Abstract
BACKGROUND
Respiratory RNA viruses are associated with bronchiolitis obliterans syndrome (BOS) in lung transplant recipients (LTRS), however the immune mechanisms that regulate airway obliteration remain incompletely understood.
METHODS
Using the mouse heterotopic tracheal transplant (HTT) model of obliterative airway disease (OAD), we studied the role of dsRNA using (polyinosinic:polycytidylic acid; poly(I:C)), a synthetic analog of viral dsRNA, in abrogating airway allograft tolerance established with donor-specific transfusion (DST) and anti-CD154 mAb therapy.
RESULTS
Wild-type (WT) B6 recipients of accepted BALB/c airway grafts demonstrated significantly reduced intragraft CD8+ T-cells with markedly impaired allospecific IFN-γ and TNF-α secretion, uncoupled from an activated phenotype and evidence of proliferation. Administration of poly(I:C) to DST/anti-CD154-treated recipients restored OAD pathology and CD8+ alloeffector responses to levels observed in untreated mice. However, B6 IFNαβR−/− recipients were resistant to the abrogation of tolerance mediated by poly(I:C) and did not develop CD8+ alloeffector responses or OAD. Further, adoptive transfers of either WT CD8+ T-cells or CD11c+ dendritic cells (DC) alone into B6 IFNαβR−/− recipients treated with poly(I:C) and DST/anti-CD154 were incapable of abrogating airway graft tolerance.
CONCLUSIONS
Together, these data indicate abrogation of DST/anti-CD154-induced airway allograft tolerance via dsRNA requires type-I IFN responsiveness for mouse airway obliteration.
Keywords: CD8+ T-cell, tolerance, BOS, respiratory viral infection, interferon
Introduction
Lung transplantation is a therapeutic option for select patients with end-stage pulmonary disorders, yet its long-term success is limited by BOS (1). BOS is the major cause of morbidity and mortality in LTRs, affecting approximately 30% and 75% of LTRs by 2.5 and 10 years, respectively (2). Recently, several studies have shown an association between community-acquired respiratory viral infections (CARVs) and increased risk of BOS in LTRs (3–5). The majority of CARVs in LTRs are enveloped RNA viruses and members of the paramyxovirus family that include respiratory syncytial virus (RSV), metapneumonvirus (MPV), parainfluenza virus or the orthomyxovirus, influenza. Despite increased recognition of CARVs as a risk factor for BOS, the immune mechanism(s) that regulate ensuing airway obliteration remain incompletely understood (6). To address this, we chose to use the mouse complete major histocompatibility complex (MHC)-mismatched HTT model in which allogeneic grafts develop OAD by day 28 following marked airway graft infiltration by alloreactive CD8+ T-cells, as this model directly evaluates airway obliteration that is spared in the mouse orthotopic lung transplant model (7–9). Because costimulation blockade is an effective strategy for establishing transplantation tolerance in several experimental transplant models including airway transplant (10–13), we first evaluated a tolerance protocol using DST and anti-CD154 therapy and observed significant lack of OAD development in airway grafts that correlated with an absence of alloreactive CD8+ T-cell responses. Several studies have demonstrated the capacity of toll-like receptor (TLR) agonists to abrogate costimulation blockade-induced allograft acceptance, including the TLR3 agonist dsRNA or poly(I:C), a functional analog of viral RNA (14, 15). Because poly(I:C) is a potent inducer of type-I interferons (IFNs) (16–18), we hypothesized poly(I:C) exposure would abrogate airway allograft acceptance in a type-I IFN-dependent manner. Herein, we show that poly(I:C) abrogates costimulation blockade-induced airway allograft acceptance via restoration of intragraft alloefffector CD8+ T-cells in WT mice. However, type-I IFN receptor-deficient (IFNαβR−/−) mice did not develop CD8+ alloeffectors or OAD with poly(I:C) exposure. Moreover, adoptive transfer of either WT CD8+ T-cells or WT CD11c+ DCs into IFNαβR−/− recipients failed to restore CD8+ alloeffector responses or OAD in the presence of poly(I:C) with costimulation blockade. Together, these findings indicate type-I IFN responsiveness is required for innate and adaptive cellular interactions necessary for mouse OAD pathology.
Materials and Methods
Mice
WT female C57BL/6 (I-ab, H-2b), and BALB/c (I-ad, H-2d) mice, 5–8 weeks old, were purchased from the National Cancer Institute (Frederick, MD). C57BL/6 IFNαβR−/− mice (C57BL/6J-Tnfsf5tm1Imx) were a kind gift from Dr. Robert Seder, NIAID, NIH. Mice were housed in the Laboratory Animal Resource Center at the Johns Hopkins University Bayview Asthma and Allergy Center or the University of Pittsburgh Division of Laboratory Animal Resources. Protocols were approved by the Animal Care and Use Committee of both institutions.
Heterotopic Tracheal Transplant and Treatment
Donor BALB/c tracheae were excised from mice euthanized via CO2 asphyxiation. Recipient mice (C57BL/6, IFNαβR−/−) were anesthetized and prepped as previously described (19). A small incision was made and tracheal grafts were placed in a subcutaneous pocket with two grafts per mouse for experiments with cellular assays and single grafts for histopathology. The wound was closed with two clips and mice were given an i.p. injection of cefazolin 25mg/kg (Apothecon, Netherlands). Mice were euthanized on day 10–14 for cellular assays, and days 28/90 for histopathology. Recipient mice were treated with either no treatment (control); DST/anti-CD154 mAb; or DST/anti-CD154/poly(I:C). DST (5×106 splenocytes) was given i.v. day -7. Anti-CD154 (0.5 mg i.p.) was administered on days -7, -4, 0, +4. Poly(I:C) (0.1 mg i.v.) was administered on days -7, 0, based upon dose titration experiments of Poly(I:C) (10, 100 µg) and the days of alloantigen exposure resulting in consistent OAD.
Reagents, Cell preparations, stimulation and cytokine detection
Cell culture medium RPMI 1640 (Mediatech, Manassas, VA) was supplemented with 10% FBS (Sigma-Aldrich, St. Louis, MO), 2mM glutamine, 1mM Sodium Pyruvate, 1% NEAA, 100 U/ml Penicillin, 100 mcg/ml Streptomycin, 50µM ß-mercaptoethanol, and 25mM HEPES. Spleen, LN, lungs, and grafts were harvested and processed on days 10–14 as previously described (20). Isolated cells from grafts, spleen or lung mononuclear cells (LMNC) were co-cultured for 5 h with/without BALB/c splenocytes (1:1). Brefeldin A (10 µg/ml) was added for the final 2 h of stimulation, followed by surface and intracellular staining.
Flow Cytometry
The following antibodies were purchased from BD PharMingen (San Diego, CA): Fluorescein Isothiocyanate (FITC)-labeled anti-CD4; Peridinin-chlorophyll-protein complex (PerCP)Cy5.5-labeled anti-CD8; FITC-, PE-labeled anti-H2Dd, PECy-7-labeled anti-CD69, Allophycocyanin (APC)-labeled anti-CD44; APC-labeled anti-IFN-γ; FITC-labeled anti-TNF-α; PE-labeled anti-granzyme B, and PE-labeled anti-CD11c+. Surface and intracellular cytokine staining (ICS) was performed as previously described (20). Flow cytometry was performed using a FACSCalibur or LSR Fortessa (Becton Dickinson, San Jose, CA), and Flowjo software for analysis (Tree Star, San Carlos, CA).
Cell proliferation
Mice were injected with bromodeoxyuridine (BrdU;1 mg i.p.) (Sigma-Aldrich) on day 0 and fed 0.8mg/ml BrdU in drinking water for 7 days before sacrifice. BrdU incorporation was assayed with BrdU-FITC Flow Kit (BD Pharmingen) per manufacturer’s protocol.
Histopathology and OAD scoring
Grafts were fixed in 10% formalin, paraffin embedded, sectioned and stained using Hematoxylin/Eosin. OAD scores were determined by 2 independent, blinded reviewers using a 4 point scale to calculate the mean degree of injury (0 = no injury, 4 = very severe) based on 4 parameters: epithelial injury, airway obliteration, collagen deposition, lymphocytic infiltration, as previously described (21).
Adoptive Cell Transfer
CD8+ (2 × 106) or CD11c+ DC (1.5 × 106) were isolated from C57BL/6 WT spleen using MACS Magnetic Cell Separation (Miltenyi Biotec, Auburn, CA) and adoptively transferred i.v. into IFNαβR−/− recipients on day 0. All isolated cells were analyzed via flow cytometry yielding purity of ≥ 90% before transfer.
Statistical analysis
Data were compared with two-tailed student’s t-test using Microsoft Excel (Redmond, WA). A p-value < 0.05 was considered statistically significant.
Results
DST/anti-CD154 therapy establishes durable airway allograft tolerance
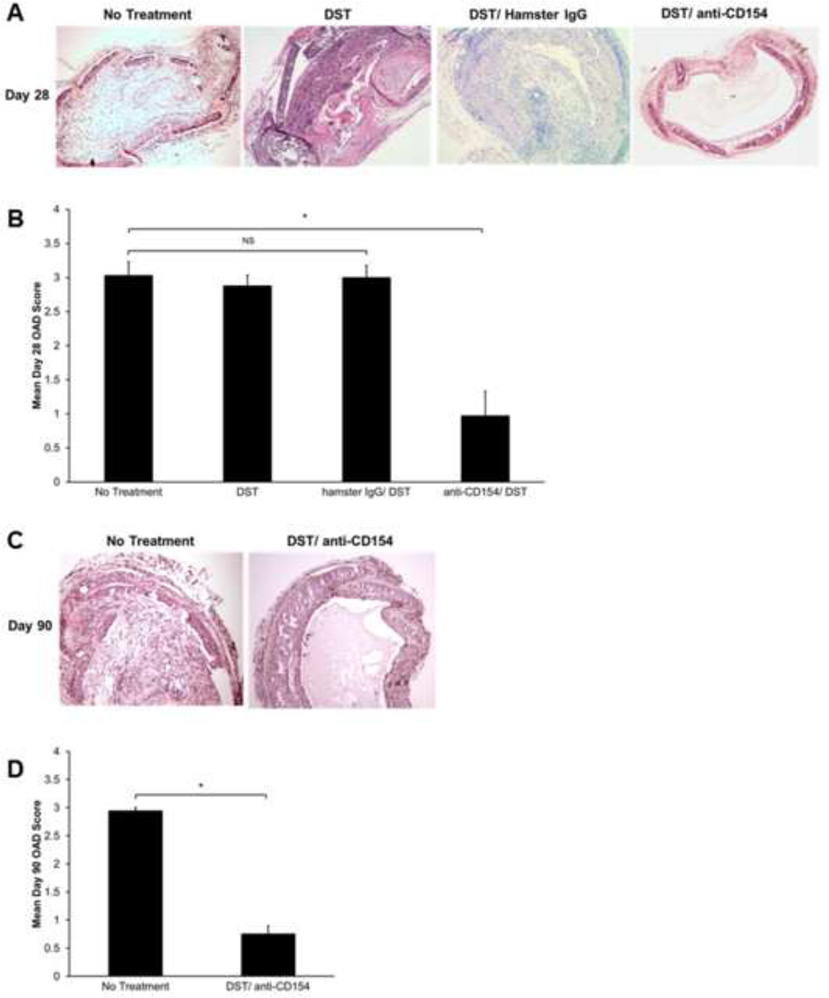
To investigate the role of the CD154/CD40 pathway in the HTT model, we compared graft histology in fully MHC-mismatched C57BL/6 recipients of BALB/c airway allografts that received DST/anti-CD154 therapy versus no treatment. Similar to our previous findings, 100% of untreated B6 recipient mice developed airway obliteration and fibrosis by day 28 posttransplant (18). In contrast, airway allografts from DST/anti-CD154-treated mice did not develop OAD, retained intact epithelial structure for 28 days and in fact, were accepted to day 90 (Figure 1A). Using a standardized scoring system for murine OAD, we observed that mice treated with DST/anti-CD154 had significantly lower OAD scores than untreated mice at days 28 and 90 (Figure 1B and D). Interestingly, DST/anti-CD154-treated mice had increased OAD scores at day 28 compared with isograft controls, primarily due to increased cellular infiltration (data not shown). To ensure neither DST nor non-specific antibody binding had an impact on OAD, we evaluated recipients of DST alone and DST with Hamster IgG and observed OAD scores comparable to untreated mice (Figure 1A and B).
Figure 1.
DST/anti-CD154 mAb therapy results in long-term graft acceptance. C57BL/6 WT recipient mice treated with DST alone, DST/Hamster IgG, and DST/anti-CD154 were transplanted with trachea from BALB/c mice and compared to untreated allogeneic transplant controls. (A) Representative histology with H&E staining on d28, comparing the four treatment groups. (B) Mean d28 OAD scores with bars representing mean vales + SEM for each treatment group, p value determined by student’s t test (*p<0.0001). Each treatment group contains (C) Representative histology with H&E staining on d90. (D) Mean d90 OAD scores with bars representing mean values + SEM for each treatment group (*p<0.0001) Each treatment group contains 8 mice.
DST/anti-CD154 therapy reduces intragraft CD8+ T-cells and abrogates allospecific effector function
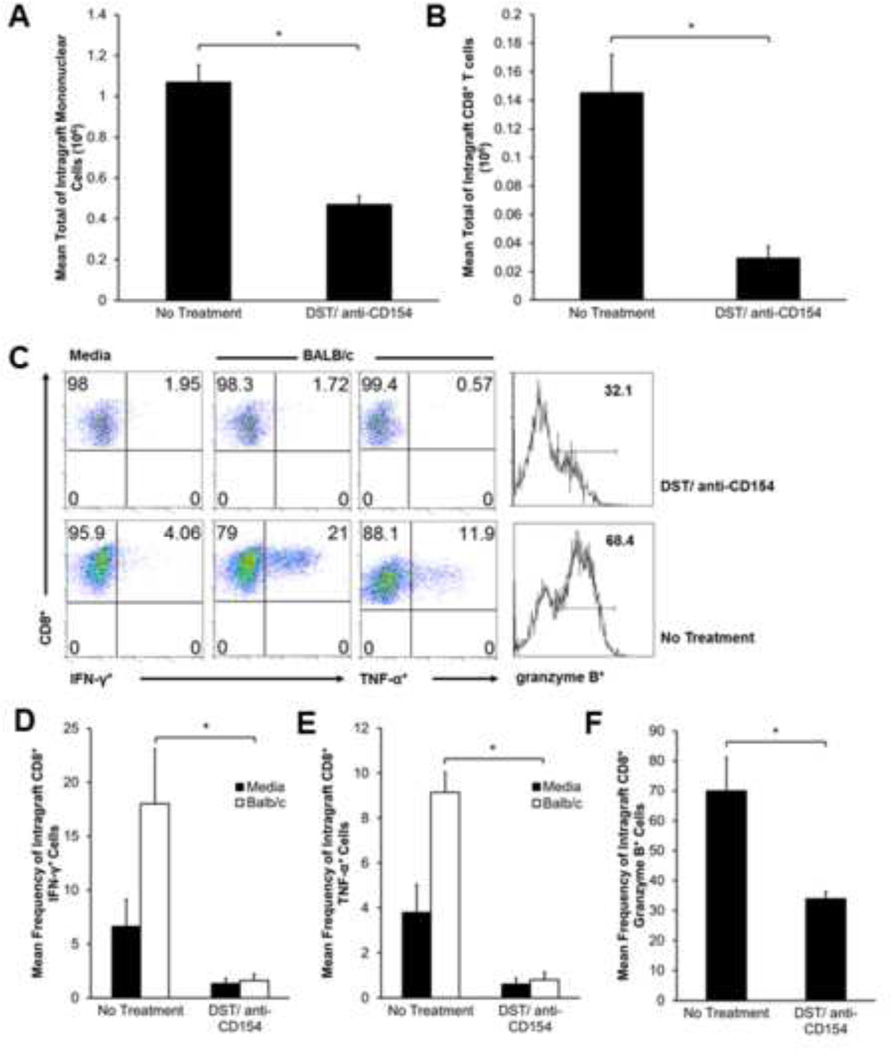
We have previously shown peak graft cellular infiltration and predominant CD8+ alloeffector responses in the HTT model by day 10–14 (18), and thus investigated whether inhibition of OAD using DST/anti-CD154 resulted in altered intragraft T-cell populations and/or effector function at this time point. At day 10, recipients treated with DST/anti-CD154 had reduced intragraft mononuclear and CD8+ T-cells (Figures 2A–B), though importantly, these cells were detectable. Notably, allografts of DST/anti-CD154 recipients had more mononuclear cells and CD8+ T-cells than isografts, which yielded too few cells to quantitate. Furthermore, the CD8:CD4 allograft ratio was diminished in DST/anti-CD154-treated mice, at ~1.3:1 versus ~2:1 in untreated mice (data not shown).
Figure 2.
Allografts of recipients treated with DST/anti-CD154 mAb have reduced CD8+ T-cells and lack allospecific functional responses. (A) Total mononuclear cell counts at d10 in no treatment vs. DST/anti-CD154 were determined by hemocytometer count after mechanical and enzymatic digestion of graft tissue (*p<0.0002). Bar values represent the average from a total of 18–20 mice. (B) Total intragraft CD8+ cells (d10) were determined by hemocytometer count of total lymphocytes, followed by fluorescent dye-conjugated anti-CD4 and anti-CD8 Ab staining and flow cytometric analysis. Total intragraft CD8+ number was calculated by multiplying CD8+ frequency in live cell gate by total lymphocyte count (*p<0.008). (C) Representative d10 ICCS for IFN-γ, TNF-α, and granzyme B in allograft mononuclear cells cultured for 5 h in medium alone or in the presence of BALB/c splenocytes (1:1 ratio); gating on H-2d negative, CD8+ T-cells in mixed cultures. (D and E) Mean d10 frequency of CD8+ IFN-γ+ and CD8+ TNF-α+ cells detected after 5 hour stimulation in the presence of medium alone or BALB/c splenocytes. Results consist of a minimum of 4 separate experiments with pooled responses from 3 to 5 mice per group (*p<0.007, *p<0.0001). (F) Mean d10 frequency of CD8+ grzB+ cells after 5 hour stimulation in medium. Results consist of a minimum of 4 separate experiments with pooled responses from 3 to 5 mice per group (*p<0.04).
To quantitate effector function at day 10, we isolated graft mononuclear cells and cultured them in vitro with/without BALB/c splenocytes (1:1 stimulator:responder ratio). We then analyzed IFN-γ, TNF-α and the cytolytic molecule granzyme B (grzB) using ICS. DST/anti-CD154 therapy strikingly reduced the frequencies of CD8+IFN-γ+, CD8+TNF-α+ and CD8+grzB+ cells compared to untreated mice (Figure 2C). Moreover, CD8+ cells from DST/anti-CD154-treated grafts failed to demonstrate allospecific responses observed in untreated mice, marked by an increase in cytokine expression upon in vitro re-stimulation with BALB/c alloantigen. Cumulatively, DST/anti-CD154 therapy resulted in a 10-fold reduction in mean intragraft allospecific CD8+IFN-γ+ and CD8+TNF-α+ frequencies (Figures 2D,E), and a 2-fold decrease in grzB expression by intragraft CD8+ T-cells (Figure 2F). As a control, no differences in intragraft T-cell numbers or effector function were detected in mice treated with DST alone versus no treatment (data not shown). Together, these data show airway allograft acceptance via DST/anti-CD154 is associated with significantly reduced intragraft CD8+ T-cells and impaired effector function.
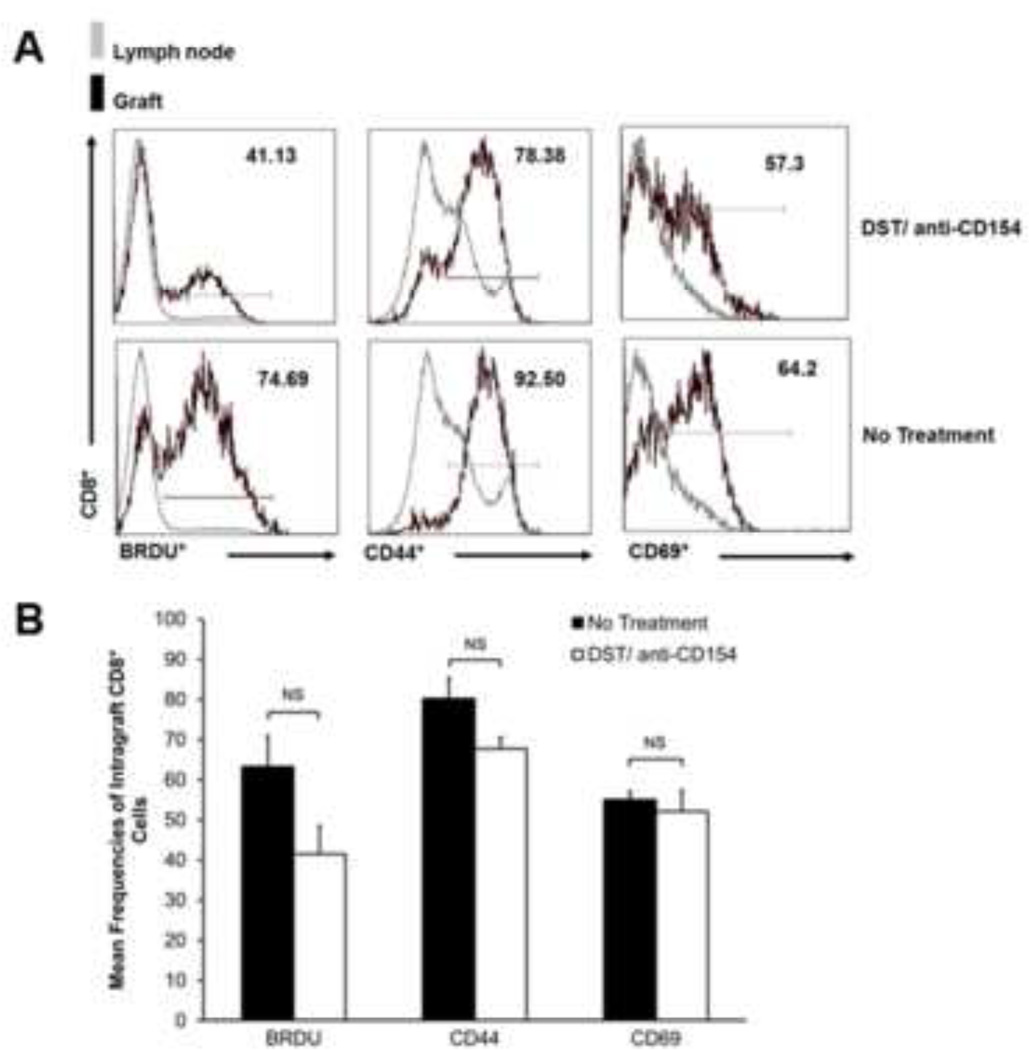
Airway graft CD8+ T-cells demonstrate an activated phenotype and proliferate in DST/anti-CD154-treated recipients
Next, we evaluated the phenotype of functionally impaired intragraft CD8+ T-cells in DST/anti-CD154-treated mice compared to controls. We evaluated surface expression of the memory and activation markers CD44, CD69, respectively, at day 14. As shown in Figure 3A–B, CD44 and CD69 expression in intragraft CD8+ T-cells from DST/anti-CD154-treated mice did not differ from controls (Figure 3A–B). We also measured incorporation of the thymidine analog, Bromodeoxyuridine (BrdU), to evaluate in vivo proliferation of endogenous CD8+ T-cells. Intragraft CD8+ T-cells from DST/anti-CD154-treated mice showed reduced, yet substantial BrdU incorporation compared to untreated mice (Figure 3B). To determine whether differences in systemic proliferation existed between the two groups, we quantified BrdU incorporation in recipient lymph nodes and detected similar frequencies that were far less than those detected in intragraft CD8+ T-cells (Figure 3A). Collectively, our data show that while intragraft CD8+ cells in DST/anti-CD154-treated recipients demonstrate impaired effector function, they still exhibit an activated phenotype and proliferate.
Figure 3.
Intragraft CD8+ T-cells retain activated and proliferative phenotype with DST/anti-CD154 mAb therapy. (A) Representative d14 flow cytometric histograms for ICCS of BrdU incorporation and surface expression of CD44 and CD69 in graft and lymph node, gating on CD8+ cells. Positive expression was determined using lymph node cells as a negative control. (B) Mean d14 expression of intragraft BrdU incorporation, CD44 expression, and CD69 expression. Mice were administered BrdU from days 6 to 14. Results encompass a minimum of 4 separate experiments with pooled responses from 3 to 5 mice per group (NS=not significant).
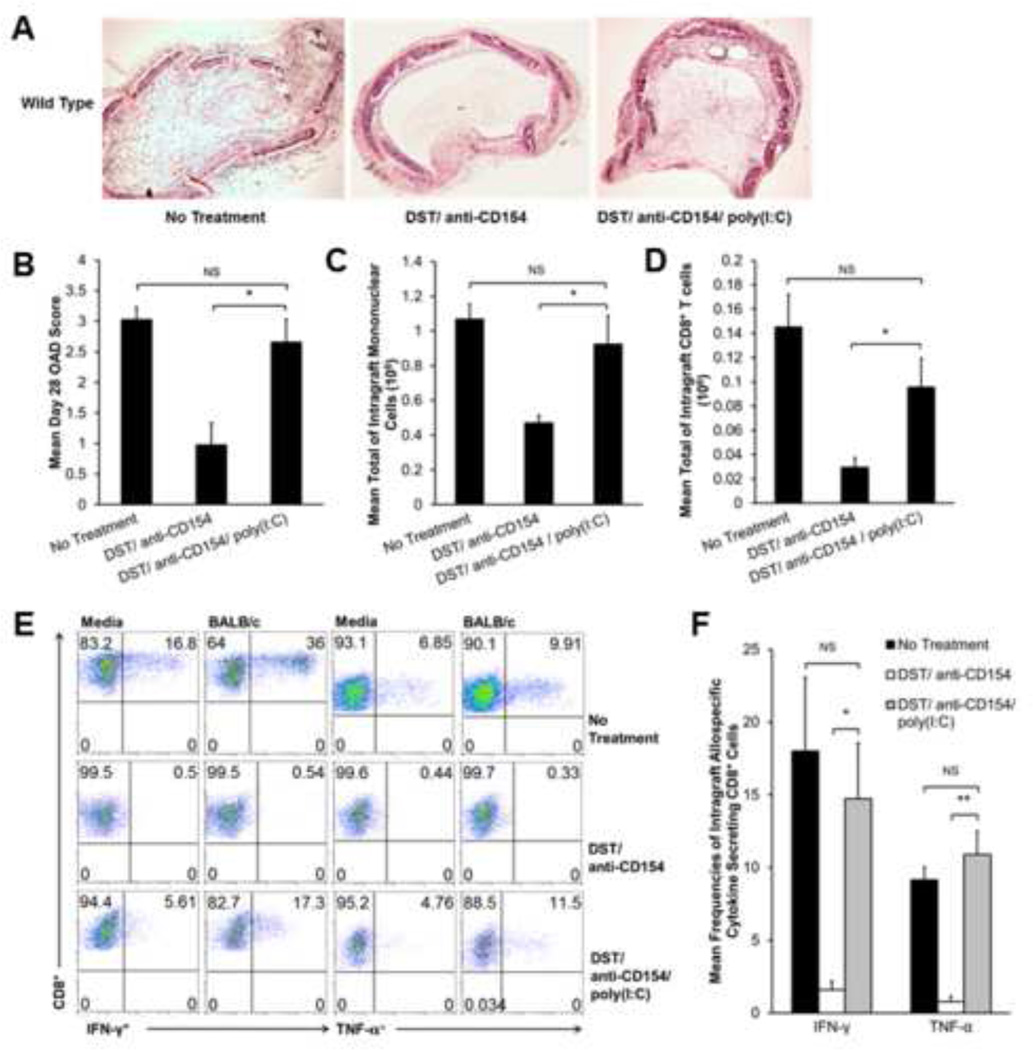
Poly(I:C) abrogrates DST/anti-CD154-induced airway allograft acceptance and restores alloeffector responses in intragraft CD8+ T-cells
Previous work has shown TLR agonists are able to abrogate costimulation blockadeinduced tolerance in different transplant models (14, 15). Given the relevance of RNA viruses in lung transplantation, we tested the effects of poly(I:C) (dsRNA), a TLR3 agonist, in our model with DST/anti-CD154 therapy. Allografts from DST/anti-CD154/poly(I:C)-treated recipients developed OAD (Figure 4A), with mean day 28 OAD scores significantly higher than DST/anti-CD154-treated mice and comparable to untreated recipients (Figure 4A–B). Furthermore, day 10 intragraft mononuclear cells and CD8+ T-cells were significantly increased in DST/anti-CD154/poly(I:C)-treated mice and comparable to untreated mice (Figure 4C–D), with restoration of the CD8:CD4 ratio (~2:1) seen in untreated mice (data not shown). Additionally, poly(I:C) administration restored day 10 allospecific effector function in intragraft CD8+ T-cells from DST/anti-CD154-treated mice. As shown in Figure 4E, DST/anti-CD154/poly(I:C)-treated mice demonstrated constitutive intragraft CD8+IFN-γ+ and CD8+TNF-α+ cells, and an allospecific increase in these responses upon in vitro re-stimulation with alloantigen. Cumulative analysis showed the addition of poly(I:C) to DST/anti-CD154 therapy resulted in a 9-fold increase in the frequency of intragraft allospecific CD8+IFN-γ+ cells and a 13-fold increase in the frequency of allospecific CD8+ TNF-α+ cells. Frequencies of intragraft allospecific IFN-γ+ and TNF-α+ secreting CD8+ cells in DST/anti-CD154/poly(I:C)-treated mice were similar to untreated mice (Figure 4F). Similarly, grzB expression was increased in poly(I:C)-treated mice compared to DST/anti-CD154 alone (data not shown). In summary, these data demonstrate that poly(I:C) abrogates DST/anti- CD154-induced tolerance by restoring the intragraft CD8+ T-cell effector responses that precede OAD pathology.
Figure 4.
Poly(I:C) abrogates tolerance and restores alloresponses in DST/anti-CD154 mAb treated mice. C57BL/6 WT recipient mice treated with DST/anti-CD154/poly(I:C) were transplanted with trachea from BALB/c mice. (A) Representative d28 histology with H&E staining comparing DST/anti-CD154/poly(I:C) grafts to previously described experimental groups. (B) Mean d28 OAD scores from each experimental group (*p<0.006). Each treatment group consists of 8–10 mice. (C) Total mononuclear cell counts on d10 in experimental groups were determined by hemocytometer count after mechanical and enzymatic digestion of graft tissue (*p<0.02). Bar values represent the mean count from a total of 18–20 mice. (D) Total intragraft CD8+ cells on d10 were determined by hemocytometer-based count of total lymphocytes, followed by fluorescent dye-conjugated anti-CD4 and anti-CD8 Ab staining and flow cytometric analysis. Total intragraft CD8+ number was calculated by multiplying CD8+ frequency in live cell gate by total lymphocyte count (*p<0.05). (E) Representative d10 ICCS for IFN-γ and TNF-α in allograft mononuclear cells cultured for 5 h in medium alone or in the presence of BALB/c splenocytes (1:1 ratio); gating on H-2d negative, CD8+ T-cells in mixed cultures. (F) Mean d10 frequency of intragraft allospecific CD8+ IFN-γ+ and CD8+ TNF-α+ cells by ICCS (*p<0.003, **p<0.0002). Results consist of a minimum of 4 separate experiments with pooled responses from 3 to 5 mice per group.
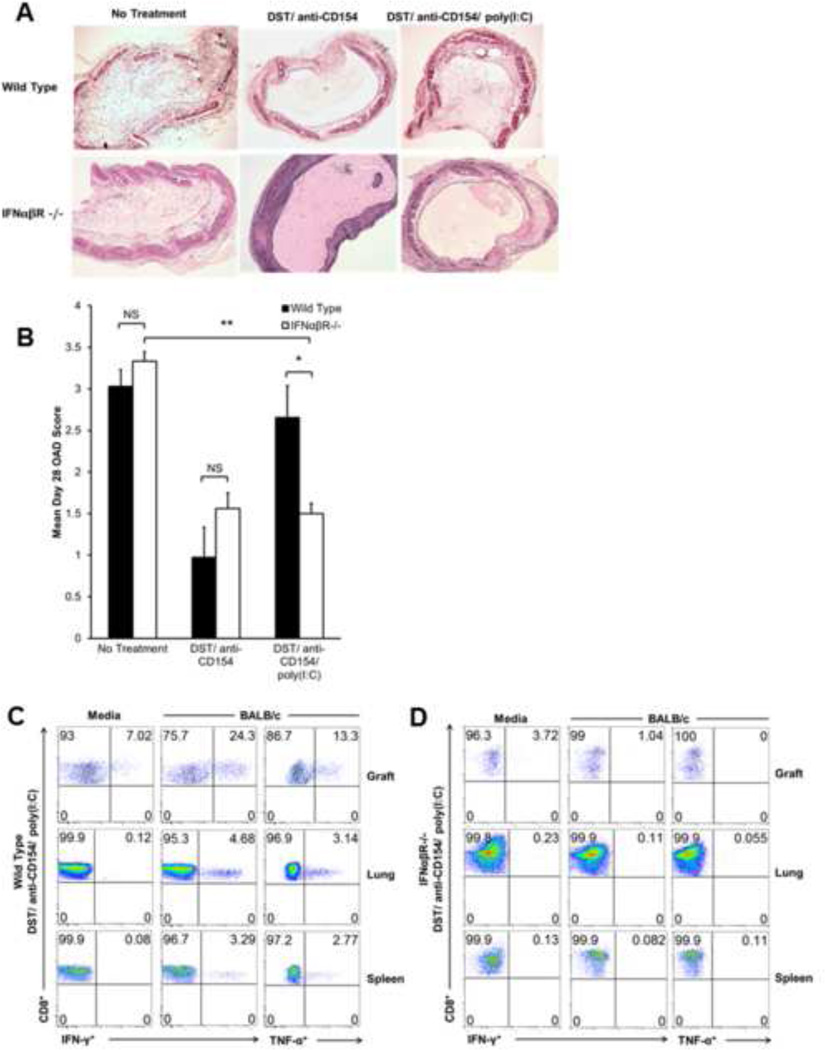
IFN-αβ-receptor deficient mice are resistant to poly(I:C)-induced OAD
Because poly(I:C) induces type-I interferon (IFN-α and IFN-β) production from antigen presenting cells (APC), we hypothesized that IFN-αβ-responsiveness was required for the abolition of tolerance associated with poly(I:C) treatment. Therefore, we evaluated OAD and allospecific CD8+ cytokine production in IFN-αβ-receptor deficient B6 (IFNαβR−/−) recipients of BALB/c grafts. As shown in Figure 5A, while OAD pathology is similar in IFNαβR−/− and WT mice receiving no treatment or mice receiving DST/anti-CD154 (significant reduction of OAD in both groups), IFNαβR−/− mice fail to develop OAD following DST/anti-CD154/poly(I:C) treatment. In addition, administration of Poly (I:C) alone to either WT or IFNαβR−/− mice resulted in severe OAD similar to untreated animals as expected (data not shown). The mean OAD score in DST/anti-CD154/poly (I:C)-treated IFNαβR−/− mice was significantly lower than similarly treated WT mice, while allografts of no treatment or DST/antiCD154 in WT and IFNαβR−/− recipients scored similarly (Figure 5B). Furthermore, DST/anti-CD154/poly(I:C)-treated IFNαβR−/− mice failed to demonstrate allospecific intragraft CD8+IFN-γ+ and CD8+TNF-α+ cells at day 10 (Figure 5D). We previously have shown systemic CD8+ alloeffector cells in the HTT model (18). To ensure that lack of intragraft allospecific responses in IFNαβR−/− mice treated with DST/anti-CD154/poly(I:C) was not due to low cell yield, we compared CD8+ responses in the lung and spleen to WT mice receiving the same treatment. IFNαβR−/− mice treated with DST/anti-CD154/poly(I:C) failed to exhibit systemic CD8+ alloeffector responses upon alloantigen rechallenge (Figure 5D), while WT mice given the same treatment demonstrate CD8+ alloeffector cells in the lung and spleen at day 10 (Figure 5C).
Figure 5.
Poly(I:C) does not attenuate OAD or restore alloresponses in IFN-αβ-receptor deficient mice treated with DST/anti-CD154 mAb. IFNαβR−/− B6 recipient mice treated with DST/Anti-CD154/poly(I:C) or DST/Anti-CD154 were transplanted with trachea from BALB/c mice and compared to WT mice that received the same treatment. (A) Representative d28 tracheal graft histology with H&E staining comparing IFNαβR−/− vs. WT type recipient mice; no treatment DST/anti-CD154, and DST/anti-CD154/poly(I:C). (B) Mean d28 OAD scores in grafts of IFNαβR−/− vs. WT recipients under no treatment, DST/anti-CD154, or DST/anti-CD154/poly(I:C) (*p<0.02, **p<0.00001). Results comprise scores from 4–6 mice. (C and D) Representative d10 ICCS for CD8+ IFN-γ+ and CD8+ TNF-α+ cells in graft, lung, and spleen in WT vs. IFNαβR−/− recipients, both treated with DST/anti-CD154/poly(I:C). Allograft mononuclear cells were cultured for 5 h in medium alone or in the presence of BALB/c splenocytes (1:1 ratio); gating on H-2d negative, CD8+ T-cells in mixed cultures.
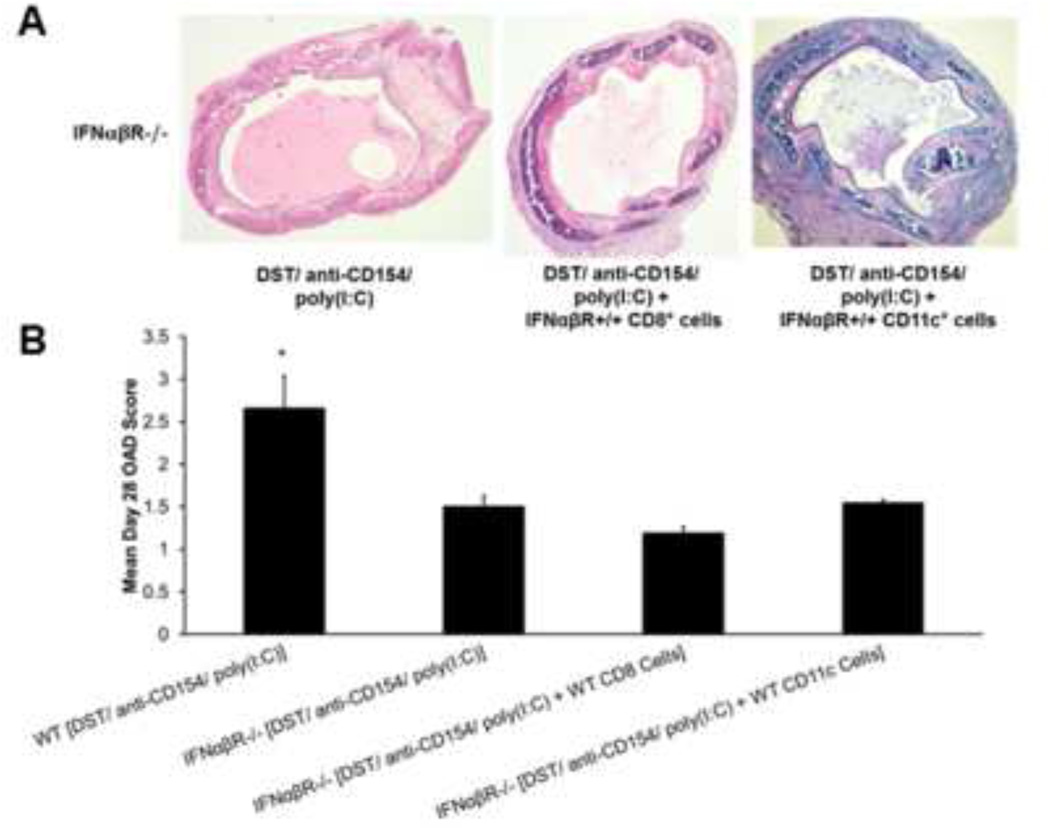
WT CD8+ cells or dendritic cells alone are insufficient to restore OAD in IFNαβR−/− mice treated with DST/anti-CD154/poly(I:C)
Next, we asked whether either WT CD8+ T-cells or APC restored allograft rejection in IFNαβR−/− recipients. For this, we adoptively transferred IFNαβR+/+ CD8+ T-cells (5 × 106) or CD11c+ DC (1.6 × 106) on day 0 into IFNαβR−/− recipients treated with DST/anti-CD154/poly(I:C). Unexpectedly, neither WT CD8+ T-cells nor DC induced OAD in IFNαβR-deficient mice by day 28, although all groups had evidence of mononuclear inflammation (Figure 6A). OAD scores in the WT DST/anti-CD154/poly(I:C) group were significantly higher than all IFNαβR−/− experimental groups (Figure 6B). Furthermore, regardless of adoptive transfer of either WT-cell population, DST/anti-CD154/poly(I:C)-treated IFNαβR−/− recipients had no detectable intragraft or systemic CD8+ alloeffector responses (data not shown). Together, these data indicate that abrogation of DST/anti-CD154-induced airway allograft tolerance via dsRNA requires broad intact type-I IFN responsiveness for alloeffector responses and OAD induction.
Figure 6.
IFNαβR+/+ CD8+ or CD11c+ cells alone are insufficient to restore rejection in IFNαβR−/− mice treated with DST/anti-CD154/poly(I:C). Either CD8+ (5 × 106) or CD11c+ (1.6 × 106) cells isolated from WT spleen were adoptively transferred into IFNαβR−/− B6 recipient mice on day 0. Recipient mice were treated with DST/anti-CD154/poly(I:C) and were transplanted with trachea from BALB/c mice. (A) Representative d28 tracheal graft histology with H&E staining of IFNα/βR−/− recipient mice with various treatments. (B) Mean d28 OAD scores in grafts of various experimental groups (*p<0.02). Results comprise scores from 4 mice.
Discussion
Respiratory RNA viruses contribute to allograft dysfunction in LTRs, and are associated with acute cellular rejection, BOS and death (3–6). Herein, we show that type-I IFN responsiveness is required for dsRNA to abrogate costimulation-induced airway allograft acceptance, as IFNαβR−/− mice are OAD-resistant, unlike WT mice, with poly(I:C) exposure. Importantly, OAD was associated with intragraft alloeffector CD8+ T-cells producing IFN-γ, TNF-α and grzB. Moreover, adoptive transfers of either WT CD8+ T-cells or CD11c+ DC failed to induce alloeffector CD8+ responses or OAD. Together, these data indicate type-I IFN responsiveness is required for the innate/adaptive cellular interactions necessary for dsRNA to disrupt DST/anti-CD154-induced airway allograft acceptance via the generation of alloeffector CD8+ responses that precede OAD.
The establishment of long-term allograft acceptance using anti-CD154 blockade with/without DST has been demonstrated in multiple experimental transplant models including bone marrow, skin, kidney, islet, heterotopic heart and airway(10, 12, 22–25). We observed a marked reduction in intragraft CD8+ T-cell numbers, consistent with previous studies implicating peripheral clonal deletion of alloreactive CD8+ T-cells using this therapy. However, we also demonstrate the presence of proliferating CD44highCD69highCD8+ T-cells in accepted grafts that are incapable of producing allospecific IFN-γ or TNF-α and exhibit reduced grzB expression. Together, our data indicate that costimulation blockade results in alloreactive CD8+ T-cells that escape deletion but have impaired effector function, consistent with anergy (26). This uncoupling of CD8+ activation/proliferation from effector function in intragraft T-cells is reminiscent of our previous findings in CD154−/− mice (20). Moreover, our findings are similar to earlier studies showing CD8+ T-cell graft-infiltration in heart and non-human primate kidney transplant models following costimulation blockade, with the former study showing impaired CD8+ function (12, 27). While some studies have found a role for regulatory T-cells in costimulation blockade-induced tolerance, we did not find this in our previous studies in this model in CD154-deficient mice (15, 20, 26). Thus, several mechanisms contribute to airway allograft acceptance following CD154/CD40 costimulation blockade.
Several studies in the skin allograft model have demonstrated the capacity of various TLR agonists to abrogate CD154/CD40 costimulation blockade-induced allograft acceptance, including dsRNA (14, 28). Collectively, these studies support alternative activation of adaptive immunity rendering alloreactive CD8+ T-cells CD154/CD40 costimulation blockade-resistant. While our findings of increased intragraft CD8+ T-cells with poly(I:C) are consistent with deletional escape via alternative activation, the inflammation associated with dsRNA may also prevent anergy in CD8+ T-cells (29). This bypassing of the CD154/CD40 pathway may occur via multiple mechanisms, including DC maturation, and induction of type-I IFNs or IL-12p70 (16–18, 30, 31). Moreover, TLR agonists, including dsRNA, are potent enhancers of antigen-specific T-cell effector responses via DC in mice and humans (32–34). Further, the CD154/CD40 pathway is dispensable for primary cytotoxic CD8+ T-cell responses during LCMV infection (35). Thus, it is plausible that elaboration of dsRNA during the replicative phase of a respiratory viral infection would result in TLR signaling and innate cytokine production that bypasses the need for CD154/CD40 costimulation. This mechanism is consistent with our findings of restoration of CD8+ alloeffector responses with DST/anti- CD154/poly(I:C) is consistent with alternative activation of APC enhancing CD8+ responses and leading to airway oblieration. However, the inability of WT CD11c+ DC infusion to restore CD8+ responses or OAD suggests the importance of type-I IFN responsiveness in T-cells, recently shown as critical for CD8+ memory during respiratory viral infection (36). Together, our data suggest broad type-I IFN responsiveness is required for dsRNA to abrogate airway tolerance via alloeffector responses necessary for OAD. However, we should point out the possibility that type-I IFN responsiveness in other cell types is required for OAD pathology to develop.
There are several caveats to our studies. While the HTT model of murine OAD bears similarities to human OB pathology, these grafts are not directly vascularized, although rapid neovascularization occurs (37). However, vascularization of allografts in other models has not been predictive of CD8+ regulation via the CD154/CD40 pathway, thus mixed results in other systems might be due to factors such as graft tissue type, different accessory-cell populations or cytokine milieus (38, 39). Nevertheless, the HTT model is useful to study intragraft alloimmune mechanisms that precede airway obliteration in a controlled manner, in the absence of environmental exposures. Because the HTT is a large airway model and BOS primarily affects the small airways in LTRs, one should exercise caution in extrapolating findings in mouse OAD to human OB, however, our studies provide plausible cellular mechanisms that may be important in LTRs (8). Lastly, though poly(I:C) is a frequently used viral analog, other factors may contribute to BOS in the setting of a replicating live viral infection, and this is an area where further studies are needed in LTRs to characterize immune responses associated with BOS progression.
In conclusion, our studies provide new insights into CD154/CD40 regulation of alloreactive CD8+ T-cells and that novel approaches to block the CD154/CD40 pathway may be beneficial in the transplant setting if this can be achieved safely. However, we also demonstrate that dsRNA is a potent barrier to costimulation blockade-induced airway allograft tolerance, with type-I IFN responsiveness being critical for this disruption. As RNA viruses are a risk factor for BOS, understanding the immune mechanisms regulating airway acceptance versus rejection may improve future strategies aimed at BOS prevention.
Acknowledgments
Funding/Acknowledgements/Disclosures: This work was supported in part by NIH grant award AI079175 (JFM) No relevant disclosures or acknowledgements.
Abbreviations
- BOS
bronchiolitis obliterans syndrome
- OAD
obliterative airway disease
- poly(I:C)
polyinosinic:polycytidylic acid
- APC
antigen presenting cell
- DC
dendritic cell
- CARV
community-acquired respiratory viral infection
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Christie JD, Edwards LB, Kucheryavaya AY, Benden C, Dobbels F, Kirk R, et al. The Registry of the International Society for Heart and Lung Transplantation: Twenty-eighth Adult Lung and Heart-Lung Transplant Report--2011. J Heart Lung Transplant. 30(10):1104–1122. doi: 10.1016/j.healun.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Weigt SS, Wallace WD, Derhovanessian A, Saggar R, Lynch JP, Belperio JA. Chronic allograft rejection: epidemiology, diagnosis, pathogenesis, and treatment. Semin Respir Crit Care Med. 31(2):189–207. doi: 10.1055/s-0030-1249116. [DOI] [PubMed] [Google Scholar]
- 3.Khalifah AP, Hachem RR, Chakinala MM, Schechtman KB, Patterson GA, Schuster DP, et al. Respiratory viral infections are a distinct risk for bronchiolitis obliterans syndrome and death. American journal of respiratory and critical care medicine. 2004;170(2):181–187. doi: 10.1164/rccm.200310-1359OC. Epub 2004/05/08. [DOI] [PubMed] [Google Scholar]
- 4.Kumar D, Erdman D, Keshavjee S, Peret T, Tellier R, Hadjiliadis D, et al. Clinical impact of community-acquired respiratory viruses on bronchiolitis obliterans after lung transplant. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2005;5(8):2031–2036. doi: 10.1111/j.1600-6143.2005.00971.x. Epub 2005/07/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Billings JL, Hertz MI, Savik K, Wendt CH. Respiratory viruses and chronic rejection in lung transplant recipients. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2002;21(5):559–566. doi: 10.1016/s1053-2498(01)00405-3. Epub 2002/05/02. [DOI] [PubMed] [Google Scholar]
- 6.Shah PD, McDyer JF. Viral infections in lung transplant recipients. Seminars in respiratory and critical care medicine. 2010;31(2):243–254. doi: 10.1055/s-0030-1249120. Epub 2010/04/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hertz MI, Jessurun J, King MB, Savik SK, Murray JJ. Reproduction of the obliterative bronchiolitis lesion after heterotopic transplantation of mouse airways. The American journal of pathology. 1993;142(6):1945–1951. Epub 1993/06/01. [PMC free article] [PubMed] [Google Scholar]
- 8.McDyer JF. Human and murine obliterative bronchiolitis in transplant. Proceedings of the American Thoracic Society. 2007;4(1):37–43. doi: 10.1513/pats.200605-107JG. Epub 2007/01/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okazaki M, Gelman AE, Tietjens JR, Ibricevic A, Kornfeld CG, Huang HJ, et al. Maintenance of airway epithelium in acutely rejected orthotopic vascularized mouse lung transplants. American journal of respiratory cell and molecular biology. 2007;37(6):625–630. doi: 10.1165/rcmb.2007-0257RC. Epub 2007/08/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Markees TG, Phillips NE, Noelle RJ, Shultz LD, Mordes JP, Greiner DL, et al. Prolonged survival of mouse skin allografts in recipients treated with donor splenocytes and antibody to CD40 ligand. Transplantation. 1997;64(2):329–335. doi: 10.1097/00007890-199707270-00026. Epub 1997/07/27. [DOI] [PubMed] [Google Scholar]
- 11.Wells AD, Li XC, Li Y, Walsh MC, Zheng XX, Wu Z, et al. Requirement for T-cell apoptosis in the induction of peripheral transplantation tolerance. Nat Med. 1999;5(11):1303–1307. doi: 10.1038/15260. [DOI] [PubMed] [Google Scholar]
- 12.Kirk AD, Burkly LC, Batty DS, Baumgartner RE, Berning JD, Buchanan K, et al. Treatment with humanized monoclonal antibody against CD154 prevents acute renal allograft rejection in nonhuman primates. Nature medicine. 1999;5(6):686–693. doi: 10.1038/9536. Epub 1999/06/17. [DOI] [PubMed] [Google Scholar]
- 13.Chalermskulrat W, McKinnon KP, Brickey WJ, Neuringer IP, Park RC, Sterka DG, et al. Combined donor specific transfusion and anti-CD154 therapy achieves airway allograft tolerance. Thorax. 2006;61(1):61–67. doi: 10.1136/thx.2005.047316. Epub 2005/10/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thornley TB, Brehm MA, Markees TG, Shultz LD, Mordes JP, Welsh RM, et al. TLR agonists abrogate costimulation blockade-induced prolongation of skin allografts. J Immunol. 2006;176(3):1561–1570. doi: 10.4049/jimmunol.176.3.1561. Epub 2006/01/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen L, Wang T, Zhou P, Ma L, Yin D, Shen J, et al. TLR engagement prevents transplantation tolerance. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2006;6(10):2282–2291. doi: 10.1111/j.1600-6143.2006.01489.x. Epub 2006/09/15. [DOI] [PubMed] [Google Scholar]
- 16.Cella M, Salio M, Sakakibara Y, Langen H, Julkunen I, Lanzavecchia A. Maturation, activation, and protection of dendritic cells induced by double-stranded RNA. The Journal of experimental medicine. 1999;189(5):821–829. doi: 10.1084/jem.189.5.821. Epub 1999/03/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kadowaki N, Antonenko S, Liu YJ. Distinct CpG DNA and polyinosinic-polycytidylic acid double-stranded RNA, respectively, stimulate CD11c-type 2 dendritic cell precursors and CD11c+ dendritic cells to produce type I IFN. J Immunol. 2001;166(4):2291–2295. doi: 10.4049/jimmunol.166.4.2291. Epub 2001/02/13. [DOI] [PubMed] [Google Scholar]
- 18.Reis e Sousa C. Toll-like receptors and dendritic cells: for whom the bug tolls. Seminars in immunology. 2004;16(1):27–34. doi: 10.1016/j.smim.2003.10.004. Epub 2004/01/31. [DOI] [PubMed] [Google Scholar]
- 19.West EE, Lavoie TL, Orens JB, Chen ES, Ye SQ, Finkelman FD, et al. Pluripotent allospecific CD8+ effector T cells traffic to lung in murine obliterative airway disease. American journal of respiratory cell and molecular biology. 2006;34(1):108–118. doi: 10.1165/rcmb.2005-0164OC. Epub 2005/10/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shah PD, West EE, Whitlock AB, Orens JB, McDyer JF. CD154 deficiency uncouples allograft CD8+ T-cell effector function from proliferation and inhibits murine airway obliteration. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2009;9(12):2697–2706. doi: 10.1111/j.1600-6143.2009.02805.x. Epub 2009/12/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belperio JA, Keane MP, Burdick MD, Lynch JP, 3rd, Xue YY, Li K, et al. Critical role for CXCR3 chemokine biology in the pathogenesis of bronchiolitis obliterans syndrome. J Immunol. 2002;169(2):1037–1049. doi: 10.4049/jimmunol.169.2.1037. [DOI] [PubMed] [Google Scholar]
- 22.Zhai Y, Meng L, Busuttil RW, Sayegh MH, Kupiec-Weglinski JW. Activation of alloreactive CD8+ T cells operates via CD4-dependent and CD4-independent mechanisms and is CD154 blockade sensitive. J Immunol. 2003;170(6):3024–3028. doi: 10.4049/jimmunol.170.6.3024. Epub 2003/03/11. [DOI] [PubMed] [Google Scholar]
- 23.Haspot F, Fehr T, Gibbons C, Zhao G, Hogan T, Honjo T, et al. Peripheral deletional tolerance of alloreactive CD8 but not CD4 T cells is dependent on the PD-1/PD-L1 pathway. Blood. 2008;112(5):2149–2155. doi: 10.1182/blood-2007-12-127449. Epub 2008/06/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Y, Li XC, Zheng XX, Wells AD, Turka LA, Strom TB. Blocking both signal 1 and signal 2 of T-cell activation prevents apoptosis of alloreactive T cells and induction of peripheral allograft tolerance. Nature medicine. 1999;5(11):1298–1302. doi: 10.1038/15256. Epub 1999/11/05. [DOI] [PubMed] [Google Scholar]
- 25.Fernandez FG, McKane B, Marshbank S, Patterson GA, Mohanakumar T. Inhibition of obliterative airway disease development following heterotopic murine tracheal transplantation by costimulatory molecule blockade using anti-CD40 ligand alone or in combination with donor bone marrow. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2005;24((7 Suppl)):S232–S238. doi: 10.1016/j.healun.2004.06.008. Epub 2005/07/05. [DOI] [PubMed] [Google Scholar]
- 26.Quezada SA, Bennett K, Blazar BR, Rudensky AY, Sakaguchi S, Noelle RJ. Analysis of the underlying cellular mechanisms of anti-CD154-induced graft tolerance: the interplay of clonal anergy and immune regulation. J Immunol. 2005;175(2):771–779. doi: 10.4049/jimmunol.175.2.771. Epub 2005/07/09. [DOI] [PubMed] [Google Scholar]
- 27.Zhai Y, Shen XD, Gao F, Coito AJ, Wasowska BA, Salama A, et al. The CD154-CD40 T cell costimulation pathway is required for host sensitization of CD8(+) T cells by skin grafts via direct antigen presentation. J Immunol. 2002;169(3):1270–1276. doi: 10.4049/jimmunol.169.3.1270. Epub 2002/07/23. [DOI] [PubMed] [Google Scholar]
- 28.Thornley TB, Phillips NE, Beaudette-Zlatanova BC, Markees TG, Bahl K, Brehm MA, et al. Type 1 IFN mediates cross-talk between innate and adaptive immunity that abrogates transplantation tolerance. J Immunol. 2007;179(10):6620–6629. doi: 10.4049/jimmunol.179.10.6620. Epub 2007/11/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Curtsinger JM, Lins DC, Mescher MF. Signal 3 determines tolerance versus full activation of naive CD8 T cells: dissociating proliferation and development of effector function. The Journal of experimental medicine. 2003;197(9):1141–1151. doi: 10.1084/jem.20021910. Epub 2003/05/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gautier G, Humbert M, Deauvieau F, Scuiller M, Hiscott J, Bates EE, et al. A type I interferon autocrine-paracrine loop is involved in Toll-like receptor-induced interleukin-12p70 secretion by dendritic cells. The Journal of experimental medicine. 2005;201(9):1435–1446. doi: 10.1084/jem.20041964. Epub 2005/04/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Curtsinger JM, Valenzuela JO, Agarwal P, Lins D, Mescher MF. Type I IFNs provide a third signal to CD8 T cells to stimulate clonal expansion and differentiation. J Immunol. 2005;174(8):4465–4469. doi: 10.4049/jimmunol.174.8.4465. Epub 2005/04/09. [DOI] [PubMed] [Google Scholar]
- 32.Lore K, Betts MR, Brenchley JM, Kuruppu J, Khojasteh S, Perfetto S, et al. Toll-like receptor ligands modulate dendritic cells to augment cytomegalovirus- and HIV-1-specific T cell responses. J Immunol. 2003;171(8):4320–4328. doi: 10.4049/jimmunol.171.8.4320. Epub 2003/10/08. [DOI] [PubMed] [Google Scholar]
- 33.Salem ML, Diaz-Montero CM, El-Naggar SA, Chen Y, Moussa O, Cole DJ. The TLR3 agonist poly(I:C) targets CD8+ T cells and augments their antigen-specific responses upon their adoptive transfer into naive recipient mice. Vaccine. 2009;27(4):549–557. doi: 10.1016/j.vaccine.2008.11.013. Epub 2008/11/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kunzmann V, Kretzschmar E, Herrmann T, Wilhelm M. Polyinosinic-polycytidylic acid-mediated stimulation of human gammadelta T cells via CD11c dendritic cell-derived type I interferons. Immunology. 2004;112(3):369–377. doi: 10.1111/j.1365-2567.2004.01908.x. Epub 2004/06/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whitmire JK, Slifka MK, Grewal IS, Flavell RA, Ahmed R. CD40 ligand-deficient mice generate a normal primary cytotoxic T-lymphocyte response but a defective humoral response to a viral infection. Journal of virology. 1996;70(12):8375–8381. doi: 10.1128/jvi.70.12.8375-8381.1996. Epub 1996/12/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kohlmeier JE, Cookenham T, Roberts AD, Miller SC, Woodland DL. Type I interferons regulate cytolytic activity of memory CD8(+) T cells in the lung airways during respiratory virus challenge. Immunity. 33(1):96–105. doi: 10.1016/j.immuni.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Belperio JA, Keane MP, Burdick MD, Gomperts B, Xue YY, Hong K, et al. Role of CXCR2/CXCR2 ligands in vascular remodeling during bronchiolitis obliterans syndrome. J Clin Invest. 2005;115(5):1150–1162. doi: 10.1172/JCI24233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo Z, Meng L, Kim O, Wang J, Hart J, He G, et al. CD8 T cell-mediated rejection of intestinal allografts is resistant to inhibition of the CD40/CD154 costimulatory pathway. Transplantation. 2001;71(9):1351–1354. doi: 10.1097/00007890-200105150-00033. Epub 2001/06/14. [DOI] [PubMed] [Google Scholar]
- 39.Jones ND, Turvey SE, Van Maurik A, Hara M, Kingsley CI, Smith CH, et al. Differential susceptibility of heart, skin, and islet allografts to T cell-mediated rejection. J Immunol. 2001;166(4):2824–2830. doi: 10.4049/jimmunol.166.4.2824. Epub 2001/02/13. [DOI] [PubMed] [Google Scholar]