Abstract
The response of montane and subalpine hay meadow plant and arthropod communities to the application of liquid manure and aerial irrigation – two novel, rapidly spreading management practices – remains poorly understood, which hampers the formulation of best practice management recommendations for both hay production and biodiversity preservation. In these nutrient-poor mountain grasslands, a moderate management regime could enhance overall conditions for biodiversity. This study experimentally assessed, at the site scale, among low-input montane and subalpine meadows, the short-term effects (1 year) of a moderate intensification (slurry fertilization: 26.7–53.3 kg N·ha−1·year−1; irrigation with sprinklers: 20 mm·week−1; singly or combined together) on plant species richness, vegetation structure, hay production, and arthropod abundance and biomass in the inner European Alps (Valais, SW Switzerland). Results show that (1) montane and subalpine hay meadow ecological communities respond very rapidly to an intensification of management practices; (2) on a short-term basis, a moderate intensification of very low-input hay meadows has positive effects on plant species richness, vegetation structure, hay production, and arthropod abundance and biomass; (3) vegetation structure is likely to be the key factor limiting arthropod abundance and biomass. Our ongoing experiments will in the longer term identify which level of management intensity achieves an optimal balance between biodiversity and hay production.
Keywords: Agriculture, arthropods, grassland management, hump-shaped model, liquid manure, vegetation heterogeneity
Introduction
Numerous studies have documented that grassland management intensification alters biodiversity, leading to decline of plant and arthropod species richness and modifying plant traits as well as community structure (e.g., Marini et al. 2008; Riedener et al. 2013; Niu et al. 2014). Similarly, but on the other extreme of the grassland management intensity gradient, abandonment occurring in steep and less accessible mountain regions leads to forest encroachment and the disappearance of many open-habitat species (MacDonald et al. 2000; Tasser et al. 2007). However, alternatives to this dichotomous trend (agriculture intensification versus abandonment) exist in the form of an intermediate intensity of management in terms of mowing regime (e.g., Tonn and Briemle 2010; Bernhardt-Romermann et al. 2011), irrigation (Jeangros and Bertola 2000), and fertilization (e.g., Pauli et al. 2002; Bowman et al. 2006). This moderate management is likely to have conjugated positive effects on plant and invertebrate diversity, hay production, and forage nutritional quality. Different theories and factors can explain why an intermediate or moderate management intensity is likely to benefit grassland flora and fauna communities. For example, based on the hump-shaped species diversity curve of Grime (1973; see also Mittelbach et al. 2001), a moderate addition of resources should enhance plant species growth and richness. This phenomenon is especially expected in nutrient-poor montane and subalpine grasslands (Peter et al. 2009). In turn, an increase in plant growth will provide more food, space, and shelters for arthropods, boosting their abundances (e.g., Haddad et al. 2000; Perner et al. 2005; Dittrich and Helden 2011; Buri et al. 2013). Higher plant species richness not only provides more potential host plants for herbivores, but also greater horizontal and vertical vegetation structure complexity, which seems to be crucial to support higher diversity and abundance of arthropods (e.g., Brown et al. 1992; Morris 2000; Woodcock et al. 2009; Dittrich and Helden 2011). A more abundant arthropod community will promote higher trophic levels up to vertebrates through a cascading process (Hunter and Price 1992; Britschgi et al. 2006). In seminatural mountain meadows, the exact management practices that would permit decent hay production without degrading the functional integrity of the system remain unknown, thus meriting further investigation.
We launched a two-way factorial experiment on the short-, mid-, and long-term effects of fertilization and irrigation on plant and arthropod communities of montane and subalpine hay meadows of the inner European Alps (Valais, SW Switzerland). The main objective of this study is to document the short-term changes that occurred just 1 year after the onset of differential experimental management treatments. While end-user management recommendations will be based on the longer-term outputs of the study, thoroughly assessing the short-term effects clarifies the ecological mechanisms at play during the temporal process of grassland intensification. More specifically, we addressed two questions: (1) What are the short-term effects of fertilization and irrigation, considered separately and in combination, on plant species richness, vegetation structure, hay production, and arthropod abundance and biomass? and (2) what is the relationship between vegetation and arthropod parameters?
Plants and arthropods were hypothesized to respond differently to the fertilization and irrigation treatments in the short-term, that is, after just 1 year of experimental manipulation, partly because plants typically have a slower reaction time than animals to changes in environmental conditions (Mortimer et al. 1998; Cole et al. 2010). More specifically, we expected slight positive effects of fertilization on plant species richness and hay production (Grime 1973), while an increase in plant growth and richness was expected to increase vegetation structure, which would in turn promote arthropod populations (Woodcock et al. 2009). On the other hand, we predicted that irrigation would have no effect on plant species richness (Riedener et al. 2013), but still positive effects on arthropod abundance through an increased phytomass productivity and protection against dessication (Nielsen 1955). Fertilization was also predicted to increase herbivorous arthropod abundances, owing to an increase in plant tissue nitrogen content (Haddad et al. 2000; Dittrich and Helden 2011). However, due to a highly diverse plant species pool among all our meadows (given that they have been extensively managed over the past decades), a high ecological stability and resistance against the experimental treatments were expected in the short term (Tilman and Downing 1994), therefore translating into few contrasted effects.
Materials and Methods
Study sites
In 2010, twelve extensively managed montane and subalpine hay meadows were selected according to their management history. The meadows had to be managed extensively for at least the last 10 years with no or very low levels of fertilization (with solid manure only) and irrigation (terrestrial only), and only a single cut per year. Their homogeneous topography and their size were also considered (>4000 m2). The study sites were situated in the inner Alps (Valais, SW Switzerland) between 790 and 1740 m above sea level, encompassing a wide gradient of altitudes and ambient temperatures (Table 1). This region experiences a continental climate with cold and wet winters, and dry and hot summers.
Table 1.
Description of the twelve study sites with altitude, exact coordinates, and quantity of fertilizer, that is, nitrogen (N), phosphorus (P), and potassium (K), applied per hectare per year. The fertilizer consisted of organic NPK pellets, and mineral K2O dissolved in water to reach the equivalent of standard-farm liquid manure
| Coordinates | Fertilizer applied [kg·ha−1·year−1] | ||||||
|---|---|---|---|---|---|---|---|
| Site | Name | Altitude [m] | Latitude | Longitude | N | P | K |
| 1 | La Garde | 980 | 46°3′45″N | 7°8′35″E | 40.0 | 33.3 | 133.3 |
| 2 | Sembrancher | 798 | 46°4′24″N | 7°8′36″E | 53.3 | 44.4 | 177.7 |
| 3 | Orsières | 1022 | 46°1′44″N | 7°9′8″E | 53.3 | 44.4 | 177.7 |
| 4 | Vens | 1373 | 46°5′7″N | 7°7′24″E | 40.0 | 33.3 | 133.3 |
| 5 | Euseigne | 1028 | 46°10′9″N | 7°25′27″E | 53.3 | 44.4 | 177.7 |
| 6 | Eison | 1768 | 46°9′18″N | 7°28′10″E | 26.7 | 22.3 | 89.0 |
| 7 | St-Martin | 1589 | 46°11′8″N | 7°26′43″E | 26.7 | 22.3 | 89.0 |
| 8 | Grimentz | 1738 | 46°11′22″N | 7°34′35″E | 26.7 | 22.3 | 89.0 |
| 9 | Arbaz | 1270 | 46°16′42″N | 7°22′47″E | 40.0 | 33.3 | 133.3 |
| 10 | Icogne1 | 1200 | 46°17′56″N | 7°26′31″E | 40.0 | 33.3 | 133.3 |
| 11 | Icogne2 | 880 | 46°17′6″N | 7°26′10″E | 53.3 | 44.4 | 177.7 |
| 12 | Cordona | 1153 | 46°19′45″N | 7°33′8″E | 40.0 | 33.3 | 133.3 |
Design
A two-way full factorial design was applied in our experiments. At each study site, that is, in each meadow, four circular plots of 20 m in diameter were established with at least 5 m between plot boundaries. The different management treatments were randomly assigned to the four plots within a given meadow. The first plot served as a control (C-plot: neither irrigation nor fertilization). The second plot was only irrigated (I-plot) at regular time intervals with sprinklers. The third plot was only fertilized (F-plot) with liquid manure, and the fourth plot was irrigated and fertilized (I + F-plot). C-plots were cut once a year, which corresponds to local standards for extensively managed meadows, while I, F, and I + F-plots were cut twice a year. Although this discrepancy deviated the design from a purely speaking two-way full factorial design, it made agronomical sense; local farmers would not irrigate or fertilize their field without doing a second cut. Treatments I and I + F were irrigated weekly from mid-May to the beginning of September, except when heavy rainfall occurred (>20 mm over the previous week). Weekly sprinkler irrigation amounted to 20 mm of water column. The fertilizer consisted of organic dried manure NPK pellets (MEOC SA, 1906 Charrat, Switzerland), and mineral potassium oxide (K2O) dissolved in water to reach the equivalent of standard-farm liquid manure (Sinaj et al. 2009), consisting namely of 2.4 kg of usable nitrogen, 2 kg of phosphate (P2O5), and 8 kg of potassium oxide (K2O) per m3 of solution. 174, 262, or 349 l of liquid manure per plot, corresponding to, respectively, 26.7, 40.0, or 53.3 kg N·ha−1 year−1, were applied three times in August 2010, May 2011, and August 2011 (Table 1). The exact amount of manure applied at each site depended on the theoretical local hay production potential calculated using pre-experimental hay yield (when extensively managed) and site elevation, and it matched the local mid-intensive management norm recommended in Sinaj et al. (2009). In each plot, a 4 × 2 m permanent rectangle subplot was established at a distance of 4 m from plot center, randomly placed along the slope axis on the right or the left side of the plot. In each subplot, we measured plant species richness, vegetation structure, hay production, and abundance and biomass of arthropods (Fig. 1).
Figure 1.
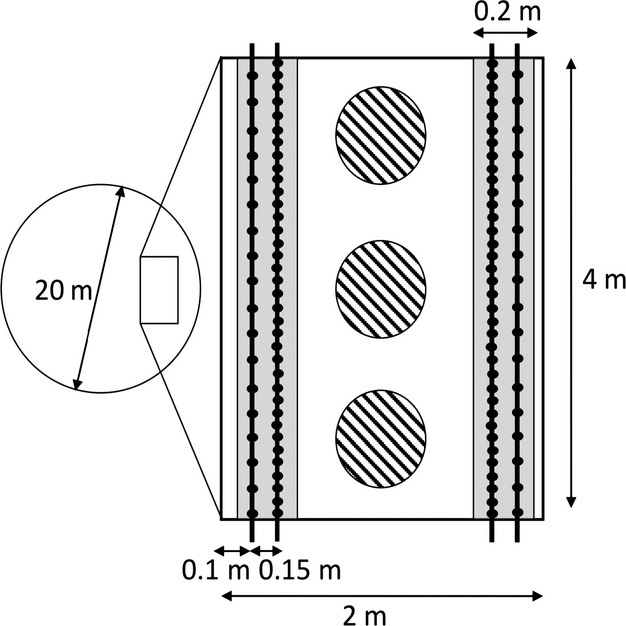
Experimental design. Four management treatments were applied at random onto 20-m-diameter circles delineated on each meadow. In each circle (excerpt), vegetation (n = 122 records per circle, black dots), hay production (gray strips), and arthropods (three dashed circles of 0.2 m2) were sampled.
Vegetation sampling
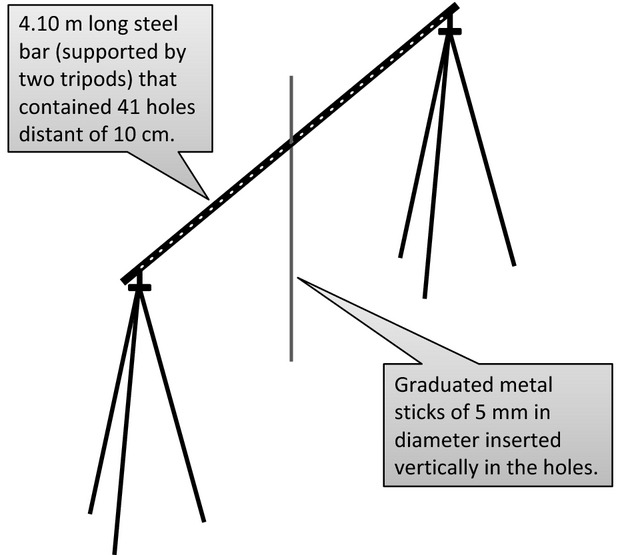
In 2011, plant species richness, vegetation structure, and hay production were assessed twice: once just before the first cut (from mid-June to end of July, at a similar vegetation stage, depending on altitude; hereafter referred to as July samples) and once just before the second cut (from August to September; hereafter August samples). Vegetation surveys were performed using the point quadrat method in order to obtain information on the vertical distribution of each plant species (Stampfli 1991). For that purpose, we developed an ad hoc device that consisted of a 4.10-m-long steel bar (supported by two tripods) that contained 41 holes distant of 10 cm (Appendix 1). Graduated metal sticks of 5 mm in diameter were inserted vertically into the holes. Each plant species touching the stick was recorded, and the height at which the plant touched the stick was noted. If the same species touched more than once a single stick, the maximal height was retained. The sampling device was positioned along each long side of the permanent rectangular subplot, first 10 cm and then 25 cm from the long edge (Fig. 1). We recorded contacts between plants and sticks at 20 and 41 holes (points) when the device was positioned at 10 cm and 25 cm from the edge, respectively. Altogether, we thus recorded 122 points in each plot. A modified Shannon–Wiener diversity index (Woodcock et al. 2009) was used to define the structure of the vegetation:
where Struct is the index for vegetation structure and pi the proportion of the number of contacts with the stick at each height i, in each subplot, at each sampling session. Thus, greater structural complexity of the vegetation results in a higher value.
Just before each grass cut, hay production was estimated by clipping two strips of grass with an area of 0.2 × 4 m along each long edge of the permanent subplot at 6 cm above the ground, exactly where the vegetation relevés had been performed (Fig. 1). The two samples from the same subplot were then pooled together. The collected plant material was dried at 105°C during 72 h and then weighed (±0.1 g) in order to quantify hay production.
Arthropod sampling
Arthropods were sampled using a suction sampler (Stihl SH 86 D; Stihl) equipped with a gauze sampling sack fixed inside the nozzle to collect arthropod items. This technique has been proved to be efficient for grassland vegetation-dwelling arthropods (Sanders and Entling 2011). All plots were sampled twice during the vegetation season, once before each grass cut. At each sampling session, three subsamples were collected at three regularly spaced locations in the middle of each permanent subplot (Fig. 1). Subsamples consisted of the vacuumed content of a metallic cylinder of 50 cm height and 50.5 cm diameter (0.2 m2 area) that was placed directly on the ground. The content of the gauze sampling sack was transferred into a sealed plastic bag stored at low temperature in an ice-cooled box. Sampling was undertaken between 11:00 and 17:00, only under dry vegetation conditions and with low or moderate wind. Arthropod specimens were then stored in the laboratory at −20°C before being classified in six main taxonomic groups: spiders, Auchenorrhyncha (i.e., plant- and leafhoppers), weevils, leaf beetles, ants, and others (other arthropods not belonging to the previous groups). The number of specimens was counted prior to drying the arthropods at 60° during 72 h. Finally, all arthropod groups stemming from one subsample were weighed (±0.1 mg). For statistical analyses, the three subsamples per plot were summed. Ants had to be discarded because suction trapping proved to be inefficient for sampling this group due to their massive local colonial aggregations.
Statistical analysis
Treatment effects were analyzed with linear mixed-effects models (LMMs) using the lmer function from the lme4 package for R (Bates et al. 2011). P-values and confidence intervals (CI) were computed with the pvals.fnc function from the languageR package using 100,000 Markov chain Monte Carlo iterations (Baayen 2011). Response variables were log-transformed plant species richness, vegetation structure, hay production, log-transformed arthropod abundance, and log-transformed arthropod biomass. As grass (Poaceae), legume (Fabaceae), and forb species may respond differently to the management treatments (e.g., Li et al. 2010), additional models on the relative cover of each functional group were run. Note that not all variables needed log-transformation prior to analysis to achieve normal distribution of residuals. The fixed effects were the treatments (C, I, F, or I + F) and the sampling sessions (July or August) which were added as a factor to take in account the fact that two measures were made per plot. For hay production, analyses were performed on the sum of the July and August (pooled samples). Thus, for this variable, fixed effects were limited to the treatments. The study sites (geographic replicates) were designated as a random effect. To better appraise differences between treatments, post hoc tests were performed using the function relevel of R to change the first reference level of the factor “treatment.”
In order to further understand the relationship between the vegetation and arthropod parameters, simple linear regressions were performed using the lm function (Crawley 2007). The log-transformed abundance and biomass of arthropods were fitted against plant species richness, vegetation structure (index Struct), and hay production. Finally, to test whether the variance in arthropod abundance and biomass (variance of the nontransformed raw data) changes with respect to vegetation structure, a homoscedasticity test (Bartlett's test) was conducted between the values obtained from the first and the third quantiles of Struct (Crawley 2007). Thus, a significant P-value would indicate that with low vegetation structure, there are only few arthropods, while with a higher vegetation structure, it is possible to have either few or many arthropods (see Fig. 4). In other words, this value indicates whether vegetation structure limits arthropod abundance and/or biomass. All statistical tests were performed using R version 2.15.3 (R Core Team 2013).
Figure 4.
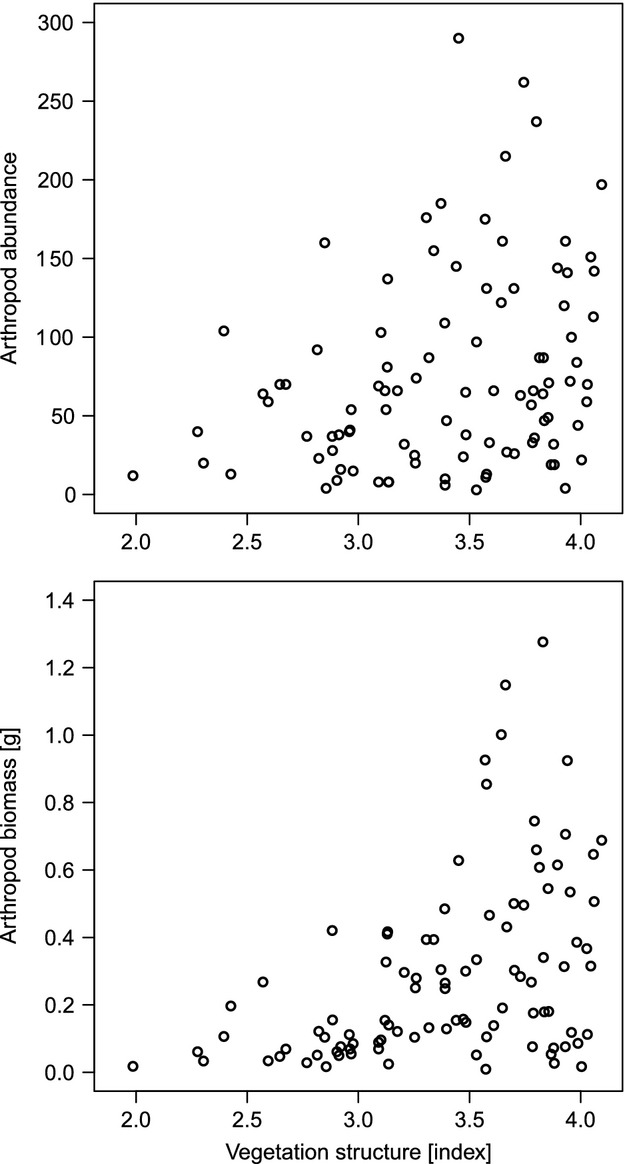
Relationships between arthropod abundance and biomass versus vegetation structure (index Struct). Greater the structure of the vegetation, higher the Struct index.
Results
Effects of irrigation and fertilization on the vegetation
In total, 194 plant species belonging to 34 families were identified during the two sampling sessions across all meadows (see Appendix 2 for a complete list of the plant species recorded). F-plots, I-plots, and I + F-plots harbored significantly more plant species than C-plots (Fig. 2; and Table A3.1 in Appendix 3 for related model outputs). Moreover, irrigated plots (I and I + F) had significantly higher species richness than F-plots, but treatment I + F was not different from I. Irrigated plots exhibited a higher vegetation structure (index Struct) than C-plots and F-plots, while treatment F did not differ from C. The greatest vegetation structure was measured in July and the lowest in August; this pattern was consistent across all treatments. Annual hay production (sum of both sampling sessions) ranged from 96.5 to 1111 g·m−2 across all plots. It was approximately three times higher in the irrigated plots compared with C-plots, but I + F treatment did not differ from treatment I. Fertilization (F) had a lower effect compared with irrigation but still gave a significantly higher hay production than C.
Figure 2.
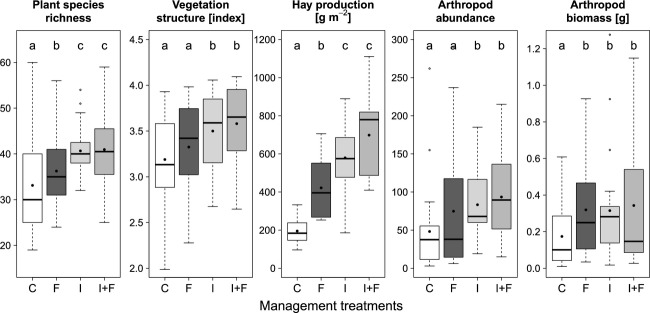
Responses of the vegetation (plant species richness, vegetation structure and hay production) and arthropod (abundance and dry biomass) variables to the different management treatments. Bold lines represent medians, solid points the means, boxes the first and third quantiles. Different letters indicate significant differences among treatments at an alpha rejection value set to 0.05. Treatments abbreviations are as follows: (C) control; (I) irrigated, (F) fertilized, and (I + F) irrigated and fertilized.
Relative cover of grasses decreased in I, F, and I + F-plots compared with the control plots, while legumes increased their cover (Fig. 3). Relative changes were all significant at a P < 0.01 level (see Table A3.2 in Appendix 3 for exact values of models outputs). Forb species cover did not differ among treatments except I + F that had significantly less cover than C (P = 0.011).
Figure 3.
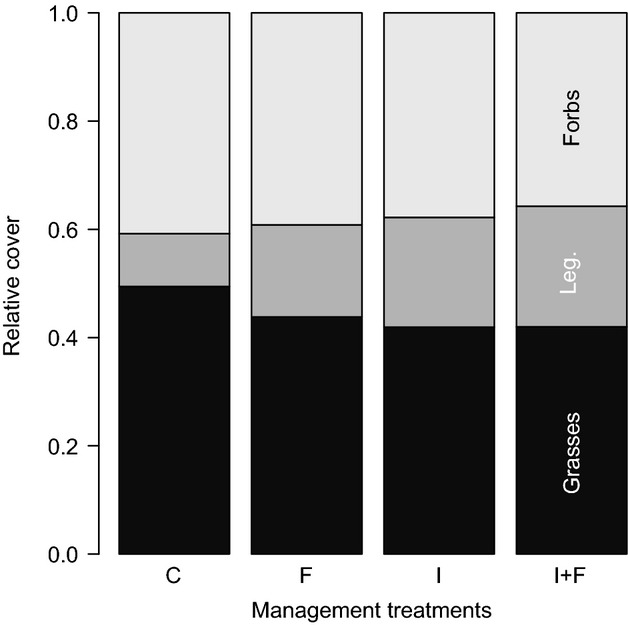
Responses of relative cover of grass (dark-gray), legume (mid-gray), and forb (light-gray) species to the different management treatments. Model outputs (including estimates, CIs, and P-values) are provided in Table A3.2 in Appendix 3. For treatment abbreviations, see legend of Fig. 2.
Effects of irrigation and fertilization on the arthropods
In total, 7198 arthropods (ants excluded) were collected across all replicates (3923 in July and 3275 in August). The samples included n individuals of the following taxa: 629 spiders (Araneae), 1869 plant- and leafhoppers (Hemiptera: Auchenorrhyncha), 562 weevils (Coleoptera: Curculionidae), 587 leaf beetles (Coleoptera: Chrysomelidae), and 3551 others. Abundance of arthropods in I-plots and I + F-plots were significantly higher than in C-plots and F-plots (Fig. 2; and Table A3.1 in Appendix 3 for related model outputs). Treatment F did not deliver a higher abundance of arthropods compared with treatment C. The only significant differences within a single arthropod group were for plant- and leafhoppers where in I + F-plots, there were more individuals compared with C-plots (MCMC mean = 0.890, 95% CI = 0.281–1.511, P MCMC = 0.005) and to F-plots (MCMC mean = 0.766, 95% CI = 0.161–1.385, P MCMC = 0.015). For spiders, abundance in I + F-plots was marginally significantly higher than in C-plots (MCMC mean = 0.375, 95% CI = −0.021–0.759, P MCMC = 0.060), while no differences were detected between I-plots and F-plots, on one side, and C-plots, on the other side.
In total, 26.92 g dry weight of arthropods was collected across all replicates (17.13 g in July and 9.79 g in August). The samples (excluding ants) included the following taxa: 1.856 g of spiders, 2.705 g of plant- and leafhoppers, 0.766 g of weevils, 0.458 g of leaf beetles, and 21.130 g for others. All treatments affected positively the biomass of arthropods (Fig. 2; and Table A3.1 in Appendix 3). The biomass of plant- and leafhoppers was significantly higher in I + F-plots than in the C-plots (MCMC mean = 0.019, 95% CI = 0.001–0.037, P MCMC = 0.038), while there were no significant biomass differences between treatments and controls in another arthropod taxonomic group.
Relationships between arthropods and vegetation
The total abundance of arthropods was positively linked to hay production (estimate = 2.60·10−3 t = 4.767, P < 0.001; adjusted R2 = 0.186, i.e. 18.6% explained variance), plant species richness (estimate = 6.79·10−2 t = 6.696; P < 0.001, R2 = 0.316), and vegetation structure (estimate = 0.572, t = 2.752, P = 0.007, R2 = 0.065). The variance in arthropod biomass was explained in about the same order of magnitude by hay production (estimate = 2.905·10−3 t = 5.085, P < 0.001, R2 = 0.207), plant species richness (estimate = 5.580·10−2 t = 4.747, P < 0.001, R2 = 0.185), and vegetation structure (estimate = 1.049, t = 5.182, P < 0.001, adjusted R2 = 0.214). Note that estimates are on the log scale. Regarding the analyses about whether vegetation structure limits arthropods, for both arthropod abundance (Bartlett's K2 = 6.933, df = 1, P = 0.008) and biomass (Bartlett's K2 = 23.145, df = 1, P < 0.001), Bartlett's test showed a greater variance at the third than at the first quantile of vegetation structure (Fig. 4).
Discussion
This study shows that among low-input montane and subalpine hay meadows, plant species richness, vegetation structure, hay production as well as arthropod abundance and biomass all immediately and positively react to moderate experimental fertilization and irrigation. It should be noted, however, that the starting conditions in our study meadows were typical of the traditional, extremely extensive management practices that have been prevailing for centuries in the inner Alps, with very low fertilizer application and limited terrestrial irrigation. It is thus not totally surprising that our experimental treatments improved both biodiversity and hay yield in the very short term. These traditional grasslands typically are poor in nitrophilous species with specialized taxa present due to a very constraining edaphic context and watering regime (Peter et al. 2009). The speed at which these changes operated in response to intensification was, however, unexpectedly rapid. A powerful advantage of our full block design approach is certainly that it allows a direct comparison of the effects of both irrigation and fertilization, which were either separated or conjugated, upon meadowland ecological communities regardless of other potentially confounding abiotic factors such as altitude, exposition, or soil properties.
Effects of fertilization and irrigation on the vegetation
Fertilizing with liquid manure and watering with sprinklers are two modern, currently spreading management practices, even in remote areas of the Alps (Riedener et al. 2013). Our treatments thus mimic the trends of modern agriculture in these areas. Although we had predicted slower effects on plant species richness, basing our predictions on the dynamics observed in most long-term studies in alpine and arctic regions (e.g., Carlen et al. 1998; Yang et al. 2011), our findings are in accordance with the predictions of the hump-shaped model of plant diversity (Grime 1973; Mittelbach et al. 2001). This model stipulates that an intermediate level of intensification must support a higher plant species richness than low- or high-input systems. Yet, we cannot exclude, given that we measured effects just 1 year after the onset of the experimental treatments, that abiotic factors, such as interannual weather variation, might have interacted with the treatment effects, amplifying the signal (Walker et al. 1994). What is certain, however, is that no plant community would ever reach an equilibrium after just 1 year of this management (Yang et al. 2011). Hence, a short-term, moderate intensification as applied here may indeed promote high plant species richness because it rapidly offers favorable conditions to nitrophilous and mesophilous species that are normally absent on nutrient-poor and dry soils. Some of the original plant species pool consisting of heliophilous species, tolerant to reduced nutrients and water supply but particularly intolerant to intensification and shade, may actually have persisted in the community merely because they were already extant. This suggests the possibility of a short-term coexistence of plants with different life-history traits and varied ecological requirements (Bowman et al. 2006). In the mid- and long run, however, one would expect that interspecific competition for resources such as light will especially increase among some species. Species exhibiting characteristics such as low growth rate could become progressively disadvantaged and possibly decline to local extinction (Rajaniemi 2002; Hautier et al. 2009).
Irrigating and fertilizing increased the relative cover of legumes, which appears to be mostly at the expense of the cover of grasses. While this seems in contradiction with most grassland fertilization studies that found the reverse pattern regarding their biomasses (e.g. DiTommaso and Aarssen 1989; Carlen et al. 1998; Li et al. 2010), it must be stressed that relative cover does not necessarily correlate with biomass, especially when comparing grasses that grow tall and thin with legumes that tend to grow wider. In addition, fertilization studies that found positive effects of intensification on grasses and negative effects on legumes usually applied mineral fertilizers, while the application of organic fertilizers is known to have slightly different influences, typically favoring legume species (e.g., Vintu et al. 2011).
In contradiction to our prediction that fertilization would have a positive short-term effect on all vegetation parameters, addition of liquid manure alone did not increase vegetation structure, while the combination of fertilization and irrigation did not elicit a greater response from vegetation parameters than did irrigation alone. This indicates that in the short term, application of fertilizer (only) might enhance the sensitivity of the vegetation to water stress (Huston 1997) or that our meadows were more likely to be limited by water supply than nitrogen supply. Indeed, the climatic context in the inner Alps is characterized by its dryness (Central Valais, around Sion-Visp, is the pole of xericity in the whole Alpine massif, with ca 500 mm annual precipitation), with even April-June 2011 slightly drier than interannual average (94 mm vs. 136 mm mean rainfall during 2006–2010 in Sion; MeteoSwiss). Plant nutrient uptake may also have been improved by water addition thus enhancing plant growth (Davis et al. 2000). Future vegetation surveys in the same study meadows will enable disentangling climatic from agronomic effects, while characterizing mid- and longer-term changes in plant communities.
Effects of fertilization and irrigation on arthropod communities
Irrigation in turn had a positive effect on arthropod species richness, as predicted. This indicates that water might be a limiting factor for arthropods (e.g., intolerance to desiccation; Nielsen 1955), or that there is an indirect effect mediated via plants onto arthropods. In contrast, fertilization per se led to no discernible effect on arthropods, corroborating previous findings in comparable montane ecosystems (Grandchamp et al. 2005). The less complex vegetation structure achieved via fertilization alone compared with irrigation means that the offer of microhabitats and the resulting ecological niche opportunities are less favorable when only fertilization is augmented (Reid and Hochuli 2007). Irrigation and fertilization were also expected to increase the rate of herbivory, that is, the abundance of plant- and leafhoppers, and as a result increase the abundance of their predators such as spiders (Kirchner 1977). However, only plant- and leafhoppers showed a numeric response to irrigation and fertilization suggesting that a steady state had not been achieved with no discernible effects being propagated to the upper trophic levels along the food chain at this stage. It is also important to note that a much smaller sample size for predator taxa than for prey taxa could have blurred the pattern due to lower statistical power.
Relationships between arthropods and vegetation
Vegetation parameters such as plant species richness, plant biomass, and vegetation structure all influence arthropod community to some extent (Knops et al. 1999; Haddad et al. 2000). There is still an ongoing debate about which factor has the greatest impact on arthropods (Perner et al. 2005), but recent studies have pointed out that vegetation structure might be the crux (Woodcock et al. 2009; Dittrich and Helden 2011). Our analyses show that all vegetation parameters influence arthropods to a certain degree. However, neither plant species richness (31.6% of explained variance for abundance/18.5% for biomass) nor hay production (18.6%/20.7%) or vegetation structure (6.5%/21.4%) individually accurately predicted arthropod abundance and biomass. This seems to contradict the view that vegetation structure is a key factor. However, there is evidence that vegetation structure did profoundly influence the number of arthropods in our meadows (Fig. 4), yet vegetation structure is more likely to act as a limiting than a predictive factor. Indeed, at low vegetation structure, low arthropod abundance and biomass always prevail, whereas at high vegetation structural diversity, arthropod abundance and biomass can either be low or high. This pattern is in line with the predictions of the habitat heterogeneity hypothesis (Brown et al. 1992). A higher entanglement of plant above-ground parts can increase the mobility of grass-dwelling arthropods (Randlkofer et al. 2009) through better vertical and horizontal connectivity while it offers a broader palette of ecological niches (Duffey 1962). Thus, if complex vegetation structure is a sine qua non condition for high arthropod abundance and biomass, it does not guarantee it. It is likely that source populations must exist in the surrounding matrix to colonize any newly emerging, highly structured vegetation patches. Moreover, new detrimental factors generated by high vegetation structure might also obliterate the ability of arthropod populations to develop, such as microclimatic conditions that adversely affect some taxa (increase moisture or shade) or altered diffusion of plant volatiles that hampers resource location (e.g. Van Wingerden et al. 1992; Finch and Collier 2000; Després et al. 2007).
Conclusion
Although plant community stability was likely not achieved after just 1 year of experimental fertilization and irrigation, our findings demonstrate that on a short-term basis, a moderate level of intensification positively affects biodiversity and hay production of low-input, extensively managed montane and subalpine meadows. Tremendous land-use changes steadily affect mountainous regions, leading either to abandonment of marginal grasslands or to intensification of fields accessible to machinery (Tasser et al. 2007). This rather dichotomous trend should be reversed, which calls for more intermediate management practices if one wants to concomitantly promote grassland biodiversity and acceptable agricultural revenue. Although this short-term study only provides insights into the mechanism of intensification within upland grasslands, the continuation of our experiments will deliver detailed prescriptions in the mid term for optimizing slurry fertilization and aerial irrigation so as to achieve the best possible compromise between hay production, biodiversity preservation, and ecosystem functioning among montane and subalpine hay meadows.
Acknowledgments
We would like to thank Markus Fischer and Jérôme Pellet for their help designing the study, Stéphane Mettaz, Jean-François Buri, and Claire Bottini for field assistance, Sébastien Nusslé for statistical advice, our colleagues at the Division of Conservation Biology, Bern University, as well as the members of the project accompanying group. We are also thankful to farmers, to the Agricultural school of Conthey, the Canton of Valais and the Swiss Federal Office of Meteorology and Climatology for logistic support.
Appendix 1: Drawing of the ad hoc device used to sample the vegetation (point quadrat method)
 |
Appendix 2: A complete list of the plant species identified during the two sampling sessions across all treatments in all meadows
Table A2.1.
In total, 194 plant species belonging to 34 families were identified during the two sampling sessions across all meadows
| Plant species name | Family | Plant species name | Family |
|---|---|---|---|
| Achillea millefolium l. | Asteraceae | Crepis conyzifolia (Gouan) | Asteraceae |
| Acinos alpinus (l.) Moench | Lamiaceae | Crepis pyrenaica (l.) Greuter | Asteraceae |
| Agrimonia eupatoria l. | Rosaceae | Crocus albiflorus Kit. | Iridaceae |
| Agrostis capillaris l. | Poaceae | Cynosurus cristatus l. | Poaceae |
| Agrostis stolonifera l. | Poaceae | Dactylis glomerata l. | Poaceae |
| Ajuga pyramidalis l. | Lamiaceae | Dactylorhiza fuchsii (Druce) sod | Orchidaceae |
| Ajuga reptans l. | Lamiaceae | Descampsia sp | Poaceae |
| Alchemilla vulgaris aggr. | Rosaceae | Elymus repens (l.) Gould. | Poaceae |
| Allium oleraceum l. | Liliaceae | Erucastrum nastrurtiifolium | Brassicaceae |
| Anthericum ramosum l. | Liliaceae | Euphorbia cyparissias l. | Euphorbiaceae |
| Anthoxanthum odoratum l. | Poaceae | Euphorbia verrucosa l. | Euphorbiaceae |
| Anthriscus sylvestris (l.) Hoffm. | Apiaceae | Euphrasia rostkoviana aggr. | Scrophulaceae |
| Anthyllis vulneraria l. | Fabaceae | festuca arundinacea schreb. | Poaceae |
| Arabis ciliata Clairv. | Brassicaceae | festuca ovina l. | Poaceae |
| Arabis hirsuta (l.) scop. | Brassicaceae | festuca pratensis Huds. | Poaceae |
| Arenaria serpyllifolia l. | Caryophyllaceae | festuca rubra l. | Poaceae |
| Arrhenatherum elatius (l.) | Poaceae | festuca valesiaca Gaudin | Poaceae |
| Asperula cynanchica l. | Rubiaceae | filipendula vulgaris Moench | Rosaceae |
| Avenella flexuosa (l.) Drejer | Poaceae | Galium anisophyllum Vill. | Rubiaceae |
| Botrychium lunaria (l.) sw. | Ophiolglossaceae | Galium boreale l. | Rubiaceae |
| Brachypodium pinnatum (l.) | Poaceae | Galium mollugo aggr. | Rubiaceae |
| Briza media l. | Poaceae | Galium pumilum Murray | Rubiaceae |
| Bromus erectus Huds. | Poaceae | Galium verum l. | Rubiaceae |
| Bunium bulbocastanum l. | Apiaceae | Gentiana acaulis l. | Gentianacees |
| Campanula glomerata l. | Campanulaceae | Gentiana campestris l. | Gentianacees |
| Campanula rhomboidalis l. | Campanulaceae | Gentiana verna l. | Gentianacees |
| Campanula rotundifolia l. | Campanulaceae | Geranium sanguineum l. | Geraniaceae |
| Campanula scheuchzeri Vill. | Campanulaceae | Geranium sylvaticum l. | Geraniaceae |
| Cardamina hirsuta | Brassicaceae | Geum montanum l. | Rosaceae |
| Carex caryophyllea latourr. | Cyperaceae | Gymnadenia conopsea (l.) r. Br. | Orchidaceae |
| Carex flacca schreb. | Cyperaceae | Helianthemum nummularium (l.) Mill. | Cistaceae |
| Carex montana l. | Cyperaceae | Helictotrichon pubescens (Huds.) Pilg. | Poaceae |
| Carex ornithopoda Willd. | Cyperaceae | Hepatica nobilis schreb. | Renonculaceae |
| Carex pallescens l. | Cyperaceae | Heracleum sphondylium l. | Apiaceae |
| Carex sempervirens Vill. | Cyperaceae | Hieracium murorum aggr. | Asteraceae |
| Carlina acaulis l. | Asteraceae | Hieracium piloselloides Vill. | Asteraceae |
| Carum carvi l. | Apiaceae | Hippocrepis comosa l. | Fabaceae |
| Centaurea jacea l. | Asteraceae | Hypericum perforatum l. | HypEricaceae |
| Centaurea scabiosa l. | Asteraceae | Hypochoeris maculata l. | Asteraceae |
| Cerastium arvense l. | Caryophyllaceae | Inula salicina l. | Asteraceae |
| Cerastium fontanum | Caryophyllaceae | Knautia arvensis (l.) Coult. | Dipsacaceae |
| Chaerophyllum hirsutum l. | Apiaceae | Knautia dipsacifolia Kreutzer | Dipsacaceae |
| Cirsium acaule scop. | Asteraceae | Koeleria pyramidata (lam.) P. Beauv. | Poaceae |
| Cirsium arvense (l.) scop. | Asteraceae | laserpitium latifolium l. | Apiaceae |
| Clinopodium vulgare l. | Lamiaceae | laserpitium siler l. | Apiaceae |
| Colchicum alpinum DC. | Liliaceae | lathyrus pratensis l. | Fabaceae |
| Colchicum autumnale l. | Liliaceae | leontodon hispidus l. | Asteraceae |
| Crepis aurea (l.) Cass. | Asteraceae | leucanthemum vulgare aggr.r | Asteraceae |
| Crepis biennis l. | Asteraceae | linaria vulgaris Mill. | Scrophulaceae |
| linum catharticum l. | Linaceae | Prunella vulgaris l. | Lamiaceae |
| listera ovata (l.) r. Br. | Orchidaceae | Pulmonaria australis (Murr) | Lamiaceae |
| lolium perenne l. | Poaceae | Pulsatilla alpina (l.) Delarbre | Renonculaceae |
| lotus corniculatus l. | Fabaceae | ranunculus acris l. | Renonculaceae |
| luzula campestris (l.) DC. | Joncaceae | ranunculus bulbosus l. | Renonculaceae |
| luzula nivea (l.) DC. | Joncaceae | ranunculus montanus aggr. | Renonculaceae |
| luzula sylvatica aggr. | Joncaceae | ranunculus tuberosus lapeyr. | Renonculaceae |
| Medicago lupulina l. | Fabaceae | rhinanthus alectorolophus (scop.) | Scrophulaceae |
| Molinia arundinacea schrank | Poaceae | rosa pendulina l. | Rosaceae |
| Molinia caerulea (l.) Moench | Poaceae | rubus caesius l. | Rosaceae |
| Myosotis arvensis Hill. | Boraginaceae | rumex acetosa l. | Polygonaceae |
| Myosotis sylvatica Hoffm. | Boraginaceae | salvia pratensis l. | Lamiaceae |
| Nardus stricta l. | Poaceae | sanguisorba minor scop. | Rosaceae |
| Onobrychis viciifolia scop. | Fabaceae | sanguisorba officinalis l. | Rosaceae |
| Ononis repens l. | Fabaceae | scabiosa columbaria l. | Dipsacaceae |
| Ononis spinosa l. | Fabaceae | securigera varia (l.) lassen | Fabaceae |
| Paradisea liliastrum (l.) Bertol. | Liliaceae | selaginella selaginoides (l.) | Selaginellaceae |
| Pastinaca sativa l. | Apiaceae | sesleria caerulea (l.) Ard. | Poaceae |
| Peucedanum oreoselinum (l.) | Apiaceae | silene nutans l. | Caryophyllaceae |
| Phleum alpinum l. | Poaceae | silene vulgaris (Moench) Garcke | Caryophyllaceae |
| Phleum pratense l. | Poaceae | soldanella alpina l. | Primulaceae |
| Phyteuma betonicifolium Vill. | Campanulaceae | stachys recta l. | Lamiaceae |
| Phyteuma orbiculare l. | Campanulaceae | Taraxacum officinale aggr. | Asteraceae |
| Phyteuma spicatum l. | Campanulaceae | Thalictrum minus aggr. | Renonculaceae |
| Picris hieracioides l. | Asteraceae | Thesium alpinum l. | Santalaceae |
| Pimpinella saxifraga l. | Apiaceae | Thesium pyrenaicum Pourr. | Santalaceae |
| Plantago atrata Hoppe | Plantaginaceae | Thymus serpyllum aggr. | Lamiaceae |
| Plantago lanceolata l. | Plantaginaceae | Tragopogon pratensis l. | Asteraceae |
| Plantago media l. | Plantaginaceae | Trifolium alpestre l. | Fabaceae |
| Poa alpina l. | Poaceae | Trifolium badium schreb. | Fabaceae |
| Poa bulbosa l. | Poaceae | Trifolium dubium sibth. | Fabaceae |
| Poa pratensis l. | Poaceae | Trifolium medium l. | Fabaceae |
| Poa trivialis l. | Poaceae | Trifolium montanum l. | Fabaceae |
| Polygala alpestris rchb. | Polygalceae | Trifolium pratense l. | Fabaceae |
| Polygala chamaebuxus l. | Polygalceae | Trifolium repens l. | Fabaceae |
| Polygala comosa schkuhr | Polygalceae | Trisetum flavescens (l.) P. Beauv. | Poaceae |
| Polygala sp. | Polygalceae | Trollius europaeus l. | Renonculaceae |
| Polygala vulgaris l. | Polygalceae | Vaccinium myrtillus l. | Ericaceae |
| Polygonatum odoratum | Liliaceae | Verbascum nigrum l. | Scrophulaceae |
| Polygonum viviparum l. | Polygonaceae | Veronica arvensis l. | Scrophulaceae |
| Potentilla aurea l. | Rosaceae | Veronica chamaedrys l. | Scrophulaceae |
| Potentilla crantzii fritsch | Rosaceae | Veronica teucrium l. | Scrophulaceae |
| Potentilla erecta (l.) raeusch. | Rosaceae | Vicia cracca l. | Fabaceae |
| Potentilla pusilla Hostr | Rosaceae | Vicia sativa l. | Fabaceae |
| Potentilla rupestris l. | Rosaceae | Vicia sepium l. | Fabaceae |
| Potentilla thuringiaca link | Rosaceae | Viola hirta l. | Violaceae |
| Primula veris l. | Primulaceae | Viola rupestris f. W. schmidt | Violaceae |
| Prunella grandiflora (l.) scholler | Lamiaceae | Viola tricolor l. | Violaceae |
Appendix 3: Outputs of the linear mixed-effects models (LMMs) carried out on: (1) the effects of fertilization and irrigation on plant species richness, vegetation structure, hay production, arthropod abundance and biomass; and (2) the effects of fertilization and irrigation on the relative cover of grass, legume, and forb species. Table A3.1 refers to figure 2, and Table A3.2 refers to figure 3
Table A3.1.
Results of the linear mixed-effects models (LMMs) carried out on the effects of fertilization and irrigation on plant species richness, vegetation structure, hay production, arthropod abundance and biomass. Table refers to Fig. 2 in the article. The fixed factors were the experimental treatments (with four levels: C = control plots; F = fertilized; I = irrigated; I + F = irrigation and fertilization combined) and the sampling sessions (two levels: July and August). Random factor were the experimental study sites. P-values and 95% confidence intervals (CI) were computed with 100,000 Markov chain Monte Carlo (MCMC) iterations. MCMC mean parameter estimates (differences between expected mean densities) are given for the paired treatments comparisons, and significant contrasts are highlighted in bold
| Response variable and comparison | MCMC mean | Lower 95% CI | Upper 95% CI | MCMC P-value |
|---|---|---|---|---|
| Plant species richness (log scale) | ||||
| F vs. C | 0.109 | 0.016 | 0.205 | 0.023 |
| I vs. C | 0.240 | 0.145 | 0.333 | <0.001 |
| I + F vs. C | 0.236 | 0.144 | 0.331 | <0.001 |
| I vs. F | 0.130 | 0.035 | 0.223 | 0.007 |
| I + F vs. F | 0.127 | 0.033 | 0.221 | 0.009 |
| I + F vs. I | −0.003 | −0.097 | 0.092 | 0.947 |
| Structure of vegetation (index) | ||||
| F vs. C | 0.136 | −0.001 | 0.272 | 0.051 |
| I vs. C | 0.311 | 0.176 | 0.450 | <0.001 |
| I + F vs. C | 0.392 | 0.255 | 0.529 | <0.001 |
| I vs. F | 0.175 | 0.039 | 0.311 | 0.012 |
| I + F vs. F | 0.256 | 0.121 | 0.395 | 0.001 |
| I + F vs. I | 0.081 | −0.054 | 0.219 | 0.247 |
| Hay production [g·m−2] | ||||
| F vs. C | 226.8 | 101.1 | 352.5 | 0.001 |
| I vs. C | 384.4 | 262.6 | 514.1 | <0.001 |
| I + F vs. C | 503.2 | 379.7 | 630.8 | <0.001 |
| I vs. F | 157.6 | 29.0 | 280.2 | 0.015 |
| I + F vs. F | 276.7 | 150.2 | 400.7 | <0.001 |
| I + F vs. I | 118.8 | −7.2 | 245.6 | 0.065 |
| Arthropod abundance (log scale) | ||||
| F vs. C | 0.403 | −0.039 | 0.845 | 0.072 |
| I vs. C | 0.935 | 0.497 | 1.378 | <0.001 |
| I + F vs. C | 1.014 | 0.579 | 1.452 | <0.001 |
| I vs. F | 0.534 | 0.087 | 0.966 | 0.018 |
| I + F vs. F | 0.612 | 0.164 | 1.044 | 0.006 |
| I + F vs. I | 0.077 | −0.365 | 0.514 | 0.730 |
| Arthropod biomass [g] (log scale) | ||||
| F vs. C | 0.829 | 0.327 | 1.303 | 0.001 |
| I vs. C | 0.824 | 0.325 | 1.306 | 0.001 |
| I + F vs. C | 0.734 | 0.237 | 1.219 | 0.004 |
| I vs. F | −0.005 | −0.501 | 0.477 | 0.983 |
| I + F vs. F | −0.094 | −0.579 | 0.397 | 0.706 |
| I + F vs. I | −0.091 | −0.587 | 0.389 | 0.716 |
Table A3.2.
Results of the linear mixed effects models (LMMs) carried out on the effects of fertilization and irrigation on the relative cover of grass, legume and forb species. Table refers to Fig. 3 in the article. The fixed factors were the experimental treatments (with four levels: C = control plots; F = fertilized; I = irrigated; I+F = irrigation and fertilization combined) and the sampling sessions (two levels: July and August). Random factors were the experimental study sites. P-values and 95% confidence intervals (CI) were computed with 100,000 Markov chain Monte Carlo (MCMC) iterations. MCMC mean parameter estimates (differences between expected mean densities) are given for the paired treatments comparisons and significant contrasts are highlighted in bold
| Response variable and comparison | MCMC mean | Lower 95% CI | Upper 95% CI | MCMC P-value |
|---|---|---|---|---|
| Grasses (Poaceae) | ||||
| F vs. C | −0.056 | −0.099 | −0.014 | 0.009 |
| I vs. C | −0.075 | −0.117 | −0.033 | 0.001 |
| I + F vs. C | −0.075 | −0.116 | −0.032 | 0.001 |
| I vs. F | −0.019 | −0.061 | 0.023 | 0.380 |
| I + F vs. F | −0.018 | −0.059 | 0.024 | 0.390 |
| I + F vs. I | 0.001 | −0.042 | 0.043 | 0.974 |
| Legumes (Fabaceae) | ||||
| F vs. C | 0.073 | 0.037 | 0.108 | <0.001 |
| I vs. C | 0.105 | 0.070 | 0.140 | <0.001 |
| I + F vs. C | 0.125 | 0.091 | 0.162 | <0.001 |
| I vs. F | 0.033 | −0.003 | 0.068 | 0.070 |
| I + F vs. F | 0.053 | 0.018 | 0.088 | 0.004 |
| I + F vs. I | 0.020 | −0.015 | 0.055 | 0.261 |
| Forbs | ||||
| F vs. C | −0.016 | −0.055 | 0.024 | 0.415 |
| I vs. C | −0.030 | −0.070 | 0.009 | 0.131 |
| I + F vs. C | −0.051 | −0.090 | −0.012 | 0.011 |
| I vs. F | −0.014 | −0.054 | 0.025 | 0.479 |
| I + F vs. F | −0.035 | −0.074 | 0.005 | 0.083 |
| I + F vs. I | −0.021 | −0.059 | 0.020 | 0.302 |
Conflict of Interest
None declared.
References
- Baayen RH. 2011. languageR: Data sets and functions with “Analyzing Linguistic Data: A practical introduction to statistics”. R package version 1.4.
- Bates D, Maechler M, Bolker B. 2011. lme4: Linear mixed-effects models using S4 classes. R package version 0.999375-42.
- Bernhardt-Romermann M, Romermann C, Sperlich S, Schmidt W. Explaining grassland biomass - the contribution of climate, species and functional diversity depends on fertilization and mowing frequency. J. Appl. Ecol. 2011;48:1088–1097. [Google Scholar]
- Bowman WD, Gartner JR, Holland K, Wiedermann M. Nitrogen critical loads for alpine vegetation and terrestrial ecosystem response: are we there yet? Ecol. Appl. 2006;16:1183–1193. doi: 10.1890/1051-0761(2006)016[1183:nclfav]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Britschgi A, Spaar R, Arlettaz R. Impact of grassland farming intensification on the breeding ecology of an indicator insectivorous passerine, the Whinchat Saxicola rubetra: lessons for overall Alpine meadowland management. Biol. Conserv. 2006;130:193–205. [Google Scholar]
- Brown VK, Gibson CWD, Kathirithamby J. Community organisation in leaf hoppers. Oikos. 1992;65:97–106. [Google Scholar]
- Buri P, Arlettaz R, Humbert J-Y. Delaying mowing and leaving uncut refuges boosts orthopterans in extensively managed meadows: evidence drawn from field-scale experimentation. Agric. Ecosyst. Environ. 2013;181:22–30. [Google Scholar]
- Carlen C, Darbellay C, Gex P. Effets à long terme de la fumure et de la fréquence des coupes sur une prairie permanente en montagne. Revue suisse Agric. 1998;30:215–221. [Google Scholar]
- Cole LJ, Pollock ML, Robertson D, Holland JP, McCracken DI, Harrison W. The influence of fine-scale habitat heterogeneity on invertebrate assemblage structure in upland semi-natural grassland. Agric. Ecosyst. Environ. 2010;136:69–80. [Google Scholar]
- Core Team R. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2013. [Google Scholar]
- Crawley MJ. The R book. West Sussex, UK: John Wiley & Sons Ltd; 2007. [Google Scholar]
- Davis MA, Grime JP, Thompson K. Fluctuating resources in plant communities: a general theory of invasibility. J. Ecol. 2000;88:528–534. [Google Scholar]
- Després L, David JP, Gallet C. The evolutionary ecology of insect resistance to plant chemicals. Trends Ecol. Evol. 2007;22:298–307. doi: 10.1016/j.tree.2007.02.010. [DOI] [PubMed] [Google Scholar]
- DiTommaso A, Aarssen LW. Resource manipulations in natural vegetation: a review. Vegetatio. 1989;84:9–29. [Google Scholar]
- Dittrich ADK, Helden AJ. Experimental sward islets: the effect of dung and fertilisation on Hemiptera and Araneae. Insect Conserv. Diversity. 2011;5:46–56. [Google Scholar]
- Duffey E. A population study of spiders in limestone grassland - The field layer fauna. Oikos. 1962;13:15–000. [Google Scholar]
- Finch S, Collier RH. Host-plant selection by insects - a theory based on ‘appropriate/inappropriate landings’ by pest insects of cruciferous plants. Entomol. Exp. Appl. 2000;96:91–102. [Google Scholar]
- Grandchamp A-C, Bergmanini A, Stofer S, Niemelä J, Duelli P, Scheidegger C. The influence of grassland management on ground beetles (Carabidae, Coleoptera) in Swiss montane meadows. Agric. Ecosyst. Environ. 2005;110:307–317. [Google Scholar]
- Grime JP. Competitive exclusion in herbaceous vegetation. Nature. 1973;242:344–347. [Google Scholar]
- Haddad NM, Haarstad J, Tilman D. The effects of long-term nitrogen loading on grassland insect communities. Oecologia. 2000;124:73–84. doi: 10.1007/s004420050026. [DOI] [PubMed] [Google Scholar]
- Hautier Y, Niklaus PA, Hector A. Competition for light causes plant biodiversity loss after eutrophication. Science. 2009;324:636–638. doi: 10.1126/science.1169640. [DOI] [PubMed] [Google Scholar]
- Hunter MD, Price PW. Playing chutes and ladders: heterogeneity and the relative roles of bottom-up and top-down forces in natural communities. Ecology. 1992;73:724–732. [Google Scholar]
- Huston MA. Hidden treatments in ecological experiments: re-evaluating the ecosystem function of biodiversity. Oecologia. 1997;110:449–460. doi: 10.1007/s004420050180. [DOI] [PubMed] [Google Scholar]
- Jeangros B, Bertola C. Effets de l'arrosage sur la végétation des prairies permanentes d'une région du Haut-Valais. Revue suisse Agric. 2000;32:189–194. [Google Scholar]
- Kirchner TB. The effects of resource enrichment on the diversity of plants and arthropods in a shortgrass prairie. Ecology. 1977;58:1334–1344. [Google Scholar]
- Knops JMH, Tilman D, Haddad NM, Naeem S, Mitchell CE, Haarstad J, et al. Effects of plant species richness on invasion dynamics, disease outbreaks, insect abundances and diversity. Ecol. Lett. 1999;2:286–293. doi: 10.1046/j.1461-0248.1999.00083.x. [DOI] [PubMed] [Google Scholar]
- Li W, Wen SJ, Hu WX, Du GZ. Root-shoot competition interactions cause diversity loss after fertilization: a field experiment in an alpine meadow on the Tibetan Plateau. J. Plant Ecol. 2010;4:138–146. [Google Scholar]
- MacDonald D, Crabtree JR, Wiesinger G, Dax T, Stamou N, Fleury P, et al. Agricultural abandonment in mountain areas of Europe: environmental consequences and policy response. J. Environ. Manage. 2000;59:47–69. [Google Scholar]
- Marini L, Fontana P, Scotton M, Klimek S. Vascular plant and Orthoptera diversity in relation to grassland management and landscape composition in the European Alps. J. Appl. Ecol. 2008;45:361–370. [Google Scholar]
- Mittelbach GG, Steiner CF, Scheiner SM, Gross KL, Reynolds HL, Waide RB, et al. What is the observed relationship between species richness and productivity? Ecology. 2001;82:2381–2396. [Google Scholar]
- Morris MG. The effects of structure and its dynamics on the ecology and conservation of arthropods in British grassland. Biol. Conserv. 2000;95:129–142. [Google Scholar]
- Mortimer SR, Hollier JA, Brown VK. Interactions between plant and insect diversity in the restoration of lowland calcareous grasslands in southern Britain. Appl. Veg. Sci. 1998;1:101–114. [Google Scholar]
- Nielsen CO. Studies on Enchytraeidae 5. Factors causing seasonal fluctuations in numbers. Oikos. 1955;6:153–169. [Google Scholar]
- Niu K, Choler P, de Bello F, Mirotchnick N, Du G, Sun S. Fertilization decreases species diversity but increases functional diversity: a three-year experiment in a Tibetan alpine meadow. Agric. Ecosyst. Environ. 2014;182:106–112. [Google Scholar]
- Pauli D, Peintinger M, Schmid B. Nutrient enrichment in calcareous fens: effects on plant species and community structure. Basic Appl. Ecol. 2002;3:255–266. [Google Scholar]
- Perner J, Wytrykush C, Kahmen A, Buchmann N, Egerer I, Creutzburg S, et al. Effects of plant diversity, plant productivity and habitat parameters on arthropod abundance in montane European grasslands. Ecography. 2005;28:429–442. [Google Scholar]
- Peter M, Gigon A, Edwards PJ, Luscher A. Changes over three decades in the floristic composition of nutrient-poor grasslands in the Swiss Alps. Biodivers. Conserv. 2009;18:547–567. [Google Scholar]
- Rajaniemi TK. Why does fertilization reduce plant species diversity? Testing three competition-based hypotheses. J. Ecol. 2002;90:316–324. [Google Scholar]
- Randlkofer B, Jordan F, Mitesser O, Meiners T, Obermaier E. Effect of vegetation density, height, and connectivity on the oviposition pattern of the leaf beetle Galeruca tanaceti. Entomol. Exp. Appl. 2009;132:134–146. [Google Scholar]
- Reid AM, Hochuli DF. Grassland invertebrate assemblages in managed landscapes: effect of host plant and microhabitat architecture. Austral Ecol. 2007;32:708–718. [Google Scholar]
- Riedener E, Rusterholz H-P, Baur B. Effects of different irrigation systems on the biodiversity of species-rich hay meadows. Agric. Ecosyst. Environ. 2013;164:62–69. [Google Scholar]
- Sanders D, Entling MH. Large variation of suction sampling efficiency depending on arthropod groups, species traits, and habitat properties. Entomol. Exp. Appl. 2011;138:234–243. [Google Scholar]
- Sinaj S, Richner W, Flisch R, Charles R. Données de base pour la fumure des grandes cultures et des herbages (DBF-GCH) Revue suisse Agric. 2009;41:1–98. [Google Scholar]
- Stampfli A. Accurate determination of vegetational change in meadows by successive point quadrat analysis. Vegetatio. 1991;96:185–194. [Google Scholar]
- Tasser E, Walde J, Tappeiner U, Teutsch A, Noggler W. Land-use changes and natural reforestation in the Eastern Central Alps. Agric. Ecosyst. Environ. 2007;118:115–129. [Google Scholar]
- Tilman D, Downing JA. Biodiversity and stability in grasslands. Nature. 1994;367:363–365. [Google Scholar]
- Tonn B, Briemle G. Minimum management intensity for maintaining and improving biodiversity of a mesotrophic semi-natural grassland. Grassl. Sci. Europe. 2010;15:747–749. [Google Scholar]
- Van Wingerden WKRE, van Kreveld AR, Bongers W. Analysis of species composition and abundance of grasshoppers (Orth. Acrididae) in natural and fertilized grasslands. J. Appl. Entomol. 1992;113:138–152. [Google Scholar]
- Vintu V, Samuil C, Sirbu C, Popovici CI, Stavarache M. Sustainable Management of Nardus stricta L. Grasslands in Romania's Carpathians. Not. Bot. Horti Agrobot. Cluj Napoca. 2011;39:142–145. [Google Scholar]
- Walker MD, Webber PJ, Arnold EH, Ebertmay D. Effects of interannual climate variation on aboveground phytomass in alpine vegetation. Ecology. 1994;75:393–408. [Google Scholar]
- Woodcock BA, Potts SG, Tscheulin T, Pilgrim E, Ramsey AJ, Harrison-Cripps J, et al. Responses of invertebrate trophic level, feeding guild and body size to the management of improved grassland field margins. J. Appl. Ecol. 2009;46:920–929. [Google Scholar]
- Yang ZL, van Ruijven J, Du GZ. The effects of long-term fertilization on the temporal stability of alpine meadow communities. Plant Soil. 2011;345:315–324. [Google Scholar]


