For comparison, 20 solutions were tested with the smallest set of grid cells required to represent all species of each indicator group based on a random collection of species, assessing their effectiveness in relation to all studied species. These sets were built to evaluate whether the performance of the selected indicator groups was higher, similar, or lower than that expected randomly, extrapolating the representation of a null model.
In addition, land cost-effective relationships were calculated according to the number of grid cells required to represent all species from each indicator group assessed. The land cost-effective values were based on the model proposed by Bode et al. (2008), which established an economic cost of 68,733 dollars by each km2 of Brazilian Atlantic Forest. Thus, it was possible to provide an economic cost estimation of the minimum effective land coverage of each indicator group.
The relationship between the number of species and the representativeness of each indicator group evaluated was correlated by linear regression analyses, using the software Ecosim 7.72 (Gotelli and Entsminger 2005). Subsequently, the average representation percentage of each indicator group was compared through an analysis of variance (ANOVA), using the software STATISTICA, version 8.0 (StatSoft, Inc 2007), where the effectiveness in capturing biodiversity represented by the relative number of species recorded was the response variable. The significance level of this analysis was 1% because even though the sets of solutions for each indicator group are unique, there may be a large overlay of the cells regarded as important, therefore reducing the independence of solutions (Lawler and White 2008). Diminishing the significance level to a more conservative value may be a way to reduce the effects of spatial autocorrelation when specific methods to control this phenomenon are not applicable or are simply unnecessary (Diniz-Filho et al. 2003; Kubota et al. 2007; Loyola 2009; Trindade-Filho and Loyola 2011).
Results
Spatial patterns of species richness
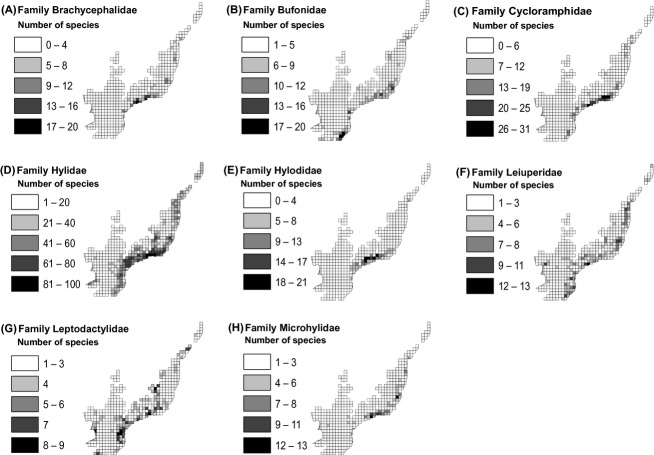
The geographical distribution of the eight potential indicator groups showed different spatial patterns of species richness among them (Fig. 1). There was greater species richness in the southeastern Brazil, mainly for Brachycephalidae, Cycloramphidae, Hylidae, Hylodidae, and Microhylidae. However, Hylidae, Leiuperidae, and Leptodactylidae also were well represented within the southern and northeastern regions (Fig. 1), so that Bufonidae was more distributed in the southern and southeastern Brazil (Fig. 1).
Figure 1.
Spatial patterns of species richness from eight potential indicator groups assessed in the Brazilian Atlantic Forest (n = 408 species). (A) Number of Brachycephalidae species. (B) Number of Bufonidae species. (C) Number of Cycloramphidae species. (D) Number of Hylidae species. (E) Number of Hylodidae species. (F) Number of Leiuperidae species. (G) Number of Leptodactylidae species. (H) Number of Microhylidae species.
Performance and efficiency of indicator groups
The use of families as overall amphibian diversity indicators represented more species than the random choice for representative areas of amphibian diversity in the Brazilian Atlantic Forest (Fig. 2). All amphibian family groups analyzed were considered as potential indicators and showed a good spatial congruence in relation to their representativeness, because all the groups considered individually accounted for more than 50% of the species pool assessed (Fig. 2, Table 1). However, some indicator group indicators were more effective than others in regard to the representation of the taxonomic groups assessed (F = 312.76; P < 0.01). Leiuperidae was considered as the best indicator group, as it showed a good performance and cost-effective, representing 71% of amphibian species in the Brazilian Atlantic Forest (i.e., 290 species) from only eight grid cells, being based on a group with a relatively low number of species (i.e., 31 species; Fig. 2, Table 1). Species richness within the indicator groups was not correlated with the mean representativeness among them (r = 0.40; P > 0.15; see Table 1).
Figure 2.
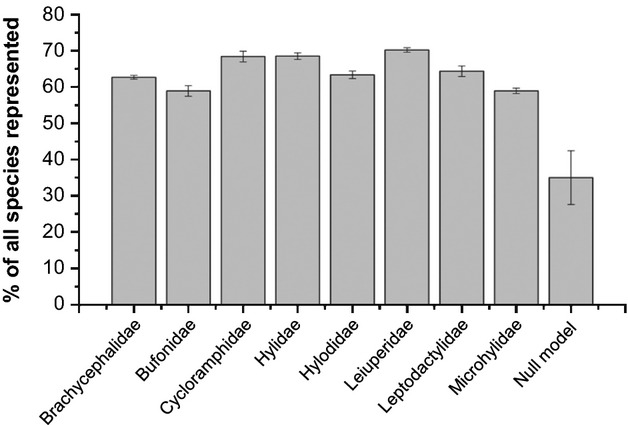
Efficiency of indicator groups to represent the amphibian species in the Brazilian Atlantic Forest. Gray bars represent the mean percentages among the 20 best solutions to represent all species as from the smallest set of grid cells necessary for each indicator group. Error bars denote standard deviations of the means.
Table 1.
Number of species, number of grid cells required to represent all species, percentage of species represented, and land cost-effective by each indicator group assessed in the Brazilian Atlantic Forest
| Indicators Groups (IG) | Number of species per IG | Number of grid cells required to represent all species from each IG | Percentage of species represented by IG (%) | Land cost-effective by IG ($) |
|---|---|---|---|---|
| Brachycephalidae | 35 | 9 | 63 | 30,929,850 |
| Bufonidae | 33 | 9 | 59 | 30,929,850 |
| Cycloramphidae | 41 | 11 | 69 | 37,803,150 |
| Hylidae | 184 | 26 | 69 | 89,352,900 |
| Hylodidae | 33 | 13 | 65 | 44,676,450 |
| Leiuperidae | 31 | 8 | 71 | 27,493,200 |
| Leptodactylidae | 30 | 11 | 65 | 37,803,150 |
| Microhylidae | 21 | 8 | 59 | 27,493,200 |
Discussion
One of the biggest challenges for tropical conservation biology is to develop precise methods for conservation planning (Becker et al. 2010). Our results indicate that sites selected from potential indicator groups can include a large part of the diversity of amphibians in the Brazilian Atlantic Forest. Similar conclusions were obtained using similar methodologies applied to other taxonomic groups (e.g., Lawler et al. 2003; Loyola et al. 2007; Lawler and White 2008; Pinto et al. 2008; Larsen et al. 2009; Trindade-Filho and Loyola 2011; Trindade-Filho et al. 2012), even though their results can be considered controversial (see Lawler et al. 2003). Some authors have argued that the efficient use of indicator groups requires the selection of large extensions of land, so that the majority of the target species can be represented (see Howard et al. 1998). However, our results showed that good indicator groups can effectively represent biodiversity from a relatively small area.
A species taxonomic group can be considered a good indicator when its geographic distribution spatially coincides with the distribution of the other groups in a given region (Gaston 1996; Flather et al. 1997; Virolainen et al. 2000). In regard to amphibians, although they have been widely promoted as indicators of environmental quality, rigorous complementarity tests are still lacking (Sewell and Griffiths 2009). In large spatial scales, the objective is not to identify areas for protected areas, but to identify regions of high value for conservation that are important in the scale in question (Moore et al. 2003). Besides representing all conservation targets, the regions selected by complementarity are constituted by the lowest possible pool of cells (i.e., minimum of resources) (Lawler et al. 2003).
The performance observed for Leiuperidae as an indicator group may be associated with the diffuse geographic distribution of their species, the lower number of grid cells required to represent all of the species of each indicator group, and the low number of species which compose this group in comparison with the other groups evaluated (see Table 1). Leiuperidae species cover a wide range of different environmental conditions (Grant et al. 2006), representing a great spatial heterogeneity. These species co-occur in common habitats as much for generalist species as for specialist species, providing the occurrence of complementary groups, which favors a greater beta diversity (Loyola et al. 2007; Lawler and White 2008; Pinto et al. 2008; Larsen et al. 2009; Trindade-Filho and Loyola 2011). However, some authors argue that only species with restricted distribution exhibit congruent geographic standards compared with other species distributed in wide spatial scales (Lamoreux et al. 2006).
Our results are relatively optimistic, because they consist of a representation of species in at least one grid cell. This is a limitation, because restricting species occurrence to a single site is similar of the old adage of putting all your eggs on a single basket (see Ricketts et al. 2005). Conservation outcomes were most sensitive to uncertainty in the land cost data, because the use of species extents of occurrence overestimates their real geographic ranges (Rondinini et al. 2006), which in turn increase the effectiveness of indicator groups whose distribution was based on such maps. One possible solution would be the utilization of species distribution modeling methods currently available (Araújo and New 2007). However, these models are known have other sources of uncertainties (Loiselle et al. 2003; Wilson et al. 2005; Diniz-Filho et al. 2009a,b2009b, 2010). Nevertheless, as we are not proposing the creation of protected areas, but suggesting that the use of indicator groups to operate as a shortcut for mapping biodiversity, the use of species extents of occurrence may still be considered a possible solution to investigate the efficacy of indicator groups (e.g., Lawler et al. 2003; Loyola et al. 2007; Rodrigues and Brooks 2007; Lawler and White 2008; Pinto et al. 2008; Larsen et al. 2009; Grantham et al. 2010; Trindade-Filho and Loyola 2011; Trindade-Filho et al. 2012).
For this purpose, future studies on species inventories could be concentrated on the groups scientifically proven as indicators of biodiversity. This suggests that taxonomists tend to concentrate their efforts in the localities that guarantee success in the collection of as many species as possible (Sastre and Lobo 2009). Optimal solutions of complementarity based on different biodiversity analyses have been successful in conservation planning at the global level (Csuti et al. 1997), including for amphibians (Diniz-Filho et al. 2006). The use of taxonomic subgroups as potential indicators of biodiversity has also been a common practice in conservation studies (e.g., Simberloff 1998; Caro and O'Doherty 1999; Andelman and Fagan 2000). In this context, biodiversity surrogate groups and indicator groups have been utilized in different ways to guide conservation strategies (Caro and O'Doherty 1999). Yet, there is an ample spectrum of circumstances that define the relative complexity of conservation planning based on the use of indicator groups (Stoms et al. 2005). Indicator groups should follow predictors of complementarity performance, such as variability between extents of occurrence, occupation of different ecoregions, variability of records of geographic distribution, and average body size in relation to the species pool considered in the analyses (Manne and Williams 2003).
Nevertheless, when we try to choose a specific target to protect other biodiversity aspects than species richness, we create a challenge to the conservation biologists. Here, we are proposing that the use of amphibian families as indicator groups of biodiversity can be a straightforward strategy to maximize the conservation value of small spatial scales. Usually, we must allocate conservation efforts to areas with higher diversity than expected by chance. However, this depends on the purpose of the conservation plan as well on the nature of the ecosystem we are interested in protect. In practice, our results carry a great deal of interest, not only because they are novel, but also because they reveal that a taxonomically defined group (i.e., Leiuperidae) can be used as a conservation shortcut of amphibian biodiversity in the Brazilian Atlantic Forest.
Even though the indicator groups presented in this study had a good performance in representing amphibian diversity in the Brazilian Atlantic Forest, it is important to note that our analyses evaluated efficacy based on a single measurement of diversity. Therefore, we did not incorporate other important aspects, such as population viability (see Carroll et al. 2003), functional diversity, and phylogenetic relationships (see Carvalho et al. 2010; Devictor et al. 2010; Trindade-Filho et al. 2012). However, this was due to the limited knowledge about the majority of the species of our data group. A recent analysis showed that the data-deficient species also seems to reflect a spatial knowledge deficiency (Brito 2010). This lack of knowledge underscores the urgent need for the development of strategies toward systematic conservation planning, which may contribute directly to the stability of the ecosystems and long-term evolutionary processes (Trindade-Filho et al. 2012). In this sense, this study helps in understanding how the spatial patterns of amphibians can be informative for the conservation planning at regional scales.
Acknowledgments
We thank the University of Barcelona (UB) and the State University of Santa Cruz (UESC) for the structural and scientific support, and the CAPES Foundation for the financial support. We are also grateful to Deborah Faria, Sharon Azzopardi, and two anonymous reviewers for the comments and suggestions on the manuscript.
Conflict of Interest
None declared.
References
- Andelman SJ, Fagan WF. Umbrellas and flagships: efficient conservation surrogates or expensive mistakes. Proc. Natl Acad. Sci. USA. 2000;97:5954–5959. doi: 10.1073/pnas.100126797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andelman S, Ball I, Davis F, Stoms D. 1999. SITES v 1.0 – An analytical toolbox for designing ecoregional conservation portfolios. Technical report, The Nature Conservancy. Available at http://www.biogeog.ucsb.edu/projects/tnc/toolbox.html (accessed 15 January 2013)
- Araújo MB, New M. Ensemble forecasting of species distributions. Trends Ecol. Evol. 2007;22:42–47. doi: 10.1016/j.tree.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Araújo MB, Humphries CJ, Densham PJ, Lampinen R, Hagemeijer WJM, Mitchell-Jones AJ, et al. Would environmental diversity be a good surrogate for species diversity? Ecography. 2001;24:103–110. [Google Scholar]
- Ball IR, Possingham HP. Marxan and relatives: software for spatial conservation prioritisation. In: Moilanen A, Wilson KA, Possingham HP, Watts M, editors. Spatial conservation prioritisation: quantitative methods and computational tools. Oxford, U.K: Oxford Univ. Press; 2009. pp. 185–195. [Google Scholar]
- Bani L, Massimino-Bottoni DL, Massa R. A multiscale method for selecting indicator species and priority conservation areas: a case study for broadleaved forests in Lombardy, Italy. Conserv. Biol. 2006;20:512–526. doi: 10.1111/j.1523-1739.2006.00331.x. [DOI] [PubMed] [Google Scholar]
- Becker CG, Fonseca CR, Haddad CFB, Batista RF, Prado PI. Habitat split and the global decline of amphibians. Science. 2007;318:1775–1777. doi: 10.1126/science.1149374. [DOI] [PubMed] [Google Scholar]
- Becker CG, Loyola RD, Haddad CFB, Zamudio KR. Integrating species life-history traits and patterns of deforestation in amphibian conservation planning. Divers. Distrib. 2010;16:10–19. [Google Scholar]
- Blackburn DC, Wake DB. Zhang Z, editor. Animal biodiversity: an outline of higher-level classification and survey of taxonomic richness. Zootaxa. 2011;3148:38–54. doi: 10.11646/zootaxa.3703.1.1. Class Amphibia Gray, 1825. [DOI] [PubMed] [Google Scholar]
- Blaustein AR, Wake DB. The puzzle of declining amphibian populations. Sci. Am. 1995;272:52–57. [Google Scholar]
- Bode M, Wilson KA, Brooks TM, Turner WR, Mittermeier RA, McBride MF, et al. Cost-effective global conservation spending is robust to taxonomic group. Proc. Natl Acad. Sci. USA. 2008;105:6498–6501. doi: 10.1073/pnas.0710705105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito D. Overcoming the linnean shortfall: data deficiency and biological survey priorities. Basic Appl. Ecol. 2010;11:709–713. [Google Scholar]
- Cabeza M, Moilanen A. Design of reserve networks and the persistence of biodiversity. Trends Ecol. Evol. 2001;16:242–248. doi: 10.1016/s0169-5347(01)02125-5. [DOI] [PubMed] [Google Scholar]
- Caro TM, O'Doherty G. On the use of surrogate species in conservation biology. Conserv. Biol. 1999;13:805–814. [Google Scholar]
- Carroll C, Noss RE, Paquet PC, Schumaker NH. Use of population viability analysis and reserve selection algorithms in regional conservation plans. Ecol. Appl. 2003;13:1773–1789. [Google Scholar]
- Carvalho RA, Cianciaruso MV, Trindade-Filho J, Sagnori MD, Loyola RD. Drafting a blueprint for functional and phylogenetic diversity conservation in the Brazilian Cerrado. Nat. Conserv. 2010;8:171–176. [Google Scholar]
- Chiarucci A, D'auria F, Bonini I. Is vascular plant species diversity a predictor of bryophyte species diversity in Mediterranean forest? Biodivers. Conserv. 2007;16:525–545. [Google Scholar]
- Church RL, Stoms DM, Davis FW. Reserve selection as a maximal covering location problem. Biol. Conserv. 1996;76:105–112. [Google Scholar]
- Clemens MA, ReVelle CS, Williams JC. Reserve design for species preservation. Eur. J. Oper. Res. 1999;112:273–283. [Google Scholar]
- Cosson JF, Ringuet S, Claessens O, De Massary JC, Dalecky A, Villiers JF, et al. Ecological changes in recent land-bridge islands in French Guiana, with emphasis on vertebrate communities. Biol. Conserv. 1999;91:213–222. [Google Scholar]
- Cowling RM, Pressey RL. Introduction to systematic conservation planning in the Cape Floristic Region. Biol. Conserv. 2003;112:1–3. [Google Scholar]
- Csuti B, Polasky S, Williams PH, Pressey RL, Camm JD, Kershawf M, et al. A comparison of reserve selection algorithms using data on terrestrial vertebrates in Oregon. Biol. Conserv. 1997;80:83–97. [Google Scholar]
- DeGarady CJ, Halbrook RS. Using anurans as bioindicators of PCB contaminated streams. J. Herpetol. 2006;40:127–130. [Google Scholar]
- Devictor V, Mouillot D, Meynard C, Jiguet F, Thuiller W, Mouquet N. Spatial mismatch and congruence between taxonomic, phylogenetic and functional diversity: the need for integrative conservation strategies in a changing world. Ecol. Lett. 2010;13:1030–1040. doi: 10.1111/j.1461-0248.2010.01493.x. [DOI] [PubMed] [Google Scholar]
- Diniz-Filho JAF, Bini LM, Hawkins BA. Spatial autocorrelation and red herrings in geographical ecology. Glob. Ecol. Biogeogr. 2003;12:53–64. [Google Scholar]
- Diniz-Filho JAF, Bini LM, Pinto MP, Rangel TFLVB, Carvalho P, Bastos RP. Anuran species richness, complementarity and conservation conflicts in Brazilian Cerrado. Acta Oecol. 2006;29:9–15. [Google Scholar]
- Diniz-Filho JAF, Bini LM, Pinto MP, Terrilile LC, Oliveira G, Vieira CM, et al. Conservation planning: a macroecological approach using the endemic terrestrial vertebrates of the Braziilan Cerrado. Oryx. 2008;42:567–577. [Google Scholar]
- Diniz-Filho JAF, Bini LM, Rangel TFLVB, Loyola RD, Hof C, Nogués-Bravo D, et al. Partitioning and mapping uncertainties in ensembles of forecasts of species turnover under climate change. Ecography. 2009a;32:897–906. [Google Scholar]
- Diniz-Filho JAF, Oliveira G, Bini LM, Loyola RD, Nabout JC, Rangel TFLVB. Conservation biogeography and climate change in the Brazilian Cerrado. Nat. Conserv. 2009b;7:100–112. [Google Scholar]
- Diniz-Filho JAF, Nabout JC, Bini LM, Loyola RD, Rangel TFLVB, Nogués-Bravo D, et al. Ensemble forecasting shifts in climatically suitable areas for Tropidacris cristata (Orthoptera: Acridoidea: Romaleidae) Insect Conserv. Divers. 2010;3:213–221. [Google Scholar]
- ESRI. Arcgis Software. 2008. Version 9.3. Available at http://www.esri.com/products/index.html (accessed 10 December 2012)
- Faith DP, Walker PA. Environmental diversity: on the best-possible use of surrogate data for assessing the relative biodiversity of sets of areas. Biodivers. Conserv. 1996a;5:399–415. [Google Scholar]
- Faith DP, Walker PA. How do indicator groups provide information about the relative biodiversity of different sets of areas? on hotspots, complementarity and pattern-based approaches. Biodivers. Lett. 1996b;3:18–25. [Google Scholar]
- Flather CH, Wilson KR, Dean DJ, McComb WC. Identifying gaps in conservation networks: of indicators and uncertainty in geographic-based analyses. Ecol. Appl. 1997;7:531–542. [Google Scholar]
- Gaston KJ. Biodiversity – congruence. Prog. Phys. Geogr. 1996;20:105–112. [Google Scholar]
- Gotelli NJ, Entsminger GL. 2005. EcoSim: Null models software for ecology. Version 7.72. Acquired Intelligence Inc. & Kesey-Bear. Available at http://homepages.together.net/∼gentsmin/ecosim.htm (accessed 15 January 2013)
- Grant T, Frost DR, Caldwell JP, Gagliardo R, Haddad CFB, Kok PJR, et al. Phylogenetic systematics of dart-poison frogs and their relatives (Amphibia: Athesphatanura: Dendrobatidae) Bull. Am. Mus. Nat. Hist. 2006;299:1–262. [Google Scholar]
- Grantham HS, Pressey RL, Wells JA, Beattie AJ. Effectiveness of biodiversity surrogates for conservation panning: different measures of effectiveness generate a kaleidoscope of variation. PLoS One. 2010;5:e11430. doi: 10.1371/journal.pone.0011430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves CR, Jensen DB, Valutis LL, Redford KH, Shaffer ML, Scott JM, et al. Planning for biodiversity conservation: putting conservation science into practice. Bioscience. 2002;52:499–512. [Google Scholar]
- Haddad CFB. Biodiversidade dos anfíbios no Estado de São Paulo. In: Castro RMC, editor. Biodiversidade do Estado de São Paulo, Brasil: síntese do conhecimento ao final do século XX. São Paulo: Editora FAPESP; 1998. pp. 17–26. [Google Scholar]
- Haddad CFB, Toledo LF, Prado C. Anfíbios da Mata Atlântica: guia dos anfíbios anuros da Mata Atlântica. São Paulo: Editora Neotropica; 2008. [Google Scholar]
- Howard PC, Viskanic P, Davenport TRB, Baltzer M, Dickinson CJ, Lwanga JS, et al. Complementarity and the use of indicator groups for reserve selection in Uganda. Nature. 1998;394:472–475. [Google Scholar]
- IUCN. 2012. IUCN Red List of Threatened Species. Version 2012.2. Available at http://www.iucnredlist.org (accessed 10 December 2012)
- van Jaarsveld AS, Freitag S, Chown SL, Muller C, Koch S, Hull H, et al. Biodiversity assessments and conservation strategies. Science. 1998;279:2106–2108. doi: 10.1126/science.279.5359.2106. [DOI] [PubMed] [Google Scholar]
- Kati V, Devillers P, Dufrêne M, Legakis A, Vokou D, Lebrun P. Hotspots, complementarity or representativeness? designing optimal small-scale reserves for biodiversity conservation. Biol. Conserv. 2004;120:471–480. [Google Scholar]
- Kelley C, Garson J, Aggarwal A, Sarkar S. Place prioritization for biodiversity reserve network design: a comparison of the SITES and ResNet software packages for coverage and efficiency. Divers. Distrib. 2002;8:297–306. [Google Scholar]
- Kirkpatrick S, Gelatt CD, Vecchi MP. Optimization by simulated annealing. Science. 1983;220:671–680. doi: 10.1126/science.220.4598.671. [DOI] [PubMed] [Google Scholar]
- Kremen C. Assessing the indicator properties of species assemblages for natural areas monitoring. Ecol. Appl. 1992;2:203–217. doi: 10.2307/1941776. [DOI] [PubMed] [Google Scholar]
- Kubota U, Loyola RD, Almeida AM, Carvalho DE, Lewinsohn TM. Body size and host range co-determinate the altitudinal distribution of Neotropical tephritid flies. Glob. Ecol. Biogeogr. 2007;16:632–639. [Google Scholar]
- Kwet A. Efeitos da contaminação das águas superficiais associadas à atividade de extração e processamento de carvão sobre anfíbios. In: Teixeira EC, Pires MJR, Di-Bernardo M, editors. Meio ambiente e carvão – Impactos da exploração e utilização. Porto Alegre: Fundação Estadual de Proteção Ambiental (FEPAM); 2002. pp. 413–422. [Google Scholar]
- Lamoreux JF, Morrison JC, Ricketts TH, Olson DM, Dinerstein E, McKnight MW, et al. Global tests of biodiversity concordance and the importance of endemism. Nature. 2006;440:212–214. doi: 10.1038/nature04291. [DOI] [PubMed] [Google Scholar]
- Larsen FW, Bladt J, Rahbek C. Indicator taxa revisited: useful for conservation planning? Divers. Distrib. 2009;15:70–79. [Google Scholar]
- Lawler JJ, White D. Assessing the mechanisms behind successful surrogates for biodiversity in conservation planning. Anim. Conserv. 2008;11:270–280. [Google Scholar]
- Lawler JJ, White D, Sifneos JC, Master LL. Rare species and the use of indicator groups for conservation planning. Conserv. Biol. 2003;17:875–882. [Google Scholar]
- Lebboroni M, Ricchiardino G, Bellavita M, Chelazzi G. Potential use of anurans as indicators of biological quality in upstreams of central Italy. Amphib.-Reptilia. 2006;27:73–79. [Google Scholar]
- Lewandowski AS, Noss RF, Parsons DR. The effectiveness of surrogate taxa for the representation of biodiversity. Conserv. Biol. 2010;24:1367–1377. doi: 10.1111/j.1523-1739.2010.01513.x. [DOI] [PubMed] [Google Scholar]
- Lima JS. Processos Biológicos e o biomonitoramento: aspectos bioquímicos e morfológicos. In: Maia NB, Martos HL, Barrela W, editors. Indicadores ambientais: conceitos e aplicações. São Paulo: EDUC; 2001. pp. 95–115. [Google Scholar]
- Loiselle BA, Howell CA, Graham CH, Goerck JM, Brooks T, Smith KG, et al. Avoiding pitfalls of using species distribution models in conservation planning. Conserv. Biol. 2003;17:1591–1600. [Google Scholar]
- Loucks C, Ricketts TH, Naidoo R, Lamoreux J, Hoekstra J. Explaining the global pattern of protected area coverage: relative importance of vertebrate biodiversity, human activities and agricultural suitability. J. Biogeogr. 2008;35:1337–1348. [Google Scholar]
- Loyola RD. Broad-scale hypotheses do not account for species richness patterns of Central American mayflies. Open Ecol. J. 2009;2:29–36. [Google Scholar]
- Loyola RD, Kubota U, Lewinsohn TM. Endemic vertebrates are the most effective surrogates for identifying conservation priorities among Brazilian ecoregions. Divers. Distrib. 2007;13:389–396. [Google Scholar]
- Mace G, Possingham HP. Prioritizing choices in conservation. In: Macdonald DW, Service K, Leader-Williams N, editors. Key topics in conservation biology. Oxford, U.K: Blackwell Oxford; 2007. pp. 17–34. [Google Scholar]
- Manne LL, Williams PH. Building indicator groups based on species characteristics can improve conservation planning. Anim. Conserv. 2003;6:291–297. [Google Scholar]
- Margules CR, Pressey RL. Systematic planning for biodiversity conservation. Nature. 2000;405:243–253. doi: 10.1038/35012251. [DOI] [PubMed] [Google Scholar]
- Margules CR, Sarkar S. Systematic conservation planning. Cambridge, U.K: Cambridge Univ. Press; 2007. [Google Scholar]
- Metzger JP. Tree functional group richness and landscape structure in a Brazilian tropical fragmented landscape. Ecol. Appl. 2000;10:1147–1161. [Google Scholar]
- Metzger JP, Martensen AC, Dixo M, Bernacci LC, Ribeiro MC, Teixeira AMG, et al. Time-lag in biological responses to landscape changes in a highly dynamic Atlantic forest region. Biol. Conserv. 2009;142:1166–1177. [Google Scholar]
- Mittermeier RA, Gill PR, Hoffmann M, Pilgrim J, Brooks J, Mittermeier CJ, et al. Hotspots revisited: earth's biologically richest and most endangered terrestrial ecoregions. Washington, DC: CEMEX; 2004. [Google Scholar]
- Moilanen A. Generalized complementarity and mapping of the concepts of systematic conservation planning. Conserv. Biol. 2008;22:1655–1658. doi: 10.1111/j.1523-1739.2008.01043.x. [DOI] [PubMed] [Google Scholar]
- Moore JL, Balmford A, Brooks T, Burgess ND, Hansen LA, Rahbek C, et al. Performance of sub-Saharan vertebrates as indicator groups for identifying priority areas for conservation. Conserv. Biol. 2003;17:207–218. [Google Scholar]
- Myers N, Mittermeier RA, Mittermeier CG, Fonseca GAB, Kent J. Biodiversity hotspots for conservation priorities. Nature. 2000;403:853–858. doi: 10.1038/35002501. [DOI] [PubMed] [Google Scholar]
- Naidoo R, Balmford A, Ferraro PJ, Polasky S, Ricketts TH, Rouget M. Integrating economic costs into conservation planning. Trends Ecol. Evol. 2006;21:681–686. doi: 10.1016/j.tree.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Pinto MP, Diniz-Filho JAF, Bini LM, Blamires D, Rangel TFLVB. Biodiversity surrogate groups and conservation priority areas: birds of the Brazilian Cerrado. Divers. Distrib. 2008;14:78–86. [Google Scholar]
- Possingham HP, Ball I. Mathematical methods for identifying representative reserve networks. In: Ferson S, Burgman M, Andelman SJ, editors. Quantitative methods for conservation biology. New York: Springer; 2000. pp. 291–305. [Google Scholar]
- Ranta P, Blom T, Niemelä J, Joensuu E, Siitonen M. The fragmented Atlantic rain forest of Brazil: size, shape and distribution of forest fragments. Biodivers. Conserv. 1998;7:385–403. [Google Scholar]
- Raven PH, Wilson EO. A fifty-year plan for biodiversity surveys. Science. 1992;258:1099–1100. doi: 10.1126/science.258.5085.1099. [DOI] [PubMed] [Google Scholar]
- Ribeiro MC, Metzger JP, Martensen AC, Ponzoni FJ, Hirota MM. The Brazilian Atlantic Forest: how much is left, and how is the remaining forest distributed? Implications for conservation. Biol. Conserv. 2009;142:1141–1153. [Google Scholar]
- Ricketts TH, Dinerstein E, Boucher T, Brooks TM, Butchart SHM, Hoffmann M, et al. Pinpointing and preventing imminent extinctions. Proc. Natl Acad. Sci. USA. 2005;102:18497–18501. doi: 10.1073/pnas.0509060102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues ASL, Brooks TM. Shortcuts for biodiversity conservation planning: the effectiveness of surrogates. Annu. Rev. Ecol. Evol. Syst. 2007;38:713–737. [Google Scholar]
- Rondinini C, Wilson KA, Boitani L, Grantham H, Possingham HP. Tradeoffs of different types of species occurrence data for use in systematic conservation planning. Ecol. Lett. 2006;9:1136–1145. doi: 10.1111/j.1461-0248.2006.00970.x. [DOI] [PubMed] [Google Scholar]
- Sarkar S. Conservation biology. In: Zalta EN, editor. The Stanford encyclopedia of philosophy. Stanford, CA: Stanford University; 2004. pp. 1–76. Available at plato.stanford.edu/archives/sum2004/entries/conservation–biology (accessed 20 January 2013) [Google Scholar]
- Sarkar S, Justus J, Fuller T, Kelley C, Garson J, Mayfield M. Effectiveness of environmental surrogates for the selection of conservation area networks. Conserv. Biol. 2005;19:815–825. [Google Scholar]
- Sastre P, Lobo JM. Taxonomist survey biases and the unveiling of biodiversity patterns. Biol. Conserv. 2009;142:462–467. [Google Scholar]
- Schmit JP, Mueller GM, Leacock PR, Mata JL, Wu QF, Huang Y. Assessment of tree species richness as a surrogate for macrofungal species richness. Biol. Conserv. 2005;121:99–110. [Google Scholar]
- Sewell D, Griffiths RA. Can a single amphibian species be a good biodiversity indicator? Diversity. 2009;1:102–117. [Google Scholar]
- Silva WGS, Metzger JP, Simões S, Simonetti C. Relief influence on the spatial distribution of the Atlantic Forest cover at the Ibiúna Plateau, SP. Braz. J. Biol. 2007;67:403–411. doi: 10.1590/s1519-69842007000300004. [DOI] [PubMed] [Google Scholar]
- Simberloff D. Flagships, umbrellas, and keystones: is single-species management passé in the landscape era? Biol. Conserv. 1998;83:247–257. [Google Scholar]
- SOS Mata Atlântica Instituto Nacional de Pesquisas Espaciais. 2008. Atlas dos remanescentes florestais da Mata Atlântica, período de 2000 a 2005. Available at http://www.sosmatatlantica.org.br (accessed 15 December 2012)
- StatSoft, Inc. 2007. STATISTICA: Data Analysis Software System. Version 8.0. Available at http://www.statsoft.com (accessed 10 January 2013)
- Stoms DM, Comer PJ, Crist PJ, Grossman DH. Choosing surrogates for biodiversity conservation in complex planning environments. J. Conserv. Plan. 2005;1:26–39. [Google Scholar]
- Stuart SN, Chanson JS, Cox NA, Young BE, Rodrigues ASL, Fischman DL, et al. Status and trends of amphibian declines and extinctions worldwide. Science. 2004;306:1783–1786. doi: 10.1126/science.1103538. [DOI] [PubMed] [Google Scholar]
- Teixeira AMG, Soares BS, Freitas SR, Metzger JP. Modeling landscape dynamics in an Atlantic rain forest region: implications for conservation. Forest Ecol. Manag. 2009;257:1219–1230. [Google Scholar]
- Tocher MD, Gascon C. Fragmentation effects on a central Amazonian frog community: a ten-year study. In: Laurence WF, Bierregaard RO Jr, Zimmerman BL, editors. Tropical forest remnants: ecology, management and conservation of fragmented communities. Chicago, IL: University of Chicago Press; 1997. pp. 124–137. [Google Scholar]
- Trakhtenbrot A, Kadmon R. Environmental cluster analysis as a tool for selecting complementary networks of conservation sites. Ecol. Appl. 2005;15:335–345. [Google Scholar]
- Trindade-Filho J, Loyola RD. Performance and consistency of indicator groups in two biodiversity hotspots. PLoS One. 2011;6:e19746. doi: 10.1371/journal.pone.0019746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trindade-Filho J, Sobral FL, Cianciaruso MV, Loyola RD. Using indicator groups to represent bird phylogenetic and functional diversity. Biol. Conserv. 2012;146:155–162. [Google Scholar]
- Underhill LG. Optimal and suboptimal reserve selection algorithms. Biol. Conserv. 1994;70:85–87. [Google Scholar]
- Vane-Wright RI, Humphries CJ, Williams PH. What to protect? Systematics and the agony of choice. Biol. Conserv. 1991;55:235–254. [Google Scholar]
- Viana VM, Tabanez AAJ. Dynamic and restoration of forest fragments in the Brazilian Atlantic moist forest. In: Laurence WF, Bierregaard RO Jr, Batista JL, editors. Tropical forest remanants: ecology, management and conservation of fragmented communities. Chicago, IL: University of Chicago Press; 1997. pp. 351–365. [Google Scholar]
- Virolainen KM, Ahlroth P, Hyvarinen E, Korkeamaki E, Mattila J, Paivinen J, et al. Hot spots, indicator taxa, complementarity and optimal networks of taiga. Proc. R. Soc. Lond. B Biol. Sci. 2000;267:1143–1147. doi: 10.1098/rspb.2000.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson KA, Westphal MI, Possingham HP, Elith J. Sensitivity of conservation planning to different approaches to using predicted species distribution data. Biol. Conserv. 2005;122:99–112. [Google Scholar]