Abstract
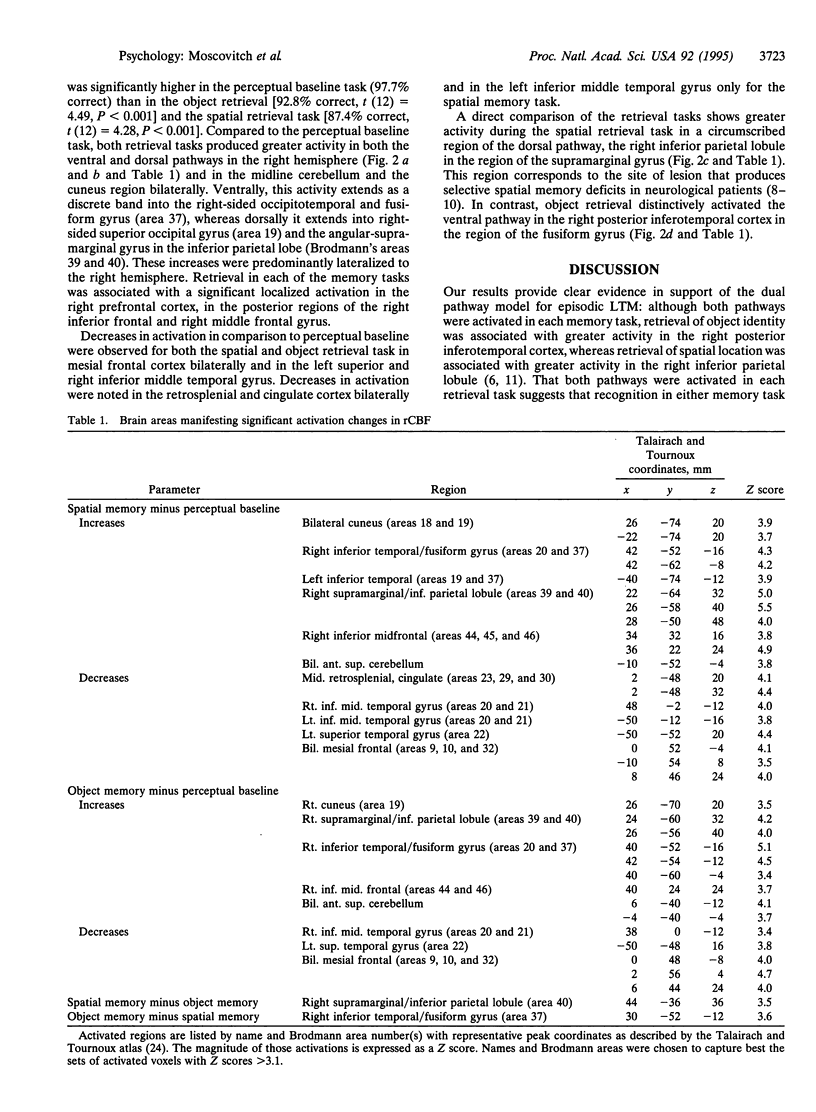
The purpose of the present study was to investigate by using positron emission tomography (PET) whether the cortical pathways that are involved in visual perception of spatial location and object identity are also differentially implicated in retrieval of these types of information from episodic long-term memory. Subjects studied a set of displays consisting of three unique representational line drawings arranged in different spatial configurations. Later, while undergoing PET scanning, subjects' memory for spatial location and identity of the objects in the displays was tested and compared to a perceptual baseline task involving the same displays. In comparison to the baseline task, each of the memory tasks activated both the dorsal and the ventral pathways in the right hemisphere but not to an equal extent. There was also activation of the right prefrontal cortex. When PET scans of the memory tasks were compared to each other, areas of activation were very circumscribed and restricted to the right hemisphere: For retrieval of object identity, the area was in the inferior temporal cortex in the region of the fusiform gyrus (area 37), whereas for retrieval of spatial location, it was in the inferior parietal lobule in the region of the supramarginal gyrus (area 40). Thus, our study shows that distinct neural pathways are activated during retrieval of information about spatial location and object identity from long-term memory.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Corbetta M., Miezin F. M., Shulman G. L., Petersen S. E. A PET study of visuospatial attention. J Neurosci. 1993 Mar;13(3):1202–1226. doi: 10.1523/JNEUROSCI.13-03-01202.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Renzi E., Faglioni P., Previdi P. Spatial memory and hemispheric locus of lesion. Cortex. 1977 Dec;13(4):424–433. doi: 10.1016/s0010-9452(77)80022-1. [DOI] [PubMed] [Google Scholar]
- Farah M. J., Hammond K. M., Levine D. N., Calvanio R. Visual and spatial mental imagery: dissociable systems of representation. Cogn Psychol. 1988 Oct;20(4):439–462. doi: 10.1016/0010-0285(88)90012-6. [DOI] [PubMed] [Google Scholar]
- Fox P. T., Mintun M. A. Noninvasive functional brain mapping by change-distribution analysis of averaged PET images of H215O tissue activity. J Nucl Med. 1989 Feb;30(2):141–149. [PubMed] [Google Scholar]
- Friston K. J., Frith C. D., Liddle P. F., Frackowiak R. S. Comparing functional (PET) images: the assessment of significant change. J Cereb Blood Flow Metab. 1991 Jul;11(4):690–699. doi: 10.1038/jcbfm.1991.122. [DOI] [PubMed] [Google Scholar]
- Goodale M. A., Milner A. D. Separate visual pathways for perception and action. Trends Neurosci. 1992 Jan;15(1):20–25. doi: 10.1016/0166-2236(92)90344-8. [DOI] [PubMed] [Google Scholar]
- Grasby P. M., Frith C. D., Friston K. J., Bench C., Frackowiak R. S., Dolan R. J. Functional mapping of brain areas implicated in auditory--verbal memory function. Brain. 1993 Feb;116(Pt 1):1–20. doi: 10.1093/brain/116.1.1. [DOI] [PubMed] [Google Scholar]
- Habib M., Sirigu A. Pure topographical disorientation: a definition and anatomical basis. Cortex. 1987 Mar;23(1):73–85. doi: 10.1016/s0010-9452(87)80020-5. [DOI] [PubMed] [Google Scholar]
- Haxby J. V., Horwitz B., Ungerleider L. G., Maisog J. M., Pietrini P., Grady C. L. The functional organization of human extrastriate cortex: a PET-rCBF study of selective attention to faces and locations. J Neurosci. 1994 Nov;14(11 Pt 1):6336–6353. doi: 10.1523/JNEUROSCI.14-11-06336.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herscovitch P., Markham J., Raichle M. E. Brain blood flow measured with intravenous H2(15)O. I. Theory and error analysis. J Nucl Med. 1983 Sep;24(9):782–789. [PubMed] [Google Scholar]
- Jonides J., Smith E. E., Koeppe R. A., Awh E., Minoshima S., Mintun M. A. Spatial working memory in humans as revealed by PET. Nature. 1993 Jun 17;363(6430):623–625. doi: 10.1038/363623a0. [DOI] [PubMed] [Google Scholar]
- Milner B., Petrides M., Smith M. L. Frontal lobes and the temporal organization of memory. Hum Neurobiol. 1985;4(3):137–142. [PubMed] [Google Scholar]
- Mishkin M., Appenzeller T. The anatomy of memory. Sci Am. 1987 Jun;256(6):80–89. doi: 10.1038/scientificamerican0687-80. [DOI] [PubMed] [Google Scholar]
- Morris R. G. Distinctive computations and relevant associative processes: hippocampal role in processing, retrieval, but not storage of allocentric spatial memory. Hippocampus. 1991 Jul;1(3):287–290. doi: 10.1002/hipo.450010318. [DOI] [PubMed] [Google Scholar]
- Newcombe F., Ratcliff G., Damasio H. Dissociable visual and spatial impairments following right posterior cerebral lesions: clinical, neuropsychological and anatomical evidence. Neuropsychologia. 1987;25(1B):149–161. doi: 10.1016/0028-3932(87)90127-8. [DOI] [PubMed] [Google Scholar]
- Pardo J. V., Fox P. T., Raichle M. E. Localization of a human system for sustained attention by positron emission tomography. Nature. 1991 Jan 3;349(6304):61–64. doi: 10.1038/349061a0. [DOI] [PubMed] [Google Scholar]
- Pardo J. V., Pardo P. J., Raichle M. E. Neural correlates of self-induced dysphoria. Am J Psychiatry. 1993 May;150(5):713–719. doi: 10.1176/ajp.150.5.713. [DOI] [PubMed] [Google Scholar]
- Pigott S., Milner B. Memory for different aspects of complex visual scenes after unilateral temporal- or frontal-lobe resection. Neuropsychologia. 1993 Jan;31(1):1–15. doi: 10.1016/0028-3932(93)90076-c. [DOI] [PubMed] [Google Scholar]
- Posner M. I., Petersen S. E. The attention system of the human brain. Annu Rev Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Raichle M. E., Fiez J. A., Videen T. O., MacLeod A. M., Pardo J. V., Fox P. T., Petersen S. E. Practice-related changes in human brain functional anatomy during nonmotor learning. Cereb Cortex. 1994 Jan-Feb;4(1):8–26. doi: 10.1093/cercor/4.1.8. [DOI] [PubMed] [Google Scholar]
- Seitz Rüdiger J., Roland Per E. Learning of Sequential Finger Movements in Man: A Combined Kinematic and Positron Emission Tomography (PET) Study. Eur J Neurosci. 1992;4(2):154–165. doi: 10.1111/j.1460-9568.1992.tb00862.x. [DOI] [PubMed] [Google Scholar]
- Sergent J., Signoret J. L. Functional and anatomical decomposition of face processing: evidence from prosopagnosia and PET study of normal subjects. Philos Trans R Soc Lond B Biol Sci. 1992 Jan 29;335(1273):55–62. doi: 10.1098/rstb.1992.0007. [DOI] [PubMed] [Google Scholar]
- Shallice T., Fletcher P., Frith C. D., Grasby P., Frackowiak R. S., Dolan R. J. Brain regions associated with acquisition and retrieval of verbal episodic memory. Nature. 1994 Apr 14;368(6472):633–635. doi: 10.1038/368633a0. [DOI] [PubMed] [Google Scholar]
- Smith M. L., Milner B. The role of the right hippocampus in the recall of spatial location. Neuropsychologia. 1981;19(6):781–793. doi: 10.1016/0028-3932(81)90090-7. [DOI] [PubMed] [Google Scholar]
- Snodgrass J. G., Vanderwart M. A standardized set of 260 pictures: norms for name agreement, image agreement, familiarity, and visual complexity. J Exp Psychol Hum Learn. 1980 Mar;6(2):174–215. doi: 10.1037//0278-7393.6.2.174. [DOI] [PubMed] [Google Scholar]
- Squire L. R., Ojemann J. G., Miezin F. M., Petersen S. E., Videen T. O., Raichle M. E. Activation of the hippocampus in normal humans: a functional anatomical study of memory. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1837–1841. doi: 10.1073/pnas.89.5.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E., Kapur S., Craik F. I., Moscovitch M., Houle S. Hemispheric encoding/retrieval asymmetry in episodic memory: positron emission tomography findings. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2016–2020. doi: 10.1073/pnas.91.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E., Markowitsch H. J., Kapur S., Habib R., Houle S. Novelty encoding networks in the human brain: positron emission tomography data. Neuroreport. 1994 Dec 20;5(18):2525–2528. doi: 10.1097/00001756-199412000-00030. [DOI] [PubMed] [Google Scholar]
- Valenstein E., Bowers D., Verfaellie M., Heilman K. M., Day A., Watson R. T. Retrosplenial amnesia. Brain. 1987 Dec;110(Pt 6):1631–1646. doi: 10.1093/brain/110.6.1631. [DOI] [PubMed] [Google Scholar]
- Wilson F. A., Scalaidhe S. P., Goldman-Rakic P. S. Dissociation of object and spatial processing domains in primate prefrontal cortex. Science. 1993 Jun 25;260(5116):1955–1958. doi: 10.1126/science.8316836. [DOI] [PubMed] [Google Scholar]



