Abstract
BACKGROUND
During the surgical repair of infants with congenital cardiac defects, there can be periods of decreased cerebral blood flow, particularly during deep hypothermic circulatory arrest (DHCA). As a result, these infants are at increased risk for seizures and long-term neuro-developmental difficulties.
METHODS
Thirty-two infants with congenital heart disease had continuous video-EEG monitoring pre-, intra-, and post-operatively for 48 hours following surgery.
RESULTS: For patients requiring DHCA (n=17)
the EEG pattern for all patients became suppressed and eventually isoelectric below 25°C. Two of the 32 infants had electrical seizures within the 48 hour monitoring window. Both required DHCA and the burst pattern during recovery had rhythmic, sharp components that were high amplitude and often asynchronous between the hemispheres. The interval between the onset of seizure activity, and initiation of the sharp burst pattern during surgery, was 29 and 40 hours. This pattern was not observed during isoelectric recovery from infants who did not develop post-operative seizures.
CONCLUSIONS
The EEG in infants during DHCA displayed predictable changes. We identified an EEG pattern following the isoelectric period that may be predictive of seizure development in the subsequent 48 hours.
Keywords: Congenital heart disease, Postoperative seizures, Deep hypothermic circulatory arrest, Intraoperative EEG monitoring
Introduction
The incidence of clinically apparent post-operative seizures in infants with complicated cardiac defects requiring surgery is 4–10%1,2. Further, when postoperative continuous EEG monitoring is used, seizure frequency increases to 26%.3 Children with complex cardiac defects are at an increased risk of neurocognitive delay, and the development of post-operative seizures may only increase that risk.4,5
Neuroprotective techniques have evolved to make cardiothoracic surgery safer by protecting vital organs and reducing potential harm to the central nervous system. Deep hypothermic circulatory arrest (DHCA) is a neuroprotective technique where circulation is arrested and the patient is cooled to 18°C during repair of the aortic arch.6 Intraoperative EEGs during the repair of congenital heart disease in infants follows a predictable pattern according to the temperature of the patient during cardiopulmonary bypass (CPB) and deep hypothermic circulatory arrest.7,8 The EEG evolves from a sedated pattern, to burst suppression with an increasing duration of the interburst interval, to completely suppressed and isoelectric. As CPB is discontinued and the patient is warmed, EEG activity gradually returns to a burst suppression pattern and then becomes more continuous.2,8
Variations from the expected EEG pattern during different phases of surgery may give insight into cerebral function in the post-operative period. Historically, the intra-operative EEG has been helpful in determining sedation levels, but it is not commonly used in the management of patients during the immediate postoperative period. We hypothesized that the use of intra-operative EEG monitoring would predict infants at risk for seizures following surgery.
Methods
Study Design
A convenience sample of infants ≤ 3 months of age admitted for cardiac surgery at the University of Rochester Medical Center was consented for participation in a prospective observational study. Exclusion criteria included pre-existing neonatal seizures, central nervous system injury not directly attributable to congenital heart disease, multiple extra-cardiac congenital anomalies, chromosomal abnormalities, or previous cardiac surgery. Pre-operative neuroimaging to exclude for neurologic defects was not performed in all patients. The study was approved by the Research Subjects Review Board at the University of Rochester Medical Center. Informed consent was obtained from the parents of each infant prior to participation. Due to the variety and complexity of the different cardiac pathologies seen, a classification system was utilized to more broadly delineate each patient’s anatomy and operative procedure: Class IBi-Ventricular infants requiring complete repair, Class II-Bi-ventricular infants requiring palliative repair, Class III-Infants with a morphologic single left or right ventricle requiring palliative repair.
Each infant had a 30 minute preoperative EEG. The scalp electrodes placed pre-operatively were kept in place and used for continuous monitoring intra-operatively and for 48 hours post-operatively, excluding the patient transfer from the operating room to the Pediatric Cardiac Intensive Care Unit. Each EEG was recorded using gold-plated electrodes affixed to the scalp with collodion and applied according to the international 10–20 system using the standard temporal, para-sagittal and midline placements with the exception of FP1 and FP2. Periodic re-gelling of electrodes was done to ensure technically adequate recordings.
EEG Analysis
Each EEG was reviewed in its entirety by a blinded electrophysiologist trained in neonatal EEG interpretation. The preoperative EEG was categorized as appropriate for age, dysmature (EEG consistent with a gestational age 2 or more weeks younger), or abnormal. The intra-operative EEG was analyzed for degree of discontinuity, voltage suppression, sharp waves (duration 70 – 200 msec), spikes (20 – <70 msec), and seizures. The post-operative EEG background was analyzed to determine if there were focal changes compared with the baseline EEG or if seizures were present. Seizures were defined by a pattern of rhythmic or semi-rhythmic activity present in more than one lead and lasting for 10 seconds or more.
At predetermined intraoperative and postoperative time points, the EEG background was evaluated and graded based on the level of continuity, where a grade of 5 was considered appropriate for age, and 1 was isoelectric (Table 1). Changes in the EEG were then correlated with phases in surgery. EEGs were evaluated at five time points during the intraoperative EEG including the start of the operation, five minutes prior to becoming isoelectric, 5 minutes from last cerebral activity, at onset of cerebral activity, at onset of burst suppression with interburst intervals <30 seconds, and end of operative recording. These times were chosen as we felt they were the points in which patients may demonstrate the most variation. In addition, the EEG was also graded at the initiation of postoperative care, and at 24, 36, and 48 hours post-operatively.
Table 1.
Grading System for EEG evaluation at specified time points
| Grade | Classification | Definition |
|---|---|---|
| 1 | Isoelectric | No brain electrical activity for ≥ 3 min, amplitude <10 μV |
| 2 | Severe burst suppression | Time of suppression between bursts ≥ 30 seconds* |
| 3 | Mild burst suppression | Time of suppression between bursts < 30 seconds* |
| 4 | Slow and continuous | EEG continuous, but with increased slower frequencies |
| 5 | Appropriate for Age | EEG normal for subject’s age or mildly dysmature |
Longest IBI at specified time point
Chart Review
Each subject’s medical record was reviewed and abstracted for the following data: demographic information, medical history, pre-surgical cardiac anatomy, duration of CPB, duration of aortic clamp time, duration of DHCA, duration of regional cerebral perfusion, and esophageal temperature (at the time of induction, incision, on CPB, aorta clamp, aorta unclamp, and off CPB).
Statistics
All data are reported as mean ± standard deviation or n (%). Comparisons between the categorical variables were carried out using Fisher’s exact test where all p values< 0.05 were considered significant. We performed Fisher’s exact testing to indicate the relative strength of this unusual finding, though we recognize its limitations in this largely descriptive study.
Results
Thirty-two infants had complete EEG data including preoperative, intraoperative, and postoperative recordings. As shown in table 2, the majority of infants were males less than 30 days of age at the time of operation with bi-ventricular anatomy requiring complete repair. No infants demonstrated focal neurologic abnormalities at the time of pre-operative examination. Head ultrasound as part of standard care was preformed on 18/32 infants preoperatively. Of these, 16 were considered normal. One of the abnormal ultrasounds showed prominent third and fourth ventricles, and the other a small right choroid plexus cyst. Neither of the abnormal ultrasounds were of infant’s with had seizures in the first 48 hours postoperatively.
Table 2.
Patient Demographics
| Non-DHCA (n=15) | DHCA without seizures (n=15) | DHCA with Seizures (n=2) | |
|---|---|---|---|
| Age at operation(days) | 10.5 ± 11.5 | 10.8 ± 7.8 | 7.0 ± 0 |
| Gestational Age at birth (weeks) | 38.5 ± 2.3 | 38.7 ± 1.2 | 37.5 ± 0.7 |
| Male Gender | 73.3% (11) | 46.7% (7) | 50% |
| Birth Weight (kg) | 3.4 ± 0.6 | 3.0 ± 0.5 | 3.5 ± 0.7 |
| Cardiac Designation | |||
| Class I | 80% (12) | 66.7% (10) | |
| Class II | 20% (3) | ||
| Class III | 33.3% (5) | 100% | |
| Intra-operative variables | |||
| CPB time (min) | 134.7 ± 48.5 | 143.9 ± 39.6 | 159.0 ± 9.9 |
| Cross Clamp Time (min) | 89.9 ± 39.8 (13) | 78.9 ± 36.3 | 71.0 ± 26.9 |
| Circulatory Arrest Time (min)n=17 | 26.1 ± 13.5 | 9.5 ± 0.7 | |
| Regional Perfusion (min) n=7 | 55.0 ± 21.3 | 66.0 ± 5.7 | |
| Lowest Temp on CPB (°C) | 33.7 ± 1.2 (14) | 21.3 ± 2.3 | 20.3 ± 0.4 |
| Duration EEG Isoelectric (min) | 97.2 ± 49.5 | 140.5 ± 36.1 | |
Abbreviations: CPB-Cardiopulmonary Bypass
Pre-operative EEGs
The pre-operative EEGs in all infants were without asymmetry or evidence of seizure activity. They were all appropriate for gestational age except in three where the EEGs were dysmature (EEG consistent with a gestational age 2 weeks younger).
Evolution of EEG during DHCA
Of the 32 infants, 17 underwent deep hypothermic circulatory arrest (DHCA) during which their core body temperature decreased to a mean of 21.2 ± 2.2°C. As expected, the duration of DHCA and regional perfusion was longest in infants with a single ventricle undergoing palliative staged repair (class III) 75.6 ± 12.5 min compared to 28.8 ± 11.3 min in infants with Bi-ventricular complete or palliative repair (classes I and II).
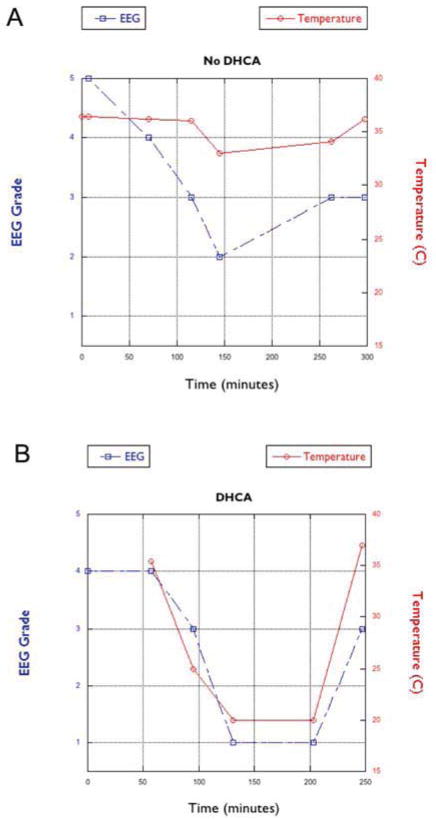
Among those patients who underwent DHCA, the EEG showed a diffuse slow wave pattern indicative of sedation, which gradually became more discontinuous as the level of sedation increased. As the subjects were cooled, the degree of EEG suppression increased, with increasing interburst intervals. The electrical bursts eventually attenuated until the recording was essentially isoelectric with EEG activity <10uV (102.3 ± 49.3 min). The EEG became isoelectric in all patients cooled to temperatures < 25°C (n=17) (Figure 1a). Patients whose body temperature was ≥ 25°C (n=15) did have suppression of the EEG during surgery, but never became isoelectric (Figure 1b).
Figure 1.
The graphs demonstrate the relationship between EEG and temperature during surgery in two representative patients (A) requiring deep hypothermic circulatory arrest (DHCA) and cardiopulmonary bypass and (B) requiring cardiopulmonary bypass without DHCA.
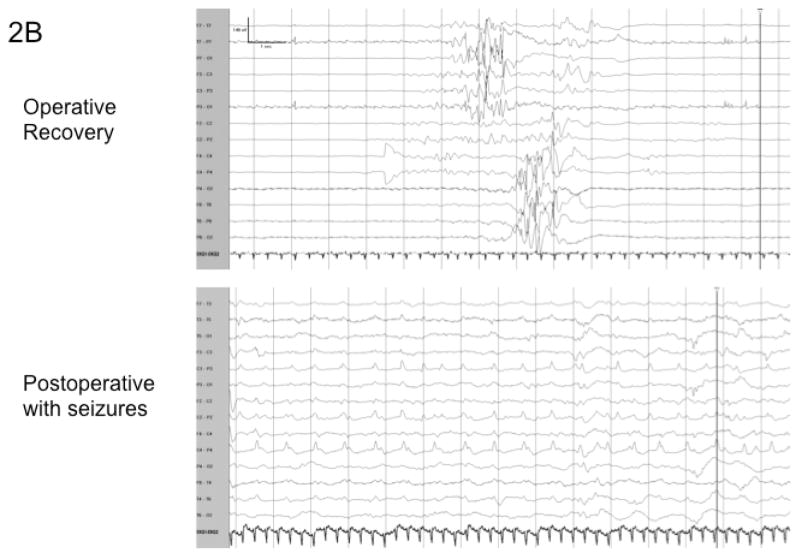
As the patients were re-warmed, the EEG gradually returned to a burst suppression pattern with a decreasing interburst interval. Of the patients who underwent DHCA 15/17 patients followed this pattern, and the return of the EEG following the isoelectric period was gradual and benign without sharp features (Figure 2a). The two patients who did not develop this pattern developed a burst pattern during recovery from DHCA with rhythmic, sharp components that were high amplitude and often asynchronous between the hemispheres.
Figure 2.
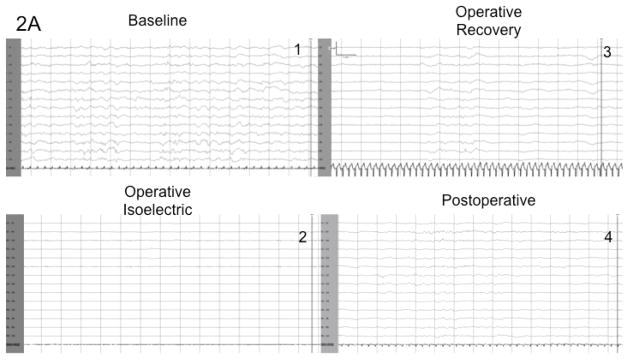
Figure 2a shows the typical progression of the EEG in a patient who underwent Deep Hypothermic Circulatory Arrest (DHCA) and did not develop postoperative seizures. Figure 2b demonstrates the EEG progression of a patient who underwent DHCA and did develop seizures within the first 48 hours postoperatively. This EEG during recovery demonstrates higher amplitude bursts with intermixed sharp features and asynchrony.
Postoperative EEG recordings
In the initial postoperative period EEGs in all patients demonstrated a diffuse slow wave pattern consistent with sedation. The majority of subjects started the postoperative period at a level 3 (mild burst suppression) and then fluctuated between level 3 and 5 during the duration of the 48-hour postoperative recording (supplementary table 1).
Of 32 patients, only 2 had electrographic seizures during the 48-hour postoperative period. Both infants had variants of single ventricle cardiac anatomy (double inlet left ventricle, mitral atresia, and aortic arch obstruction; and unbalanced atrio-ventricular septal defect). The seizures occurred at 25 hours in patient #30 and 29 at hours in patient #31. Patient #31 had clinical and electrographic seizures with electroclinical dissociation; while patient #30 only had electrographic seizures that were not clinically recognized.
Seizures occurred repeatedly for both patients and were prolonged. The total duration of seizure activity for patient #30 was 79 and for patient #31 was 293 minutes. The seizures in both patients were focal in onset and occurred independently in each hemisphere. Patient #30 had ictal onset in the posterior quadrants while patient #31 had onset in the central regions. The ictal EEG morphology in both patients primarily consisted of rhythmic sharp waves or slow waves occurring at a frequency of 1 –2 Hz.
Significantly, the occurrence of seizures in both of these patients was heralded by the atypical burst EEG patterns occurring more than 24 hours earlier during the intraoperative EEG recordings (p=0.005). In these two patients as the burst-suppression pattern returned after the isoelectric period, the bursts had unique features not seen in the other patients. The electrical activity within the bursts were more than two times the amplitude than in non-seizure patients. In addition, the electrical activity contained sharper features with epileptiform morphology, and at times contained rhythmic components. Furthermore, the bursts often were asynchronous between the hemispheres (Figure 2b). By contrast, the infants who underwent DHCA ± regional perfusion who did not have seizures had electrical recovery from the isoelectric period characterized by synchronous bursts without sharp components (Figure 2a).
Discussion
The results of the present study indicate that continuous EEG monitoring during the surgical repair of congenital heart defects in infants may be useful in identifying patients at risk for postoperative seizures. We identified EEG patterns during the recovery phase from DHCA in 2 patients that predicted the occurrence of major seizures more than 24 hours later.
The evolution of the EEG during cardiac surgery with DHCA has been described in the past and follows a typical pattern7,8. The 2 patients in our series who developed postoperative seizures deviated from this typical pattern, exhibiting a burst-suppression pattern with high amplitude epileptiform-like activity within the bursts. This EEG finding was seen during the recovery phase of the intraoperative EEG, following the period of DHCA and isoelectric EEG (Figure 2b). This activity was not seen in any of the other patients, all of whom followed the typical pattern of recovery described in the literature.
Our data also highlight additional possible risk factors for the development of postoperative seizures. Both of the patients who developed seizures were infants with single ventricle cardiac anatomy. Consequently, they required longer than average operative times, during which they were subjected to cooling with longer periods with isoelectric EEG (Table 2).
Our series of patients had a lower incidence of postoperative seizures than observed in previous studies.4,9 One possible reason for this is the use of regional cerebral perfusion in patients that required more complicated repair, decreasing the amount of cerebral ischemia. For this reason, it has been suggested that selective cerebral perfusion may improve neurologic outcome.10,11 More studies are needed to evaluate whether regional cerebral perfusion does decrease seizures in the postoperative period, or improve long-term developmental outcomes.
A particular limitation of this study was that a convenience sample was used and therefore many types of cardiac anatomy were included limiting our ability to calculate a true incidence of postoperative seizures. Only half the subjects underwent more complicated procedures requiring DHCA and regional perfusion, which further limited our numbers. Additional subjects are necessary to see if this electrical finding is consistent across a larger population of infants undergoing DHCA.
In conclusion, both patients with postoperative seizures had an electrophysiologic finding during surgery that was not seen in the other patients. More than 24 hours elapsed between when this EEG finding was seen during surgery and the onset of seizures. In the future, using intraoperative EEG monitoring may help to determine the children who are at highest risk for seizures in the immediate postoperative period.
Supplementary Material
Acknowledgments
The authors wish to thank Dr. Cecilia Meagher, Dr. Jill Cholette, Dr. Dawn Sweeney, Ms. Gina Cable and the EEG technicians for their help. This was supported in part by K12 NS 066098.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ehyai A, Fenichel GM, Bender HW., Jr Incidence and prognosis of seizures in infants after cardiac surgery with profound hypothermia and circulatory arrest. Jama. 1984 Dec 14;252(22):3165–3167. [PubMed] [Google Scholar]
- 2.Brunberg JA, Reilly EL, Doty DB. Central nervous system consequences in infants of cardiac surgery using deep hypothermia and circulatory arrest. Circulation. 1974 Aug;50(2 Suppl):II60–68. [PubMed] [Google Scholar]
- 3.Newburger JW, Jonas RA, Wernovsky G, et al. A comparison of the perioperative neurologic effects of hypothermic circulatory arrest versus low-flow cardiopulmonary bypass in infant heart surgery. N Engl J Med. 1993 Oct 7;329(15):1057–1064. doi: 10.1056/NEJM199310073291501. [DOI] [PubMed] [Google Scholar]
- 4.Rappaport LA, Wypij D, Bellinger DC, et al. Relation of seizures after cardiac surgery in early infancy to neurodevelopmental outcome. Boston Circulatory Arrest Study Group. Circulation. 1998 Mar 3;97(8):773–779. doi: 10.1161/01.cir.97.8.773. [DOI] [PubMed] [Google Scholar]
- 5.Clancy RR, Sharif U, Ichord R, et al. Electrographic neonatal seizures after infant heart surgery. Epilepsia. 2005 Jan;46(1):84–90. doi: 10.1111/j.0013-9580.2005.22504.x. [DOI] [PubMed] [Google Scholar]
- 6.Ohye RG, Goldberg CS, Donohue J, et al. The quest to optimize neurodevelopmental outcomes in neonatal arch reconstruction: the perfusion techniques we use and why we believe in them. J Thorac Cardiovasc Surg. 2009 Apr;137(4):803–806. doi: 10.1016/j.jtcvs.2008.10.046. [DOI] [PubMed] [Google Scholar]
- 7.Miller G, Rodichok LD, Baylen BG, Myers JL. EEG changes during open heart surgery on infants aged 6 months or less: relationship to early neurologic morbidity. Pediatr Neurol. 1994 Mar;10(2):124–130. doi: 10.1016/0887-8994(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 8.Reilly EL, Brunberg JA, Doty DB. The effect of deep hypothermia and total circulatory arrest on the electroencephalogram in children. Electroencephalogr Clin Neurophysiol. 1974 Jun;36(6):661–667. doi: 10.1016/0013-4694(74)90233-8. [DOI] [PubMed] [Google Scholar]
- 9.Helmers SL, Wypij D, Constantinou JE, et al. Perioperative electroencephalographic seizures in infants undergoing repair of complex congenital cardiac defects. Electroencephalogr Clin Neurophysiol. 1997 Jan;102(1):27–36. doi: 10.1016/s0013-4694(96)95079-8. [DOI] [PubMed] [Google Scholar]
- 10.Oppido G, Pace Napoleone C, Turci S, et al. Moderately hypothermic cardiopulmonary bypass and low-flow antegrade selective cerebral perfusion for neonatal aortic arch surgery. Ann Thorac Surg. 2006 Dec;82(6):2233–2239. doi: 10.1016/j.athoracsur.2006.06.042. [DOI] [PubMed] [Google Scholar]
- 11.Andropoulos DB, Easley RB, Brady K, et al. Neurodevelopmental Outcomes After Regional Cerebral Perfusion With Neuromonitoring for Neonatal Aortic Arch Reconstruction. Ann Thorac Surg. 2012 Jul 3; doi: 10.1016/j.athoracsur.2012.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.