Abstract
Antipsychotic drugs regulate gene transcription in striatal neurons by blocking dopamine D2-like receptors. Little is known about the underlying changes in chromatin structure, including covalent modifications at histone N-terminal tails that are epigenetic regulators of gene expression. We show that treatment with D2-like antagonists rapidly induces the phosphorylation of histone H3 at serine 10 and the acetylation of H3-lysine 14 in bulk chromatin from striatum and in nuclei of striatal neurons. We find that, in vivo, D2-like antagonist-induced H3 phospho-acetylation is inhibited by the NMDA receptor antagonist MK-801 and by the protein kinase A (PKA) inhibitor Rp-adenosine 3c’,5c’-cyclic monophosphorothioate triethylammonium salt but increased by the PKA activator Sp-adenosine 3c’,5c’-cyclic monophosphorothioate triethylammonium salt. Furthermore, in dissociated striatal cultures which lack midbrain and cortical pre-synaptic inputs, H3 phospho-acetylation was induced by glutamate, l-type Ca2+ channel agonists and activators of cAMP-dependent PKA but inhibited by NMDA receptor antagonists or PKA antagonists. The dual modification, H3pS10-acK14, was enriched at genomic sites with active transcription and showed the kinetics of the early response. Together, these results suggest that histone modifications and chromatin structure in striatal neurons are dynamically regulated by dopaminergic and glutamatergic inputs converging on the cellular level. Blockade of D2-like receptors induces H3 phospho-acetylation, H3pS10-acK14, through cAMP-dependent PKA, and post-synaptic NMDA receptor signaling.
Keywords: basal ganglia, caudate-putamen, histone acetyltransferase, nucleosome, psychosis, schizophrenia
Striatal dopamine receptors are an important target of antipsychotic drugs that directly interact with dopamine D2-like receptors. The molecular cascades linking blockade of D2-like receptors to reduction of psychosis are unknown but transcriptional mechanisms appear to be involved (Robertson et al. 1992; Gunther et al. 2003). Upon inhibition of D2-like signaling, striatal and pallidal gene expression is altered in an orchestrated and sequential fashion, starting with the transient expression of early response genes (Dragunow et al. 1990; Robertson et al. 1991; Nguyen et al. 1992; Deutch et al. 1996) followed by increased transcription of neuropeptides, second and third messenger molecules, receptors, ion channels and other neurotransmission-related molecules (Merchant and Dorsa 1993; Fox et al. 1994; Delfs et al. 1995; Fitzgerald et al. 1995; Laprade and Soghomonian 1995; Schoots et al. 1995; Doucet et al. 1996; Moratalla et al. 1996; Eastwood et al. 1997; Healy and Meador-Woodruff 1997; Mijnster et al. 1998; Atkins et al. 1999; Nakahara et al. 2001; Chong et al. 2002; Lau et al. 2003; Lipska et al. 2003; McCullumsmith et al. 2003).
The magnitude of antipsychotic drug-induced gene expression is likely to require profound molecular adaptations in nuclei of striatal neurons. In eukaryotes, the rate-limiting biochemical response that leads to activation of gene expression involves alterations in chromatin structure (Felsenfeld and Groudine 2003). Dynamic changes in chromatin structure and accessibility of transcription factors are mediated by the chemical modification of residues located at the amino-terminal tails of the histones. Specifically, a set of covalent modifications of specific arginine, lysine and serine residues at the histone N-terminal tails defines a ‘histone code’ that is differentially regulated in chromatin at sites of active gene expression, in comparison to inactive and silenced chromatin (Jenuwein and Allis 2001; Turner 2002).
At present, nothing is known about the regulation of striatal chromatin. To examine chromatin and histone–DNA interactions in striatum in the context of pharmacological manipulation of D2-like receptors, we monitored dynamic changes in acetylation, methylation and phosphorylation of specific tail residues of two core histones, H3 and H4, and used chromatin-immunoprecipitation assays (Kuo and Allis 1999) to study three aspects of antipsychotic drug-induced chromatin changes: (i) the type(s) of covalent histone modifications regulated by D2-like signaling, including the underlying kinetics; (ii) the genomic sites targeted by histone-modifying enzymes and (iii) the signal transduction pathways that link blockade of D2-like receptors to the histone modification machinery in the nucleus.
We report here for the first time that, in vivo, treatment with D2-like receptor antagonists and antipsychotics induces the phospho-acetylation of histone H3 in striatal chromatin both on a global level and at defined genomic sequences. Moreover, we show that both dopaminergic and glutamatergic input converge at a cellular level to regulate chromatin structure in striatal neurons and that antipsychotic drug-induced H3 phospho-acetylation is mediated through cAMP-protein kinase A (PKA) and NMDA receptor pathways.
Materials and methods
In vivo experiments
All animal experiments were approved by the University of Massachusetts animal care committee.
Mice
For each experiment, 5–10-week-old male and female outbred mice (predominantly 129/SvJ) were used. Mice received i.p. injections of vehicle (saline) (0.1 mL/10 g body weight) with or without: (i) the D2-like agonist quinpirole (2 mg/kg); (ii) the D2-like antagonist S(–)-raclopride tartrate (10 mg/kg) (drugs purchased from Sigma-RBI, St Louis, MO, USA); (iii) the D2-like antagonist and antipsychotic haloperidol lactate (1 mg/kg) (Ortho-McNeil, Fort Washington, PA, USA); (iv) the atypical antipsychotic, risperidone hydrochloride (Janssen Pharmaceuticals, Beerse Belgium) or (v) the NMDA receptor antagonist, MK-801 (Sigma-RBI) (1 mg/kg). Each drug-treated animal was processed together with a saline-treated littermate control of the same gender. For each group, the male : female ratio was 1 : 1 and the minimum n was 6.
Survival times
Mice treated with quinpirole, raclopride or risperi-done were killed 120 min after a single injection and haloperidol-treated mice were killed at various timepoints (15, 30, 120 and 480 min) after a single injection or 120 min after a 1-, 3-, 5- or 10-day course of twice daily injections at 08:00 and 16:00 h. Animals that were treated for 5 days with haloperidol received the last injection at a higher dose (4 mg/kg), otherwise the haloperidol dose for each treatment was 1 mg/kg. Mice that received a single dose of MK-801 were treated 15 min after the MK-801 dose with haloperidol or saline and were killed 30 min after the second treatment. Mice were either decapitated and the striatum dissected or, under deep anesthesia, perfusion-fixed with 4% phosphate-buffered paraformaldehyde.
Rats
Adult Sprague-Dawley rats (350–450 g) were used. Animals were anesthesized by 4% isoflurane inhalation, placed into a stereotaxic frame and then one of the following drugs was infused over a period of 5 min in a total volume of 1 lL into the center of the left striatum: (i) phosphate-buffered saline (0.1 mol/L, pH 6.9) as vehicle or the cAMP analogs (ii) Rp-adenosine 3c’,5c’-cyclic monophosphorothioate triethylammonium salt (100 nmol) or (iii) Sp-adenosine 3c’,5c’-cyclic monophosphorothioate triethylammonium salt (100 nmol) (both drugs from Sigma-RBI). Animals were allowed to recover and 30 min after the intrastriatal injection animals received a systemic dose of either haloperidol (1.5 mg/kg) or saline. At 30 min after haloperidol or saline, animals were then killed and the striata were dissected and further processed for immunoblotting as described below. Additional series of animals were treated with a systemic dose of MK-801 (1 mg/kg) or saline followed after 15 min by a systemic dose of haloperidol (1.5 mg/kg) or saline and animals were killed 30 min after the second treatment. For each treatment group, the minimum n was 5.
In vitro experiments
Primary striatal cultures were prepared as described previously, with minor modifications (Konradi et al. 1996; Rajadhyaksha et al. 1998). Striata from 18-day-old Sprague Dawley rat fetuses were dissected and resuspended in defined medium [50% F12/Dulbecco's modified Eagle's medium and 50% Dulbecco's modified Eagle's medium (Gibco-Invitrogen, Grand Island, NY, USA) with the following supplements/L of medium: 4 g of dextrose, 1 × B27, 10 mL of penicillin-streptomycin liquid (Gibco-Invitrogen) and 25 mm HEPES]. Cells were resuspended in defined medium to 1.2 × 106 cells/mL and plated in 12-well plates (Costar, Cambridge, MA, USA) at 2 × 106 cells/well. Plates were pre-treated with 1 mL of a 1 : 500-diluted sterile solution of polyethylenimine in water for 18 h, washed twice with sterile water, coated with 2.5% serum-containing phosphate-buffered saline solution for at least 4 h and aspirated just before plating. All experiments were performed in triplicate with cells that were in culture for 6 days and repeated at least once in an independent dissection. As determined by HPLC analysis, glutamate levels in the medium on the day of the experiments ranged from 1 to 5 μm. The neuron : astroglia ratio was below 25 : 1, as established by immunocytochemical staining with the glial fibrillary acid protein (Dako, Carpinteria, CA, USA) and counterstaining with 1% cresyl violet.
All drugs used for the In vitro experiments were purchased from Sigma. On the day of the experiment, cells were pre-treated with 2 μm tetrodotoxin citrate for a period of 120 min before harvest to suppress transneuronal activity. At 60 min before harvest cells were treated with one of the following drugs (Treatment 1): (1.1) vehicle (10 μL dimethylsulfoxide); (1.2) the PKA inhibitor N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide dihydrochlo-ride (H89; 20 μm) or (1.3) the NMDA receptor antagonist [(+)MK-801 hydrogen maleate; MK-801; 2 μm]. At 30 min before harvest, cells were treated with one of the following drugs (Treatment 2): (2.1) vehicle (10 μL dimethylsulfoxide); (2.2) glutamate (50 μm); (2.3) the adenylyl cyclase-activating drug forskolin (1 and 10 μm) or (2.4) the l-type Ca2+ channel agonist, 2,5-dimethyl-4-[2-(phenylmethyl)benzoyl]-1H-pyrrole-3-carboxylic acid methylester (FPL 64176; 20 lm).
Anti-histone antibodies
To profile the pattern of histone acetylation, methylation and phosphorylation, we used a panel of nine antibodies selectively recognizing modifications at specific amino acids of the N-terminal tail of histones H3 and H4. The following antibodies (Upstate, Charlottesville, VA, USA) were used (with the working dilution for immunoblotting experiments in parentheses): anti-acetyl-histone H3 (Lys14), H3acK14 (1 : 250); anti-acetyl-histone H3 (Lys 9 and 14), H3acK9/14 (1 : 2000); anti-acetyl-histone H4 (Lys 8), H4acK8 (1 : 5000); anti-acetyl-histone H4 (Lys 12), H4acK12 (1 : 2000); anti-dimethyl-histone H3 (Lys4), H3meK4 (1 : 1000); anti-dimethyl-histone H3 (Lys9), H3meK9 (1 : 500); anti-dimethyl-histone H3 (Arg17), H3meR17 (1 : 250); anti-phospho-histone H3 (Ser 10), H3pS10 (1 : 1000); an antibody that recognizes phospho-acetylated H3, defined by phospho (Ser10) in conjunction with acetyl (Lys14), H3pS10-acK14 (1 : 500).
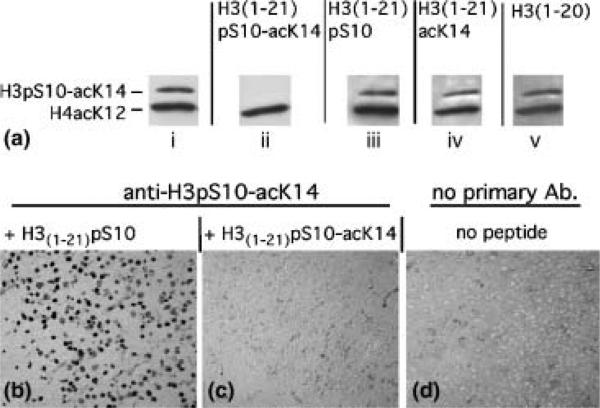
To control for the specificity of the antibody recognizing the dual modification, H3pS10-acK14, blots were processed in the presence of 1.5 μg/mL of a synthetic peptide containing the sequence of 21 residues at the N-terminal tail of H3 and the epitope defined by the dual modification, phospho-serine 10 plus acetyl-lysine 14. When this peptide was added to the primary antibody incubation medium, immunoreactivity was completely abolished (Fig. 1a). In contrast, the addition of synthetic peptides containing the same sequence and epitopes defined by the single modifications, phospho-serine 10 or acetyl-lysine 14, did not inhibit H3pS10-acK14 immunoreactivity (Fig. 1a). The specificity of the anti-H3pS10-acK14 antibody was maintained in paraformaldehyde-fixed tissue processed for immunohistochemistry (Figs 1b–d). In addition to the experiments shown in Fig. 1, blocking experiments with synthetic peptides were conducted to confirm the specificity of the following antibodies: anti-H3pS10, anti-H3acK14, anti-H3acK9/14, anti-H3meK4, anti-H3meK9, anti-H4acK8 and anti-H4acK12 (data not shown).
Fig. 1.
Specificity of anti-histone antibodies. (a) Film autoradiograms from immunoblots on mouse cerebral cortex probed with anti-H3pS10-acK14 antibody, and then with anti-H4acK12 antibody as loading control. Membranes (i-v) were incubated with primary antibody in presence or absence of synthetic peptides (1.5 μg/ml) containing the first 21 residues of histone H3 and a site-specific modification: (i) no peptide; (ii) H3(1-21)pS10-acK14; (iii) H3(1-21) pS10; (iv) H3(1-21)acK14; (v) H3(1-20) without a covalent modification. Notice the selective loss of H3pS10-acK14 immunoreactivity in (ii), due to addition of H3(1-21)pS10-acK14 peptide to primary antibody incubation solution. Antibody working dilutions: anti-H3pS10-acK14, 1:500; anti-H4acK12, 1:4000. (b-d) Digitized images from DAB/immunoperoxidase-stained sections from adult piriform cortex were incubated with (b,c) or without (d) anti-H3pS10-acK14 antibody. Peptides containing a single modification (H3(1-21)pS10) did not block immunoreactivity when added to the primary antibody solution (b), in contrast to competitor peptides containing the dual modification, H3(1-21)pS10-acK14 (c). Notice selective complete loss of immunoreactivity in (c) due to competitor antigen, similar to negative control (d) that was processed without primary antibody. Images in (b–d) taken at 20x10 magnification.
Immunoblots
Striata were dounced in 0.2 n H2SO4 to extract basic proteins including histones. Acid-soluble proteins were precipitated with trichloroacetic acid (final concentration 33%), washed in 100% acetone/0.05 m HCl and 100% acetone and resuspended in H2O. Samples were then eluted in 1 × Laemmli buffer and 20 μg/ sample were run on 10–20% polyacrylamide gradient Tris.HCl gels and immunoblotted on polyvinylidene difluoride membranes (Bio-Rad Laboratories, Hercules, CA, USA). Tissue culture samples were directly dissolved in 1 × Laemmli buffer without prior acid extraction. The membranes were incubated for 4–8 h with site-specific anti-histone antibodies, washed and further processed with horseradish peroxidase-conjugated secondary antibodies to reveal immunocomplexes by enhanced chemiluminescence (Pierce, Rockford, IL, USA). Quantity One software (Bio-Rad Laboratories) was used for densitometry of film autoradiograms, including custom-made standards. Furthermore, membranes were processed for H3 and H4 immunoreactivity allowing direct comparison of H3- and H4-immunoreactive bands (Fig. 1a). Equal loading of the samples was also checked by gel Coomassie blue stain revealing the characteristic histone banding patterns (Wan et al. 2001).
Immunohistochemistry
Coronal sections (18 μm), cut from blocks containing striatum and adjacent cerebral cortex, were processed free-floating for immunoperoxidase-based staining and immunofluorescence using standard protocols (Akbarian et al. 2002), the anti-H3pS10-acK14 antibody (1 : 250) and an anti-NeuN antibody (1 : 300; Chemicon, Temecula, CA, USA) to label neurons. Sections were examined with an Axiovert microscope (Carl Zeiss MicroImaging, Inc. Thornwood, NJ, USA) and digitized images were obtained with OpenLab software (Improvision, Lexington, MA, USA). Quantification of immunolabeled nuclei in 3’3-diaminobenzidine tetrahydrochloride (DAB)-stained sections from dorso-lateral striatum was done with the × 20 objective and with a 7 × background threshold for the software to count intensely labeled nuclei for each digitized image covering an area of 0.14 mm2. Data were expressed as percentage of total nuclei/image.
Chromatin immunoprecipitation
For each chromatin immunoprecipitation experiment, bilateral striata from three mice were pooled. Nuclei from dissected striata were isolated in ice-cold 10 mm Tris.HCl (pH 7.5)/10 mm NaCl/3 mm MgCl2/0.1% NP-40, washed and resuspended in 2 mL ice-cold buffer EB [20 mm HEPES, pH 7.5, 100 mm NaCl, 1 mm dithiothreitol, 1 mm EDTA, 5% glycerol, 0.05% Triton X-100, 5 mm sodium butyrate, one tablet of protease inhibitor cocktail; Roche, Mannheim, Germany and 500 μL phosphatase inhibitor cocktail (Upstate)/50 mL buffer]. The nuclei were cross-linked with 1% formaldehyde for 10 min at room temperature on a rotator. Cross-linking was stopped with 1 mm glycine and, after repeated washings, chromatin was sonicated in 400 μL EB buffer to an average of 500 bp (Branson, Danbury, CT, USA). From each sonicated sample, 10% was used as the input control for immunoprecipitated fragments at a DNA concentration of approximately 0.5 mg/mL. The remaining 90% of each sonicated sample was incubated overnight in a total volume of 600 μL EB buffer containing 4 μL of anti-H3pS10-acK14 antibody or rabbit IgG as control. Antibody complexes were bound to G-sepharose (Amersham, Piscataway, NJ, USA), washed and then eluted in 50 mm sodium bicarbonate/1% sodium dodecyl sulfate at room temperature for 30 min under constant vortexing and then incubated in proteinase K (100 μg/mL). From each striatal sample, the DNA from immunoprecipitated chromatin and the input DNA (see above) were purified (phenol/chloroform), ethanol precipitated and resuspended in 10 μL H2O. Samples were amplified in duplicate and at two different final dilutions (1 : 25 and 1 : 50) by low cycle PCR (25 cycles) using the following primer pairs [gene/accession no./bp amplified fragment upstream of 5’UTR/forward primer/backward primer: c-fos/NW_000053.1/(−380)-(−63)/ACACAGGATGTCCATA TTA/TGGAGTAGTAGGCGCCTCAGC; GluR2/AF250875/(−569)-(−382)/ TTTGGGAGTTGTCCCTTCAG/GGAAGCCGAACTGCT AATTG; GFAP/NT_039521/(−396)-(−203)/ GTGAGAGCCAGGAA GTCTGC/GGAACCCCCTTTCTGGTAAA; β-globin locus control region/AF071080, bp 61551–61767/ACTGCATCTGCAAGCCTT TT/GTGCCTGATTCCGGGTACTA]. The specificity of PCR products from each experiment was controlled by 1.8% agarose gel electrophoresis and Southern blot using sequence-verified subclones labeled with psoralen-biotin (Ambion, Austin, TX, USA) for hybridization, in conjunction with chemiluminescence (BrightStar; Ambion) and film autoradiography. Quantification of film auto-radiograms of PCR experiments with no yield in negative controls (minus DNA) was done by densitometry and Quantity One software (Bio-Rad).
Quantitative RT-PCR
Striata from three mice were pooled and RNA was extracted with the TRIzol reagent (Invitrogen, Carlsbad, CA, USA). For each sample, 5 μg of total RNA were reverse transcribed with AMV-reverse transcriptase and amplified by PCR using the c-fos relative RT-PCR kit (Ambion) (c-fos primers containing the sequences forward primer, AGAGCGCAGAGCATCGGCAG and reverse primer, CCCTAGAACGTCCGTCCAGC) with amplified 18s rRNA transcripts as loading control and a 1 : 4 ratio of 18s primer/competimer to adjust for the abundance of 18s rRNA, according to the manufacturer's instructions. The specificity of PCR products was examined by Southern blotting with sequence-verified c-fos and 18s cDNA probes. Real-time PCR was then used to quantify c-fos and 18s transcripts from reverse-transcribed RNA (iQ SYBR Green Supermix and MyiQ Single Color Detection System; Bio-Rad).
Statistical analyses
As the data are compiled from many different cell counts, Southern blots and real-time RT-PCR experiments, all data are normalized to untreated, internal controls. Data are, therefore, semiquantitative and not based on absolute numbers. Data were analysed with one-way anovas and two-tailed t-tests using Statview software (SAS Institute, Cary, NC, USA).
Results Blockade of D2-like receptors induces global H3 phospho-acetylation in striatum
In dividing cells, histone modifications are highly regulated both on a global level and at defined genomic regions. Global changes occur when a particular histone modification is altered in a coordinated manner across a large number of genomic sites. Once a certain threshold from baseline is reached, these changes then become apparent in bulk chromatin. Examples include the phosphorylation of histone H3 at serine 10 across entire chromosomes of dividing cells in prophase that is then followed by global dephosphorylation upon exit from mitosis (Prigent and Dimitrov 2003). Furthermore, a dual modification at the N-terminal tail of H3, defined by phospho-serine 10 and acetyl-lysine 14 (H3pS10-acK14) is rapidly induced on a global level in cultured cells treated with hormones, growth factors or toxins. The dimodified H3 molecules, H3pS10-acK14, are associated with transcriptional activation (Cheung et al. 2000; Clayton et al. 2000; Salvador et al. 2001; Li et al. 2002).
To examine global histone modification changes in the striatum in vivo, we treated adult mice with a single dose of the D2-like antagonist and conventional antipsychotic, haloperidol (1 mg/kg, i.p.). Littermates received saline as control. Mice were killed 120 min after treatment and histone modification patterns of striatal nuclei were profiled by immunoblotting with a panel of nine antibodies recognizing site-specific modifications at the N-terminal tails of histones H3 and H4. Each epitope was defined by acetylation, methylation or phosphorylation of specific residues (Fig. 2a). Each of these histone modifications has been studied extensively in cultured, non-neuronal cells and is part of a combinatorial set of histone modifications differentiating between open chromatin at sites of actual or potential transcription (upward arrow in Fig. 2a) and inactive, silenced and condensed chromatin (downward arrow in Fig. 2a) (Jenuwein and Allis 2001; Turner 2002).
Fig. 2.
The conventional anti-psychotic, haloperidol, increases H3 phospho-acetylation in bulk chromatin. (a) The nine site-specific modifications at N-terminal tails of histone H3 and H4 that were examined in the present study. Ac, acetylation; me, methylation; p, phosphorylation. Single letter code: K, lysine; R, arginine; S, serine. Upward arrows mark association with open chromatin and the transcriptional state, downward arrows mark assocation with silenced and inactive chromatin, combined arrows mark more generalized function independent of transcription (Jenuwein and Allis, 2001). (b) Representative immunoblots from acid extracted proteins from striatum of mice killed 120 min after a single dose of haloperidol or saline, showing immunoreactivities for the total of nine site- and modificationspecific histone epitopes shown in (a). Notice increased H3 phosphoacetylation (H3pS10-acK14) in striatum of haloperidol-treated animals (top), but no differences to controls for H3 acetylation (H3acK9/14 and H3acK14), methylation (H3meK4, H3meK9, H3meR17) and phosphorylation (H3pS10) and for H4 acetylation, (H4acK8, H4acK12). (c) Levels of immunoreactivity (mean ± S.E.M.) for each of the nine histone epitopes in striatum of mice 120 min after a single dose of haloperidol. Levels are normalized to saline-treated littermate controls. Notice 2-fold increase in H3pS10-acK14 in striatum of haloperidol-treated animals, but no difference to controls for the remaining eight H3/H4 epitopes.
The H3 epitope defined by the dual modification, phospho-serine 10 in conjunction with acetyl-lysine 14 (H3pS10-acK14), was consistently and on average 2.2-fold increased in the haloperidol-treated animals (Figs 2b top and c). None of the other eight epitopes/histone modifications that we examined were differentially regulated on a global level in haloperidol-treated animals in comparison to controls (Figs 2b and c). These included two H3 epitopes defined by lysine acetylation (H3acK9/14 and H3acK14), two H4 epitopes defined by lysine acetylation (H4-acK8 and H4-acK12, Figs 2a–c), three H3 epitopes defined by methylated lysine and arginine residues (H3meK4, H3meK9 and H3meR17, Figs 2a–c) and one H3 epitope defined by serine phosphorylation (H3Sp10) (Figs 2a–c). Notably, H3pS10 and H3acK14 comprise together, when residing on the same H3 tail, phospho-acetylated H3, H3pS10-acK14. Therefore, our experiments demonstrate that, in bulk chromatin from striatum, H3pS10-acK14 is up-regulated in haloperidol-treated animals but, when H3-phospho-serine 10 and H3-acetyl-lysine 14 are analysed separately, no changes are detectable when bulk chromatin is assayed. This result was not unexpected because differential regulation of the dimodified H3 epitope and the two monomodified H3 epitopes in bulk chromatin has been previously observed in non-neuronal cell culture (Cheung et al. 2000; Salvador et al. 2001; Li et al. 2002) and in hippocampal neurons in vivo (Crosio et al. 2003). As H3 molecules with dimodified tails, H3pS10-acK14, comprise only a minor fraction of the total H3 pool, a change in H3pS10-acK14 immunoreactivity, as observed in this study, may not be accompanied by similar alterations in immunoblots for H3pS10 and H3acK14 if H3 tails harboring these modifications separately comprise a much larger fraction of the total H3 in bulk chromatin (Cheung et al. 2000; Clayton et al. 2000; Salvador et al. 2001; Thomson et al. 2001). To confirm that the increase in striatal H3pS10-acK14 after treatment with haloperidol is caused by blockade of D2-like signaling, we treated mice with another D2-like antagonist, raclopride (10 mg/kg). Levels of H3pS10-acK14 in raclopride-treated animals were increased consistently and, on average, 1.8-fold above baseline (p < 0.05) (Figs 3a and b). Another group of mice was treated with the atypical antipsychotic risperidone, an antagonist to dopamine D2-like receptors and to serotonin 5-HT2 receptors. A single dose (1 mg/kg) resulted in a robust and, on average, twofold increase in phosphoacetylated H3 in striatum 120 min after treatment (p < 0.05) (Fig. 3b).
Fig. 3.

Striatal H3 phospho-acetylation is selectively induced by D2-like antagonists. (a) Striatal immunoblots from mice killed 120 min after a single dose of D2-like agonist (quinpirole) or D2-like antagonist (raclopride) or saline. Blots were processed first for H3pS10-acK14 and then for H4acK12 immunoreactivity. Corresponding gel coomassie-blue stains for loading control show characteristic banding pattern of the four core histones, including H3, approximately 14.5 kDa, and H4, approximately 10.5 kDa. Notice increase in H3pS10-acK14, but not H4acK12 immunoreactivity in raclopride-treated animals. Notice that H3pS10-acK14 is unchanged in quinpirole-treated animals, in comparison to saline-treated littermates. (b) Levels of striatal H3pS10-acK14 immunoreactivity (mean ± S.E.M.) in D2-agonist (quinpirole)-treated animals, and in animals treated with raclopride or the atypical anti-psychotic risperidone. Levels are normalized to saline-treated littermate controls. There is an increase in striatal H3pS10-acK14 in raclopride- and risperidone-, but not in quinpirole-treated animals (*p < 0.05).
To examine if stimulation of D2-like receptors differentially affects striatal H3pS10-acK14, mice were treated with a single dose of the D2-like agonist quinpirole (2 mg/kg) and no changes from baseline were observed (Figs 3a and b). We conclude that the increase in H3pS10-acK14 in bulk chromatin of striatum is specific for drugs acting as D2-like antagonists.
Drug-induced striatal H3 phospho-acetylation shows the kinetics of the early response and desensitization after repeated treatment
To monitor the dynamic regulation of H3 phospho-acetylation after blockade of D2-like signaling, we killed animals at 15, 30, 120 and 480 min after a single dose of haloperidol (1 mg/kg) or saline.
There was a significant, approximately twofold increase in H3pS10-acK14 within the first 15 min after haloperidol treatment and these levels were maintained for at least 120 min (p < 0.01–0.05) (Figs 4a and b). In striatum of animals killed 480 min after treatment, levels of phosphoacetylated H3 had returned to baseline (Figs 4a and b). Therefore, the kinetics of the haloperidol-induced up-regulation of striatal H3pS10-acK14 bears a resemblance to the immediate-early gene response because immediate-early gene proteins, including fos and other AP-1 transcription factors, characteristically peak 60– 120 min after acute haloperidol treatment in striatum (Dragunow et al. 1990).
Fig. 4.
Blockade of D2-like signaling induces H3 phospho-acetylation in striatum with kinetics that are characteristic of the early response. (a) Representative immunoblots from striatum of haloperidol-treated mice at 15, 30 and 480 min after a single dose (1mg/kg), and after regular treatments twice daily (1mg/kg) for a period of 10 days. Notice increased levels of H3pS10-acK14 in striatum at 15 and 30 min after acute haloperidol treatment. (b) Kinetics of H3 phospho-acetylation in striatum of haloperidol-treated mice. Data are normalized to levels of saline-treated controls and expressed as mean ± S.E.M. (*p < 0.05, anova with Fisher's paired least significant difference (PLSD) corrected for multiple comparisons). There is a significant increase in striatal H3pS10-acK14 at 15, 30 and 120 min after a single dose, and return to baseline levels at 480 min. Notice normal levels of phospho-acetylated H3 in striatum of animals subject to repeated haloperidol treatment for 1, 3, 5 or 10 days. Animals that were subject to repeated treatments were killed 120 min after the last treatment.
After regular and repeated exposure to dopaminergic drugs and antipsychotics, the transcriptional activation of immediate-early genes is subject to desensitization (Atkins et al. 1999 and references therein). To examine if striatal H3 phospho-acetylation is subject to desensitization in the context of extended haloperidol treatment, we injected mice twice daily at 08:00 and 16:00 h with haloperidol (1 mg/kg) for a period of 1, 3 or 10 days and then killed the animals 120 min after the final treatment. For each of these treatment groups, levels of phospho-acetylated H3 in striatum were not different from saline-treated controls (Fig. 4b). To examine the possibility that this lack of H3pS10-acK14 induction was the result of a shift in the haloperidol dose–response curve, mice were treated for 4 days with 1 mg/kg haloperidol twice daily and on the fifth day with a much higher dose of 4 mg/kg. In striatum of these mice, levels of H3pS10-acK14 remained unaltered from controls (Fig. 4b). Therefore, H3 phospho-acetylation is up-regulated in response to acute blockade of D2-like signaling but this mechanism is subject to desensitization when the duration of treatment is extended.
D2-like antagonists up-regulate striatal H3pS10-acK14 through activation of the cyclic AMP-protein kinase A pathway
Blockade of D2-like receptors removes the D2-mediated inhibition of adenylyl cyclases in striatum (Sibley 1995). The resulting activation of cAMP-dependent PKA (cAMP-PKA) is essential for transcriptional activation after treatment with antipsychotics blocking D2-like receptors (Adams et al. 1997; Brandon et al. 1998). To determine whether the cAMP-PKA pathway is involved in D2-like antagonist-induced chromatin remodeling, including the up-regulation of striatal H3pS10-acK14, we conducted in vivo experiments. We injected cAMP-analog drugs into the striatum of adult rats and monitored the resulting changes in H3pS10-acK14. We first examined the effect of Rp-adenosine 3c’,5c’-cyclic monophosphorothioate triethyl-ammonium salt, a cAMP analog that inhibits PKA. Rats received Rp-adenosine 3c’,5c’-cyclic monophosphorothioate triethylammonium salt (100 nmol) into the left striatum, followed after 30 min by a systemic dose of haloperidol (1.5 mg/kg, i.p.). After an additional period of 30 min, animals were then killed and striatal histones were profiled by immunoblotting. Levels of H3pS10-acK14 in the left striatum were consistently and, on average, 30% decreased in comparison to levels in the (untreated) right striatum (Figs 5a and d). This difference was significant (p < 0.05). In contrast, animals that received vehicle (phosphate-buffered saline) into the left striatum showed, after haloperidol treatment, a robust increase in H3pS10-acK14 in striatum bilaterally (Figs 5a and d). We conclude that haloperidol-induced H3 phosphoacetylation in striatum is significantly inhibited when cAMP-PKA is blocked.
Fig. 5.
D2-like antagonists induce striatal H3 phospho-acetylation by activating cAMP-dependent PKA. (a, b) Immunoblots from left (L) and right (R) striatum of rats showing H3pS10-acK14 and H4acK12 immunoreactivity, and gel coomassie blue stain as additional loading control. Animals shown in (a) received PBS or Rp-cAMPs into the left striatum, followed 30 min later by a systemic dose of haloperidol or saline. Blots show striatal histones at 30 min after the systemic treatment. Notice robust increase in H3pS10-acK14 in left and right striatum of haloperidol-treated animals that received PBS into the left striatum. Notice attenuated response in left striatum of haloperidol-treated animals that received Rp-cAMPs into the left striatum. (b) Striatal immunoblots from animals 60 min after an infusion of PBS or Sp-cAMPs into the left striatum. There is a robust increase in H3pS10-acK14 in left striatum of Sp-cAMPs treated animals. (c) Blots from primary striatal cultures, treated with vehicle, forskolin or H89 followed by forskolin. Note increased levels of H3pS10-acK14 in forskolin-treated cultures that is completely blocked by H89. The cultures were treated with forskolin for a period of 30 min with or without pre-treatment with H89 for 30 min; pre-treatment with H89 was for 30 minutes. (d) Levels of H3pS10-acK14 immunoreactivity (mean ± S.E.M.) of in vivo experiments with cAMP analogue drugs. Gray bar, left striatum; checkered bar, right striatum. Notice that Rp-cAMPs induced a significant decrease in left striatum of haloperidol-treated animals, while Sp-cAMPs induced a significant increase from baseline. *= p < 0.05. (e) Graph summarizing levels of H3pS10-acK14 immunoreactivity (mean ± S.E.M.) of In vitro experiments, notice significant increase from baseline in forskolin-treated cultures and significance decrease from baseline when cultures were pre-treated with H89.
Next, we examined changes in striatal H3pS10-acK14 after activation of cAMP-PKA. Rats received the cAMP analog and PKA activator drug Sp-adenosine 3c’,5c’-cyclic monophosphorothioate triethylammonium salt (100 nmol) into the left striatum and, after a period of 60 min, striatal histones were extracted for immunoblotting. When compared with vehicle-treated animals, Sp-adenosine 3c’,5c’-cyclic monophosphorothioate triethylammonium salt induced a significant, approximately 2.5-fold increase in H3pS10-acK14 in the left striatum (Figs 5b and d). Furthermore, levels in H3pS10-acK14 in the left striata treated with Spadenosine 3c’,5c’-cyclic monophosphorothioate triethylammonium salt were approximately twofold increased, in comparison to the right striata of the same animals (Figs 5b and d). These differences were significant (p < 0.05). Therefore, in vivo activation of cAMP-PKA induces the phospho-acetylation of H3 in striatum.
To confirm that cAMP-PKA signaling in striatal neurons is essential for H3 phospho-acetylation and to exclude the possibility that in vivo effects of the cAMP analog drugs are due to altered synaptic transmission and other variables, we monitored H3 phospho-acetylation after pharmacological manipulation of the cAMP-PKA pathway In vitro, using dissociated striatal cultures which lack midbrain and cortical pre-synaptic inputs. Neuronal transmission was blocked with tetrodotoxin citrate (2 lm) starting 120 min prior to cell harvest. Treatment of striatal cultures with forskolin (1 μm), a drug that activates adenylyl cyclases, induced a 2.4-fold increase in H3pS10-acK14 within 30 min (p < 0.05) (Figs 5c and e). Next, we treated striatal cultures with the PKA inhibitor drug H89 (20 μm) for a period of 30 min before adding forskolin (1 μm) for another 30 min. We observed that H89 completely suppressed forskolin-induced H3 phospho-acetylation (Figs 5c and e). This effect was significant (p < 0.05). Additional cultures were treated with a higher forskolin dose (10 μm) and results were indistinguishable when compared with cultures treated with the lower dose (1 μm) (data not shown). We conclude from these studies that activation of adenylyl cyclase and cAMP-PKA in striatal neurons induces H3 phospho-acetylation.
Post-synaptic NMDA receptors modulate D2-like antagonist-induced H3 phospho-acetylation in striatal neurons
Previous studies demonstrated that blockade of D2-like receptors in striatal neurons induces gene expression through synergistic interaction of cAMP-PKA signaling and glutamatergic input emanating from the cerebral cortex and subcortical areas (Boegman and Vincent 1996; Konradi et al. 1996; Chesselet et al. 1998; de Souza and Meredith 1999; Hussain et al. 2001). It is thought that glutamatergic signaling activates NMDA receptors that, in turn, regulate voltage-operated Ca2+ channels (Rajadhyaksha et al. 1999). Ca2+-dependent enzymes then mediate the phospho-activation of the transcription factor cAMP response element-binding protein (Rajadhyaksha et al. 1999). To examine whether NMDA receptor activity is required for D2-like antagonist-induced histone modification changes in striatum, we treated mice and rats with a systemic dose of the NMDA receptor antagonist MK-801 (1 mg/kg) or saline as control, followed after 15 min by a systemic dose of haloperidol (mice, 1 mg/ kg; rats, 1.5 mg/kg, i.p.) or saline. After an additional 30 min, striata were then dissected and histones profiled by immuno-blotting. Animals that were treated first with saline and then with haloperidol showed a twofold increase in striatal H3pS10-acK14 (Figs 6a–c). In contrast, animals that received MK-801 and then haloperidol showed an attenuated response and levels of striatal H3pS10-acK14 were decreased by 30–40% in comparison to animals treated first with saline and then with haloperidol (Figs 6b and c). These differences were significant (p < 0.05). We conclude that, after NMDA receptor blockade, the D2-like antagonist-induced H3 phospho-acetylation in striatum is partially inhibited.
Fig. 6.
NMDA receptor- and Ca2+-dependent signaling pathways are required for D2-like antagonist-induced striatal H3 phospho-acetylation. (a) Immunoblots showing levels of H3pS10-acK14 and H4acK12 in striatum of rats treated first with saline or the NMDA receptor antagonist MK-801, followed after 15 min by a single dose of haloperidol or saline. Animals were sacrificed 30 min after the second injection. Notice that pre-treatment with MK-801 blocks the haloper-idol-induced increase in H3pS10-acK14. (b, c) Bar graphs summarizing levels of H3pS10-acK14 (mean ± S.E.M.) in (b) mice and (c) rats treated first with MK801 or saline followed by haloperidol or saline. (d) Representative immunoblots of primary striatal cultures treated with vehicle or glutamate for 30 min, with or without pre-treatment with MK-801 for 30 minutes. Notice that glutamate treatment increases levels of H3pS10-acK14, and notice that this effect is blocked by MK801. (e) Representative immunoblot of dissociated striatal culture treated with vehicle or the l-type Ca2+ channel agonist FPL 64176. (f) Levels of H3pS10-acK14 immunoreactivity in striatal cultures after treatment with vehicle, glutamate (±pre-treatment with MK-801) and FPL 64176. Notice that glutamate treatment upregulates H3pS10-acK14, and this is blocked by pre-treatment with MK-801. Notice further that the l-type Ca2+ channel agonist FPL 64176 upregulates H3pS10-acK14.
This partial inhibition of haloperidol-induced chromatin changes by MK-801 in vivo suggests that glutamatergic input and dopaminergic transmission converge to induce H3 phospho-acetylation in striatal neurons. We further tested this hypothesis in dissociated striatal cultures pre-treated with tetrodotoxin citrate to suppress trans-synaptic signaling. We first examined the effects of glutamate treatment (50 μm) on H3pS10-acK14 and observed, at 30 min after treatment, a twofold increase from baseline (p < 0.05) (Figs 6d and f). In contrast, cultures that were treated with MK-801 (2 μm) for a period of 30 min prior to the addition of glutamate did not show a significant change from baseline (Figs 6d and f). We conclude that glutamate induces H3 phospho-acetylation in striatal neurons through post-synaptic NMDA receptors. Previous studies have shown that l-type Ca2+ channels are essential for NMDA receptor-mediated gene transcription in striatum (Rajadhyaksha et al. 1999). Furthermore, l-type Ca2+ channel currents are suppressed after activation of D2-like receptors in striatal neurons (Hernandez-Lopez et al. 2000). Therefore, we asked whether l-type Ca2+ channel activity induces changes in striatal H3 phospho-acetylation. To address this question, we treated striatal cultures with the l-type Ca2+ channel agonist FPL 64176 (20 μm) for 30 min and observed a 2.3-fold increase in H3pS10-acK14 from baseline (p < 0.01) (Figs 6e and f). We conclude from these experiments that D2-like antagonist-induced H3 phosphoacetylation in striatal neurons depends on post-synaptic NMDA receptors that then activate Ca2+-dependent signaling pathways.
D2-like antagonists induce H3 phospho-acetylation in neuronal nuclei
To determine if D2-like blockade changes histone modification patterns in nuclei of striatal neurons, we examined the cellular distribution of H3pS10-acK14 by immunohisto-chemistry. Coronal, 18-lm thick sections cut through the caudate-putamen of mice killed 120 min after a single dose of haloperidol, risperidone or saline were examined. Staining for H3pS10-acK14 was confined to nuclei and, in DAB/ immunoperoxidase-stained sections, was visibly much more intense in the risperidone- and haloperidol-treated animals (Figs 7a, c and e) compared with saline-treated controls (Figs 7b, d and f). The overall distribution, shape and size of the immunostained nuclei were consistent with a neuronal distribution pattern (Figs 7a–f). We used a semiquantitative approach to measure the haloperidol-induced increase in H3pS10-acK14 immunoreactivity in striatal nuclei using digitized images and OpenLab software. We compared sections from drug- and saline-treated animals that were processed in parallel. Sections from drug-treated animals showed a significant 70% increase in dark, DAB-stained immunopositive nuclei, operationally defined by an arbitrary threshold of 7 × background in digitized images (Fig. 7l). This finding is in agreement with the immunoblotting experiments described above.
Fig. 7.
Anti-psychotic drugs induce H3 phospho-acetylation in the nuclei of striatal neurons. (a–f) Coronal sections through dorsolateral striatum and adjacent cerebral cortex (a,b) were processed for H3pS10-acK14 immunoreactivity and stained with immunoperoxidase/ diaminobenzidine. Sections from an animal killed 120 min after a single dose of risperidone (a) or haloperidol (c,e) (1mg/kg). (b,d,f) Show sections from saline-treated controls. Notice increased numbers of dark stained nuclei in striatum (ST), but not cerebral cortex (CC) in risperidone-treated animal (a). Notice increased numbers of dark stained nuclei in striatum after haloperidol treatment (c,e). (g–i) Show digitized images from immunofluorescence-stained sections through dorsolateral striatum at 120 min after drug administration. Sections are triple-labeled, (g) shows NeuN immunopositive neuronal nuclei, (h) shows H3pS10-acK14 immunoreactivity and (i) 4,6,-diamidino-2-phenylindole dihydrochloride (DAPI) counterstain in order to identify nuclei. Arrows mark identical nuclei in corresponding images. Notice high levels of phospho-acetylated H3 in a subset of neuronal nuclei. (j, k) show higher resolution photomicrograph of neuronal nucleus. (j) DAPI stain, (k) H3pS10-acK14 immunolabeling. Notice compartmentalized distribution of H3pS10-acK14 in neuronal nucleus (k), with sparing of condensed chromatin (arrows) as defined by DAPI stain (j). Images taken at magnification (a,b) 4x10, 10x10 (c,d), 60x10 (e–i), 100x10 (j,k). Bar graph in (l) shows percentage of DAB/immunoperoxidase-stained nuclei (mean ± S.E.M.) expressing high H3pS10-acK14 immunoreactivity (operationally defined as > 7- fold increase above background) in dorso-lateral striatum of haloperidol-treated mice at 120 min after drug injection, in comparison to saline-treated controls. (m) Shows fraction of NeuN+ positive nuclei expressing high H3pS10-acK14 immunofluorescence >7x background. *p < 0.05. Notice significant increase in H3pS10-acK14 immunoreactivity in neuronal nuclei of haloperidol-treated animals.
To further confirm that the haloperidol-induced increase in striatal H3pS10-acK14 includes neuronal nuclei, we double labeled sections for immunofluorescence for phospho-acetylated H3 and NeuN, a neuron-specific nuclear protein (Muller et al. 1992). A subset of NeuN + nuclei showed intense H3pS10-acK14 immunofluorescence (Fig. 7g–i). To estimate the difference between drug- and saline-treated animals, we determined the fraction of NeuN + nuclei with H3pS10-acK14 immunofluorescence exceeding the arbitrary threshold of 7 × background in digitized images. There was a significant, threefold increase in immunofluorescence in the haloperidol-treated animals in comparison to controls (Fig. 7m). Together, the results of our immunohistochemical experiments demonstrate that treatment with antipsychotics and D2-like antagonists induces the phospho-acetylation of H3 in nuclei of striatal neurons.
Notably, the distribution of H3pS10-acK14 within each neuronal nucleus was not uniform and, when the labeling pattern of H3pS10-acK14 was compared with the staining pattern of the nucleophilic dye 4,6,-diamidino-2-phenylin-dole dihydrochloride (DAPI), it was apparent that H3pS10-acK14 is enriched in nuclear areas comprised of less condensed and loose chromatin but absent from nuclear regions comprised of condensed (hetero-)chromatin (Figs 7j and k). Therefore, the enrichment of H3pS10-acK14 in nuclear subregions defined by a low content of condensed chromatin provides further evidence that this histone modification is in striatal neurons associated with open chromatin, as has been previously reported for non-neuronal cells In vitro (Cheung et al. 2000; Clayton et al. 2000) and for hippocampal neurons in vivo (Crosio et al. 2003).
H3 phospho-acetylation is dynamically regulated at the c-fos promoter
To examine the dynamics of H3 phospho-acetylation at regulatory sequences of early reponse genes in comparison to other genes, we used chromatin immunoprecipitation assays to measure H3 phospho-acetylation at defined genomic sites distinguished by different levels of transcriptional activity. We focused on chromatin around the promoters of the following genes: (i) c-fos, an early response gene that, in striatum, shows several-fold induction from baseline after blockade of D2-like signaling (Dragunow et al. 1990; Nguyen et al. 1992); (ii) GluR2, an α-amino-3-hydroxy-5-methylisoxazole-4-propionate receptor subunit expressed at high levels in adult forebrain neurons, including striatum (Brene et al. 1998); (iii) GFAP, a phenotypic marker for a subset of astrocytes and (iv) the locus control region of the erythroid-specific β-globin genes, which are silenced and heterochromatic in brain (Tolhuis et al. 2002).
Using the anti-H3pS10-acK14 antibody or rabbit IgG as control, we immunoprecipitated striatal chromatin of mice killed 15 or 120 min after a single dose of haloperidol (1 mg/ kg) or saline. We quantified the abundance of the regulatory sequences of the four genomic regions of interest in chromatin immunoprecipitation fractions by quantitative PCR in conjunction with Southern blot in order to ensure the specificity of the reaction product (Fig. 8a). We found that, 120 min after haloperidol treatment, phospho-acetylated H3 in chromatin around the c-fos promoter was increased 2.7-fold in comparison to chromatin from saline-treated littermates (p < 0.05) (Fig. 8b). When striatal chromatin was examined at 15 min after haloperidol treatment, levels of H3pS10-acK14 at the site of the c-fos promoter differed less than 30% in comparison to saline-treated controls. This difference was not significant (Fig. 8b). In chromatin at GluR2, GFAP and β-globin regulatory sequences, levels of phospho-acetylated H3 did not differ significantly between haloperidol- and saline-injected animals at 15 or 120 min after treatment (Fig. 8b). Therefore, the newly phospho-acetylated H3 molecules are not randomly distributed in striatal chromatin but enriched in chromatin around a subset of genes, including the early response gene, c-fos. Next, we compared the kinetics of H3 phospho-acetylation in striatal nucleosomes located at the c-fos promoter with corresponding changes in c-fos mRNA levels. In striatum of haloperidol-treated mice, levels of H3pS10-acK14 were increased at 30 min, peaked at 120 min and returned to baseline 480 min after treatment (Fig. 8c). In contrast, c-fos transcript was increased within 15 min, peaked at 30 min and then declined towards baseline levels at 120 min after treatment (Fig. 8c), which is consistent with previous studies (Merchant and Dorsa 1993; Adams et al. 1997). We conclude that there is a rapid and transient increase in H3 phospho-acetylation at the c-fos promoter, consistent with the kinetics of the early response. Notably, our results suggest that a substantial portion of H3 molecules in chromatin at the c-fos promoter becomes phospho-acetylated sequential to the increase in striatal c-fos transcripts (Fig. 8c).
Fig. 8.
Drug-induced H3 phospho-acetylation of striatal chromatin is differentially regulated at defined genomic sequences. (a) Representative Southern blots from PCR-amplified soluble striatal chromatin immunoprecipitated with the anti-H3pS10-acK14 antibody or rabbit IgG as control. Blots show amplification products for 5’ regulatory sequences of c-fos, GluR2, GFAP and β-globin genes. S-in, input saline-treated controls; H-in, input haloperidol-treated animals; S-ch, immunoprecipitated chromatin (ChIP) from saline-treated mice; H-ch, ChIP from haloperidol-treated mice. Blots are from mice killed 15 min (left) or 120 min (right) after a single dose of haloperidol (1mg/kg). Notice low levels of c-fos and β-globin DNA, and high levels of GluR2 and GFAP DNA in chromatin immunoprecipitated with anti-H3pS10-acK14 antibody. Notice increased levels of c-fos DNA in ChIP from animals killed 120 min after haloperidol treatment. (b) Bar graphs show, for chromatin of each of the 4 genes, levels of phospho-acetylated H3, expressed as ChIP-to-input ratio (mean ± S.E.M.) in haloperidol-treated animals. Data were normalized to saline-treated littermates. Notice significant increase in c-fos DNA in ChIP at 120 min after haloperidol treatment (p<0.05). (c) Line graph shows dynamic changes in H3 phospho-acetylation at c-fos promoter (filled dots) and c-fos transcript (open triangles) 15-480 min after a single dose of haloperidol. Data are normalized to controls and expressed as mean ± S.E.M. The drug-induced increase both in c-fos promoter H3 phosphoacetylation and c-fos gene expression is transient. In addition, the kinetics of H3 phospho-acetylation appear to be slower, in comparison to the more rapid change in transcript levels. (d) Southern blots of anti-H4acK8 ChIP from striata of mice killed 30 min after haloperidol or saline treatment. Notice increased levels of c-fos DNA in ChIP from drug-treated animals.
To determine if haloperidol treatment induces additional histone modifications at the c-fos promoter, we conducted chromatin immunoprecipitation experiments with an anti-H4acK8 antibody (Fig. 8d). The acetylation of H4-lysine 8 is, like the phospho-acetylation of H3, H3pS10-acK14, associated with open chromatin and actual transcription (Jenuwein and Allis 2001; Turner 2002). When striatal chromatin was examined 30 min after haloperidol treatment, levels of H4acK8 at the c-fos promoter were increased twofold in comparison to saline-treated littermates (Fig. 8d). This difference was significant (p < 0.05). Levels of H4acK8 at the GluR2, GFAP and β-globin regulatory sequences were similar in haloperidol- and saline-treated animals (Fig. 8d). We conclude that acute blockade of D2-like signaling induces both H3 phospho-acetylation and H4 acetylation in chromatin surrrounding c-fos. Furthermore, we observed that levels of both H3pS10-acK14 and H4acK8 are high in chromatin around regulatory sequences of GluR2 and GFAP but very low at the β-globin locus control region (Figs 8a and d). This finding is in agreement with the observation that H3pS10-acK14 and H4acK8 are associated with open chromatin because the GluR2 and GFAP genes are expressed at high levels in striatum, while chromatin of the entire β-globin locus, including the locus control region, is silenced (Tolhuis et al. 2002) and subject to histone hypoacetylation in brain (Forsberg et al. 2000).
Discussion
We report that D2-like antagonists and antipsychotic drugs induce chromatin modifications in nuclei of striatal neurons. Blockade of D2-like receptors induced a rapid increase in dually modified N-terminal tails of histone H3, defined by phospho-serine 10 in conjunction with acetyl-lysine 14 (H3pS10-acK14). These changes from baseline were of sufficient magnitude to become detectable in immunochemical stainings of bulk chromatin and neuronal nuclei, which suggests that drug-induced H3 phospho-acetylation affects chromatin across a considerable portion of the genome. Furthermore, we demonstrated, in a series of experiments conducted in vivo and In vitro, that H3 phospho-acetylation in striatal neurons in response to D2-like receptor blockade requires the activation of cAMP-PKA and NMDA receptor pathways. The phospho-acetylation of H3 in striatal chromatin after D2-like receptor blockade showed the kinetics of the early response and repeated treatment resulted in desensitization. Consistent with this observation, chromatin-immunoprecipitation assays on striatal extracts showed a rapid but transient increase in H3 phospho-acetylation and H4 acetylation in chromatin around regulatory sequences of the immediate-early gene c-fos. Together, these results suggest that dopaminergic and glutamatergic transmission dynamically regulate chromatin structure in striatal neurons. By blocking D2-like signaling, antipsychotic drugs induce H3 phospho-acetylation, H3pS10-acK14, a dual H3 modification that is associated with transcriptional activation and epigenetic regulation of gene expression (Turner 2002).
Chromatin modification — a molecular mechanism mediating antipsychotic drug-induced growth and plasticity in striatum?
Our experiments show that treatment in vivo with D2-like antagonists and antipsychotic drugs induces, within the first 15 min, an increase in phospho-acetylated H3, H3pS10-acK14, in nuclei of striatal neurons that is then sustained in bulk chromatin for several hours. A rapid and transient increase in H3pS10-acK14 was previously reported for dividing cells exposed In vitro to stimuli with a dramatic effect on cell growth and differentiation, such as mitogenic growth factors and hormones (Cheung et al. 2000; Salvador et al. 2001; Thomson et al. 2001). By analogy, increased H3 phospho-acetylation in the nucleosomes of striatal neurons could reflect an early adaptation that ultimately leads to profound changes in neuronal function. Notably, D2-like antagonists and antipsychotic drugs, such as haloperidol, have a remarkable growth-promoting effect on adult striatum, as shown by the increased size of neuronal somata, dendrite calibers and axon terminals (Benes et al. 1985; Uranova et al. 1991), proliferative changes in post-synaptic densities (Kerns et al. 1992; Meshul et al. 1992), increased spine densities (Meredith et al. 2000) and altered functional connectivity (Onn and Grace 1995). These adaptations are of sufficient magnitude to result in gross morphological alterations of the basal ganglia, as shown by the increase in striatal volume of patients and animals chronically treated with antipsychotics (Heckers et al. 1991; Jernigan et al. 1991; Chakos et al. 1998; Gur et al. 1998). Importantly, our study provides evidence that striatal chromatin is remodeled after acute treatment. However, it remains to be determined if long-term treatment with antipsychotics induces lasting adaptations of striatal chromatin, including changes in H3 phospho-acetylation at genes that are expressed at increased levels after chronic treatment (Konradi and Heckers 2001).
Co-regulation of H3 phospho-acetylation and early response genes
The kinetics of striatal H3 phospho-acetylation bears resemblance to the surge of early response proteins after activation of D1 or blockade of D2-like receptors (Dragunow et al. 1990; Graybiel et al. 1990; Robertson et al. 1992; Deutch et al. 1996; Gerfen et al. 1998; Missale et al. 1998). Considering that the dual histone modification, H3pS10-acK14, defines open chromatin and activation of gene expression in yeast, mammals and Drosophila (Cheung et al. 2000; Clayton et al. 2000; Lo et al. 2000; Thomson et al. 2001), its coordinated regulation in concert with immediate-early transcription factors, including fos (Curran and Morgan 1995), makes biological sense; levels of c-fos protein in striatum and global levels of H3pS10-acK14 in striatal chromatin are transiently increased after acute treatment and may operate synergistically to enhance transcription of selected genes.
Furthermore, our results suggest that dynamic changes in H3pS10-acK14 at the c-fos promoter are involved in the regulation of c-fos transcription. According to our results, the increase in H3pS10-acK14 in chromatin around the c-fos promoter becomes detectable shortly after the rise in striatal c-fos transcripts after D2-like antagonist treatment. However, we cannot rule out the possibility that our chromatinimmunoprecipitation assays lack the sensitivity to detect a subtle increase in H3 phospho-acetylation at c-fos chromatin that may have occurred in parallel with or even prior to the increase in c-fos transcription. Alternatively, the early phase of transcriptional activation at the c-fos promoter may be associated with histone modifications other than H3 phospho-acetylation. Thus, while the present study provides evidence for drug-induced H3 phospho-acetylation and H4 acetylation in chromatin at the c-fos promoter, the functional significance of these histone modifications in relation to c-fos transcription remains to be clarified.
Our observation that the up-regulation of striatal H3 phospho-acetylation after acute blockade of D2-like receptors is detectable in immunoblots on bulk chromatin indicates that chromatin is affected at multiple sites of the genome. Through these multiple chromatin imprints, D2-like receptor blockade could modulate the expression of a large number of genes with only a small pool of transcription factors. Furthermore, by coupling D2-like receptor blockade to histone modifications involved in the epigenetic regulation of gene expression, including H3pS10-acK14 (Turner 2002), striatal neurons could stabilize striatal transcription, making it less dependent on rapidly fluctuating changes in neurotransmission and intracellular Ca2+ or cAMP levels (Liu and Graybiel 1996).
Cyclic AMP and NMDA receptor pathways: cellular transducers linking dopaminergic signaling to chromatin-remodeling and histone-modifying enzymes
Drugs blocking D2-like receptors regulate striatal gene expression through the cAMP and NMDA receptor pathways. cAMP-PKA is critical to activation of gene expression by D2-like antagonists because PKA-deficient mice fail to up-regulate gene expression in response to D2-like blockade (Adams et al. 1997; Brandon et al. 1998). Therefore, one would predict that cAMP-PKA is required for D2-like antagonist-induced striatal H3 phospho-acetylation, which is, indeed, in line with our observations. Furthermore, studies in striatal cultures, which lack the in vivo circuitry, have shown that an intraneuronal interaction between cAMP pathways and NMDA receptor-mediated signal transduction pathways is required to induce the phospho-activation of the transcription factor, cAMP response element-binding protein, and early response gene expression after D2-like receptor blockade (Rajadhyaksha et al. 1999; Leveque et al. 2000). Therefore, one would predict that glutamatergic input and NMDA receptor pathways regulate H3 phospho-acetylation in striatum not only in vivo but also in dissociated striatal culture In vitro, as observed in this study. Thus, two cellular signaling pathways induce chromatin changes in striatal neurons in response to blockade of D2-like receptors. One pathway involves adenylyl cyclases and cAMP-PKA and the other involves glutamatergic input and NMDA receptor-regulated, Ca2+-dependent signal transduction. By using these two partially interdependent signaling pathways (Rajadhyaksha et al. 1999; Leveque et al. 2000) and additional molecular messengers (Fienberg et al. 1998; Bibb et al. 1999; Hernandez-Lopez et al. 2000; Miyakawa et al. 2003; Moghaddam 2004) in variable combinations and intensities, striatal neurons and their dopaminergic and glutamatergic afferents could adjust and fine-tune the activity of chromatin-remodeling complexes and histone-modifying enzymes, which may greatly increase the response repertoire of the brain when it is exposed to D2-like antagonists and other antipsychotic drugs.
Acknowledgements
This work was supported by National Institutes of Health grants K08 DA00479 (SA) and R01 DA007134 (CK), the Janssen Research Foundation and the Rett Syndrome Research Foundation.
Abbreviations used
- cAMP-PKA
cAMP-dependent protein kinase A
- Rp-cAMPs
Rp-Adenosine 3c’,5c’-cyclic monophosphorothioate triethylammonium salt
- Sp-cAMPs
Sp-Adenosine 3c’,5c’-cyclic monophosphorothioate triethylammonium salt
References
- Adams MR, Brandon EP, Chartoff EH, Idzerda RL, Dorsa DM, McKnight GS. Loss of haloperidol induced gene expression and catalepsy in protein kinase A-deficient mice. Proc. Natl Acad. Sci. USA. 1997;94:12157–12161. doi: 10.1073/pnas.94.22.12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbarian S, Rios M, Liu RJ, et al. Brain-derived neurotrophic factor is essential for opiate-induced plasticity of noradrenergic neurons. J. Neurosci. 2002;22:4153–4162. doi: 10.1523/JNEUROSCI.22-10-04153.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins JB, Chlan-Fourney J, Nye HE, Hiroi N, Carlezon WA, Jr, Nestler EJ. Region-specific induction of deltaFosB by repeated administration of typical versus atypical antipsychotic drugs. Synapse. 1999;33:118–128. doi: 10.1002/(SICI)1098-2396(199908)33:2<118::AID-SYN2>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Benes FM, Paskevich PA, Davidson J, Domesick VB. The effects of haloperidol on synaptic patterns in the rat striatum. Brain Res. 1985;329:265–273. doi: 10.1016/0006-8993(85)90532-3. [DOI] [PubMed] [Google Scholar]
- Bibb JA, Snyder GL, Nishi A, et al. Phosphorylation of DARPP-32 by Cdk5 modulates dopamine signalling in neurons. Nature. 1999;402:669–671. doi: 10.1038/45251. [DOI] [PubMed] [Google Scholar]
- Boegman RJ, Vincent SR. Involvement of adenosine and glutamate receptors in the induction of c-fos in the striatum by haloperidol. Synapse. 1996;22:70–77. doi: 10.1002/(SICI)1098-2396(199601)22:1<70::AID-SYN8>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Brandon EP, Logue SF, Adams MR, Qi M, Sullivan SP, Matsumoto AM, Dorsa DM, Wehner JM, McKnight GS, Idzerda RL. Defective motor behavior and neural gene expression in RIIbeta-protein kinase A mutant mice. J. Neurosci. 1998;18:3639–3649. doi: 10.1523/JNEUROSCI.18-10-03639.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brene S, Messer C, Nestler EJ. Expression of messenger RNAs encoding ionotropic glutamate receptors in rat brain: regulation by haloperidol. Neuroscience. 1998;84:813–823. doi: 10.1016/s0306-4522(97)00490-9. [DOI] [PubMed] [Google Scholar]
- Chakos MH, Shirakawa O, Lieberman J, Lee H, Bilder R, Tamminga CA. Striatal enlargement in rats chronically treated with neuroleptic. Biol. Psychiat. 1998;44:675–684. doi: 10.1016/s0006-3223(98)00029-8. [DOI] [PubMed] [Google Scholar]
- Chesselet MF, Delfs JM, Mackenzie L. Dopamine control of gene expression in basal ganglia nuclei: striatal and nonstriatal mechanisms. Adv. Pharmacol. 1998;42:674–677. doi: 10.1016/s1054-3589(08)60838-8. [DOI] [PubMed] [Google Scholar]
- Cheung P, Tanner KG, Cheung WL, Sassone-Corsi P, Denu JM, Allis CD. Synergistic coupling of histone H3 phosphorylation and acetylation in response to epidermal growth factor stimulation. Mol. Cell. 2000;5:905–915. doi: 10.1016/s1097-2765(00)80256-7. [DOI] [PubMed] [Google Scholar]
- Chong VZ, Young LT, Mishra RK. cDNA array reveals differential gene expression following chronic neuroleptic administration: implications of synapsin II in haloperidol treatment. J. Neurochem. 2002;82:1533–1539. doi: 10.1046/j.1471-4159.2002.01104.x. [DOI] [PubMed] [Google Scholar]
- Clayton AL, Rose S, Barratt MJ, Mahadevan LC. Phosphorylation of histone H3 on c-fos- and c-jun associated nucleosomes upon gene activation. EMBO J. 2000;19:3714–3726. doi: 10.1093/emboj/19.14.3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosio C, Heitz E, Allis CD, Borrelli E, Sassone-Corsi P. Chromatin remodeling and neuronal response: multiple signaling pathways induce specific histone H3 modifications and early gene expression in hippocampal neurons. J. Cell Sci. 2003;116:4905–4914. doi: 10.1242/jcs.00804. [DOI] [PubMed] [Google Scholar]
- Curran T, Morgan JI. Fos: an immediate-early transcription factor in neurons. J. Neurobiol. 1995;26:403–412. doi: 10.1002/neu.480260312. [DOI] [PubMed] [Google Scholar]
- Delfs JM, Anegawa NJ, Chesselet MF. Glutamate decarboxylase messenger RNA in rat pallidum: comparison of the effects of haloperidol, clozapine and combined haloperidolscopolamine treatments. Neuroscience. 1995;66:67–80. doi: 10.1016/0306-4522(94)00572-m. [DOI] [PubMed] [Google Scholar]
- Deutch AY, Lewis DA, Whitehead RE, Elsworth JD, Iadarola MJ, Redmond DE, Jr, Roth RH. Effects of D2 dopamine receptor antagonists on Fos protein expression in the striatal complex and entorhinal cortex of the nonhuman primate. Synapse. 1996;23:182–191. doi: 10.1002/(SICI)1098-2396(199607)23:3<182::AID-SYN7>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Doucet JP, Nakabeppu Y, Bedard PJ, et al. Chronic alterations in dopaminergic neurotransmission produce a persistent elevation of deltaFosB-like protein(s) in both the rodent and primate striatum. Eur. J. Neurosci. 1996;8:365–381. doi: 10.1111/j.1460-9568.1996.tb01220.x. [DOI] [PubMed] [Google Scholar]
- Dragunow M, Robertson GS, Faull RL, Robertson HA, Jansen K. D2 dopamine receptor antagonists induce fos and related proteins in rat striatal neurons. Neuroscience. 1990;37:287–294. doi: 10.1016/0306-4522(90)90399-o. [DOI] [PubMed] [Google Scholar]
- Eastwood SL, Heffernan J, Harrison PJ. Chronic haloperidol treatment differentially affects the expression of synaptic and neuronal plasticity-associated genes. Mol. Psychiat. 1997;2:322–329. doi: 10.1038/sj.mp.4000238. [DOI] [PubMed] [Google Scholar]
- Felsenfeld G, Groudine M. Controlling the double helix. Nature. 2003;421:448–453. doi: 10.1038/nature01411. [DOI] [PubMed] [Google Scholar]
- Fienberg AA, Hiroi N, Mermelstein PG, et al. DARPP-32: regulator of the efficacy of dopaminergic neurotransmission. Science. 1998;281:838–842. doi: 10.1126/science.281.5378.838. [DOI] [PubMed] [Google Scholar]
- Fitzgerald LW, Deutch AY, Gasic G, Heinemann SF, Nestler EJ. Regulation of cortical and subcortical glutamate receptor subunit expression by antipsychotic drugs. J. Neurosci. 1995;15:2453–2461. doi: 10.1523/JNEUROSCI.15-03-02453.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg EC, Downs KM, Christensen HM, Im H, Nuzzi PA, Bresnick EH. Developmentally dynamic histone acetylation pattern of a tissue-specific chromatin domain. Proc. Natl Acad. Sci. USA. 2000;97:14494–14499. doi: 10.1073/pnas.97.26.14494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox CA, Mansour A, Watson SJ., Jr The effects of haloperidol on dopamine receptor gene expression. Exp. Neurol. 1994;130:288–303. doi: 10.1006/exnr.1994.1207. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Keefe KA, Steiner H. Dopamine-mediated gene regulation in striatum. Adv. Pharmacol. 1998;42:670–673. doi: 10.1016/s1054-3589(08)60837-6. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Moratalla R, Robertson H. Amphetamine and cocaine induce drug-specific activation of the c-fos gene in striosome-matrix compartments and limbic subdivisions of the striatum. Proc. Natl Acad. Sci. USA. 1990;87:6912–6916. doi: 10.1073/pnas.87.17.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther EC, Stone DJ, Gerwien RW, Bento P, Heyes MP. Prediction of clinical drug efficacy by classification of drug-induced genomic expression profiles in vitro. Proc. Natl Acad. Sci. USA. 2003;100:9608–9613. doi: 10.1073/pnas.1632587100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur RE, Maany V, Mozley PD, Swanson C, Bilker W, Gur RC. Subcortical MRI volumes in neuroleptic-naïve and treated patients with schizophrenia. Am. J. Psychiat. 1998;155:1711–1717. doi: 10.1176/ajp.155.12.1711. [DOI] [PubMed] [Google Scholar]
- Healy DJ, Meador-Woodruff JH. Clozapine and haloper-idol differentially affect AMPA and kainate receptor subunit mRNA levels in rat cortex and striatum. Mol. Brain Res. 1997;47:331–338. doi: 10.1016/s0169-328x(97)00064-8. [DOI] [PubMed] [Google Scholar]
- Heckers S, Heinsen H, Heinsen Y, Beckmann J. Cortex, white matter and basal ganglia in schizophrenia: a volumetric postmortem study. Biol. Psychiat. 1991;29:556–566. doi: 10.1016/0006-3223(91)90091-y. [DOI] [PubMed] [Google Scholar]
- Hernandez-Lopez S, Tkatch T, Perez-Garci E, Galarraga E, Bargas J, Hamm H, Surmeier DJ. D2 dopamine receptors in striatal medium spiny neurons reduce L-type Ca2+ currents and excitability via a novel PLC[beta]1-IP3-calcineurin-signaling cascade. J. Neurosci. 2000;20:8987–8995. doi: 10.1523/JNEUROSCI.20-24-08987.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain N, Flumerfelt BA, Rajakumar N. Glutamatergic regulation of haloperidol-induced c-fos expression in the rat striatum and nucleus accumbens. Neuroscience. 2001;102:391–399. doi: 10.1016/s0306-4522(00)00487-5. [DOI] [PubMed] [Google Scholar]
- Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Zisook S, Heaton RK, Moranville JT, Hesselink JR, Braff DL. Magnetic resonance imaging abnormalities in lenticular nuclei and cerebral cortex in schizophrenia. Arch. Gen. Psychiat. 1991;48:881–890. doi: 10.1001/archpsyc.1991.01810340013002. [DOI] [PubMed] [Google Scholar]
- Kerns JM, Sierens DK, Kao LC, Klawans HL, Carvey PM. Synaptic plasticity in the rat striatum following chronic haloperidol treatment. Clin. Neuropharmacol. 1992;15:488–500. doi: 10.1097/00002826-199212000-00006. [DOI] [PubMed] [Google Scholar]
- Konradi C, Heckers S. Antipsychotic drugs and neuroplasticity: insights into the treatment and neurobiology of schizophrenia. Biol. Psychiat. 2001;50:729–742. doi: 10.1016/s0006-3223(01)01267-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konradi C, Leveque JC, Hyman SE. Amphetamine and dopamine-induced immediate early gene expression in striatal neurons depends upon postsynaptic NMDA receptors and calcium. J. Neurosci. 1996;16:4231–4239. doi: 10.1523/JNEUROSCI.16-13-04231.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo MH, Allis CD. In vivo cross-linking and immuno-precipitation for studying dynamic Protein:DNA associations in a chromatin environment. Methods. 1999;19:425–433. doi: 10.1006/meth.1999.0879. [DOI] [PubMed] [Google Scholar]
- Laprade N, Soghomonian JJ. Differential regulation of mRNA levels encoding for the two isoforms of glutamate decarboxylase (GAD65 and GAD67) by dopamine receptors in the rat striatum. Mol. Brain Res. 1995;34:65–74. doi: 10.1016/0169-328x(95)00139-j. [DOI] [PubMed] [Google Scholar]
- Lau YS, Petroske E, Meredith GE, Wang JQ. Elevated neuronal nitric oxide synthase expression in chronic haloperidol-treated rats. Neuropharmacology Dec. 2003;45(7):986–994. doi: 10.1016/s0028-3908(03)00314-9. [DOI] [PubMed] [Google Scholar]
- Leveque J-C, Macías W, Rajadhyaksha A, et al. Intracellular modulation of NMDA receptor function by antipsychotic drugs. J. Neurosci. 2000;20:4011–4020. doi: 10.1523/JNEUROSCI.20-11-04011.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Chen P, Sinogeeva N, Gorospe M, Wersto RP, Chrest FJ, Barnes J, Liu Y. Arsenic trioxide promotes histone H3 phosphoacetylation at the chromatin of CASPASE-10 in acute promyelocytic leukemia cells. J. Biol. Chem. 2002;277:49504–49510. doi: 10.1074/jbc.M207836200. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Lerman DN, Khaing ZZ, Weickert CS, Weinberger DR. Gene expression in dopamine and GABA systems in an animal model of schizophrenia: effects of antipsychotic drugs. Eur. J. Neurosci. 2003;18:391–402. doi: 10.1046/j.1460-9568.2003.02738.x. [DOI] [PubMed] [Google Scholar]
- Liu FC, Graybiel AM. Spatiotemporal dynamics of CREB phosphorylation: transient versus sustained phosphorylation in the developing striatum. Neuron. 1996;17:1133–1144. doi: 10.1016/s0896-6273(00)80245-7. [DOI] [PubMed] [Google Scholar]
- Lo WS, Trievel RC, Rojas JR, Duggan L, Hsu JY, Allis CD, Marmorstein R, Berger SL. Phosphorylation of serine 10 in histone H3 is functionally linked in vitro and in vivo to Gcn5-mediated acetylation at lysine 14. Mol. Cell. 2000;5:917–926. doi: 10.1016/s1097-2765(00)80257-9. [DOI] [PubMed] [Google Scholar]
- McCullumsmith RE, Stincic TL, Agrawal SM, Meador-Woodruff JH. Differential effects of antipsychotics on haloperidol-induced vacuous chewing movements and subcortical gene expression in the rat. Eur. J. Pharmacol. 2003;477:101–112. doi: 10.1016/j.ejphar.2003.08.018. [DOI] [PubMed] [Google Scholar]
- Merchant KM, Dorsa DM. Differential induction of neurotensin and c-fos gene expression by typical and atypical antipsychotics. Proc. Natl Acad. Sci. USA. 1993;90:3447–3451. doi: 10.1073/pnas.90.8.3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith GE, De Souza IE, Hyde TM, Tipper G, Wong ML, Egan MF. Persistent alterations in dendrites, spines, and dynorphinergic synapses in the nucleus accumbens shell of rats with neuroleptic-induced dyskinesias. J. Neurosci. 2000;20:7798–7806. doi: 10.1523/JNEUROSCI.20-20-07798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshul CK, Janowsky A, Casey RK, Stallbaumer RK, Taylor B. Effect of haloperidol and clozapine on the density of ‘perforated’ synapses in caudate, nucleus accumbens and medial pre-frontal cortex. Psychopharmacology. 1992;106:45–52. doi: 10.1007/BF02253587. [DOI] [PubMed] [Google Scholar]
- Mijnster MJ, Schotte A, Docter GJ, Voorn P. Effects of risperidone and haloperidol on tachykinin and opioid precursor peptide mRNA levels in the caudate-putamen and nucleus accumbens of the rat. Synapse. 1998;28:302–312. doi: 10.1002/(SICI)1098-2396(199804)28:4<302::AID-SYN6>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol. Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- Miyakawa T, Leiter LM, Gerber DJ, Gainetdinov RR, Sotnikova TD, Zeng H, Caron MG, Tonegawa S. Conditional calcineurin knockout mice exhibit multiple abnormal behaviors related to schizophrenia. Proc. Natl Acad. Sci. USA. 2003;100:8987–8992. doi: 10.1073/pnas.1432926100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B. Bringing order to the glutamate chaos in schizophrenia. Neuron. 2003;40:881–884. doi: 10.1016/s0896-6273(03)00757-8. [DOI] [PubMed] [Google Scholar]
- Moratalla R, Xu M, Tonegawa S, Graybiel AM. Cellular responses to psychomotor stimulant and neuroleptic drugs are abnormal in mice lacking the D1 dopamine receptor. Proc. Natl Acad. Sci. USA. 1996;93:14, 928–14933. doi: 10.1073/pnas.93.25.14928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- Nakahara T, Gotoh L, Motomura K, Kawanami N, Ohta E, Hirano M, Uchimura H. Acute and chronic haloperidol treatments increase parkin mRNA levels in the rat brain. Neurosci. Lett. 2001;306:93–96. doi: 10.1016/s0304-3940(01)01880-8. [DOI] [PubMed] [Google Scholar]
- Nguyen TV, Kosofsky BE, Birnbaum R, Cohen BM, Hyman SE. Differential expression of c-fos and zif268 in rat striatum after haloperidol, clozapine, and amphetamine. Proc. Natl Acad. Sci. USA. 1992;89:4270–4274. doi: 10.1073/pnas.89.10.4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onn SP, Grace AA. Repeated treatment with haloperidol and clozapine exerts differential effects on dye coupling between neurons in subregions of striatum and nucleus accumbens. J. Neurosci. 1995;15:7024–7036. doi: 10.1523/JNEUROSCI.15-11-07024.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigent C, Dimitrov S. Phosphorylation of serine 10 in his-tone H3, what for? J. Cell Sci. 2003;116:3677–3685. doi: 10.1242/jcs.00735. [DOI] [PubMed] [Google Scholar]
- Rajadhyaksha A, Leveque J, Macias W, Barczak A, Konradi C. Molecular components of striatal plasticity: the various routes of cyclic AMP pathways. Dev. Neurosci. 1998;20:204–215. doi: 10.1159/000017314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajadhyaksha A, Barczak A, Macias W, Leveque JC, Lewis SE, Konradi C. L-Type Ca(2+) channels are essential for glutamate-mediated CREB phosphorylation and c-fos gene expression in striatal neurons. J. Neurosci. 1999;19:6348–6359. doi: 10.1523/JNEUROSCI.19-15-06348.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson GS, Vincent SR, Fibiger HC. D1 and D2 dopamine receptors differentially regulate c-fos expression in striatonigral and striatopallidal neurons. Neuroscience. 1992;49:285–296. doi: 10.1016/0306-4522(92)90096-k. [DOI] [PubMed] [Google Scholar]
- Robertson HA, Paul ML, Moratalla R, Graybiel AM. Expression of the immediate early gene c-fos in basal ganglia: induction by dopaminergic drugs. Can. J. Neurol. Sci. 1991;18:380–383. doi: 10.1017/s0317167100032480. [DOI] [PubMed] [Google Scholar]
- Salvador LM, Park Y, Cottom J, et al. Follicle-stimulating hormone stimulates protein kinase A-mediated histone H3 phos phorylation and acetylation leading to select gene activation in ovarian granulosa cells. J. Biol. Chem. 2001;276:40146–40155. doi: 10.1074/jbc.M106710200. [DOI] [PubMed] [Google Scholar]
- Schoots O, Seeman P, Guan HC, Paterson AD, Van Tol HH. Long-term haloperidol elevates dopamine D4 receptors by 2-fold in rats. Eur. J. Pharmacol. 1995;289:67–72. doi: 10.1016/0922-4106(95)90169-8. [DOI] [PubMed] [Google Scholar]
- Sibley DR. Molecular biology of dopamine receptors. In: Ariano MA, Surmeier DJ, editors. Molecular and Cellular Mechanisms of Neostriatal Function. Landes, Austin, TX.: 1995. pp. 255–272. [Google Scholar]
- de Souza IE, Meredith GE. NMDA receptor blockade attenuates the haloperidol induction of Fos protein in the dorsal but not the ventral striatum. Synapse. 1999;32:243–253. doi: 10.1002/(SICI)1098-2396(19990615)32:4<243::AID-SYN1>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Thomson S, Clayton AL, Mahadevan LC. Independent dynamic regulation of histone phosphorylation and acetylation during immediate-early gene induction. Mol. Cell. 2001;8:1231–1241. doi: 10.1016/s1097-2765(01)00404-x. [DOI] [PubMed] [Google Scholar]
- Tolhuis B, Palstra RJ, Splinter E, Grosveld F, de Laat W. Looping and interaction between hypersensitive sites in the active beta-globin locus. Mol. Cell. 2002;10:1453–1465. doi: 10.1016/s1097-2765(02)00781-5. [DOI] [PubMed] [Google Scholar]
- Turner BM. Cellular memory and the histone code. Cell. 2002;111:285–291. doi: 10.1016/s0092-8674(02)01080-2. [DOI] [PubMed] [Google Scholar]
- Uranova NA, Orlovskaya DD, Apel K, Klintsova AJ, Haselhorst U, Schenk H. Morphometric study of synaptic patterns in the rat caudate nucleus and hippocampus under haloperidol treatment. Synapse. 1991;7:253–259. doi: 10.1002/syn.890070402. [DOI] [PubMed] [Google Scholar]
- Wan M, Zhao K, Francke U. MECP2 truncating mutations cause histone H4 hyperacetylation in Rett syndrome. Hum. Mol. Genet. 2001;10:1085–1092. doi: 10.1093/hmg/10.10.1085. [DOI] [PubMed] [Google Scholar]