Abstract
Objective
Describe safety and efficacy of a supervised, low-to-moderate intensity strength training program adopted during pregnancy among women at increased risk for back pain.
Methods
32 women adopted strength training twice per week for 12 weeks. Data on musculoskeletal injuries, symptoms, blood pressure, and the absolute external load used for 5 of 6 exercises were obtained during each session. A submaximal lumbar extension endurance exercise test was performed at weeks 5, 10, and 13.
Results
The mean (± SD) exercise session attendance rate was 80.5% (± 11.3%). No musculoskeletal injuries occurred. Potentially adverse symptoms (eg, dizziness) were infrequent (2.1% of sessions). Repeated-measures ANOVA showed large increases in the external load across 12 weeks (all P values < .001) and the percentage increases in external load from weeks 1 to 12 were 36% for leg press, 39% for leg curl, 39% for lat pull down, 41% for lumbar extension and 56% for leg extension. Training was associated with a 14% increase in lumbar endurance. Blood pressure was unchanged following acute exercise sessions and after 12 weeks of exercise training.
Conclusion
The adoption of a supervised, low-to-moderate intensity strength training program during pregnancy can be safe and efficacious for pregnant women.
Keywords: blood pressure, exercise, physical activity, resistance training, symptoms, weight lifting
Physical inactivity during pregnancy reduces fitness and appears to increase fetal and maternal risk for health problems such as diabetes.1–4 Low-to-moderate intensity aerobic-type exercise performed during pregnancy is safe, improves maternal fitness and appears to improve several aspects of fetal and maternal health.5–7 Relatively little is known, however, about the influence of strength training on fitness- or health-related outcomes in pregnant women.1,2,8 Rubber tubing has been used to provide resistance in some studies;2 consequently, the progression in exercise intensity (eg, the increase in external load across training) has not been quantified precisely.
Though relatively few injuries occur during recreational strength training,9 the injury rate among women of child bearing age has increased as the number of women participating has increased.10 The injury rate among the ~3% to 4% of pregnant women in the United States who lift weights11 is unknown but their risk for musculoskeletal injury might be higher than that of nonpregnant women. Pregnancy elevates tissue levels of the peptide hormone relaxin which could increase the risk of musculoskeletal injury during strength training.12 Obstetricians recommend resistance exercise less frequently than aerobic exercise modes, perhaps because of theoretical safety concerns such as an increased risk of musculoskeletal injuries or aortic distensions.13 When weight lifting exercises were incorporated into an aerobic training program the safety and efficacy of the outcomes appeared to be uninfluenced by the weight lifting14 and Canadian experts have recommended strength training exercises for pregnant women without complications.15 Other experts have not recommended strength training, possibly because of safety concerns.5 There is a need for more data concerning the safety and efficacy of resistance training for pregnant women.
The purpose of this paper is to describe the progression of a supervised, low-to-moderate intensity strength training program adopted by pregnant women and summarize whether the program was associated with musculoskeletal injuries or changes in lumbar muscular endurance, symptoms (eg, dizziness) or resting blood pressure. Resting blood pressure was of interest because hypertension is a leading cause of maternal and fetal morbidity and it is unknown whether the adoption of a progressive resistance training program by pregnant women alters resting blood pressure. Effectiveness data on primary outcomes of the intervention will be reported elsewhere.
Methods
Participants
We recruited healthy women primarily through Athens-Clarke County area midwives and obstetricians, advertisements in a local magazine for parents and word of mouth by some study participants. The participants were required to be at low risk for pregnancy-related complications, between 18 to 38 years, between 21 to 25 weeks gestation and with back pain or a history of back pain. Excluded were those who self-reported that they regularly (≥ twice per week during the past month) performed strength training,16 had an orthopedic or cardiovascular limitation,17 had an uncontrolled psychiatric disorder,18 or had in the current or a prior pregnancy any of the following: (a) 2 or more miscarriages, (b) premature labor, (c) placental previa, (d) poor fetal growth, (e) low prepregnancy body weight (BMI < 18), (f) prepregnancy obesity (BMI > 30), (g) a multiple birth pregnancy, (h) preeclampsia, (i) preterm rupture of membranes, (j) uterine growth retardation, (k) incompetent cervix/cerclage, (l) recurrent vaginal bleeding, (m) anemia, or (n) diabetes. Participants read and signed an informed consent previously approved by the University of Georgia Institutional Review Board.
Training
Participants were asked to perform low-to-moderate intensity strength training twice per week for 12 weeks. The strength training was supervised by experienced exercise specialists. On the first day of the exercise program, and as needed after that, the participants were instructed on proper breathing such as avoiding the Valsalva maneuver (ie, attempting to forcibly exhale against a closed airway) and training techniques so as to minimize injuries. Water was available ad libitum during each exercise session.
Participants completed a preexercise questionnaire that inquired about pregnancy-related symptoms of potential relevance to that day’s exercise prescription. Blood pressure was assessed after 5 minutes of seated rest. The presence of severe hypertension (systolic pressure ≥ 180 mmHg or diastolic pressure ≥ 100 mmHg) was an absolute contraindication to that day’s resistance exercise and it occurred in one participant before her final planned exercise session.
Participants performed a whole body warm up by walking on a treadmill for ~5 minutes. Next, 6 resistance exercises were performed in the following order: dual leg extension, dual leg press, dual arm lat pull, dual leg curl, lumbar extensions, and an abdominal exercise aimed at activating the transverse abdominis muscles.
Dual leg extension, dual leg press, and dual arm lat pull down were performed in a seated position on a Universal Gym. Dual leg curls were performed in a seated position on a Cybex Eagle. Lumbar extension was performed in a seated position using a MedX lumbar extension machine.19 For these 5 exercises the number of sets (n = 2) and the number of repetitions per set (n = 15) were held constant throughout training. These exercises were performed at a low-to-moderate velocity (~2 second concentric and ~2 second eccentric) and with a moderate rest period between sets (~1 minute) and exercises (~2 minutes).
The initial external loads for the 5 exercises were selected to achieve low-to-moderate intensity based on ratings of perceived exertion (RPE). The 6 to 20 RPE scale was used.20 On this scale, exercise rated as a 13 (verbal anchor = “somewhat hard”) represents a moderate intensity and ratings that are 11 (“fairly light”) or less represent low intensity exercise. Participants were taught how to provide RPE at the training outset. Standardized instructions emphasized reporting the effort put into each 2-set task. Ratings were obtained after the second set. The external load was progressively increased based on RPE responses to each exercise. When RPE was lower in an exercise session compared with the prior session, the external load was allowed to increase in the next session, usually by the smallest amount permitted by the machine.
Abdominal exercise typically was performed in a standing position. The participants were taught first to exhale then to attempt to draw in their belly button toward their spine as if they were trying to button up pants that were too tight in the waist. In nonpregnant women this preferentially activates the transverse abdominis.21 To avoid breath holding, the participants were asked to talk while learning to perform this exercise. Repetitions per set for this exercise were held constant at 8 throughout training. The duration of each repetition was ~8 seconds. Exercise progression involved an increase in the number of sets performed. There was a rest period of ~1 minute between sets.
Approximately 5 minutes after the final exercise in the ~45 minute exercise sessions, seated blood pressure was measured and the participants completed a questionnaire addressing potentially problematic symptoms that might have been experienced during the exercise session.
Adherence
Attendance was determined from exercise logs and expressed as the percentage of sessions attended out of 24 possible sessions.
Outcome Measures
Musculoskeletal Injury
Immediately after each session, the participants indicated by questionnaire whether they had been injured during that day’s workout and if so to describe the injury.
Potentially Problematic Symptoms
The participants were asked via questionnaire about the presence of: chest, pelvic or abdominal pain, sudden swelling of hands or feet, headache or visual disturbances, irregular heartbeats, dizziness, or unexpected vaginal bleeding/leaking.
Progression of Exercise Intensity
Progression of exercise intensity was described in both absolute (weight in kilograms) and relative (perceived exertion) units.
Blood Pressure
Blood pressure was measured while seated before and after exercise via auscultation after sitting for ~5 minutes.22
Lumbar Endurance
Sixteen of 32 participants completed all 3 3-minute lumbar extension endurance exercise test at weeks 5, 10, and 13. The weight used at the first test (17.2 ± 2.9 kg) was held constant for the 3 tests for each individual. The speed at which the repetitions were performed was not paced. Participants were instructed to perform the repetitions at a controlled and safe pace. Immediately after the test, RPEs were reported. To account for test-to-test variations in power output, the RPE data at each trial were divided by the number of repetitions performed at each trial to yield a criterion score used for analysis.
Statistical Analysis
Raw data were entered into SPSS Statistical Software. Weekly averages were used for analysis of training data. When data for one session was missing in a given week, the data for the other session that week was used as the criterion score. When data for both sessions in a week were missing, data were imputed using the average from the prior week. 2.7% of the exercise session data were imputed (21 / 768 = 2.7%; 768 = 32 participants × 24 workouts). The conclusion of this paper is unchanged whether the imputations were included or excluded from the analysis.
The statistical significance of the main effect across Time was determined from F-statistics using either one-way or two-way repeated-measures ANOVAs. The one-way analysis examined changes across 12 weeks. The two-way analysis examined main and interaction effects of an Acute Exercise Time factor (from pre- to postexercise) and a Chronic Exercise Time factor (across 12 weeks) on resting blood pressure. A sample size of 32 provided statistical power of ≥ .80 for detecting a 0.5 SD change (5 mmHg) across time in resting blood pressure given an alpha of .05 and an observed between trial correlation in blood pressures of 0.7.23 Effect sizes for F-statistics were expressed as partial eta squared (η2 ). Paired sample t tests were used as post hocs to test for simple effects. The family-wise error rate was controlled using the Bonferroni adjustment. Percent change and Cohen’s d were reported to provide additional measures of effect size. Data are reported as mean (± SD) in the text.
Results
Age, height and weight at week 1 was 29 (± 4) yrs, 166 (±6) cm, and 76 (±2) kg, respectively. The average weight gain across 12 weeks was 3 (±1) kg. The attendance rate across 12 weeks was 81% (±11%). One women reported preeclampsia and no other negative pregnancy outcomes were reported.
No musculoskeletal injuries occurred during strength training. Potentially problematic symptoms were infrequent. Symptoms associated with an acute bout of resistance exercise were reported a total of 13 times across a total of 768 exposures to resistance exercise (1.7%). Palpitations or chest pains were never reported. The most common symptoms were dizziness (8/13) and abdominal/pelvic pain (4/13). One person who did not have a headache at the beginning of one exercise session did report a headache after that exercise session. Three of the 13 symptoms were reported during the first 3 workouts. Zero to two symptoms was reported weekly across weeks 3 to 12.
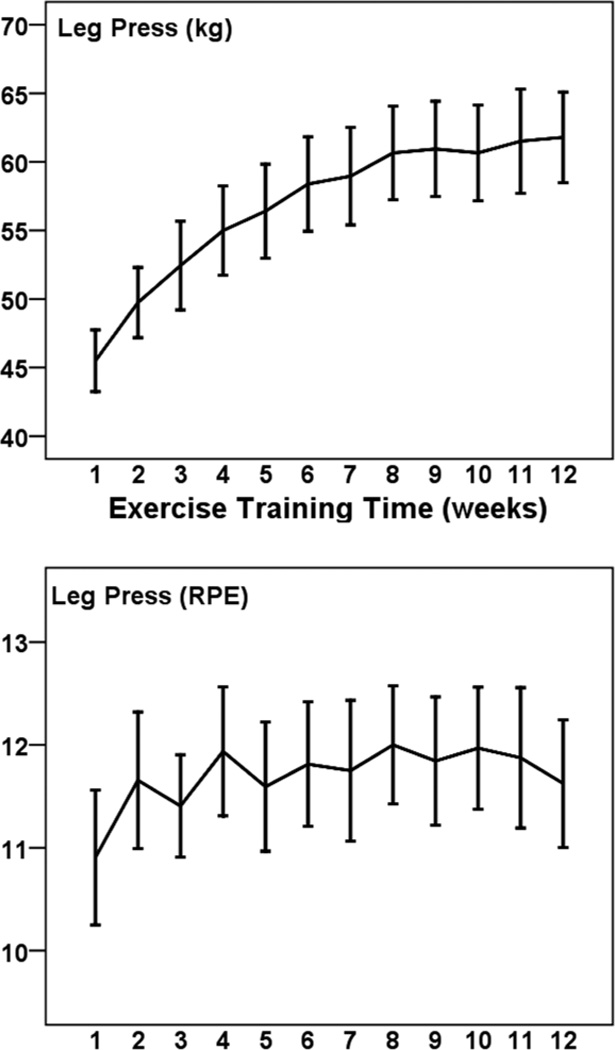
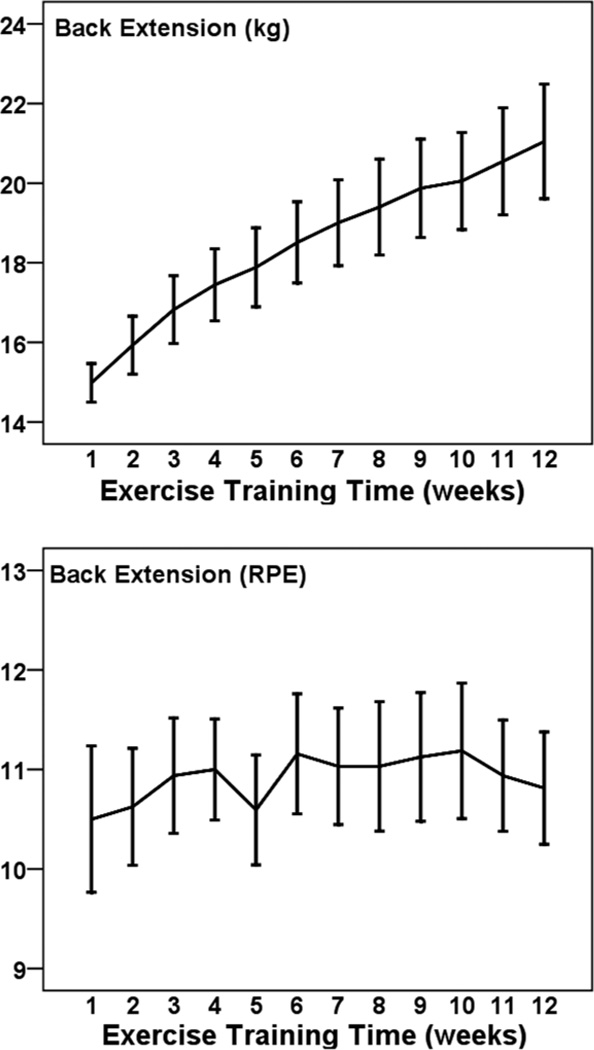
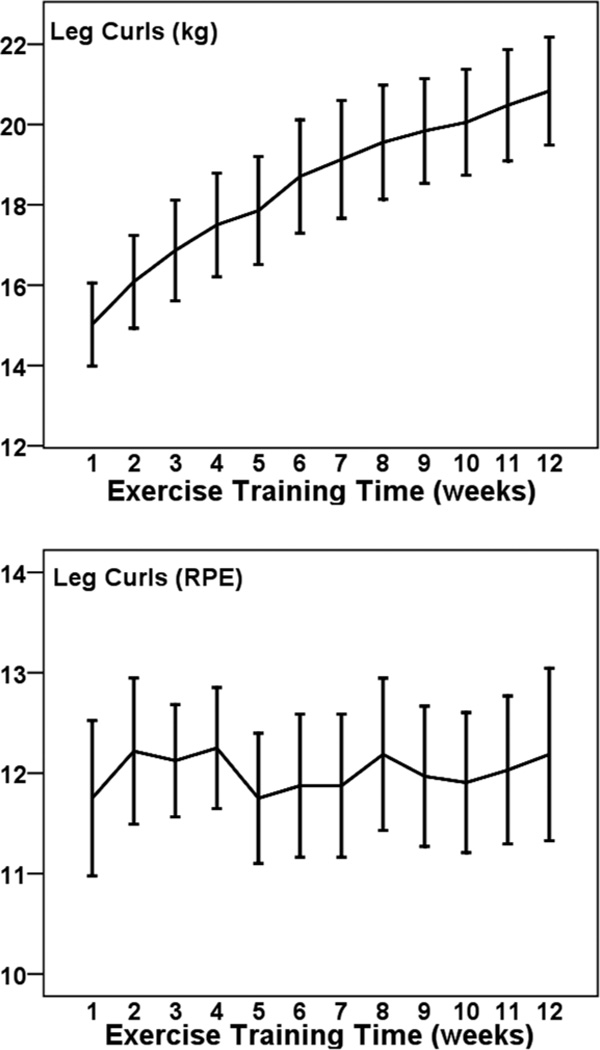
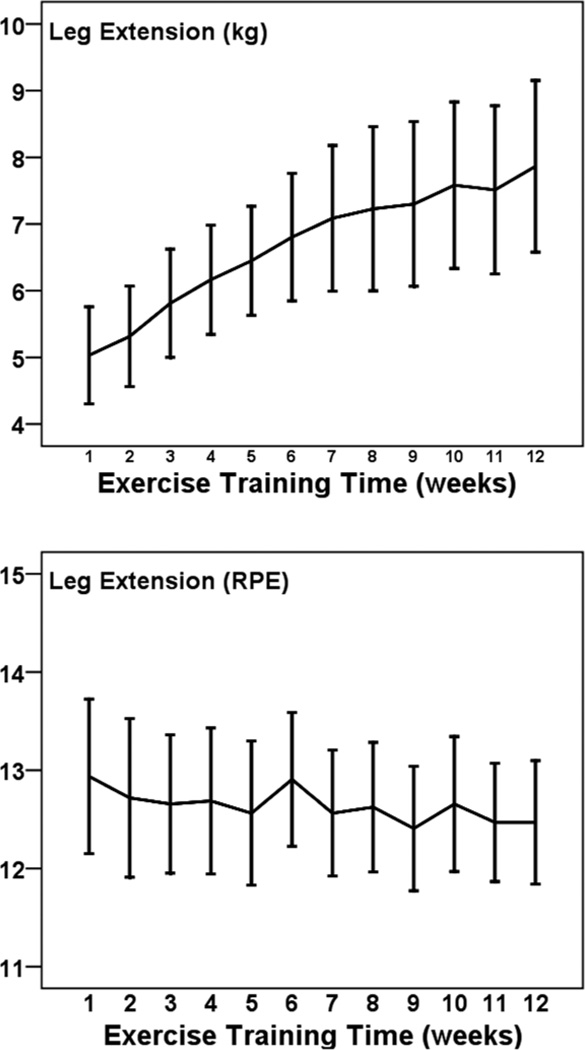
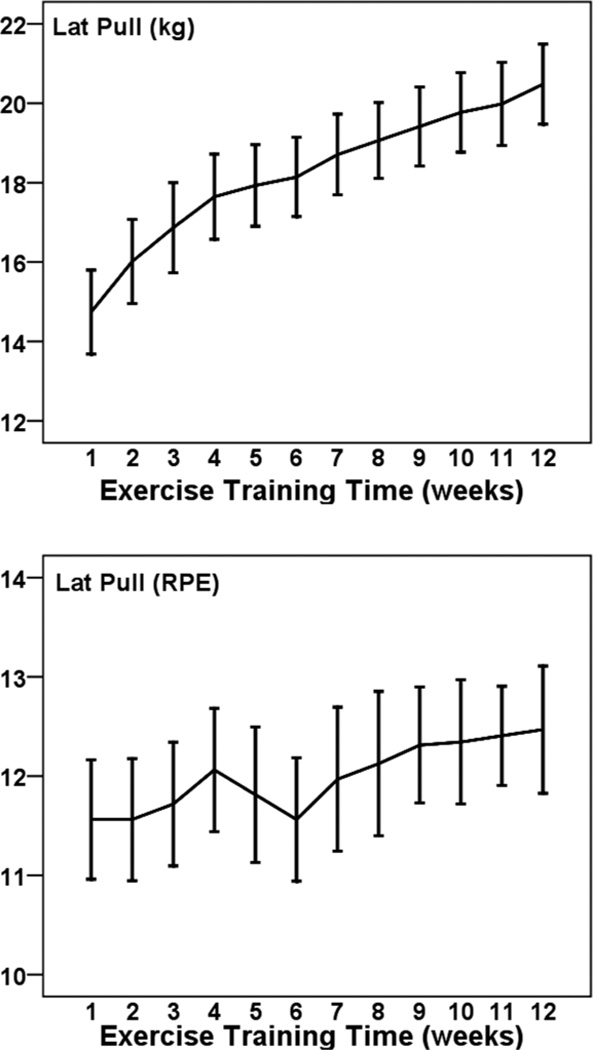
Figures 1 to 5 illustrate the progression of the intensity of strength training for the 5 exercises performed in a seated position. For each exercise there was a statistically significant increase in the external load used across 12 weeks (η2 of .431 to .691; all P < .001). The percentage increases from weeks 1 to 12 were 36% for leg press (Cohen’s d = 2.1), 39% for leg curl (d = 1.7), 39% for lat pull down (d = 2.0), 41% for lumbar extension (d = 2.2), and 56% for leg extension (d = 1.0). The exercises were performed at a low-to-moderate perceived intensity (mean RPEs of 10.5 to 12.9; 11 = fairly light and 13 = somewhat hard). RPE did not change significantly across 12 weeks for these exercises (p from .083 to .717).
Figure 1.
— Mean (± SE) progression of absolute (kg; Top panel) and perceived (RPE; Bottom panel) training intensity for leg press across 12 weeks among 32 pregnant women.
Figure 5.
— Mean (± SE) progression of absolute (kg; Top Panel) and perceived (RPE; Bottom Panel) training intensity for lumbar extension across 12 weeks among 32 pregnant women.
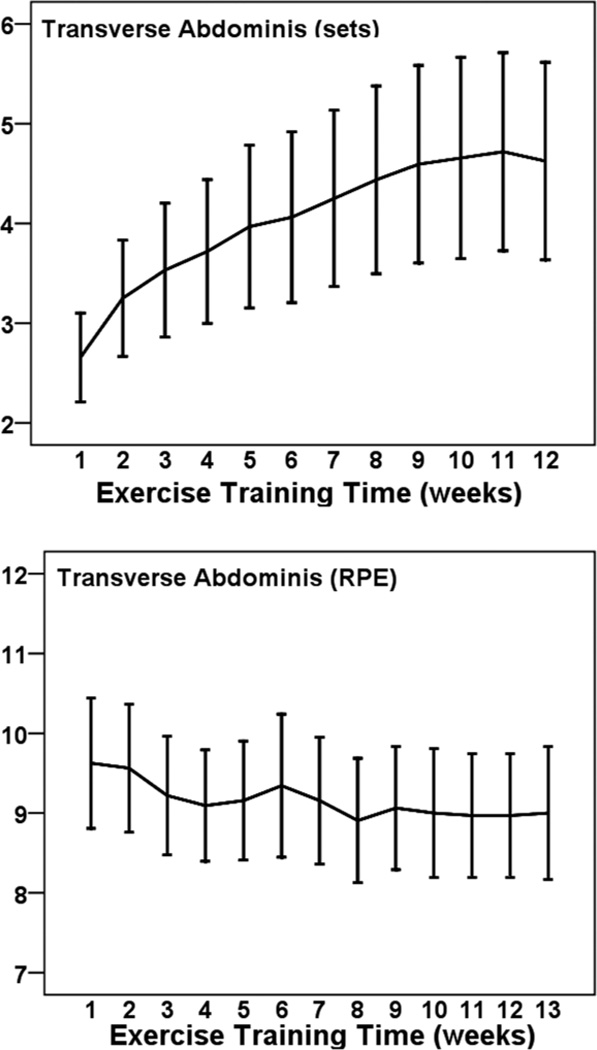
Figure 6 illustrates the progression of sets and RPE values for abdominal actions. The number of sets increased by 2 (from 2.6 to 4.6, a 77% increase, d = 1.0) from week 1 to 12. This exercise was performed at a low perceived intensity that ranged between 8.9 and 9.6 (9 = very light) and did not change significantly across weeks (P > .05).
Figure 6.
— Mean (± SE) progression of sets (Top Panel) and perceived training intensity (RPE; Bottom Panel) transverse abdominis muscle actions across 12 weeks among 32 pregnant women.
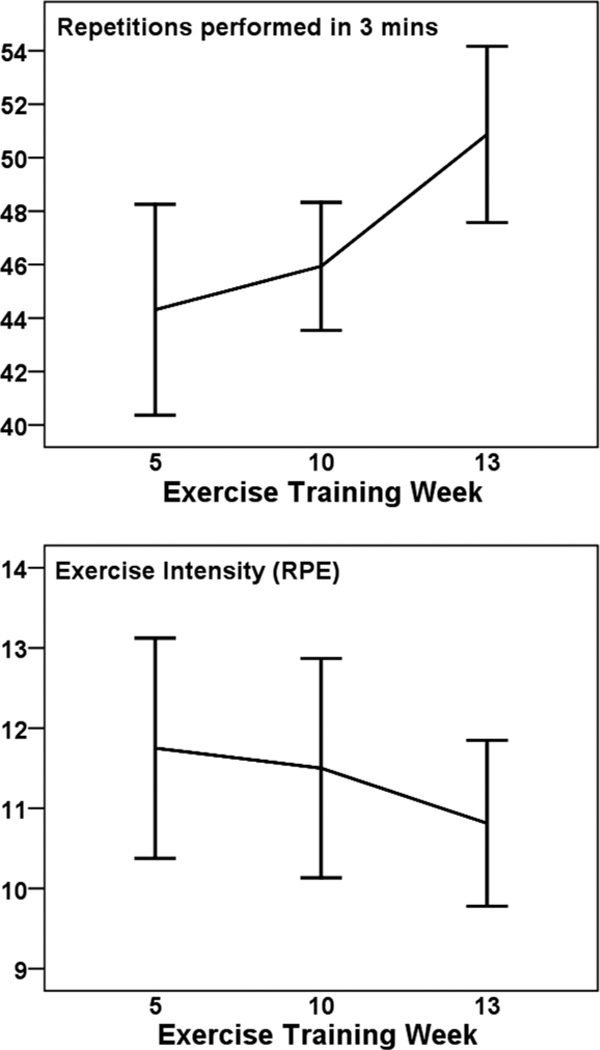
Figure 7 illustrates the results from the lumbar endurance test. The number of repetitions performed during the 3-minute exercise test changed over time (F2,30 = 8.179, P = .001, η2 = .353). Post hoc tests showed that number of repetitions increased at week 13 compared with both week 5 (t = −3.59, df = 15, P < .003) and week 10 (t = −3.06, df = 15, P < .008). The average increase in repetitions from week 5 to 13 was 6 repetitions (13.6%). The effort associated with the exercise tests also changed over time (F2,30 = 15.774, P < .0001, η2 = .513, RPE adjusted for repetitions). Post hoc tests showed that adjusted RPE was lower at week 13 compared with both week 5 (t = 5.065, df = 15, P < .0001) and week 10 (t = 5.072, df = 15, P < .0001). The unadjusted mean reduction in RPE scores from week 5 to 13 was 1 raw score unit.
Figure 7.
— Mean (± SE) repetitions (Top Panel) and unadjusted perceived exertion (RPE; Bottom Panel) scores associated with the 3-min lumbar extension endurance test among 16 pregnant women.
Neither acute (preexercise compared with postexercise across all sessions) nor chronic (week 1 compared with week 12) exercise was associated with a significant change in systolic or diastolic blood pressure (all P > .10). Systolic and diastolic blood pressure at week 12 (113.9 ± 10.0 / 73.3 ± 7.1 mmHg) did not differ from week 1 (113.5 ± 8.4 / 71.9 ± 6.8 mmHg). Averaged across all exercise sessions, systolic blood pressure before and after exercise was 113.5 and 112.5 mmHg, respectively. Averaged across all exercise sessions, diastolic blood pressure before and after exercise was 71.6 and 71.7 mmHg, respectively.
Discussion
Because some physiological responses to pregnancy theoretically could increase the risk of musculoskeletal injury, professionals have emphasized being aware of the increased potential for musculoskeletal injury when prescribing exercise for pregnant women.5 One finding of this investigation was that the adoption of a supervised, low-to-moderate intensity strength training program during pregnancy did not cause any musculoskeletal injuries among women at increased risk for low back pain. The low incidence of musculoskeletal injuries probably was due in part to the low-to-moderate intensity and careful supervision, including education about proper technique. These factors are thought to be important in preventing musculoskeletal injuries caused by strength training.24
Blood pressure was unchanged immediately after the exercise sessions compared with before the sessions, and the 12-week strength training program was not associated with any change in blood pressure. In nonpregnant samples, blood pressure is sometimes,25 but not always,26 reduced after a single low-to-moderate intensity bout of resistance exercise. This postexercise hypotension could have clinical importance if it helps to cause an adaptation resulting in a sustained reduction of blood pressure. Quantitative reviews have established that among body weight stable, nonpregnant normotensives, resting systolic and diastolic blood pressures typically are reduced after short duration strength training programs (median duration of 12 weeks), albeit by only ~3 mmHg.27 The most relevant data available from pregnant women have shown that the risk of preeclampsia is reduced by 35% among women who reported performing any physical activity either before becoming pregnant or during the first 20 weeks of pregnancy compared with physically inactive women.4 A reduced risk of preeclampsia also was found with stair climbing, an activity that can have a relatively high resistance.4 The single case of preeclampsia in this study was expected based on the population prevalence of preeclampsia.
Another important finding of the present investigation concerned potentially problematic symptoms reported immediately after each exercise session. Symptoms are often used to judge whether the relative exercise intensity is too high or if an individual either is not feeling well or is experiencing an adverse event. We obtained data on several symptoms that have been recommended be used to terminate an exercise session during pregnancy.5 The rate of potentially problematic symptoms was generally low. Given that we recruited participants with back pain or a history of back pain, it was not surprising that abdominal/pelvic pain was one of the two most common symptoms reported. The most common symptom reported, however, was dizziness. Dizziness is known to occur if cardiac output is reduced during exercise, especially when the exercise is accompanied by the Valsalva maneuver.28 The presence of dizziness in even a few participants underscores the importance of monitoring for dizziness and teaching pregnant exercisers to avoid the Valsalva maneuver during resistance exercise.29 Our findings also highlight the importance of being vigilant in monitoring symptoms during the first few exercise sessions when people are learning proper breathing and training techniques.
Untrained pregnant women who adopted a low-tomoderate intensity strength training program demonstrated a large progression in the absolute training load even though the relative perceived exercise intensity during training sessions was maintained at a low-tomoderate level. The participants were encouraged to continually progress to higher external loads within the framework of not exceeding a moderate level of perceived exercise intensity. Prior strength training investigations with pregnant women often have not carefully described the progression of the external loads.2 The progression in external training load found here is consistent with what has been reported in other exercise programs aimed at improving muscular strength and endurance for fitness and health purposes among nonpregnant samples.29–31 The strength training performed here also was associated with improved endurance of the lumbar extensor muscle group. The magnitude of the improvement (13.6%) was less than the increased muscular strength that has been observed in nonpregnant samples involved in more strenuous strength training of the lumbar extension musculature.32 The present strength training program emphasized safe progression and de-emphasized maximal strength or endurance gains or progression as fast as possible. The improvement in lumbar endurance cannot be attributed solely to the strength training program, however, because of the absence of control participants who completed the endurance tests. We elected to not have the controls complete the endurance tests for practical reasons associated with our facility (eg, parking) and to minimize injury risk.
The exercises used in this investigation targeted muscle groups thought to play an important role in back pain and function. Back pain is common among pregnant women—the 9-month prevalence has been estimated between 49% and 59%.33–35 Back pain patients have an increased recruitment of latissimus dorsi muscle fibers contralateral to the side characterized by pain.36 Transverse abdominis muscles play a key role in lumbar spine stabilization.37 Inflexibility and weakness in the hamstrings, quadriceps and lumbar musculature are associated with low back pain.38–40 As a consequence of our focus on resistance exercises aimed at improving low back function and pain, the present findings may not generalize to training programs that involve a broader mix of resistance exercises (eg, those including more upper body exercises).
Noteworthy also is that a large progression in external loads and an associated improvement in muscular endurance is unlikely to be realized among pregnant women who are unable to adhere to a twice weekly strength training program. The small corpus of available research aimed at understanding exercise adherence among pregnant women suggests that perceived lack of time is a key barrier to their exercise participation and that the presence or absence of social support (husband/partner/family) influences exercise behavior during pregnancy.41
Among pregnant women who are sufficiently active to meet physical activity guidelines recommended for health and fitness, weight lifting is the third most common leisure time physical activity engaged in after walking and swimming.11 Nevertheless, because of safety concerns health care providers often have been unwilling to recommend strength training to pregnant women. The results of the present investigation show that adoption of a supervised, low-to-moderate intensity strength training program during an uncomplicated, singleton pregnancy can be safe and efficacious.
Figure 2.
— Mean (± SE) progression of absolute (kg; Top Panel) and perceived (RPE; Bottom Panel) training intensity for leg curls across 12 weeks among 32 pregnant women.
Figure 3.
— Mean (± SE) progression of absolute (kg; Top Panel) and perceived (RPE; Bottom Panel) training intensity for leg extension across 12 weeks among 32 pregnant women.
Figure 4.
— Mean (± SE) progression of absolute (kg; Top Panel) and perceived (RPE; Bottom Panel) training intensity for lat pull across 12 weeks among 32 pregnant women.
Acknowledgments
Appreciation is expressed to the pregnant women participants, study administrator Tameka Gude, exercise specialist Cheryl Maldonado, project assistants Emily Davis and Sarah Reynolds, obstetrical consultant Thomas W. Goggin, M.D., exercise specialist and founder of Atlanta, Georgia based FitFor2, Inc., Lisa Stone, UGA Fitness Center Director Harry DuVal, and the participating obstetricians and midwives in the Athens, Georgia area. This project was supported by a grant from the National Institutes of Health (RO1 NR008131).
Contributor Information
Patrick J. O’Connor, O’Connor is with the Dept of Kinesiology, University of Georgia, Athens, GA
Melanie S. Poudevigne, Poudevigne is with the Dept of Natural Sciences, Clayton State University, Morrow, GA
M. Elaine Cress, Cress is with the Dept of Kinesiology, University of Georgia, Athens, GA.
Robert W. Motl, Motl is with the Dept of Kinesiology, University of Illinois, Urbana, IL
James F. Clapp, III, Clapp is with the Dept of Obstetrics and Gynecology, MetroHealth Medical Center, Cleveland, OH.
References
- 1.American College of Sports Medicine. Impact of physical activity during pregnancy and postpartum on chronic disease risk. Med Sci Sports Exerc. 2006;38:989–1006. doi: 10.1249/01.mss.0000218147.51025.8a. [DOI] [PubMed] [Google Scholar]
- 2.Brankston GN, Mitchell BF, Ryan EA, Okun NB. Resistance exercise decreases the need for insulin in overweight women with gestational diabetes mellitus. Am J Obstet Gynecol. 2004;190:188–193. doi: 10.1016/s0002-9378(03)00951-7. [DOI] [PubMed] [Google Scholar]
- 3.Poudevigne MS, O’Connor PJ. A review of physical activity patterns in pregnant women and their relationship to psychological health. Sports Med. 2006;36:19–38. doi: 10.2165/00007256-200636010-00003. [DOI] [PubMed] [Google Scholar]
- 4.Sorensen TK, Williams MA, Lee IM, Dashow EE, Thompson ML, Luthy DA. Recreational physical activity during pregnancy and risk of pre-eclampsia. Hypertension. 2003;41:1273–1280. doi: 10.1161/01.HYP.0000072270.82815.91. [DOI] [PubMed] [Google Scholar]
- 5.Artal R, O’Toole M. Guidelines of the American College of Obstetricians and Gynecologists for exercise during pregnancy and the postpartum period. Br J Sports Med. 2003;37:6–12. doi: 10.1136/bjsm.37.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clapp JF. 3rd Exercise during pregnancy: a clinical update. Clin Sports Med. 2000;19:273–286. doi: 10.1016/s0278-5919(05)70203-9. [DOI] [PubMed] [Google Scholar]
- 7.Kramer MS, McDonald SW. Aerobic exercise for women during pregnancy. Cochrane Database Syst Rev. 2006;19(3):CD000180. doi: 10.1002/14651858.CD000180.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pivarnik JM, Perkins CD, Moyerbrailean T. Athletes and pregnancy. Clin Obstet Gynecol. 2003;46:403–414. doi: 10.1097/00003081-200306000-00019. [DOI] [PubMed] [Google Scholar]
- 9.Powell KE, Heath GW, Kresnow MJ, Sacks JJ, Branche CM. Injury rates from walking, gardening, weightlifting, outdoor bicycling, and aerobics. Med Sci Sports Exerc. 1998;30:1246–1249. doi: 10.1097/00005768-199808000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Jones CS, Christenen C, Young M. Weight training injury trends: a 20-year survey. Phys Sportsmed. 2000;28:61–72. doi: 10.3810/psm.2000.07.1086. [DOI] [PubMed] [Google Scholar]
- 11.Evenson AR, Savitz DA, Huston SL. Leisure-time physical activity among pregnant women in the US. Paediatr Perinat Epidemiol. 2004;18:400–407. doi: 10.1111/j.1365-3016.2004.00595.x. [DOI] [PubMed] [Google Scholar]
- 12.Borg-Stein J, Dugan SA, Gruber J. Musculoskeletal aspects of pregnancy. Am J Phys Med Rehabil. 2005;84:180–192. doi: 10.1097/01.phm.0000156970.96219.48. [DOI] [PubMed] [Google Scholar]
- 13.Entin PL, Munhall KM. Recommendations regarding exercise during pregnancy made by private/small group practice obstetricians in the USA. J Sports Sci Med. 2006;5:449–458. [PMC free article] [PubMed] [Google Scholar]
- 14.Hall DC, Kaufmann DA. Effects of aerobic and strength conditioning on pregnancy outcomes. Am J Obstet Gynecol. 1987;157:1199–1203. doi: 10.1016/s0002-9378(87)80294-6. [DOI] [PubMed] [Google Scholar]
- 15.Davies GA, Wolfe LA, Mottola MF, MacKinnon C. Joint SOGC/CSEP clinical practice guideline: exercise in pregnancy and the postpartum period. Can J Appl Physiol. 2003;28(3):330–341. [PubMed] [Google Scholar]
- 16.Chasan-Taber L, Schmidt MD, Roberts LE, Hosmer D, Markenson G, Freedson PS. Development and validation of a pregnancy physical activity questionnaire. Med Sci Sports Exerc. 2004;36:1750–1760. doi: 10.1249/01.mss.0000142303.49306.0d. [DOI] [PubMed] [Google Scholar]
- 17.Adams R. Revised physical activity readiness questionnaire. Can Fam Physician. 1999;45:1004–1005. [PMC free article] [PubMed] [Google Scholar]
- 18.Zimmerman M, Mattia JL. The psychiatric diagnostic screening questionnaire: development, reliability and validity. Compr Psychiatry. 2001;42:175–189. doi: 10.1053/comp.2001.23126. [DOI] [PubMed] [Google Scholar]
- 19.Pollock M, Leggett SH, Graves JE, Jones A, Fulton M, Cirulli J. Effect of resistance training on lumbar extension strength. Am J Sports Med. 1989;17:624–628. doi: 10.1177/036354658901700506. [DOI] [PubMed] [Google Scholar]
- 20.Borg G. Borg’s perceived exertion and pain scales. Champaign, IL: Human Kinetics; 1998. [Google Scholar]
- 21.Richardson C, Jull G, Hodges P, Hides J. Therapeutic exercise for spinal segmental stabilization in low back pain. Scientific basis and clinical approach. Edinburgh: Churchill Livingstone; 1999. [Google Scholar]
- 22.Kirkendall WM, Feinleib M, Freis Ed, Mark AL. Recommendations for human blood pressure determination by sphygmomanometers. Hypertension. 1981;13:510A–519A. [PubMed] [Google Scholar]
- 23.D’Amico EJ, Neilands TB, Zambarano R. Power analysis for multivariate and repeated measures designs: a flexible approach using the SPSS MANOVA procedure. Behav Res Methods Instrum Comput. 2001;33(4):479–484. doi: 10.3758/bf03195405. [DOI] [PubMed] [Google Scholar]
- 24.Mazur LJ, Yetman RJ, Risser WL. Weight training injuries: common injuries and preventative methods. Sports Med. 1993;16:57–63. doi: 10.2165/00007256-199316010-00005. [DOI] [PubMed] [Google Scholar]
- 25.MacDonald JR, MacDougall JD, Interisano SA, et al. Hypotension following mild bouts of resistance exercise and submaximal dynamic exercise. Eur J Appl Physiol Occup Physiol. 1999;79:148–154. doi: 10.1007/s004210050488. [DOI] [PubMed] [Google Scholar]
- 26.O’Connor PJ, Bryant CX, Veltri JP, Gebhardt SM. State anxiety and ambulatory blood pressure following resistance exercise in females. Med Sci Sports Exerc. 1993;25:516–521. [PubMed] [Google Scholar]
- 27.Cornelissen VA, Fagard RH. Effect of resistance training on resting blood pressure: a meta-analysis of randomized controlled trials. J Hypertens. 2005;23:251–259. doi: 10.1097/00004872-200502000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Gaffney FA, Sjogaard G, Saltin B. Cardiovascular and metabolic responses to static contraction in man. Acta Physiol Scand. 1990;138:249–258. doi: 10.1111/j.1748-1716.1990.tb08844.x. [DOI] [PubMed] [Google Scholar]
- 29.Williams MA, Haskell WL, Ades PA, et al. Resistance exercise in individuals with and without cardiovascular disease: 2007 update. Circulation. 2007;116:572–584. doi: 10.1161/CIRCULATIONAHA.107.185214. [DOI] [PubMed] [Google Scholar]
- 30.Feigenbaum MS, Pollock ML. Prescription of resistance training for health and disease. Med Sci Sports Exerc. 1999;31:516–521. doi: 10.1097/00005768-199901000-00008. [DOI] [PubMed] [Google Scholar]
- 31.Winett RA, Carpinelli RN. Potential health-related benefits of resistance training. Prev Med. 2001;33:503–513. doi: 10.1006/pmed.2001.0909. [DOI] [PubMed] [Google Scholar]
- 32.Graves JE, Pollock ML, Foster DN. Effect of training frequency and specificity on isometric lumbar extension strength. Spine (Phila Pa 1976) 1990;15:504–509. doi: 10.1097/00007632-199006000-00014. [DOI] [PubMed] [Google Scholar]
- 33.Berg G, Hammar M, Moller-Nielsen J, Linden U, Thorblad J. Low back pain during pregnancy. Obstet Gynecol. 1988;71(1):71–75. [PubMed] [Google Scholar]
- 34.Fast A, Weiss L, Ducommun EJ, Medina E, Butler JG. Low-back pain in pregnancy. Abdominal muscles, sit-up performance, and back pain. Spine (Phila Pa 1976) 1990;15(1):28–30. doi: 10.1097/00007632-199001000-00008. [DOI] [PubMed] [Google Scholar]
- 35.Ostgaard HC, Andersson GB, Karlsson K. Prevalence of back pain in pregnancy. Spine (Phila Pa 1976) 1991;16(5):549–552. doi: 10.1097/00007632-199105000-00011. [DOI] [PubMed] [Google Scholar]
- 36.Sparto PJ, Parnianpour M, Barria EA, Jaqadeesh JM. Wavelet analysis of electromyography for back muscle fatigue detection during isokinetic constant-torque exertions. Spine (Phila Pa 1976) 1999;24:1791–1798. doi: 10.1097/00007632-199909010-00008. [DOI] [PubMed] [Google Scholar]
- 37.McGill SM. Low back stability: from formal description to issues for performance and rehabilitation. Exerc Sport Sci Rev. 2001;29:26–31. doi: 10.1097/00003677-200101000-00006. [DOI] [PubMed] [Google Scholar]
- 38.Balagué F, Troussie B, Salminen J. Non-specific low back pain in children and adolescents: risk factors. Eur Spine J. 1999;8:429–438. doi: 10.1007/s005860050201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carpenter DM, Nelson BW. Low back strengthening for the prevention and treatment of low back pain. Med Sci Sports Exerc. 1999;31:18–24. doi: 10.1097/00005768-199901000-00005. [DOI] [PubMed] [Google Scholar]
- 40.Stevenson JM, Weber CL, Smith JT, Dumas G, Albert A. Longitudinal study of the development of low back pain in an industrial population. Spine (Phila Pa 1976) 2001;6:1370–1377. doi: 10.1097/00007632-200106150-00022. [DOI] [PubMed] [Google Scholar]
- 41.Symons-Downs D, Hausenblas H. Women’s exercise beliefs and behaviors during their pregnancy and postpartum. J Midwifery Womens Health. 2004;49:138–144. doi: 10.1016/j.jmwh.2003.11.009. [DOI] [PubMed] [Google Scholar]