Abstract
With its wide distribution in soft and hard connective tissues, collagen is the most abundant of animal proteins. In vitro, natural collagen can be formed into highly organized, three-dimensional scaffolds that are intrinsically biocompatible, biodegradable, non-toxic upon exogenous application, and endowed with high tensile strength. These attributes make collagen the material of choice for wound healing and tissue engineering applications. In this article, we review the structure and molecular interactions of collagen in vivo; the recent use of natural collagen in sponges, injectables, films and membranes, dressings, and skin grafts; and the on-going development of synthetic collagen mimetic peptides as pylons to anchor cytoactive agents in wound beds.
Keywords: biocompatible, biodegradable, catgut, extracellular matrix, regenerative medicine
Introduction
Collagen has served humanity in myriad ways. This most abundant of animal proteins is the principle component of leathers, glues, gelatin for food and pharmaceutical capsules, and strings for musical instruments and tennis rackets. No other protein has had as much practical utility.
The use of collagen as a modern biomaterial began in 1881.1 In that year, Joseph Lister, who founded modern surgery, and his former student William Macewen reported independently on the advantages of a biodegradable suture termed “catgut”, a collagen-rich biomaterial prepared from the small intestine of a sheep (Figure 1).2,3 Over the ensuing years, countless innovations have extended the reach of collagen in the engineering and repair of soft tissue.4-7
Figure 1.

Sir Joseph Lister (English) and Sir William Macewen (Scottish), pioneers in the use of exogenous collagen in medicine.2,3,1 The image of Lister is from an oil painting by Walter William Ouless (courtesy of the Wellcome Library, London); the image of Macewen is from an oil painting by Charles R. Dowell (courtesy of the Royal College of Physicians and Surgeons of Glasgow). The catgut in oil was prepared by Lister in 1875 (courtesy of King's College London).
In most soft and hard connective tissues, collagen fibrils and their networks comprise the majority of the extracellular matrix (ECM) and form a highly organized, three-dimensional scaffold that surrounds the cells. Collagen also plays a dominant role in maintaining the biological and structural integrity of the ECM, and is a dynamic and flexible material that undergoes constant re-modeling to refine cellular behavior and tissue function.8 Collagen is surface-active and is capable of penetrating a lipid-free interface.9 Biodegradable and non-toxic, exogenous collagen is more biocompatible than other natural polymers, and only weakly antigenic.10 Collagen can form fibers with high tensile strength and stability via cross-linking and self-aggregation. These fibers can be formulated into numerous scaffolds of high utility (Table 1), which have arisen from an in-depth understanding of collagen structure and function. Here, we review modern collagen-based biomaterials for wound healing, and we highlight on-going challenges and unmet needs.
Table 1.
Commercial forms of reconstituted collagen.
| Collagen Form | Name (Company) |
|---|---|
| Partially Purified Skin | Gelfoam (Pfizer) |
| Collagen Sponge | Helistat (Integra LifeSciences) Instat (Johnson & Johnson) ActiFoam (MedChem) SkinTemp (BioCor) |
| Collagen Fiber | Helitene (Integra LifeScience) Instat Fibrillar (Johnson & Johnson) Avitene (Medichem) |
| Collagen Powder | BioCore (Medifil) |
| Collagen Composite Dressing | Fibracol (Johnson & Johnson) Biobrane (UDL Laboratories) |
| Hydrolyzed Collagen | Chronicure (Derma Sciences) |
The Collagen Molecule
Collagen accounts for about 1/3 of the protein of humans and ¾ of the dry weight of skin. To date, 29 different types of collagen have been identified (type XXIX belongs to the class of collagens containing von Willebrand factor type A domains11), and all of them display a triple-helical tertiary structure. Types I, II, III, V, and XI have fibrillar quaternary structures.
Collagen molecules are comprised of three polypeptide chains.5 These chains, aligned in a parallel manner and coiled in a left-handed polyproline II-type (PPII) helix, wrap around each other to form a right-handed triple helix that is stabilized by interstrand hydrogen bonds and intrastrand n→π* interactions (Figure 2).12 In animals, individual collagen triple helices (tropocollagen) form macroscopic fibers and networks in tissue, bone, and basement membrane.
Figure 2.
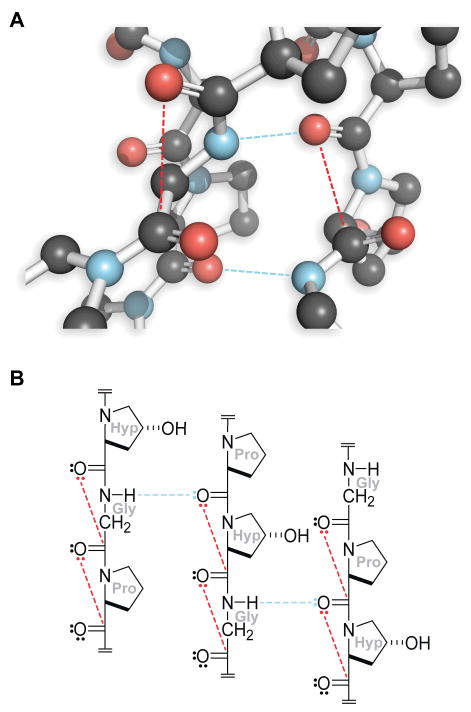
Structure of triple-helical collagen.5 Hydrogen bonds, blue dashed lines; n→π* interactions, red dashed lines. Panel A is a cross-section from PDB entry 1v4f.12
Each polypeptide chain in a collagen triple helix is composed of a thousand or so amino-acid residues. Every third residue is a glycine (Gly), resulting in a Xaa-Yaa-Gly repeat unit, where Xaa and Yaa can be any amino acid (Table 2). The repetitive presence of Gly ensures a tight packing of the three strands in a tropocollagen triple helix. The Xaa and Yaa positions are often occupied by two amino acids discovered by Emil Fischer: (2S)-proline (Pro)13 and (2S,4R)-4-hydroxyproline (Hyp),14 making Pro-Hyp-Gly the most common triplet in collagen.15 The 29 different types of collagen are composed of approximately twenty-five different chains, assembled in combination. Although the three chains in a triple helix can be identical, heterotrimeric triple helices are more prevalent than are homotrimeric ones.
Table 2.
Amino-acid composition of the two polypeptide chains that form the α1(I)·α1(I)·α2(I) triple helix of human type I collagen.55
| Amino Acid | α1(I) chain | α2(I) chain |
|---|---|---|
| Alanine | 124 | 111 |
| Arginine | 53 | 56 |
| Asparagine | 13 | 23 |
| Aspartic Acid | 33 | 24 |
| Glutamic Acid | 52 | 46 |
| Glutamine | 27 | 24 |
| Glycine | 345 | 346 |
| Histidine | 3 | 8 |
| Hydroxylysine | 4 | 9 |
| Hydroxyproline | 114 | 99 |
| Isoleucine | 9 | 18 |
| Leucine | 22 | 33 |
| Lysine | 34 | 21 |
| Methionine | 7 | 4 |
| Phenylalanine | 13 | 15 |
| Proline | 127 | 108 |
| Serine | 37 | 35 |
| Threonine | 17 | 20 |
| Tyrosine | 5 | 4 |
| Valine | 17 | 34 |
Of the various types of collagen, only a few are used in the production of collagen-based biomaterials. In fibrillar collagen, tropocollagen triple helices assemble into fibrils, which agglomerate to form fibers (Figure 3). These fibril-forming collagens have large sections of homologous sequences,16 and constitute the most commonly used forms of collagen-based biomaterials for wound healing and tissue engineering purposes. Type I collagen is the most abundant type in animals and is the type most often used in medicine.
Figure 3.
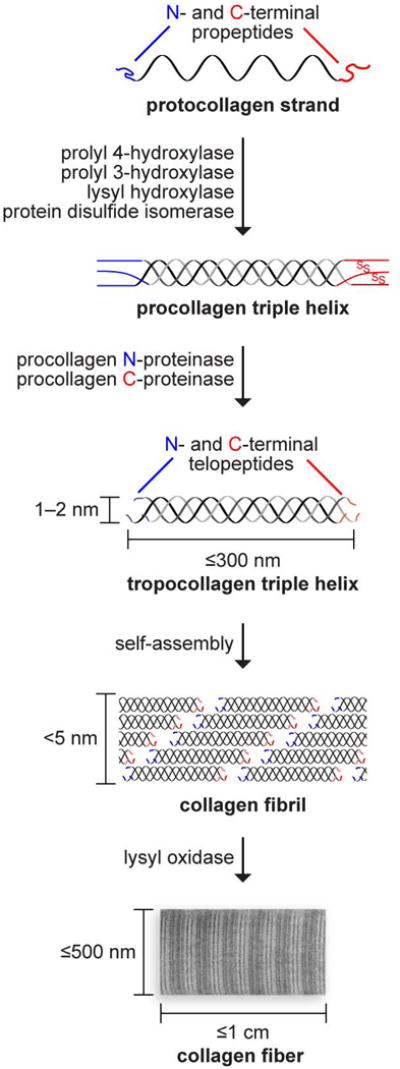
Biosynthetic route to collagen fibers. Size and complexity increase upon post-translational modification and self-assembly. The oxidation of lysine side chains leads to the spontaneous formation of hydroxylysyl pyridinoline and lysyl pyridinoline cross-links.
Collagen In Vivo
Collagen can be extracted from the tissue of any animal, even long-extinct dinosaurs.17,18 Common sources for biomedical application include bovine skin and tendons; porcine skin, intestine, or bladder mucosa; and rat tail.19 The properties of the extracted collagen differ depending on the animal and tissue. The use of collagen derived from animal sources can be complicated by allergic reaction and pathogen transmission.10,20 As an alternative, collagen can be produced by heterologous expression in mammalian, insect, and yeast cells.21 Collagen produced in Escherichia coli has special promise.22,23
Many cell-surface proteins bind to collagen.24 Cell–collagen interactions are mediated by four different kinds of proteins: (1) receptors (like glycoprotein VI) that recognize peptide sequences containing the Pro-Hyp-Gly unit,25 (2) receptors of the integrin family and discoidin domain receptor 1 and 2, which bind to the Phe-Hyp-Gly sequence, (3) receptors of the integrin-type that recognize cryptic motifs within collagen, and (4) receptors with affinity for non-collagenous domains. Many proteins (like decorin and laminin) that contain RGD or a similar integrin-recognition sequences can bind to both collagen and integrins, promoting cell adhesion and proliferation.26
Biodegradability of Collagen
As befits the primary structural protein in the body, collagen is resistant to proteolysis. Although the peptide bonds in a triple helix are occluded from enzymic active sites, single-stranded regions are cleaved by matrix metalloproteinases (MMPs). Specifically, types I–III are hydrolyzed by MMP-1, MMP-2, MMP-8, MMP-13, and MMP-14, which are collagenases.27-30 The ensuing collagen fragments are degraded further by gelatinases and non-specific proteases. Other MMPs, such as MMP-3 and MMP-9, bind to type I collagen but do not participate in its degradation.31,32
High biocompatibility and intrinsic biodegradability by endogenous collagenases makes exogenous collagen ideal for use in biomedical applications. Exogenous collagen can, however, elicit a complex response. For example, some of the degradation products of collagen types I–III induce chemotaxis of human fibroblasts,33 and such degradation is thought to promote restoration of tissue structure and functionality.34
Cross-Linking of Collagen
The proteolytic resistance and high tensile strength of natural collagen can be attributed largely to its covalent cross-links.35 Damage to such linkages upon extraction and over time weakens reconstituted forms of collagen (e.g., sponges, hydrogels, films, and membranes), which can then disintegrate on handling or under the pressure of surrounding tissues in vivo. Hence, efforts have been made to control the rate of degradation as well as in vivo absorption by generating new cross-links. Typically, the in vitro cross-linking of collagen enlists its amino and carboxyl groups to form new covalent bonds. The techniques can be grouped into three types, as follows.
Chemical cross-linking
Collagen has been cross-linked with formaldehyde,36 glutaraldehyde,37,38 carbodiimides,39,40 polyepoxy compounds,41 acyl azides,42 and hexamethylene diisocyanate.43 Although chemical cross-linking can enhance stability, residual electrophilic reagents and compounds formed upon degradation in vivo can be cytotoxic.44,45 In an alternative approach, collagen biomaterials are stabilized by exploiting the large number of amino groups on polycationic molecules like chitosan, thereby increasing the cross-linking efficiency of glutaraldehyde and minimizing its use and its potential cytotoxicity.46 Non-toxic chitosan is mixed with collagen just before lyophilization, simplifying the process.
Physical cross-linking using ultraviolet (UV) light or dehydrothermal treatment (DHT)
Exposure to either light at 254 nm or DHT increases the temperature for collagen shrinkage, tensile strength of the fibers, and resistance to proteolytic degradation.47 UV-irradiation requires only 15 min, whereas DHT treatment takes 3–5 days. UV-irradiation increases proteolytic resistance.35 DHT treatment increases the sensitivity of collagen to trypsin but lowers the propensity for degradation by pepsin and lysosomal cathepsins.48
Cross-linking with enzymes
Transglutaminase, in particular, can enhance the tensile strength and hydrolytic resistance of collagen-based biomaterials.49 This method is benign, generating no cytotoxic byproducts.
Preparation of Collagen-Based Materials
Natural collagen-based biomaterials can be classified into two categories based on the extent of their purification: de-cellularized collagen matrices that maintain the original tissue properties and ECM structure; and more refined scaffolds prepared via extraction, purification, and collagen polymerization. De-cellularizing collagen entails a combination of physical (snap freezing or high pressure), chemical (acid or alkali treatment, chelation with EDTA, or treatment with detergents or solutions of high osmolarity), and enzymatic (digestion with trypsin) methods to produce the biomaterial.50 Collagen in this form is used often as sutures, cardiac valves, and ligamentary prostheses. Generating collagen-based scaffolds involves processing collagen solutions with other biomolecules, such as elastin,51 glycosaminoglycans (GAG),52 and chitosan.38 Different applications require different formulations (Table 3). Production of such biomaterials requires the extraction and purification of collagen from natural tissues. The dissolution of collagen is, however, impeded by the low solubility of natural collagen due to its covalent cross-links. Natural collagen is insoluble in organic solvents but can dissolve in aqueous solutions, depending on the extant cross-linking. The most common solvent systems include a neutral salt solution (0.15–0.20 M NaCl),53 dilute acid solution (0.5 M acetic acid), or a solution of proteolytic enzymes, as the collagen triple-helical domain is resistant to proteases like pepsin, chymotrypsin, or ficin below ∼20 °C.54 The telopeptide ends are, however, vulnerable. Pepsin at a 1:10 weight ratio of enzyme to dry tissue in dilute acetic acid provides a medium in which collagen can be swollen and dissolved readily.55
Table 3.
Biomedical Applications of Collagen.
| Composition | Biomaterial Form | Applications |
|---|---|---|
| Collagen | Gel | Cosmetic Skin Defects Drug Delivery Vitreous Replacement Surgery Coating of Bioprostheses |
| Sponge | 3D Cell Culture Wound Dressing Hemostatic Agent Skin Replacement Drug Delivery |
|
| Hollow Fiber Tubing | Cell Culture Nerve Regeneration |
|
| Sphere | Micro-carrier for Cell Culture Drug Delivery |
|
| Membrane | Wound Dressing Dialysis Tissue Regeneration Corneal Shields Skin Patches |
|
| Rigid Form | Bone Repair | |
| Sponge | 3D Cell Culture Wound Dressing Skin Replacement |
|
| Collagen + GAG | Membrane | Tissue Regeneration Skin Patches |
| Collagen + Hydroxyapatite | Powder Sponge | Bond-Filling and Repair Drug Delivery (BMP) |
Sponges
Commercial collagen sponges are insoluble forms of the protein derived from animals like cows, horses, and pigs. The sponges are prepared by lyophilizing aqueous acid- or alkali-swollen collagen solutions containing 0.1–5% w/v dry matter. Their porosity is controlled by varying the collagen content and freezing rate. These sponges are capable of absorbing large amounts of tissue exudate, adhere smoothly to a wet wound bed, and maintain a moist environment, while shielding against mechanical trauma and bacterial infection.56 They are used routinely as a wound dressing for severe burns, pressure sores, donor sites, and leg ulcers, and in in vitro experiments.57 Collagen sponges have been combined with elastin, fibronectins, and GAGs to impart resilience and fluid-binding capacity.58,59 These materials can be cross-linked further with glutaraldehyde and conjugated with polymers like poly(hydroxyethylmethacrylate) to produce hydrophilic matrices with increased mechanical strength. Three-dimensional collagen lattices loaded physiologically with fibroblasts have been developed as an in vitro model for wound healing.60 Collagen promotes cellular motility, and inflammatory cells actively invade the porous scaffold.61 A highly vascularized granulation tissue forms that, in turn, stimulates the formation of new granulation tissue and epithelial layers. Sponge implantation in burn wounds causes a rapid recovery of the skin due to an intense infiltration of neutrophils in the sponge.62
Collagen sponges are especially useful in wound healing because their wet-strength allows their suturing to soft tissue and provides a template for new tissue growth. Collagen-based implants have been used as vehicles for delivery of cultured keratinocytes and drugs for skin replacement and burn-wound treatment.63,64,62 Implanted collagen sponges are infiltrated by amorphous connective tissue containing GAGs, fibronectin, and new collagen, followed by various cells, primarily fibroblasts and macrophages. When cells are bound to an extracellular matrix, such as an implanted collagen sponge, there is an increase in the production of new collagen.33 Depending on the degree of cross-linking, the collagen sponge is degraded by collagenases into peptide fragments and amino acids in 3–6 weeks, and the implant is then replaced by native type I collagen produced by fibroblasts. Chemical composites with other biomaterials, and acetylated, succinylated, or methylated collagen have also been used to immobilize therapeutic enzymes or control drug delivery. One such modification of interest is biotinylation.65 After the covalent attachment of biotin, a model protein (horseradish peroxidase) is bound via a pendant avidin. Biotinylation of collagen has also been used to attach peptide growth factors like heparin-binding growth factor and epidermal growth factor, modulating the healing of full-thickness wounds.66
Collagen-based sponges are an effective scaffold for the application of exogenous growth factors to wounds. Type I collagen sponges can expedite wound healing by promoting the deposition of nascent large-diameter collagen fibers parallel to the fibers in the sponge, thereby increasing tensile strength in large open dermal wounds. When these sponges are seeded with fibroblasts or coated with basic fibroblast growth factor (FGF) prior to implantation in a guinea-pig dermal wound model, they promote both early dermal and epidermal wound healing.67 Platelet-derived growth factor (PDGF), upon introduction into the wound matrix via a collagen sponge scaffold, increases fibroblast influx into the wounds and enhances capillary formation in comparison to control treatments.68
Collagen sponges are suitable for short-term delivery of antibiotics in wound bed. Sponges soaked with solutions of gentamicin, cefotaxim, fusidic acid, clindamycin, or vancomycin release 99.9% of these antibiotic agents after two days in vitro.69 Local infection is contained by a gentamicin-containing collagen matrix placed on a septic focus in rat abdomen.70 These sponges do not exhibit any unwanted side-effects and are absorbed into tissue after a few days.71
Apart from acting as a scaffold for growth factors and antibiotics, porous collagen sponges are also of use for cell culture, either for tissue engineering purposes ex vivo or as a direct implant. They have been used to promote the formation of cartilage via chondrocytes (with or without FGF),72,73 abdominal walls via myoblasts,74 and axons in spinal cord from Schwann cells.75 Dense type I collagen matrices can also act as a scaffold for in vitro fibroblast cell culture76 and analyses of angiogenesis.77 A combination of collagen biomaterials and mesenchymal stem cells could provide a useful strategy to treat wounds.78,79 A modified sponge that can act as an artificial skin graft has been developed by combining fibrillar collagen with gelatin,80 which is then stabilized via DHT cross-linking. A similar sponge incorporating gelatin has been used as a carrier matrix for mesenchymal stem cells used for cartilage stem cell therapy.81
Sponges formed from synthetic collagen are also known. A freeze-dried sponge composed of a heterogeneous polymer of Pro-Hyp-Gly triplets embedded subcutaneously into the dorsal area of a rat degrades at the same rate as does bovine type I atelocollagen, and promotes greater epithelialization of a full-thickness wound on the ear pad of a rabbit.82 Incorporation of the fibronectin-derived peptides GRGDS and PHSRN into the sponge enhances the adhesion, migration, and stratification of NIH3T3 cells.83
Injectables and Hydrogels
For several decades, dermatological defects have been treated with subcutaneous injections of collagen solutions. This application is a commercial success, particularly in the area of plastic and reconstructive surgery. An extensive study showed that treating reconstituted pepsin-solubilized bovine corium collagen dispersions with glutaraldehyde has a significant impact on physiochemical stability and that the biological response is a function of the degree of cross-linking.84,64 At low glutaraldehyde concentrations, the response is characterized by an influx of fibroblasts, neovascularization, and little inflammation. Treatment of the collagen dispersions with higher concentrations of glutaraldehyde causes a foreign body/giant cell reaction and calcification. The solubility of such treated fibrils decreases at high temperatures, but the proteolytic stability increases compared to non–cross-linked fibers. Cross-linking increases the solution viscosity,84,85 making the injection of cross-linked formulations into affected tissue difficult. The addition of hyaluronic acid at 0.3–0.5% w/v eased the injection process. A patent indicates that small molecules like maltose and neutral polymers like dextran can be used as lubricants to facilitate injection into tissue.86
The delivery of local anesthetics and central analgesics can be prolonged by 5- to 30-fold upon formulation with collagen.87 This increase could be due to a decrease in the rate of diffusion of the drug due to the high microviscosity of the collagen, or to an affinity of the drug molecule for collagen. Subsequent work shows that fibrillar collagen is capable of moderating the release-rate only of large proteins, like fibrinogen, and significant amounts of non-fibrillar content is necessary to regulate the diffusion of small proteins, like chymotrypsinogen.88
The scope for using injectable collagen formulations for the delivery of growth factors and consequent cellular regeneration and tissue repair is vast. In a porcine model, intestinal wound repair is expedited by treatment with collagen suspensions carrying FGF or transforming growth factor-β. These formulations also partially reverse the steroid-induced impairment of breaking-load in intestinal-wound models.89 Investigations of cellular function, migration, proliferation, and differentiation in collagen gels has led to further understanding of the mechanism and kinetics of transport, as well as the influence of growth factors, laminin, and fibronectin.90-92
Collagen gels positioned between the stumps of a transected spinal cord cause axons to emerge from the interface with the spinal tissue and then grow into the implanted collagen gel within a month.93 The tensile strength and durability of the collagen implant is strengthened by co-precipitation with chondroitin-6-sulfate or cross-linking by carbodiimide, which also regulates the normal scarring process, and promotes axon growth93 and fibroblast proliferation.94 The efficacy of injectable fluid collagen solution into the lesion that self-assembles in situ has been compared to an implanted collagen gel.95 Corticospinal tract (CST) axons have been visualized in the matrix, along with an influx of astroglial and microglial cells into the collagen. On the other hand, a collagen gel, implanted pre-assembled, does not show any axon growth or the influx of astroglial and microglial cells.
Collagen hydrogels present a large, uniform surface area, and can serve as a drug delivery system. A common practice has been to combine natural and synthetic polymers with synergistic properties, thereby imparting higher mechanical strength to the natural polymers and biocompatibility to their synthetic counterparts. Synthetic polymers like poly(vinyl alcohol) and poly(acrylic acid) are blended with natural polymers like collagen and hyaluronic acid, and formulated into hydrogels, films, and sponges that are then loaded with growth hormone.96 These formulations provide a controlled-release of growth hormone from the collagen hydrogel. Gels have also been formulated with atelocollagen, produced by the removal of telopeptide ends using pepsin, and used for the delivery of chondrocytes to repair cartilage defects.97
Liposomes sequestered in a collagen gel can release insulin or growth hormone into circulation in a controlled manner.98 The collagen decreases lipid peroxidation, as well as the permeability of neutral or negatively charged liposomes, resulting in the slow diffusion of the encapsulated compound over a period of 3–5 days.98,99 A patent suggests that collagen can also be used as an additive in oil-based suspensions, to sustain the release of proteins that had been lyophilized and suspended in a lipophilic liquid.100 This technology could have potential as a topical treatment for surgical and non-surgical wounds and burns.
Films and Membranes
Collagen films have been used in wound healing and tissue engineering, primarily as a barrier. Films of ∼0.1–0.5 mm thickness can be cast from collagen solutions and air-dried in a manner similar to ophthalmological shields. As an added advantage, films made from biodegradable materials like telopeptide-free reconstituted collagen demonstrate a slow release of encapsulated drugs.101 The loaded films afford easy sterilization and become pliable after hydration, without compromise to their mechanical strength.
Collagen membranes have been used for wound dressings, dural closures, reinforcement of compromised tissues, and guided tissue regeneration. Wound healing in diabetic db/db mice has been modulated by a sustained release of human growth hormone encapsulated in collagen films.102 Likewise, films made with collagen–poly(vinyl alcohol) mixtures cross-linked with glutaraldehyde vapors have been tested as a formulation for delivering recombinant human growth hormone.96 A patent describes single and multilayer collagen films as vehicles for the sustained release of pharmaceuticals, especially growth factors.103 Individual collagen films are attached together by applying gentle pressure to form multilayer membranes, and PDGF is released from these films at a constant rate for up to 100 h, improving wound healing in vivo.
Biodegradable collagen membranes can be scaffolds for viable fibroblasts.104 A blend of collagen and another polymer, such as an atelocollagen matrix, added on the surface of polyurethane films promotes the attachment and proliferation of fibroblasts, supports their growth, and enhances long-term survival.105 In addition, recombinant type I collagen from Pichia pastoris yeast has been used to formulate films for tissue engineering and guided tissue regeneration after dental surgery.106
Wound Dressings
Collagen plays a pivotal role in many pre- and post-operative surgical procedures. Due to its low antigenicity and inherent biocompatibility with most endogenous tissue, natural collagen has often been used for surgical repair.107 Wound dressings based on collagen are practical and easily remodeled due to their simple structure, relative uniformity, and abundant availability. These attributes have led to the development of novel surgical adhesives synthesized from porcine collagen and poly(glutamic acid).108 These adhesives have been used to prevent air from leaking out of damaged lungs. The absorption of such collagen-based adhesives can be regulated by altering the collagen content of the system.
Collagen-based wound dressings have long been used to cover burn wounds and treat ulcers.109,34,58 They have a distinctive practical and economic advantage compared to growth-factor and cell-based treatment of full-thickness wounds, and have been formulated in a number of different ways (Table 1). An unconventional form consisting of powdered avian collagen is effective at expediting chronic wound healing.110 The powder promotes cellular recruitment, activation of the inflammation phase of wound healing, and support for new tissue growth—similar in function to collagen sponges.
Some common, commercial skin, dermal substitutes, and dressings like Alloderm™ (human dermis), Amniograph™ (amniotic membrane), Integra® (acellular collagen-GAG scaffold), and Oasis™ (porcine skin), are used for medical applications. A combination of collagen with alginate has also been successful in promoting the inflammatory phase of wound healing, while imparting mechanical strength—a characteristic of collagen fibrils.
Collagen dressings have been prepared with a semi-occlusive polymer film attached to its outer surface.111 Such occlusive films are resistant to bacterial attack as well as further mechanical trauma, and provide proper air and vapor permeability. They reduce contraction and scarring, and increase the rate of epithelialization. One such dressing used extensively in burn care is Biobrane®, which consists of a silicone membrane knitted with a nylon membrane, both of which are impregnated with fragments of porcine collagen. Used as a temporary dressing, this composite promotes granulation and acts as an adjunct therapy for full-thickness wounds.112,113
Skin Replacement
Due to its mechanical strength and biocompatibility, reconstituted type I collagen can replace damaged skin directly.114 For example, a full-thickness excision wound in a porcine model has been used to study the effects of a collagen matrix implant on granulation tissue formation, wound contraction, and re-epithelialization.63 Wounds with implants show enhanced granulation tissue formation and re-epithelialization; contraction is reduced significantly, showing a bias towards wound regeneration and cosmetic utility.
Cultured skin substitutes from cryo-preserved skin cells have been used to cure chronic diabetic wounds.115 In lieu of pathological skin, the contracted collagen lattice serves as a support for epithelial cell growth and differentiation.116 Collagen implants have also been used in corneal healing, and corneal cells have a normal appearance when cultured individually on a synthetic collagen matrix.117
Corneal scaffolds have been constructed with recombinant human collagen118 and can induce collagen secretion by fibroblasts.119 Microbial baggage control has been attempted by the addition of antimicrobial drugs, such as amikacin, to bovine skin collagen.120 Cutaneous models with melanocytes,121 dendritic cells,122 and adipose tissue79 have been developed as well.
Cultured skin substitutes exhibit delayed keratinization after grafting in comparison to native skin autografts.123 To address this issue, collagen-based systems have been modified with other proteins, such as fibrin, and with GAGs. Human epidermal keratinocytes have been cultured on membranes composed of GAGs and collagen.62 Keratinocytes and fibroblasts have been attached to those membranes, which can be cross-linked to reduce their rate of biodegradation.124,37 Incubating collagen-substitutes at low humidity in vitro stimulates restoration of a functional epidermis.123 Similarly, cultured cells are best grafted in combination with a thin layer of either collagen or fibrin, but not both.125
Acellular bilayer artificial skin with an outer later composed of silicone and an inner layer composed of collagen matrix is compatible with long-term post-operative tissue.126 A bilayered-collagen gel seeded with human fibroblasts in the lower part and human keratinocytes in the upper layer has been used as the “dermal” matrix of an artificial skin. This product has been commercialized by Organogenesis as Apligraf®, and in 1998 became the first bio-engineered skin to receive FDA approval. Organogenesis is developing other collagen-based products, including Revitix™ (a topical cosmetic product), VCTO1™ (a bilayered bio-engineered skin), and Forta-Derm™ Antimicrobial (an anti-microbial wound dressing).
Collagen Mimetic Peptides
Synthetic peptides have provided much insight on the atomic underpinnings of collagen structure and stability.127,5,128-131,7,132,133 Using such peptides in wound-healing therapies requires their assembly into larger entities. Peptide–amphiphile conjugates provide a simple means to achieve that end. A short collagen mimetic peptide self-assembles into cylindrical nanofibers that are ∼7 nm in diameter and several micrometers in length due to alkyl chains at its N terminus.134,135 The peptide domain can display useful functionality on its fibrous surface. For example, a phosphoserine residue stimulates formation of calcium phosphate minerals and an RGD segment promotes the adhesion and growth of cells.
The self-assembly of amphiphilic peptides in a physiological medium can cross-link nanofibrillar collagen gels.136 The gels have a high water content and a matrix composed of interwoven nanofibers that are ∼10 nm in diameter with ∼200-nm pores.137 These gels maintain the morphology of differentiated chondrocytes and develop a cartilage-like ECM rich in proteoglycans and type II collagen, thereby showing potential for cartilage repair.138
Native chemical ligation has been used to polymerize collagen mimetic peptide in aqueous solution.139 The resulting 103-kDa strands form fiber-like structures that are micrometers in length. The presence of cysteine residues in these peptides can be exploited for cross-linking and covalent modification. Collagen-like supramolecules have also been created by the self-assembly of strands mediated by Coulombic forces140,141 or the hydrophobic effect,142,143 or fragments in which strands are cross-linked by disulfide bonds.144,145
Peptoid-containing collagen mimetic peptides interact with epithelial cells and fibroblasts when immobilized on a synthetic surface.146 Cell-binding requires a minimum of nine Gly-Pro-Nleu triplets, and such peptides are not cytotoxic. Amine-functionalized latex nanoparticles functionalized with ten Gly-Pro-Hyp triplets are capable of inducing human platelet aggregation with a potency close to that of type I collagen,147 and these Gly-Pro-Hyp triplets represent functional platelet–collagen receptor recognition motifs within collagen.25 Thus, these peptides can play active roles in the wound-healing process. A short peptide, (Pro-Pro-Gly)5, has also been established as a potent chemo-attractant for alveolar macrophages that induces the migration of polymorphonuclear leucocytes (PMNL) into lungs.148-150 Coating with peptides mimicking segments of type I collagen promoted mesenchymal cell adhesion to a hydroxyapatite surface and improved bone formation.151
A desirable attribute of collagen mimetic peptides is their ability to anneal to endogenous collagen strands but not triple helices (Figure 5). Collagen mimetic peptides containing Pro-Hyp-Gly triplets bind to collagen films152 and show promise for imaging and wound-healing studies.153 Gold-nanoparticles functionalized with (Pro-Hyp-Gly)n peptides bind to the “gap” regions of native collagen.154 (Pro-Hyp-Gly)n peptides have, however, a bias to be in a homotrimeric triple helix at room temperature, mandating pre-heating to high temperatures (∼80 °C) to generate single strands. Photodeprotection circumvents this limitation, enabling the imaging of (presumably) denatured collagen in tissues that are either undergoing normal renewal processes or suffering from pathological conditions, such as tumor progression or Marfan syndrome, which is a musculoskeletal disease.155
Figure 5.
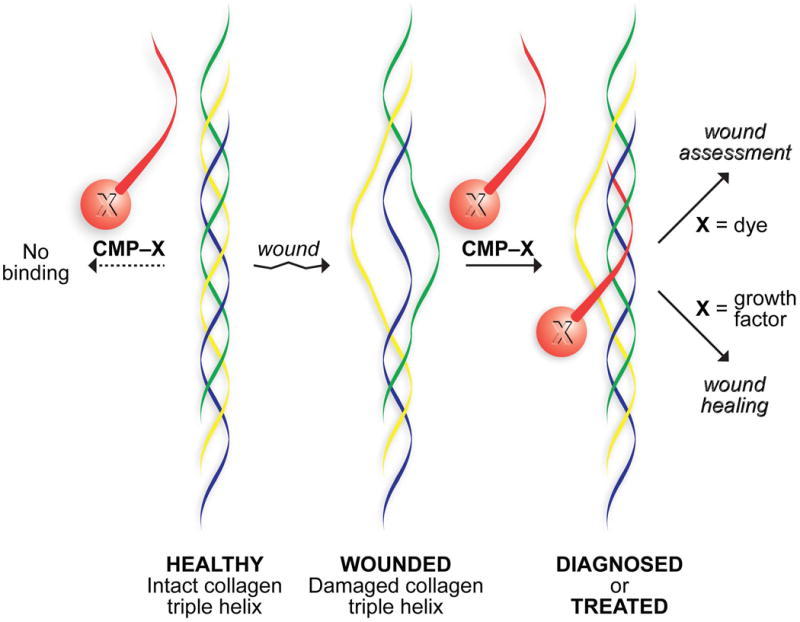
Strategy for anchoring molecules in a wound bed. A natural strand (yellow) in weak or damaged collagen is displaced by a synthetic collagen mimetic peptide (CMP; red) that forms a hyperstable triple helix. A pendant dye enables wound assessment;163 a pendant growth factor expedites wound healing.164
The conformational stability of a synthetic collagen triple helix can be increased by having (2S,4S)-4-fluoroproline (flp) and (2S,4R)-4-fluoroproline (Flp) in the Xaa and Yaa positions of the Xaa-Yaa-Gly repeat unit.156,157 Interestingly, the stability endowed by Flp promotes the adhesion and spreading of melanoma cells.158 The increased stability derived from non-natural Pro-Flp-Gly or flp-Pro-Gly triplets arises from an F–C–C–N gauche effect, which preorganizes pyrrolidine ring pucker and thus main-chain dihedral angles (Figure 4).159,160,157,161 Remarkably, (flp-Flp-Gly)n peptides, unlike (Pro-Flp-Gly)n or (flp-Pro-Gly)n peptides, do not form stable homotrimeric triple helices due to interstrand steric clashes between fluoro groups within the same cross-section of a triple helix.162 Natural collagen lacks fluoro groups in all of its triplets and Hyp in most (Table 2). Thus, (flp-Flp-Gly)7–dye conjugates anneal strongly to (presumably) damaged collagen both in vitro and ex vivo (Figure 5).163 No pre-heating or photodeprotection of the peptide is necessary, and a (flp-Flp-Gly)7–dye conjugate at ≥0.1 mM is not toxic to human fibroblast cells.
Figure 4.
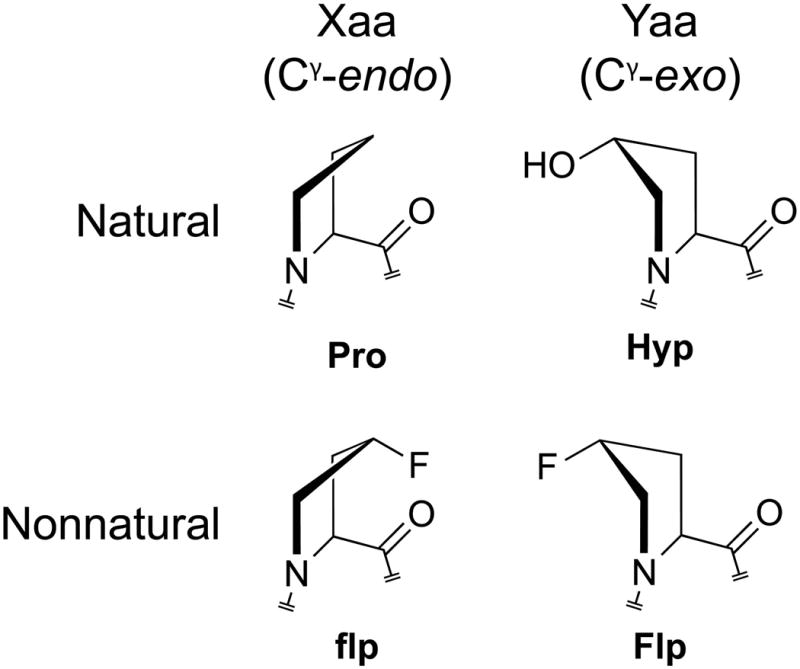
Pyrrolidine ring puckers preferred in the Xaa and Yaa positions of collagen strands, and natural and non-natural derivatives of proline with those preferences.5 The pyrrolidine rings actually prefer a twist rather than envelope conformation. As Cγ typically experiences the largest out-of-plane displacement in these twisted rings, we refer to pyrrolidine ring conformations simply as “Cγ-exo” and “Cγ-endo”.
Finally, we note that a collagen mimetic peptide can act as a pylon, anchoring a pendant molecule in damaged tissue (Figure 5). This strategy has been used to promote wound healing in mice.164 The administration of exogenous cytoactive factors by subcutaneous or intraperitoneal injection or by topical application is compromised by natural lavation that rapidly dilutes and ultimately drains soluble molecules. In contrast, the one-time application of a collagen mimetic peptide–Substance P conjugate enhances wound healing compared to unconjugated Substance P and other controls, and does so with extensive re-epithelialization and mitigated inflammatory activity. These data validate a simple and general strategy for re-engineering wound beds by the integration of beneficial cytoactive factors.
Conclusions
An area of foremost importance and urgency in health care research is the development of biomaterials that are accessible, persistent, and versatile. Collagen by virtue of its ubiquity, low immunogenicity, and ability to be molded into strong, biocompatible scaffolds, meets these criteria and thus plays a leading role in wound care. Moreover, collagen-based materials are adroitly at the interface of natural and synthetic macromolecules.
Collagen is likewise important in a related area of wound care—the controlled-release of bioactive molecules. Complementary to the use of heterogeneous collagen composites, the development and use of collagen mimetic peptides as a potent system for targeted delivery of therapeutic molecules to the wound site, can expedite progress in the field of wound healing and tissue regeneration.'
Acknowledgments
Contract grant sponsor: NIH
Contract grant number: R01 AR044276
References
- 1.Gibson T. Br J Surg. 1990;77:824–825. doi: 10.1002/bjs.1800770736. [DOI] [PubMed] [Google Scholar]
- 2.Macewen W. Br Med J. 1881;1:150–151. doi: 10.1136/bmj.1.1048.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lister J. Br Med J. 1881;1:183–185. doi: 10.1136/bmj.1.1049.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stenzel KH, Miyata T, Rubin AL. Annu Rev Biochem. 1974:231–253. doi: 10.1146/annurev.bb.03.060174.001311. [DOI] [PubMed] [Google Scholar]
- 5.Shoulders MD, Raines RT. Annu Rev Biochem. 2009;78:929–958. doi: 10.1146/annurev.biochem.77.032207.120833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parenteau-Bareil R, Gauvin R, Berthod F. Materials. 2010;3:1863–1887. [Google Scholar]
- 7.Yu SM, Li Y, Kim D. Soft Matter. 2011;7:7927–7938. doi: 10.1039/C1SM05329A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aszódi A, Legate KR, Nakchbandi I, Fässler R. Annu Rev Cell Dev Biol. 2006;22:591–621. doi: 10.1146/annurev.cellbio.22.010305.104258. [DOI] [PubMed] [Google Scholar]
- 9.Fonseca JM, Alsina AM, Francesca R. Biochim Biophys Acta Biomembr. 1996;1279:259–265. doi: 10.1016/0005-2736(95)00265-0. [DOI] [PubMed] [Google Scholar]
- 10.Maeda M, Tani S, Sano A, Fujioka K. J Control Release. 1999;62:313–324. doi: 10.1016/s0168-3659(99)00156-x. [DOI] [PubMed] [Google Scholar]
- 11.Söderhäll C, Marenholz I, Kerscher T, Rüschendorf F, Esparza-Gordillo J, Worm M, Gruber C, Mayr G, Albrecht M, Rohde K, Schulz H, Wahn U, Hubner N, Lee YA. PLoS Biol. 2007;5:1952–1961. doi: 10.1371/journal.pbio.0050242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okuyama K, Hongo C, Fukushima R, Wu G, Narita H, Noguchi K, Tanaka Y, Nishino N. Biopolymers. 2004;76:367–377. doi: 10.1002/bip.20107. [DOI] [PubMed] [Google Scholar]
- 13.Fischer E. Z Physiol Chem. 1901;33:151–176. [Google Scholar]
- 14.Fischer E. Ber. 1902;35:2660–2665. [Google Scholar]
- 15.Ramshaw JAM, Shah NK, Brodsky B. J Struct Biol. 1998;122:86–91. doi: 10.1006/jsbi.1998.3977. [DOI] [PubMed] [Google Scholar]
- 16.Timpl R. In: Extracellular Matrix Biochemistry. Piez KA, Reddi AH, editors. Elsevier; New York: 1984. pp. 159–190. [Google Scholar]
- 17.Asara JM, Schweitzer MH, Freimark LM, Phillips M, Cantley LC. Science. 2007;316:280–285. doi: 10.1126/science.1137614. [DOI] [PubMed] [Google Scholar]
- 18.Schweitzer MH, Zheng W, Organ CL, Avci R, Suo Z, Freimark LM, Lebleu VS, Duncan MB, Vander Heiden MG, Neveu JM, Lane WS, Cottrell JS, Horner JR, Cantley LC, Kalluri R, Asara JM. Science. 2009;324:626–631. doi: 10.1126/science.1165069. [DOI] [PubMed] [Google Scholar]
- 19.Badylak SF. Transplant Immunol. 2004;12:367–377. doi: 10.1016/j.trim.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 20.Koide T. Phil Trans R Soc B: Biol Sci. 2007;362:1281–1291. doi: 10.1098/rstb.2007.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olsen D, Yang C, Bodo M, Chang R, Leigh S, Baez J, Carmichael D, Perälä M, Hämäläinen ER, Jarvinen M, Polarek J. Adv Drug Deliv Rev. 2003;55:1547–1567. doi: 10.1016/j.addr.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 22.Buechter DD, Paolella DN, Lelie BS, Brown MS, Mehos KA, Gruskin EA. J Biol Chem. 2003;278:645–650. doi: 10.1074/jbc.M209364200. [DOI] [PubMed] [Google Scholar]
- 23.Pinkas DM, Ding S, Raines RT, Barron AE. ACS Chem Biol. 2011;6:320–324. doi: 10.1021/cb100298r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orgel JP, San Antonio JD, Antipova O. Connect Tissue Res. 2011;52:2–17. doi: 10.3109/03008207.2010.511353. [DOI] [PubMed] [Google Scholar]
- 25.Smethurst PA, Onley DJ, Jarvis GE, O'Connor MN, Knight CG, Herr AB, Ouwehand WH, Farndale RW. J Biol Chem. 2007;282:1296–1304. doi: 10.1074/jbc.M606479200. [DOI] [PubMed] [Google Scholar]
- 26.Fiedler LR, Schönherr E, Waddington R, Niland S, Seidler DG, Aeschlimann D, Eble JA. J Biol Chem. 2008;283:17406–17415. doi: 10.1074/jbc.M710025200. [DOI] [PubMed] [Google Scholar]
- 27.Fields GB. J Theor Biol. 1991;153:585–602. doi: 10.1016/s0022-5193(05)80157-2. [DOI] [PubMed] [Google Scholar]
- 28.Aimes RT, Quigley JP. J Biol Chem. 1995;270:5872–5876. doi: 10.1074/jbc.270.11.5872. [DOI] [PubMed] [Google Scholar]
- 29.Ohuchi E, Imai K, Fujii Y, Sato H, Seiki M, Okada Y. J Biol Chem. 1997;272:2446–2451. doi: 10.1074/jbc.272.4.2446. [DOI] [PubMed] [Google Scholar]
- 30.Lauer-Fields JL, Juska D, Fields GB. Biopolymers. 2002;66:19–32. doi: 10.1002/bip.10201. [DOI] [PubMed] [Google Scholar]
- 31.Allan JA, Hembry RM, Angal S, Reynolds JJ, Murphy G. J Cell Sci. 1991;99:789–795. doi: 10.1242/jcs.99.4.789. [DOI] [PubMed] [Google Scholar]
- 32.Allan JA, Docherty AJ, Barker PJ, Huskisson NS, Reynolds JJ, Murphy G. Biochem J. 1995;309:299–306. doi: 10.1042/bj3090299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Postlethwaite AE, Seyer JM, Kang AH. Proc Natl Acad Sci USA. 1978;75:871–875. doi: 10.1073/pnas.75.2.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yannas I, Burke J, Orgill D, Skrabut E. Science. 1982;215:174–176. doi: 10.1126/science.7031899. [DOI] [PubMed] [Google Scholar]
- 35.Weadock KS, Miller EJ, Keuffel EL, Dunn MG. J Biomed Mat Res. 1996;32:221–226. doi: 10.1002/(SICI)1097-4636(199610)32:2<221::AID-JBM11>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 36.Ruderman RJ, Wade CWR, Shepard WD, Leonard F. J Biomat Med Res. 1973;7:263–265. doi: 10.1002/jbm.820070212. [DOI] [PubMed] [Google Scholar]
- 37.Harriger MD, Supp AP, Warden GD, Boyce ST. J Biomed Mat Res. 1997;35:137–145. doi: 10.1002/(sici)1097-4636(199705)35:2<137::aid-jbm1>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 38.Wu X, Black L, Santacana-Laffitte G, Patrick CW. J Biomat Mat Res. 2007;81A:59–65. doi: 10.1002/jbm.a.31003. [DOI] [PubMed] [Google Scholar]
- 39.Powell HM, Boyce ST. Biomaterials. 2006;27:5821–5827. doi: 10.1016/j.biomaterials.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 40.Powell HM, Boyce ST. Biomaterials. 2007;28:1084–1092. doi: 10.1016/j.biomaterials.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 41.Tu R, Lu CL, Thyagarajan K, Wang E, Nguyen H, Shen S, Hata C, Quijano RC. J Biomed Mat Res. 1993;27:3–9. doi: 10.1002/jbm.820270103. [DOI] [PubMed] [Google Scholar]
- 42.Petite H, Rault I, Huc A, Menasche P, Herbage D. J Biomed Mat Res. 1990;24:179–187. doi: 10.1002/jbm.820240205. [DOI] [PubMed] [Google Scholar]
- 43.Zeugolis DI, Paul GR, Attenburrow G. J Biomed Mat Res. 2009;89A:895–908. doi: 10.1002/jbm.a.32031. [DOI] [PubMed] [Google Scholar]
- 44.Speer DP, Chvapil M, Eskelson CD, Ulreich J. J Biomed Mat Res. 1980;14:753–764. doi: 10.1002/jbm.820140607. [DOI] [PubMed] [Google Scholar]
- 45.van Luyn MJA, van Wachem PB, Damink LHHO, Dijkstra PJ, Feijen J, Nieuwenhuis P. Biomaterials. 1992;13:1017–1024. doi: 10.1016/0142-9612(92)90153-f. [DOI] [PubMed] [Google Scholar]
- 46.Maa L, Gaoa C, Maoa Z, Zhoua J, Shena J, Hub X, Han C. Biomaterials. 2003;24:4833–4841. doi: 10.1016/s0142-9612(03)00374-0. [DOI] [PubMed] [Google Scholar]
- 47.Weadock KS, Miller EJ, Bellincampi LD, Zawadsky JP, Dunn MG. J Biomed Mat Res. 1995;29:1373–1379. doi: 10.1002/jbm.820291108. [DOI] [PubMed] [Google Scholar]
- 48.Gorham SD, Light ND, Diamond AM, Willins MJ, Bailey AJ, Wess TJ, Leslie NJ. Int J Biol Macromol. 1992;14:129–138. doi: 10.1016/s0141-8130(05)80002-9. [DOI] [PubMed] [Google Scholar]
- 49.Khew ST, Yang QJ, Tong YW. Biomaterials. 2008;29:3034–3045. doi: 10.1016/j.biomaterials.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 50.Gilbert TW, Sellaro TL, Badylak SF. Biomaterials. 2006;27:3675–3683. doi: 10.1016/j.biomaterials.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 51.Buijtenhuijs P, Buttafoco L, Poot AA, Daamen WF, van Kuppevelt TH, Dijkstra PJ, de Vos RAI, Sterk LM, Geelkerken BRH, Feijen J, Vermes I. Biotechnol Appl Biochem. 2004;39:141–149. doi: 10.1042/BA20030105. [DOI] [PubMed] [Google Scholar]
- 52.Ellis DL, Yannas IV. Biomaterials. 1996;17:291–299. doi: 10.1016/0142-9612(96)85567-0. [DOI] [PubMed] [Google Scholar]
- 53.Fielding AM. In: The Methodology of Connective Tissue Research. Hall DA, editor. Joynson–Bruvvers; Oxford, UK: 1976. pp. 9–12. [Google Scholar]
- 54.Piez KA. In: Extracellular Matrix Biochemistry. Piez KA, Reddi AH, editors. Elsevier; New York: 1984. pp. 1–40. [Google Scholar]
- 55.Piez KA. Encyclopedia of Polymer Science and Engineering. Wiley; New York, NY: 1985. pp. 699–727. [Google Scholar]
- 56.Yannas IV. Angew Chem Int Ed. 1990;29:20–35. [Google Scholar]
- 57.Geesin JC, Brown LJ, Liu Z, Berg RA. J Biomed Mat Res. 1996;33:1–8. doi: 10.1002/(SICI)1097-4636(199621)33:1<1::AID-JBM1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 58.Doillon CJ, Silver FH. Biomaterials. 1986;7:3–8. doi: 10.1016/0142-9612(86)90080-3. [DOI] [PubMed] [Google Scholar]
- 59.Lefebvre F, Gorecki S, Bareille R, Amedee J, Bordenave L, Rabaud M. Biomaterials. 1992;13:28–33. doi: 10.1016/0142-9612(92)90091-2. [DOI] [PubMed] [Google Scholar]
- 60.Carlson MA, Longaker MT. Wound Rep Regen. 2004;12:134–147. doi: 10.1111/j.1067-1927.2004.012208.x. [DOI] [PubMed] [Google Scholar]
- 61.Chvapil M, Chvapil TA, Owen JA. J Surg Res. 1986;41:410–418. doi: 10.1016/0022-4804(86)90055-7. [DOI] [PubMed] [Google Scholar]
- 62.Boyce ST, Christianson DJ, Hansbrough JF. J Biomed Mat Res. 1988;22:939–957. doi: 10.1002/jbm.820221008. [DOI] [PubMed] [Google Scholar]
- 63.Leipziger LS, Glushko V, DiBernardo B, Shafaie F, Noble J, Nichols J, Alvarez OM. J Am Acad Dermatol. 1985;12:409–419. doi: 10.1016/s0190-9622(85)80004-9. [DOI] [PubMed] [Google Scholar]
- 64.McPherson JM, Sawamura S, Armstrong R. J Biomed Mat Res. 1986;20:93–107. doi: 10.1002/jbm.820200109. [DOI] [PubMed] [Google Scholar]
- 65.Boyce ST, Stompro BE, Hansbrough JF. J Biomed Mat Res. 1992;26:547–553. doi: 10.1002/jbm.820260410. [DOI] [PubMed] [Google Scholar]
- 66.Stompro BE, Hansbrough JF, Boyce ST. J Surg Res. 1989;46:413–421. doi: 10.1016/0022-4804(89)90153-4. [DOI] [PubMed] [Google Scholar]
- 67.Marks MG, Doillon C, Silvert FH. J Biomed Mat Res. 1991;25:683–696. doi: 10.1002/jbm.820250510. [DOI] [PubMed] [Google Scholar]
- 68.Lepistö J, Kujari H, Niinikoski J, Laato M. Eur Surg Res. 1994;26:267–272. doi: 10.1159/000129345. [DOI] [PubMed] [Google Scholar]
- 69.Wachol-Drewek Z, Pfeiffer M, Scholl E. Biomaterials. 1996;17:1733–1738. doi: 10.1016/0142-9612(96)87654-x. [DOI] [PubMed] [Google Scholar]
- 70.Vaneerdeweg W, Bresseleers T, Du Jardin P, Lauwers P, Pauli S, Thyssens K, Van Marck E, Elseviers M, Eyskens E. Eur J Surg. 1998;164:617–621. doi: 10.1080/110241598750005723. [DOI] [PubMed] [Google Scholar]
- 71.Stemberger A, Grimm H, Bader F, Rahn HD, Ascherl R. Eur J Surg. 1997;578:17–26. [PubMed] [Google Scholar]
- 72.Fujisato T, Sajiki T, Liu Q, Ikada Y. Biomaterials. 1996;17:155–162. doi: 10.1016/0142-9612(96)85760-7. [DOI] [PubMed] [Google Scholar]
- 73.Toolan BC, Frenkel SR, Pachence JM, Yalowitz L, Alexander H. J Biomed Mat Res. 1996;31:273–280. doi: 10.1002/(SICI)1097-4636(199606)31:2<273::AID-JBM15>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 74.van Wachem PB, van Luyn MJA, Ponte da Costa ML. J Biomed Mat Res. 1996;30:353–360. doi: 10.1002/(SICI)1097-4636(199603)30:3<353::AID-JBM9>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 75.Paíno CL, Fernandez-Valle C, Bates ML, Bunge MB. J Neurocytol. 1994;23:433–452. doi: 10.1007/BF01207115. [DOI] [PubMed] [Google Scholar]
- 76.Sung KE, Su G, Pehlke C, Trier SM, Eliceiri KW, Keely PJ, Friedl A, Beebe D. J Biomaterials. 2009;30:4833–4841. doi: 10.1016/j.biomaterials.2009.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cross VL, Zheng Y, Won Choi N, Verbridge SS, Sutermaster BA, Bonassar LJ, Fischbach C, Stroock AD. Biomaterials. 2010;31:8596–8607. doi: 10.1016/j.biomaterials.2010.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Altman AM, Matthias N, Yan Y, Song YH, Bai X, Chiu ES, Slakey DP, Alt EU. Biomaterials. 2008;29:1431–1442. doi: 10.1016/j.biomaterials.2007.11.026. [DOI] [PubMed] [Google Scholar]
- 79.Trottier V, Marceau-Fortier G, Germain L, Vincent C, Fradette J. Stem Cells. 2008;26:2713–2723. doi: 10.1634/stemcells.2008-0031. [DOI] [PubMed] [Google Scholar]
- 80.Koide M, Osaki K, Konishi J, Oyamada K, Katakura T, Takahashi A, Yoshizato K. J Biomed Mat Res. 1993;27:79–87. doi: 10.1002/jbm.820270111. [DOI] [PubMed] [Google Scholar]
- 81.Ponticiello MS, Schinagl RM, Kadiyala S, Barry FP. J Biomed Mat Res. 2000;52:246–255. doi: 10.1002/1097-4636(200011)52:2<246::aid-jbm2>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 82.Tanihara M, Kajiwara K, Ida K, Suzuki Y, Kamitakahara M, Ogata SI. J Biomed Mater Res A. 2007;85:133–139. doi: 10.1002/jbm.a.31496. [DOI] [PubMed] [Google Scholar]
- 83.Shibasaki Y, Hirohara S, Terada K, Ando T, Tanihara M. Biopolymers (Pept Sci) 2011;96:302–315. doi: 10.1002/bip.21551. [DOI] [PubMed] [Google Scholar]
- 84.McPherson JM, Ledger PW, Sawamura S, Conti A, Wade S, Reihanian H, Wallace DG. J Biomed Mat Res. 1986;20:79–92. doi: 10.1002/jbm.820200108. [DOI] [PubMed] [Google Scholar]
- 85.Wallace DG, Rhee W, Reihanian H, Ksander G, Lee R, Braun WB, Weiss BA, Pharriss BB. J Biomed Mat Res. 1989;23:931–945. doi: 10.1002/jbm.820230809. [DOI] [PubMed] [Google Scholar]
- 86.Wallace DG, Reihanian H, Pharriss BB, Braun WG. US Pat 4803075. 1989
- 87.Horáková Z, Krajícek M, Chvapil M, Boissier J. Therapie. 1967;22:1455–1460. [PubMed] [Google Scholar]
- 88.Rosenblatt J, Rhee W, Wallace D. J Control Release. 1989;9:195–203. [Google Scholar]
- 89.Slavin J, Nash JR, Kingsnorth AN. Br J Surg. 1992;79:69–72. doi: 10.1002/bjs.1800790124. [DOI] [PubMed] [Google Scholar]
- 90.Saltzman WM, Parkhurst MR, Parsons-Wingerter P, Zhu WH. Ann NY Acad Sci. 1992;665:259–273. doi: 10.1111/j.1749-6632.1992.tb42590.x. [DOI] [PubMed] [Google Scholar]
- 91.Parkhurst MR, Saltzman WM. Biophys J. 1992;61:306–315. doi: 10.1016/S0006-3495(92)81838-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Parkhurst MR, Saltzman WM. Cell Immunol. 1994;156:77–94. doi: 10.1006/cimm.1994.1154. [DOI] [PubMed] [Google Scholar]
- 93.Marchand R, Woerly S, Bertrand L, Valdes N. Brain Res Bull. 1993;30:415–422. doi: 10.1016/0361-9230(93)90273-e. [DOI] [PubMed] [Google Scholar]
- 94.Docherty R, Forrester JV, Lackie JM, Gregory DW. J Cell Sci. 1989;92:263–270. doi: 10.1242/jcs.92.2.263. [DOI] [PubMed] [Google Scholar]
- 95.Joosten EAJ, Bär PR, Gispen WH. J Neurosci Res. 1995;41:481–490. doi: 10.1002/jnr.490410407. [DOI] [PubMed] [Google Scholar]
- 96.Cascone MG, Sim B, Sandra D. Biomaterials. 1995;16:569–574. doi: 10.1016/0142-9612(95)91131-h. [DOI] [PubMed] [Google Scholar]
- 97.Uchio Y, Ochi M, Matsusaki M, Kurioka H, Katsube K. J Biomed Mat Res. 2000;50:138–143. doi: 10.1002/(sici)1097-4636(200005)50:2<138::aid-jbm7>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 98.Weiner AL, Carpenter-Green SS, Soehngen EC, Lenk RP, Popescu MC. J Pharm Sci. 1985;74:922–925. doi: 10.1002/jps.2600740903. [DOI] [PubMed] [Google Scholar]
- 99.Pajean M, Herbage D. Int J Pharm. 1993;91:209–216. [Google Scholar]
- 100.Yamahira Y, Fujioka K, Sato S, Yoshida N. Eur Pat EP0140255. 1985
- 101.Rubin AL, Stenzel KH, Miyata T, White MJ, Dunn M. J Clin Pharmacol. 1973;13:309–312. doi: 10.1002/j.1552-4604.1973.tb00217.x. [DOI] [PubMed] [Google Scholar]
- 102.Maeda M, Kadota K, Kajihara M, Sano A, Fujioka K. J Control Release. 2001;77:261–272. doi: 10.1016/s0168-3659(01)00512-0. [DOI] [PubMed] [Google Scholar]
- 103.Song SZ, Morawiecki A, Pierce GF, Pitt CG. US Pat 5418222. 1995
- 104.Rosenthal FM, Kohler G. Anticancer Res. 1997;17:1179–1186. [PubMed] [Google Scholar]
- 105.Park JC, Hwang YS, Lee JE, Park KD, Matsumura K, Hyon SH, Suh H. J Biomed Mat Res. 2000;52:669–677. doi: 10.1002/1097-4636(20001215)52:4<669::aid-jbm11>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 106.Yang C, Hillas PJ, Báez JA, Nokelainen M, Balan J, Tang J, Spiro R, Polarek JW. BioDrugs. 2004;18:103–119. doi: 10.2165/00063030-200418020-00004. [DOI] [PubMed] [Google Scholar]
- 107.Van der Laan JS, Lopez GP, van Wachem PB, Nieuwenhuis P, Ratner BD, Bleichrodt RP, Schakenraad JM. Int J Artif Organs. 1991;14:661–666. [PubMed] [Google Scholar]
- 108.Sekine T, Nakamura T, Shimizu Y, Ueda H, Matsumoto K, Takimoto Y, Kiyotani T. J Biomed Mater Res. 2001;54:305–310. doi: 10.1002/1097-4636(200102)54:2<305::aid-jbm18>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 109.Peters WJ. Ann Plast Surg. 1980;4:133–137. doi: 10.1097/00000637-198002000-00010. [DOI] [PubMed] [Google Scholar]
- 110.Whitaker K, Barrow P, Feree K. Ostomy Wound Manage. 1992;38:36–44. [PubMed] [Google Scholar]
- 111.Zitelli JA. Adv Dermatol. 1987;2:243. [PubMed] [Google Scholar]
- 112.Smith DJ., Jr J Burn Care Res. 1995;16:317–320. doi: 10.1097/00004630-199505000-00018. [DOI] [PubMed] [Google Scholar]
- 113.Lal S, Barrow RE, Wolf SE, Chinkes DL, Hart DW, Heggers JP, Herndon DN. Shock. 2000;14:314–319. doi: 10.1097/00024382-200014030-00013. [DOI] [PubMed] [Google Scholar]
- 114.Rao KP. J Biomater Sci. 1996;7:623–645. doi: 10.1163/156856295x00526. [DOI] [PubMed] [Google Scholar]
- 115.Boyce S. Med Biol Eng Comput. 1998;36:791–800. doi: 10.1007/BF02518886. [DOI] [PubMed] [Google Scholar]
- 116.Yannas IV, Lee E, Orgill DP, Skrabut EM, Murphy GF. Proc Natl Acad Sci USA. 1989;86:933–937. doi: 10.1073/pnas.86.3.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Orwin EJ, Hubel A. Tissue Eng. 2000;6:304–319. doi: 10.1089/107632700418038. [DOI] [PubMed] [Google Scholar]
- 118.Griffith M, Jackson WB, Lagali N, Merrett K, Li F, Fagerholm P. Eye. 2009;23:1985–1989. doi: 10.1038/eye.2008.409. [DOI] [PubMed] [Google Scholar]
- 119.Carrier P, Deschambeault A, Talbot M, Giasson CJ, Auger FA, Guérin SL, Germain L. Invest Ophthalmol Vis Sci. 2008;49:1376–1385. doi: 10.1167/iovs.07-0904. [DOI] [PubMed] [Google Scholar]
- 120.Boyce ST, Supp AP, Warden GD, Holder IA. Antimicrob Agents Chemother. 1993;37:1890–1895. doi: 10.1128/aac.37.9.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Régnier M, Staquet MJ, Schmitt D, Schimdt R. J Invest Dermatol. 1997;109:510–512. doi: 10.1111/1523-1747.ep12336627. [DOI] [PubMed] [Google Scholar]
- 122.Bechetoille N, Dezutter-Dambuyant C, Damour O, André V, Orly I, Perrier E. Tissue Eng. 2007;13:2667–2679. doi: 10.1089/ten.2006.0405. [DOI] [PubMed] [Google Scholar]
- 123.Supp AP, Wickett RR, Swope VB, Harriger MD, Hoath SB, Boyce ST. Wound Rep Regen. 1999;7:226–237. doi: 10.1046/j.1524-475x.1999.00226.x. [DOI] [PubMed] [Google Scholar]
- 124.Boyce ST, Goretsky MJ, Greenhalgh DG, Kagan RJ, Rieman MT, Warden GD. Ann Surg. 1995;222:743–752. doi: 10.1097/00000658-199512000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lam PK, Chan ESY, Liew CT, Lau CH, Yen SC, King WWK. Ann Plast Surg. 1999;43:523–528. doi: 10.1097/00000637-199911000-00010. [DOI] [PubMed] [Google Scholar]
- 126.Suzuki S, Kawai K, Ashoori F, Morimoto N, Nishimura Y, Ikada Y. Br J Plast Surg. 2000;53:659–666. doi: 10.1054/bjps.2000.3426. [DOI] [PubMed] [Google Scholar]
- 127.Koide T. Philos Trans R Soc, B. 2007;362:1281–1291. doi: 10.1098/rstb.2007.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Woolfson DN. Biopolymers. 2010;94:118–127. doi: 10.1002/bip.21345. [DOI] [PubMed] [Google Scholar]
- 129.Fields GB. Org Biomol Chem. 2010;8:1237–1258. doi: 10.1039/b920670a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fallas JA, O'Leary LE, Hartgerink JD. Chem Soc Rev. 2010;39:3510–3527. doi: 10.1039/b919455j. [DOI] [PubMed] [Google Scholar]
- 131.Przybyla DE, Chmielewski J. Biochemistry. 2010;49:4411–4419. doi: 10.1021/bi902129p. [DOI] [PubMed] [Google Scholar]
- 132.Siebler C, Erdmann RS, Wennemers H. Chimia. 2013;67:891–895. doi: 10.2533/chimia.2013.891. [DOI] [PubMed] [Google Scholar]
- 133.He L, Theato P. Eur Polym J. 2013;49:2986–2997. [Google Scholar]
- 134.Hartgerink JD, Beniash E, Stupp SI. Science. 2001;294:1684–1688. doi: 10.1126/science.1063187. [DOI] [PubMed] [Google Scholar]
- 135.Hartgerink JD, Beniash E, Stupp SI. Proc Natl Acad Sci USA. 2002;99:5133–5138. doi: 10.1073/pnas.072699999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhang S. Nat Biotechnol. 2003;21:1171–1178. doi: 10.1038/nbt874. [DOI] [PubMed] [Google Scholar]
- 137.Marini DM, Hwang W, Lauffenburger DA, Zhang S, Kamm RD. Nano Lett. 2002;2:295–299. [Google Scholar]
- 138.Kisiday J, Jin M, Kurz B, Hung H, Semino C, Zhang S, Grodzinsky AJ. Proc Natl Acad Sci USA. 2002;99:9996–10001. doi: 10.1073/pnas.142309999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Paramonov SE, Gauba V, Hartgerink JD. Macromolecules. 2005;38:7555–7561. [Google Scholar]
- 140.O'Leary LE, Fallas JA, Bakota EL, Kang MK, Hartgerink JD. Nat Chem. 2011;3:821–828. doi: 10.1038/nchem.1123. [DOI] [PubMed] [Google Scholar]
- 141.Fallas JA, Hartgerink JD. Nat Commun. 2011;3:1087. doi: 10.1038/ncomms2084. [DOI] [PubMed] [Google Scholar]
- 142.Rele S, Song Y, Apkarian RP, Qu Z, Conticello VP, Chaikof EL. J Am Chem Soc. 2007;129:14780–14787. doi: 10.1021/ja0758990. [DOI] [PubMed] [Google Scholar]
- 143.Cejas MA, Kinney WA, Chen C, Vinter JG, Almond J, H R, Balss KM, Maryanoff CA, Schmidt U, Breslav M, Mahan A, Lacy E, Maryanoff BE. Proc Natl Acad Sci USA. 2008;105:8513–8518. doi: 10.1073/pnas.0800291105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Koide T, Homma DL, Asada S, Kitagawa K. Bioorg Med Chem Lett. 2005;15:5230–5233. doi: 10.1016/j.bmcl.2005.08.041. [DOI] [PubMed] [Google Scholar]
- 145.Kotch FW, Raines RT. Proc Natl Acad Sci USA. 2006;103:3028–3033. doi: 10.1073/pnas.0508783103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Johnson G, Jenkins M, McLean KM, Griesser HJ, Kwak J, Goodman M, Steele JG. J Biomed Mat Res. 2000;51:612–624. doi: 10.1002/1097-4636(20000915)51:4<612::aid-jbm9>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 147.Cejas MA, Chen C, Kinney WA, Maryanoff BE. Bioconjugate Chem. 2007;18:1025–1027. doi: 10.1021/bc070105s. [DOI] [PubMed] [Google Scholar]
- 148.Laskin DL, Soltys RA, Berg RA, Riley DJ. Am J Respir Cell Mol Biol. 1990;2:463–470. doi: 10.1165/ajrcmb/2.5.463. [DOI] [PubMed] [Google Scholar]
- 149.Laskin DL, Soltys RA, Berg RA, Riley DJ. Am J Respir Cell Mol Biol. 1994;10:58–64. doi: 10.1165/ajrcmb.10.1.8292381. [DOI] [PubMed] [Google Scholar]
- 150.Inoue O, Suzuki-Inoue K, Shinoda D, Umeda Y, Uchino M, Takasaki Si, Ozaki Y. FEBS Lett. 2009;583:81–87. doi: 10.1016/j.febslet.2008.11.026. [DOI] [PubMed] [Google Scholar]
- 151.Hennessy KM, Pollot BE, Clem WC, Phipps MC, Sawyer AA, Culpepper BK, Bellis SL. Biomaterials. 2009;30:1898–1909. doi: 10.1016/j.biomaterials.2008.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang AY, Mo X, Chen CS, Yu SM. J Am Chem Soc. 2005;127:4130–4131. doi: 10.1021/ja0431915. [DOI] [PubMed] [Google Scholar]
- 153.Wang AY, Foss CA, Leong S, Mo X, Pomper MG, Yu SM. Biomacromolecules. 2008;9:1755–1763. doi: 10.1021/bm701378k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mo X, An Y, Yun CS, Yu SM. Angew Chem Int Ed. 2006;45:2267–2270. doi: 10.1002/anie.200504529. [DOI] [PubMed] [Google Scholar]
- 155.Li Y, Foss CA, Summerfield DD, Doyle JJ, Torok CM, Dietz HC, Pomper MG, Yu SM. Proc Natl Acad Sci USA. 2012;109:14767–14772. doi: 10.1073/pnas.1209721109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Holmgren SK, Taylor KM, Bretscher LE, Raines RT. Nature. 1998;392:666–667. doi: 10.1038/33573. [DOI] [PubMed] [Google Scholar]
- 157.Hodges JA, Raines RT. J Am Chem Soc. 2003;125:9262–9263. doi: 10.1021/ja035881z. [DOI] [PubMed] [Google Scholar]
- 158.Malkar NB, Lauer-Fields JL, Borgia JA, Fields GB. Biochemistry. 2002;41:6054–6064. doi: 10.1021/bi012071w. [DOI] [PubMed] [Google Scholar]
- 159.Eberhardt ES, Panasik N, Jr, Raines RT. J Am Chem Soc. 1996;118:12261–12266. doi: 10.1021/ja9623119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bretscher LE, Jenkins CL, Taylor KM, DeRider ML, Raines RT. J Am Chem Soc. 2001;123:777–778. doi: 10.1021/ja005542v. [DOI] [PubMed] [Google Scholar]
- 161.Shoulders MD, Satyshur KA, Forest KT, Raines RT. Proc Natl Acad Sci USA. 2010;106:559–564. doi: 10.1073/pnas.0909592107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hodges JA, Raines RT. J Am Chem Soc. 2005;127:15923–15932. doi: 10.1021/ja054674r. [DOI] [PubMed] [Google Scholar]
- 163.Chattopadhyay S, Murphy CJ, McAnulty JF, Raines RT. Org Biomol Chem. 2012;10:5892–5897. doi: 10.1039/c2ob25190f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chattopadhyay S, Guthrie KM, Teixeira L, Murphy CJ, Dubielzig RR, McAnulty JF, Raines RT. J Tissue Eng Regen Med. 2014;8 doi: 10.1002/term.1886. [DOI] [PMC free article] [PubMed] [Google Scholar]


