Abstract
Background
The neurotransmitter dopamine (DA), acting in various mesolimbic brain regions, is well-known for its role in promoting motivated behaviors, including ethanol drinking. Indirect evidence, however, suggests that DA in the perifornical lateral hypothalamus (PF/LH) has differential effects on ethanol consumption, depending on whether it acts on the DA 1 (D1) or DA 2 (D2) receptor subtype, and that these effects are mediated in part by local peptide systems, such as orexin/hypocretin (OX) and melanin-concentrating hormone (MCH), known to stimulate the consumption of ethanol.
Methods
The present study in brain-cannulated Sprague-Dawley rats measured the effects of dopaminergic compounds in the PF/LH on drinking behavior in animals trained to consume 7% ethanol and also on local peptide mRNA expression using digoxigenin-labeled in situ hybridization in ethanol-naïve animals.
Results
Experiments 1 and 2 showed that the D1 agonist SKF81297 (10.8 nmol/side) in the PF/LH significantly increased food intake, while tending to increase ethanol intake, and the D1 antagonist SCH23390 significantly decreased ethanol intake without affecting food intake. In contrast, the D2 agonist quinelorane (6.2 nmol/side) in the PF/LH significantly reduced ethanol consumption, while the D2 antagonist sulpiride increased it. Experiments 3 and 4 revealed differential effects of PF/LH injection of the DA agonists on local OX mRNA, which was increased by the D1 agonist and decreased by the D2 agonist. These DA agonists had no impact on MCH expression.
Conclusions
These results support a stimulatory role of the PF/LH D1 receptor in promoting the consumption of both ethanol and food, in contrast to a suppressive effect of the D2 receptor on ethanol drinking. They further suggest that these receptors affect consumption, in part, through local OX-expressing neurons. These findings provide new evidence for the function of PF/LH DA receptor subtypes in controlling ethanol and food intake.
Keywords: Dopamine, Orexin, Melanin-Concentrating Hormone, Ethanol, Lateral Hypothalamus
INTRODUCTION
Dopamine (DA), a classical neurotransmitter implicated in reward, plays an important role in alcoholism (Wise, 2004). The DA system as it relates to alcohol drinking has been extensively investigated in the mesolimbic areas (Liu & Weiss, 2002; Spanagel & Weiss, 1999; Thanos et al., 2005). For example, in the nucleus accumbens (NAc) acute exposure to ethanol increases extracellular levels of DA (Yan et al., 2005), and the activation of DA receptors enhances ethanol drinking (Bahi & Dreyer, 2012). Whereas recent preclinical studies have shown regions outside the mesolimbic circuit to have a role in ethanol consumption (Morganstern et al., 2011), the functions of DA in these areas remain unclear.
An area of the hypothalamus important in promoting ethanol drinking is the perifornical lateral hypothalamus (PF/LH), where injections of orexigenic peptides stimulate this behavior (Schneider et al., 2007). The possibility that DA acts in this area to affect ethanol drinking is suggested by evidence that exposure to ethanol affects the levels of DA transporter binding in this region (Jiao et al., 2006) and that DA 1 receptor blockade reverses the renewal of alcohol drinking associated with increased c-Fos in PF/LH neurons (Hamlin et al., 2007). Whereas studies of DA’s effects in the PF/LH on ethanol intake are lacking, investigations of feeding behavior provide evidence that DA in this area, rather than increasing motivated behaviors, has the opposite effect. This is demonstrated by investigations showing PF/LH injection of DA agonists to reduce food intake and DA antagonists to increase it (Leibowitz & Rossakis, 1979; Parada et al., 1988). With evidence showing food and ethanol intake to be similarly affected by various pharmacological agents (Barson et al., 2010; Schneider et al., 2007), it is possible that DA in the PF/LH affects ethanol consumption in a manner similar to its effect on food intake.
The actions of DA involve two receptor subtypes, DA 1-like (D1) receptors and DA 2-like (D2) receptors. These receptors, both of which exist in the PF/LH (Fetissov et al., 2002; Meador-Woodruff et al., 1991; Wamsley et al., 1989), are distinct in their function (Beaulieu & Gainetdinov, 2011). This raises the possibility that DA in the PF/LH may have different functions in controlling consummatory behavior, depending on whether it acts through the D1 or D2 receptors. Whereas little is known about the role of DA receptors in the PF/LH in controlling ethanol drinking, studies in the PF/LH show D1 receptor blockade to reduce the establishment of flavor preference learning and attenuate taste aversion (Caulliez et al., 1996; Touzani et al., 2009), suggesting a role for this receptor in stimulating consumption and reward processing. Conversely, blockade of D2 receptors in the PF/LH causes various behavioral changes related to ethanol drinking, including an increase in feeding behavior, conditioned approach for sucrose, and locomotor activity (Leibowitz & Rossakis, 1979; Morutto & Phillips, 1998; Parada et al., 1988), whereas activation of D2 receptor reduces conditioned approach behavior for sucrose (Morutto & Phillips, 1999), suggesting a function of this receptor in suppressing consumption. These studies of food intake lead us to propose that ethanol drinking may be similarly affected by DA in the PF/LH, with increased intake after D1 receptor activation and decreased intake after D2 activation.
If this prediction is correct regarding DA’s behavioral effects in the PF/LH, the next question is through which local neurochemical systems the D1 and D2 receptors act to control ethanol consumption. Densely expressed in neurons of the PF/LH are two orexigenic peptides, orexin/hypocretin (OX) and melanin-concentrating hormone (MCH), which are known to be important in consummatory behavior. The peptide OX, which is expressed primarily in the LH and PF (de Lecea et al., 1998; Sakurai et al., 1998), has a stimulatory effect on feeding and arousal (de Lecea et al., 1998; Sakurai et al., 1998). It also increases ethanol drinking when administered directly into the PF/LH (Schneider et al., 2007), and its endogenous expression is stimulated by ethanol exposure and ethanol-paired cues (Dayas et al., 2008; Morganstern et al., 2010a). The possibility that the DA receptor subtypes in the PF/LH act with distinct effects on the local OX system is suggested by the finding that stimulation of the D1 or D2 receptors differentially affect the excitability of nearby neurons expressing MCH (Conductier et al., 2011), which in the LH and zona incerta (ZI) have also been implicated in controlling ethanol consumption (Morganstern et al., 2010b). In contrast to the clear relationship between OX and ethanol, mixed results have been obtained in studies of MCH, with ethanol intake increased by cerebroventricular injection of this peptide (Duncan et al., 2005) but also by deletion of the MCH receptor gene (Duncan et al., 2007), suggesting a more complex relationship between ethanol and this peptide system. Studies of D1 and D2 agonist effects on these two PF/LH peptide systems should help to elucidate how DA acts in this area to modulate ethanol drinking.
Building on this evidence, the present study examined the function of D1 and D2 receptors in the PF/LH area in terms of their impact on both the consumption of ethanol and the endogenous expression of local peptides. In Experiments 1 and 2 using brain-cannulated rats, the effects of PF/LH injections of D1 and D2 agonists or antagonists on ethanol consumption, along with food and water intake, were examined. Then, in Experiments 3 and 4, in situ hybridization was used to determine whether injection of D1 or D2 agonists in this area, at a dose that alters ethanol drinking, can also influence OX and MCH expression to investigate whether the DA-induced changes in ethanol drinking might operate through these peptide systems.
MATERIALS AND METHODS
Subjects
Adult male Sprague-Dawley rats (225 to 250 g at the start of the experiment) were obtained from Taconic Farms (Germantown, NY). Rats were individually housed in hanging wire cages (Experiments 1–2) or plastic shoebox cages (Experiments 3–4) and maintained on a reversed 12:12-hour light–dark cycle (lights off from 6:00 am). Subjects had ad libitum access to LabDiet Rodent Chow 5001 (St Louis, MO) prior to ethanol training and ad libitum access to water throughout the experiment. All animals were allowed 1 week to acclimate to the facility before experiments began. In the present study, 62 rats were included in the analysis. All procedures were approved by the Princeton University Institutional Animal Care and Use Committee and The Rockefeller University Animal Care and Use Committee, and conformed to the National Institutes of Health guidelines on the ethical use of animals.
Ethanol Training
Subjects were acclimated to unsweetened ethanol by using a variant of the 2-bottle choice procedure (Martinetti et al., 2000). To encourage animals to drink and adapt to the unsweetened ethanol, the concentration of ethanol was gradually increased every 4 days, from 1, 2, 4, to 7% (v/v). Animals had access to ethanol solutions for 12 h per day, starting 3 h into the dark period, as described in recent publications (Chen et al., 2013; Morganstern et al., 2010b). Chow was also provided along with ethanol for 12 h per day during most of the dark period when a majority of consumption normally occurs, as shown by our preliminary observations (12 h: 78 ± 5 kcal vs 24 h: 82 ± 7 kcal) and published findings (Agabio et al., 1996). This procedure of limiting access to 12 h per day has been found to increase ethanol drinking in outbred Sprague-Dawley rats, leading to daily intake of approximately 2.5 g/kg and blood ethanol levels of 40 mg/dl (Morganstern et al., 2010a). In Experiments 1 and 2, animals received cannulation surgery after at least 4 days of access to 7% ethanol.
Surgery
Subjects were anesthetized using ketamine (80 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.), supplemented with ketamine when necessary. Stainless steel 21-gauge guide shafts (10 mm in length) were implanted bilaterally, aimed at the PF/LH (Experiments 1–2: A −2.9, L ±1.6, V 3.9 mm; Experiments 3–4: A −2.9, L ±1.6, V 3.5 mm), with reference to bregma, the midsaggital sinus, and the level skull surface. In Experiments 3–4, the cannulas were implanted for injection immediately dorsal to the target region to avoid tissue damage and allow for analysis of peptide expression. Subjects had 1 week to recover before testing. Stainless steel stylets were left in the guide shafts between injections to prevent occlusion.
Microinjection Procedures
Drugs were delivered through 26-gauge stainless steel microinjectors with fused-silica tubing inside (74 µm ID, 154 µm OD, Polymicro Technologies, Phoenix AZ) that extended beyond the stainless steel to reach the region of interest (Experiments 1–2: V 8.4 mm; Experiments 3–4: V 8.0 mm). Doses were based on previous studies examining the effect of dopaminergic ligands on consummatory behavior (Parada et al., 1988; Parada et al., 1990) and on pilot tests. The drugs and doses used for tests of ethanol intake were as follows: (i) the D1 receptor agonist SKF81297 (5.4 nmol, 10.8 nmol per side); (ii) the D1 receptor antagonist SCH23390 (7.7 nmol, 15.4 nmol per side); (iii) the D2 receptor agonist quinelorane dihydrochloride (3.1 nmol, 6.2 nmol per side) and (iv) the D2 receptor antagonist sulpiride (11.7 nmol, 23.4 nmol per side). The drugs SKF81297 and SCH23390 were purchased from Tocris Bioscience Co. (Ellisville, MO) and quinelorane dihydrochloride and sulpiride were purchased from Sigma-Aldrich Co. (St Louis, MO). Sulpiride was dissolved using HCl and diluted in H2O and the pH adjusted to 7.4 with NaOH. SCH23390 was dissolved in 50% DMSO/50% saline solution (Parada et al., 1990). All the other drugs were dissolved in preservative-free 0.9% NaCl solution (Hospira Inc., Lake Forest, IL) immediately prior to microinjection. To test the anatomical specificity of the effects, additional injections of SCH23390 (15.4 nmol/side) and sulpiride (23.4 nmol/side) were made 2 mm dorsal to the PF/LH.
Behavioral Tests
For Experiments 1 and 2, injections were counterbalanced so that each rat (n = 7–11/group) received vehicle or drug in counterbalanced order on 2 consecutive days. Animals were handled on an almost daily basis throughout their ethanol training. Injections were made immediately prior to daily ethanol access using a syringe pump which delivered 0.5 µl during 47 seconds with microinjectors left in place for an additional 47 seconds to allow for diffusion. The intake of ethanol, food, and water was measured at 1 h, 2 h, and 4 h after injection, based on published evidence showing the effects of DA compounds on consummatory behavior to occur within the first 4 h (Leibowitz & Rossakis, 1979; Nowend et al., 2001).
Digoxigenin-Labeled In Situ Hybridization Histochemistry
In Experiments 3 and 4, OX and MCH mRNA expression was measured with digoxigenin (DIG)-labeled in situ hybridization (ISH) histochemistry in animals receiving either vehicle, SKF81297, or quinelorane immediately dorsal to the PF/LH (n = 5–6/group), using the highest behaviorally effective dose identified in Experiments 1 and 2. These injections were given 30 min after removal of the food, with the animals sacrificed 30 min after injection. This time period was chosen based on our finding here, that these dopaminergic agonists affect consumption during the first hour post-injection, and on published evidence revealing significant alterations in peptide expression within 30 min after experimental treatment (Ida et al., 2000). Frozen brains were cut into 30 µm coronal sections with a cryostat. DIG-labeled cRNA probes of OX and MCH were synthesized by in vitro transcription as previously described (Wortley et al., 2003). The antisense and sense OX RNA probes were generously donated by Dr. Luis de Lecea (Stanford University), and the MCH RNA probes were donated by Dr. Nicholas A. Tritos (Harvard University). These DIG-labeled cRNA probes and 30 µm free-floating sections were used for ISH histochemistry as previously described (Chang et al., 2008). Gene expression level was measured by semiquantification with Image-Pro Plus software (Version 4.5, Media Cybernetics Inc., Silver Spring, MD) as described (Leibowitz et al., 2007) and expressed as the density of mRNA-containing cells, “cells/µm2.” Data presented in the figures represent percentage change compared to saline vehicle. The values obtained represent an average of measurements taken from 3 to 5 sections per animal from Bregma −2.8 to −3.1 mm. The OX neurons lateral to the fornix were considered to be in the LH, and all OX neurons located dorsal and 0.4 mm medial to the fornix were considered to be in the PF. The MCH neurons lateral to the fornix were considered to be in the LH, while all MCH neurons dorsal to the fornix were considered to be in the ZI. The analysis of average cell density was performed by an observer blind to the treatment groups.
Histological Analysis
The sites of the microinjections were determined after sacrifice. For the rats used in Experiments 1 and 2, the brains were sliced at 40 µm coronal sections and examined microscopically. The rats receiving microinjections in Experiments 3 and 4, in which the brains were sliced at 30 µm coronal sections, had their cannulae sites examined visually under the microscope during the analysis of peptide expression. The few animals (1–2 rats/group) with probes 0.5 mm or farther from the PF/LH were discarded from the analysis.
Statistical Data Analysis
In Experiments 1–2, ethanol, food, and water intake was analyzed using a two-way repeated measures analysis of variance (ANOVA), with drug treatment and time as within-subject factors. With our findings demonstrating very similar effects at the lower and higher doses of the drugs as compared to vehicle (see Figs. 2 and 3), we included only the higher dose in our data analyses. Also, with prior central injection studies of consummatory behavior generally showing the drug effects on intake to be relatively short-lived, mostly lasting 1–2 hours (Barson et al., 2010; Leibowitz & Rossakis, 1979; Parada et al., 1988), we made an a priori decision to follow up a significant main effect on intake with tests of simple effects only during the first two hours, using paired 2-tailed t-tests. As the present study focused on the role of DA in ethanol-drinking animals, all data from animals consuming little or no ethanol (less than 0.25 g/kg during 4 hours) were excluded from Experiment 1 (n = 2 from SKF81297 injection; n = 4–6 from each SCH23390 injection) and Experiment 2 (n = 3 from each quinelorane injection; n = 1 from sulpiride injection), since such low drinking values fail to significantly raise levels of blood ethanol (Barson et al., 2010; Morganstern et al., 2010a). For Experiments 3–4, changes in peptide expression were analyzed using a one-way ANOVA. Values of p < 0.05 were considered significant.
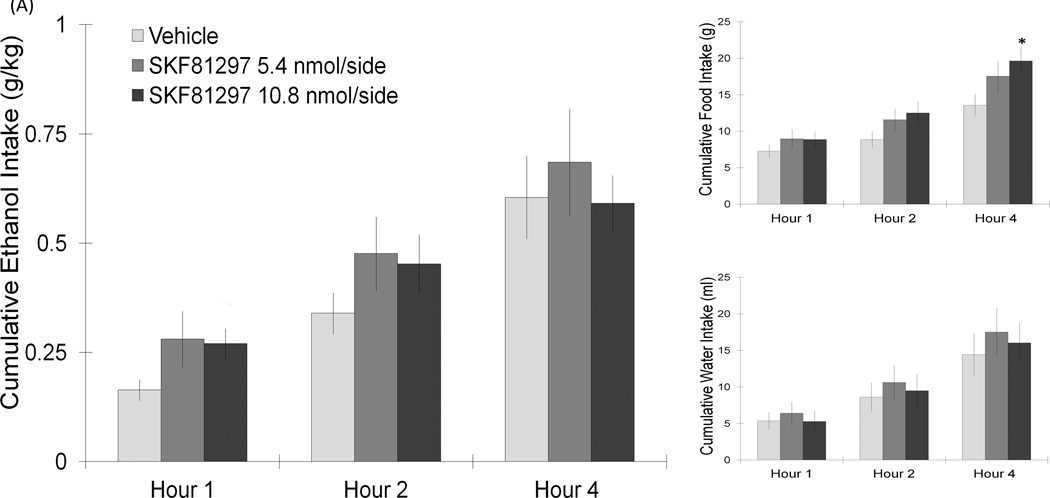
Figure 2.
(A) PF/LH injection of the D1 agonist, SKF81297, at the dose of 10.8 nmol/side, significantly increased food intake at 4 h post-injection (10.8 nmol: n = 8/group). (B) Injection of the D1 antagonist, SCH23390, in the PF/LH significantly reduced ethanol intake for 2 h without significantly affecting food or water intake (15.4 nmol: n = 8/group). Values are mean ± S.E.M. The vehicle intake presented is the average from the 2 sets of injections, *p < 0.05 vs. individual paired saline injection at specific time points with the higher dose.
RESULTS
Experiment 1: D1 receptor stimulation in the PF/LH increases food intake, while the antagonist reduces ethanol drinking
This first experiment directly examined how D1 receptor-mediated signaling in the PF/LH affects the consumption of ethanol, as well as food and water (n = 7–11/group). With injections made directly into the PF/LH area at sites depicted in the diagram of Fig. 1, the D1 agonist, SKF81297, as compared to vehicle injection caused a significant increase in food intake, with a tendency to increase ethanol intake failing to reach statistical significance (Fig. 2A). A two-way repeated measures ANOVA revealed a significant main effect of SKF81297 (10.8 nmol/side) on food intake (F(1,7) = 6.34, p < 0.04), a slight trend on ethanol intake (F(1,7) = 3.79, p = 0.09), and no change in water intake (not significant, n.s.). An interaction effect between drug and food intake over time was also noted (F(2,14) = 6.85, p < 0.008), with follow up pair-wise comparisons at specific time points showing this drug to produce a significant increase in food consumption at the 4 h time period (t = 3.33, df = 7, p < 0.05). In contrast to these stimulatory effects of the D1 agonist on consumption, injection of the D1 antagonist, SCH23390 (15.4 nmol/side), while producing no main or interaction effects on food (n.s.) or water (n.s.) intake, caused a significant reduction in ethanol drinking behavior (F(1,7) = 5.37, p < 0.05) (Fig. 2B). Planned follow-up comparisons demonstrated that this reduction in ethanol intake occurred at both the 1 h (−61%) (t = 3.1, df = 7, p < 0.05) and 2 h (−43%) (t = 2.5, df = 7, p < 0.05) time periods. Injections of SCH23390 (15.4 nmol/side) outside the PF/LH, which located 2 mm dorsal to this target region, did not significantly change ethanol, water or food intake (n.s.). Taken together, these results demonstrate that activation of D1 receptors in the PF/LH stimulates food intake, while blockade of these receptors has a very different effect of reducing drinking behavior.
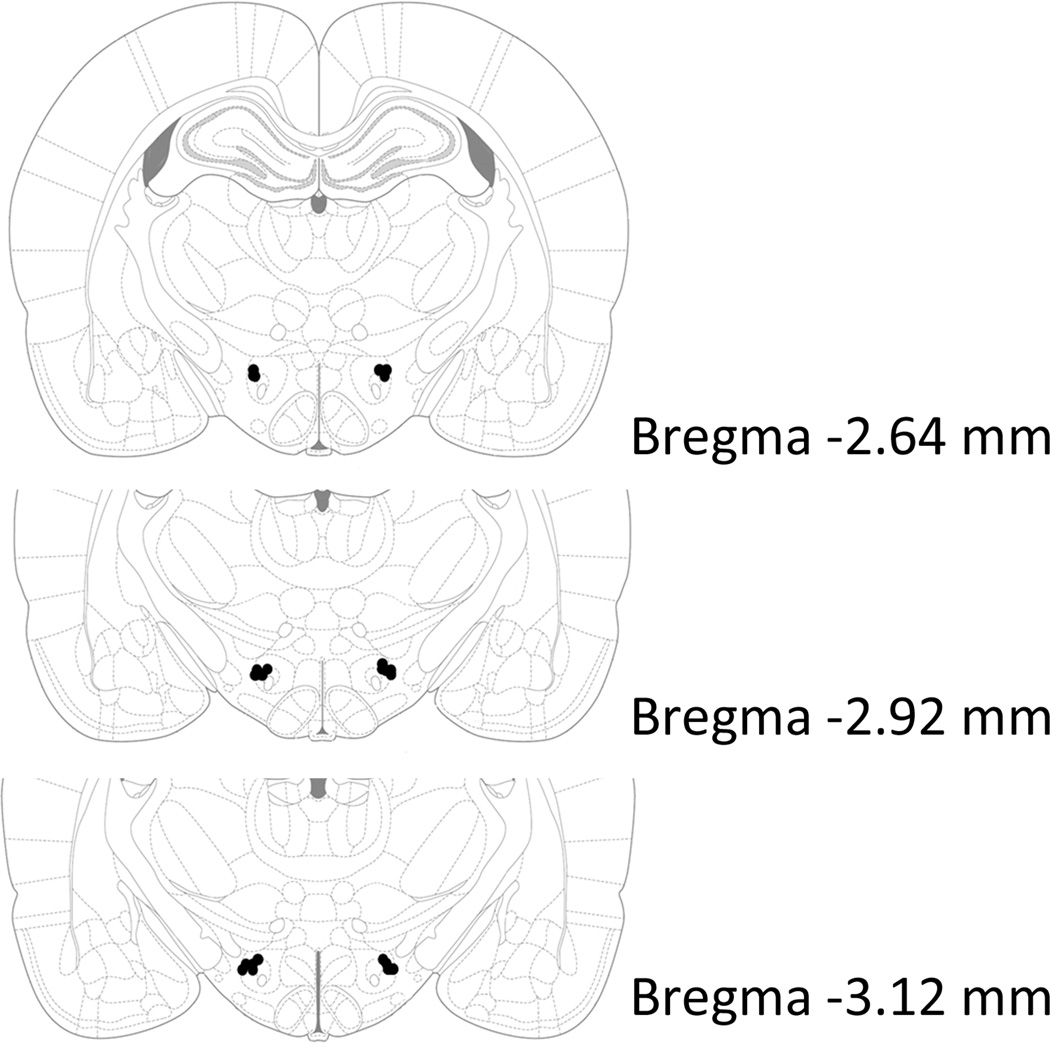
Figure 1.
Sites for PF/LH injections are indicated by black dots. Adapted from The Rat Brain, compact 6th edition, G. Paxinos and C. Watson, Copyright 2007.
Experiment 2: D2 receptor stimulation in the PF/LH suppresses ethanol drinking
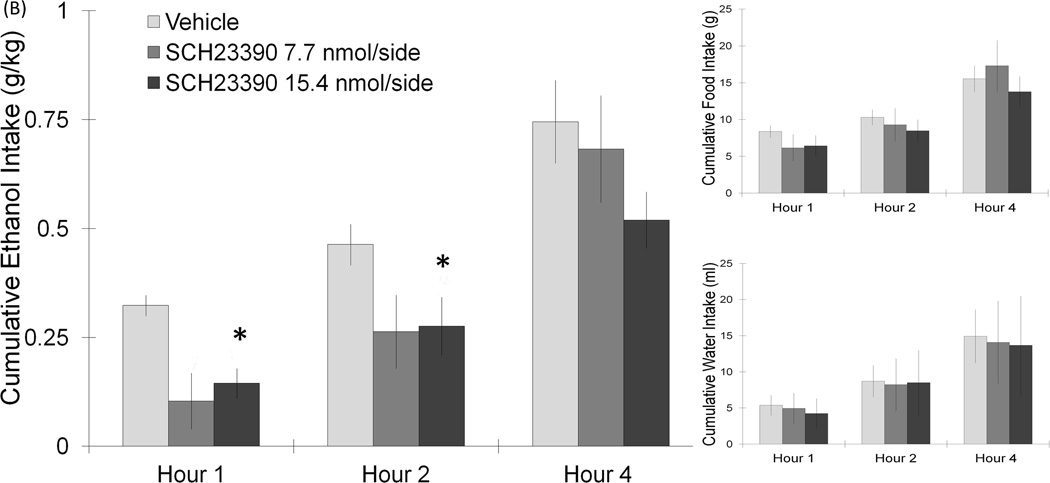
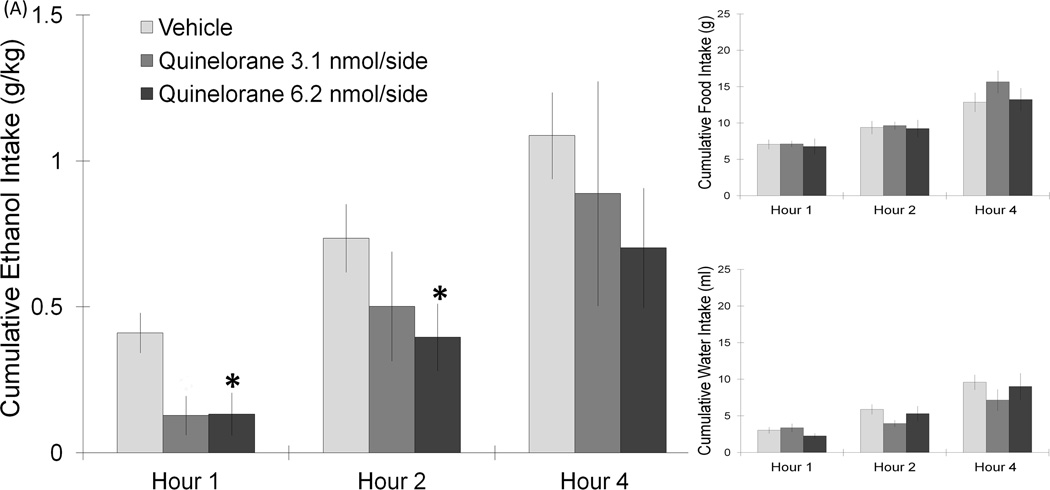
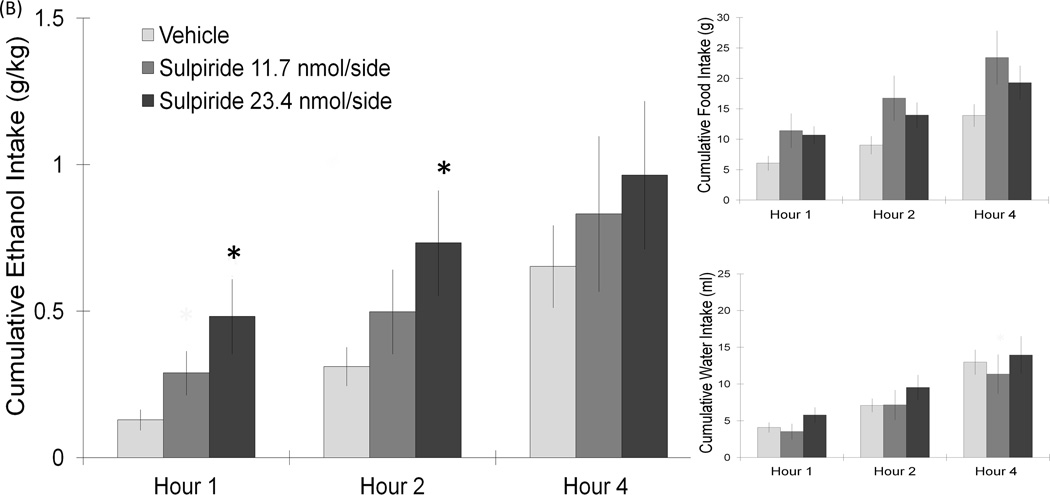
This next experiment tested the impact of D2 receptor activation on ethanol drinking behavior. In a new set of rats (n = 7–10/group), injection of the D2 receptor-specific agents in the PF/LH revealed an opposite effect to that observed with the D1 receptor compounds. Using a two-way repeated measures ANOVA, a significant main effect on ethanol intake was found with injection of the D2 agonist, quinelorane (6.2 nmol/side) (F(1,9) = 10.03, p < 0.05), with this drug reducing ethanol drinking behavior. While no interaction effects between drug and time on intake were noted (n.s.), the planned comparisons revealed a significant suppression of ethanol intake during the first 2 h post-injection (−45%) (t = 3.6, df = 9, p < 0.05) (Fig. 3A). The measures of food and water intake remained unaffected, with no significant main or interaction effects detected (n.s.). In contrast to quinelorane, PF/LH injection of the D2 antagonist, sulpiride (23.4 nmol/side), stimulated the consumption of ethanol (Fig. 3B). A significant main effect of this drug on ethanol intake was obtained (F(1,6) = 7.61, p < 0.05), which significantly increased ethanol drinking at both 1 h (307%) (t = 2.6, df = 6, p < 0.05) and 2 h (142%) (t = 3.2, df = 6, p < 0.05) post-injection. In contrast to ethanol intake, sulpiride had no impact on food or water intake (n.s.) in these ethanol-drinking rats. Injections of sulpiride outside the PF/LH, located 2 mm dorsal to this target region, did not significantly alter ethanol, food or water intake (n.s.). These tests with PF/LH injections reveal that D2 receptor activation suppresses while D2 receptor blockade stimulates ethanol intake in a dose-dependent manner.
Figure 3.
(A) Injection of the D2 agonist, quinelorane, in the PF/LH significantly suppressed ethanol intake. Food and water intake remained unaffected (6.2 nmol: n = 10/group). (B) PF/LH injection of the D2 antagonist, sulpiride, at the dose of 23.4 nmol/side can significantly elevate ethanol intake for 2 h without affecting food or water intake (23.4 nmol: n = 7/group). Values are mean ± S.E.M.. The vehicle intake presented is the average from the 2 sets of injections, *p < 0.05 vs. individual paired saline injection at specific time points with the higher dose.
Experiment 3: D1 receptor agonist stimulates expression of OX in the PF/LH
To determine if the increase in ethanol intake induced by D1 receptor stimulation is associated with changes in local neuropeptides known to stimulate drinking, gene expression of OX and MCH in the PF/LH was measured using DIG-ISH in animals (n = 5–6/group) injected with either vehicle or the D1 agonist, SKF81297 (10.8 nmol/side). The injections were made immediately dorsal to the target region, to avoid damaging the areas where the peptide-expressing neurons are most concentrated. A one-way ANOVA of data from the PF/LH subregions showed D1 receptor activation to significantly affect OX mRNA. As illustrated in Figs. 4A and 4B, administration of SKF81297 compared to vehicle produced a significant, 29% increase in the density of OX neurons specifically in the LH (0.86 ± 0.06 vs. 0.67 ± 0.03 cells/µm2×10−4) (F(1,10) = 10.18, p < 0.05), along with a smaller but significant, 13% increase in OX neurons of the PF (1.41 ± 0.05 vs. 1.25 ± 0.04 cells/µm2×10−4) (F(1,10) = 5.93, p < 0.05). A similar analysis of MCH-expressing neurons following D1 receptor stimulation compared to vehicle revealed no effect on the expression of this peptide in the specific areas of the LH (1.72 ± 0.11 vs. 1.74 ± 0.07 cells/µm2×10−4) (F(1,11) = 0.03, p = 0.86) or ZI (1.99 ± 0.09 vs. 2.17 ± 0.12 cells/µm2×10−4) (F(1,11) = 1.39, p = 0.26) (Figs. 4C and 4D). These results demonstrate that activation of the D1 receptors in the PF/LH preferentially stimulates OX-expressing neurons, with the strongest effect observed in the LH.
Figure 4.
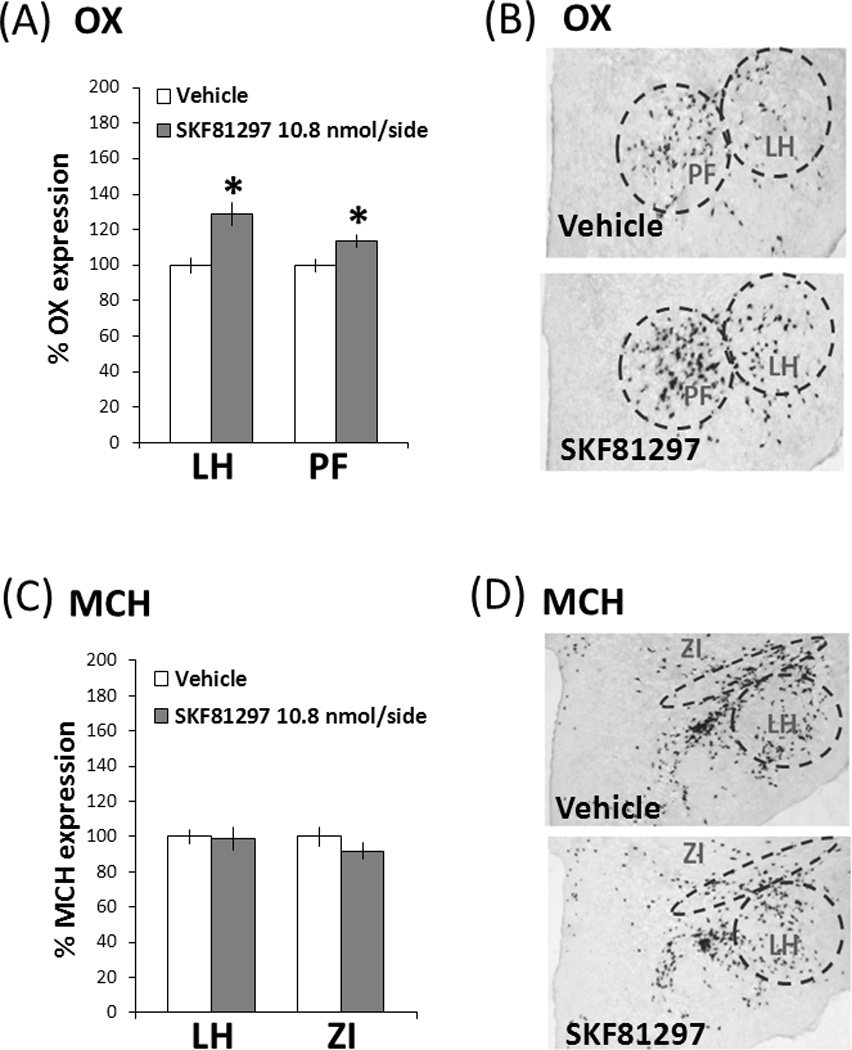
(A) Measurements with a DIG-labeled probe showed SKF81297 (10.8 nmol/side) to significantly increase OX expression in both the LH and PF subregions (n = 5–6/group). (B) Photomicrographs of OX neurons using DIG-labeled in situ hybridization. (C) SKF81297 (10.8 nmol/side) did not affect MCH expression in either the LH or ZI subregion using a DIG-labeled probe. (D) Photomicrographs of MCH neurons using DIG-labeled in situ hybridization. Values are mean ± S.E.M., expressed as percentage change from vehicle. *p < 0.05 vs. vehicle injection.
Experiment 4: D2 receptor agonist reduces expression of OX in the PF/LH
To determine if the suppression of ethanol drinking produced by D2 receptor stimulation in the PF/LH is similarly accompanied by changes in the local peptide systems, the gene expression of OX and MCH was measured using DIG-ISH in rats (n = 5–6/group) injected with either vehicle or quinelorane (6.2 nmol/side) in this region. A one-way ANOVA revealed that quinelorane compared to vehicle caused a significant reduction in OX expression (−26%), specifically in the LH (0.74 ± 0.04 vs. 0.99 ± 0.11 cells/µm2×10−4) (F(1,11) = 4.92, p = 0.05) but not in the PF (1.39 ± 0.11 vs. 2.08 ± 0.41 cells/µm2×10−4) (F(1,11) = 2.65, p = 0.14) (Figs. 5A and B). In contrast, the expression of MCH was unaffected by D2 receptor stimulation, both in the LH (1.44 ± 0.06 vs. 1.60 ± 0.07 cells/µm2×10−4) (F(1,11) = 3.25, p = 0.10) or the ZI (1.59 ± 0.08 vs. 1.75 ± 0.03 cells/µm2×10−4) (F(1,11) = 3.29, p = 0.10) (Figs. 5C and D). These results demonstrate that, while not affecting MCH expression, actvation of the D2 receptors in the PF/LH causes a reduction in the density of OX-expressing cells, an effect that is specific to the LH and opposite to that measured with D1 receptor stimulation.
Figure 5.
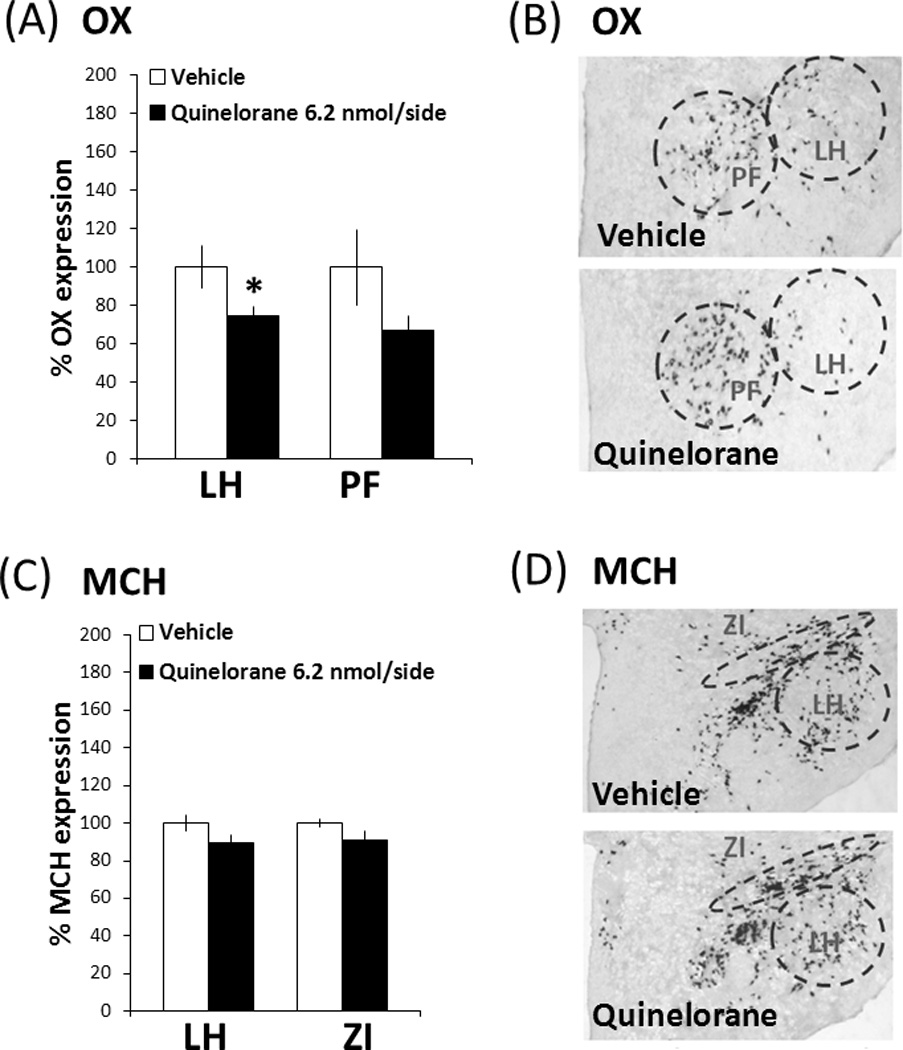
(A) Measurements with a DIG-labeled probe showed quinelorane (6.2 nmol/side) to significantly increase OX expression in the subregion of LH, but not the PF (n = 5–6/group). (B) Photomicrographs of OX neurons using DIG-labeled in situ hybridization. (C) Quinelorane (6.2 nmol/side) did not affect MCH expression in either the LH or ZI subregion, as measured using a DIG-labeled probe. (D) Photomicrographs of MCH neurons using DIG-labeled in situ hybridization. Values are mean ± S.E.M., expressed as percentage change from vehicle. *p < 0.05 vs. vehicle injection.
DISCUSSION
Results from the present study, examining the role of DA in the PF/LH in relation to ethanol and food consumption and endogenous expression of the local orexigenic peptides, OX and MCH, indicate that DA in this area acts through D1 and D2 receptors to differentially affect these parameters. In particular, activation of the D1 receptors significantly stimulates food consumption with a trend towards increasing ethanol drinking, while D1 receptor blockade significantly reduces only ethanol intake. In contrast, activation of the D2 receptor in the PF/LH significantly reduces ethanol drinking, while the blockade of this receptor subtype stimulates this behavior. With regard to peptide gene expression, PF/LH injection of the D1 agonist stimulates OX mRNA in the PF and somewhat more strongly in the LH, while injection of the D2 agonist increases OX mRNA only in the LH. In contrast to OX, the orexigenic peptide MCH in the PF/LH exhibited no change in response to the D1 and D2 agonists. Together, these findings support opposing functions for the DA receptor subtypes in the PF/LH in controlling ethanol and food intake and suggest a possible mediating role for local OX-expressing neurons in the PF and LH.
Role of D1 in the PF/LH in stimulating ethanol drinking and food intake
Dopaminergic transmission in the limbic system is well known for its stimulatory effect on consummatory and reward-related behaviors (Wellman, 2005; Wise, 2004). This effect is believed to be mediated by the D1 receptor, with peripheral administration of a D1 antagonist in rats found to produce anorexia and reduce operant responding for food (Rusk & Cooper, 1994; Zarrindast et al., 1991). Our findings confirm this role of D1 receptors in mediating consummatory responses, including ethanol and food intake, and identify the PF/LH as a specific hypothalamic site involved in this function of DA. They demonstrate that activation of D1 in the PF/LH significantly stimulates feeding behavior while showing a trend towards increasing ethanol drinking, and blockade of this receptor significantly reduces ethanol drinking behavior without affecting food or water intake. This evidence, supporting a role for the D1 receptor in the PF/LH in stimulating consummatory behavior, both ethanol and food intake, is consistent with other studies relating the activity of this receptor subtype to drug reward and addiction. These reports show that administration of a D1 agonist can reinstate cocaine place preference (Graham et al., 2007) and a D1 antagonist can attenuate the renewal of seeking for beer, heroin and cocaine (Bossert et al., 2007; Crombag et al., 2002; Hamlin et al., 2007).
Role of D2 in the PF/LH in suppressing ethanol drinking
Our findings in the PF/LH of ethanol-drinking rats clearly differentiate the function of D2 receptors from that of D1 receptors, suggesting that the former primarily suppresses ethanol intake while the latter stimulates both ethanol and food intake. This role of D2 receptors in attenuating ethanol drinking behavior is consistent with both clinical (Blum et al., 1990; Tupala et al., 2003; Volkow et al., 1996) and preclinical (Bice et al., 2008; Delis et al., 2013; Thanos et al., 2004) evidence revealing the importance of this receptor in alcoholism. The possibility that this D2 regulation is tonically active in ethanol-drinking rats is supported by our finding that the specific D2 receptor antagonist, sulpiride, stimulates consumption of ethanol. The observed effect of D2 receptor activation in reducing ethanol drinking may be due to a change in the reinforcing effects of ethanol, as injection of a D2 agonist in this area can reduce conditioned approach for sucrose while a D2 receptor antagonist enhances conditioned place preference with sucrose (Morutto & Phillips, 1998, 1999). It may also be attributed to a more general effect on consummatory behavior, as DA injection into the LH reduces food intake while a D2 antagonist elicits intense feeding (Leibowitz & Rossakis, 1979; Parada et al., 1988). Since different feeding conditions are found to alter dopaminergic activity in the LH, including the release of DA, neuronal responsiveness to DA, and expression of the DA receptor (Fetissov et al., 2002; Meguid et al., 1995; Zippel et al., 2003), it will be interesting to determine whether they also affect dopaminergic control of ethanol drinking, with the effects of DA-specific agents on ethanol intake, for example, differing in rats hungry at dark onset compared to those seen in fully sated animals.
Involvement of LH OX neurons in DA-induced changes in ethanol drinking
Measurements of endogenous gene expression of OX after D1 and D2 receptor activation provide evidence for a role of this orexigenic peptide in mediating the opposing effects of these receptor subtypes on ethanol intake. The results from our ISH analyses revealed a stimulatory effect on OX gene expression with D1 receptor agonist injection in the PF/LH, which also increased food intake and showed a tendency to increase ethanol drinking, and a suppressive effect on OX expression with D2 receptor agonist injection, which reduced ethanol drinking. These distinct effects of D1 and D2 stimulation on OX appeared to be more prominent in the LH subregion, which is crucial in reward processing (Harris & Aston-Jones, 2006), than they were in the PF subregion, which is more closely related to arousal (Harris & Aston-Jones, 2006). The opposing effects of these receptors and their possible interaction are consistent with electrophysiological findings, showing that a high concentration of DA which specifically activates the D2-like receptor may counteract the effect of D1-like receptor stimulation on the activity of hypothalamic peptide neurons (Conductier et al., 2011). While the precise mechanisms underlying these DA modulations of OX neurons has yet to be determined, the findings that DA can directly hyperpolarize OX neurons (Yamanaka et al., 2003) and local GABA neurons express DA receptors (Conductier et al., 2011) indicate that they may result from either direct activation of D2 receptors on OX neurons or indirectly from presynaptic modulation of D1 receptors on GABA interneurons or D2 receptors on glutamatergic neurons. This suggests that microinjection of a D2 receptor antagonist such as sulpiride in the LH may increase OX expression, providing a neurochemical explanation for this receptor’s stimulatory effect on ethanol intake.
The possibility that alterations in OX expression levels underlie the DA-mediated changes in ethanol drinking is further supported by the evidence that direct infusion of OX in the LH increases ethanol intake (Schneider et al., 2007) and administration of an OX antagonist attenuates ethanol consumption (Lawrence et al., 2006). In contrast to OX, the present study did not find a change in MCH gene expression after D1 or D2 receptor activation, despite previous evidence suggesting a possible interaction between DA and the MCH system (Conductier et al., 2011; Smith et al., 2005). This may be due to the possibility that DA affects MCH indirectly, via a recently proposed presynaptic mechanism (Conductier et al., 2011), which requires more time than that allotted in the present experiment to influence expression levels. Taken together, these findings suggest that the two distinct classes of DA receptors regulate ethanol drinking by interacting directly and specifically with the OX peptide system in the LH.
Conclusion
Our results demonstrate that D1 and D2 receptors in the PF/LH play differential roles in modulating consummatory behavior, with D1 receptor activation eliciting ethanol drinking and feeding behavior in association with an increase in OX expression in the PF and LH regions and D2 receptor activation suppressing ethanol drinking while reducing OX expression specifically in the LH region. These analyses of behavioral and neurochemical systems in the PF/LH shed light on the intrinsic dopaminergic system and receptor subtypes of the PF/LH that may be involved in mediating the effects of DA antagonists on eating behavior and alcohol consumption reported in humans (Gothelf et al., 2002; Ray et al., 2011).
ACKNOWLEDGEMENTS
This research was supported by USPHS Grant AA12882 and the E. H. Lane Foundation. We thank Dr. Luis de Lecea (Stanford University) and Dr. Nicholas A. Tritos (Harvard University) for kindly providing the RNA probes used in this study.
Support: USPHS Grant AA12882 and the E. H. Lane Foundation.
REFERENCES
- Agabio R, Cortis G, Fadda F, Gessa GL, Lobina C, Reali R, Colombo G. Circadian drinking pattern of Sardinian alcohol-preferring rats. Alcohol Alcohol. 1996;31:385–388. doi: 10.1093/oxfordjournals.alcalc.a008166. [DOI] [PubMed] [Google Scholar]
- Bahi A, Dreyer JL. Involvement of nucleus accumbens dopamine D1 receptors in ethanol drinking, ethanol-induced conditioned place preference, and ethanol-induced psychomotor sensitization in mice. Psychopharmacology (Berl) 2012 doi: 10.1007/s00213-011-2630-8. [DOI] [PubMed] [Google Scholar]
- Barson JR, Carr AJ, Soun JE, Sobhani NC, Rada P, Leibowitz SF, Hoebel BG. Opioids in the hypothalamic paraventricular nucleus stimulate ethanol intake. Alcohol Clin Exp Res. 2010;34:214–222. doi: 10.1111/j.1530-0277.2009.01084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 2011;63:182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- Bice PJ, Liang T, Zhang L, Strother WN, Carr LG. Drd2 expression in the high alcohol-preferring and low alcohol-preferring mice. Mamm Genome. 2008;19:69–76. doi: 10.1007/s00335-007-9089-2. [DOI] [PubMed] [Google Scholar]
- Blum K, Noble EP, Sheridan PJ, Montgomery A, Ritchie T, Jagadeeswaran P, Nogami H, Briggs AH, Cohn JB. Allelic association of human dopamine D2 receptor gene in alcoholism. JAMA. 1990;263:2055–2060. [PubMed] [Google Scholar]
- Bossert JM, Poles GC, Wihbey KA, Koya E, Shaham Y. Differential effects of blockade of dopamine D1-family receptors in nucleus accumbens core or shell on reinstatement of heroin seeking induced by contextual and discrete cues. J Neurosci. 2007;27:12655–12663. doi: 10.1523/JNEUROSCI.3926-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulliez R, Meile MJ, Nicolaidis S. A lateral hypothalamic D1 dopaminergic mechanism in conditioned taste aversion. Brain Res. 1996;729:234–245. [PubMed] [Google Scholar]
- Chang GQ, Gaysinskaya V, Karatayev O, Leibowitz SF. Maternal high-fat diet and fetal programming: increased proliferation of hypothalamic peptide-producing neurons that increase risk for overeating and obesity. J Neurosci. 2008;28:12107–12119. doi: 10.1523/JNEUROSCI.2642-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YW, Barson JR, Chen A, Hoebel BG, Leibowitz SF. Opioids in the perifornical lateral hypothalamus suppress ethanol drinking. Alcohol. 2013;47:31–38. doi: 10.1016/j.alcohol.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conductier G, Nahon JL, Guyon A. Dopamine depresses melanin concentrating hormone neuronal activity through multiple effects on alpha2-noradrenergic, D1 and D2-like dopaminergic receptors. Neuroscience. 2011;178:89–100. doi: 10.1016/j.neuroscience.2011.01.030. [DOI] [PubMed] [Google Scholar]
- Crombag HS, Grimm JW, Shaham Y. Effect of dopamine receptor antagonists on renewal of cocaine seeking by reexposure to drug-associated contextual cues. Neuropsychopharmacology. 2002;27:1006–1015. doi: 10.1016/S0893-133X(02)00356-1. [DOI] [PubMed] [Google Scholar]
- Dayas CV, McGranahan TM, Martin-Fardon R, Weiss F. Stimuli linked to ethanol availability activate hypothalamic CART and orexin neurons in a reinstatement model of relapse. Biol Psychiatry. 2008;63:152–157. doi: 10.1016/j.biopsych.2007.02.002. [DOI] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, 2nd, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis F, Thanos PK, Rombola C, Rosko L, Grandy D, Wang GJ, Volkow ND. Chronic mild stress increases alcohol intake in mice with low dopamine D2 receptor levels. Behav Neurosci. 2013;127:95–105. doi: 10.1037/a0030750. [DOI] [PubMed] [Google Scholar]
- Duncan EA, Proulx K, Woods SC. Central administration of melanin-concentrating hormone increases alcohol and sucrose/quinine intake in rats. Alcohol Clin Exp Res. 2005;29:958–964. doi: 10.1097/01.alc.0000167741.42353.10. [DOI] [PubMed] [Google Scholar]
- Duncan EA, Sorrell JE, Adamantidis A, Rider T, Jandacek RJ, Seeley RJ, Lakaye B, Woods SC. Alcohol drinking in MCH receptor-1-deficient mice. Alcohol Clin Exp Res. 2007;31:1325–1337. doi: 10.1111/j.1530-0277.2007.00427.x. [DOI] [PubMed] [Google Scholar]
- Fetissov SO, Meguid MM, Sato T, Zhang LH. Expression of dopaminergic receptors in the hypothalamus of lean and obese Zucker rats and food intake. Am J Physiol Regul Integr Comp Physiol. 2002;283:R905–R910. doi: 10.1152/ajpregu.00092.2002. [DOI] [PubMed] [Google Scholar]
- Gothelf D, Falk B, Singer P, Kairi M, Phillip M, Zigel L, Poraz I, Frishman S, Constantini N, Zalsman G, Weizman A, Apter A. Weight gain associated with increased food intake and low habitual activity levels in male adolescent schizophrenic inpatients treated with olanzapine. Am J Psychiatry. 2002;159:1055–1057. doi: 10.1176/appi.ajp.159.6.1055. [DOI] [PubMed] [Google Scholar]
- Graham DL, Hoppenot R, Hendryx A, Self DW. Differential ability of D1 and D2 dopamine receptor agonists to induce and modulate expression and reinstatement of cocaine place preference in rats. Psychopharmacology (Berl) 2007;191:719–730. doi: 10.1007/s00213-006-0473-5. [DOI] [PubMed] [Google Scholar]
- Hamlin AS, Newby J, McNally GP. The neural correlates and role of D1 dopamine receptors in renewal of extinguished alcohol-seeking. Neuroscience. 2007;146:525–536. doi: 10.1016/j.neuroscience.2007.01.063. [DOI] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Arousal and reward: a dichotomy in orexin function. Trends Neurosci. 2006;29:571–577. doi: 10.1016/j.tins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Ida T, Nakahara K, Murakami T, Hanada R, Nakazato M, Murakami N. Possible involvement of orexin in the stress reaction in rats. Biochem Biophys Res Commun. 2000;270:318–323. doi: 10.1006/bbrc.2000.2412. [DOI] [PubMed] [Google Scholar]
- Jiao X, Pare WP, Tejani-Butt SM. Alcohol consumption alters dopamine transporter sites in Wistar-Kyoto rat brain. Brain Res. 2006;1073(1074):175–182. doi: 10.1016/j.brainres.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Lawrence AJ, Cowen MS, Yang HJ, Chen F, Oldfield B. The orexin system regulates alcohol-seeking in rats. Br J Pharmacol. 2006;148:752–759. doi: 10.1038/sj.bjp.0706789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibowitz KL, Chang GQ, Pamy PS, Hill JO, Gayles EC, Leibowitz SF. Weight gain model in prepubertal rats: prediction and phenotyping of obesity-prone animals at normal body weight. Int J Obes (Lond) 2007;31:1210–1221. doi: 10.1038/sj.ijo.0803634. [DOI] [PubMed] [Google Scholar]
- Leibowitz SF, Rossakis C. Pharmacological characterization of perifornical hypothalamic dopamine receptors mediating feeding inhibition in the rat. Brain Res. 1979;172:115–130. doi: 10.1016/0006-8993(79)90899-0. [DOI] [PubMed] [Google Scholar]
- Liu X, Weiss F. Reversal of ethanol-seeking behavior by D1 and D2 antagonists in an animal model of relapse: differences in antagonist potency in previously ethanol-dependent versus nondependent rats. J Pharmacol Exp Ther. 2002;300:882–889. doi: 10.1124/jpet.300.3.882. [DOI] [PubMed] [Google Scholar]
- Martinetti MP, Andrzejewski ME, Hineline PN, Lewis MJ. Ethanol consumption and the matching law: a choice analysis using a limited-access paradigm. Exp Clin Psychopharmacol. 2000;8:395–403. doi: 10.1037//1064-1297.8.3.395. [DOI] [PubMed] [Google Scholar]
- Meador-Woodruff JH, Mansour A, Healy DJ, Kuehn R, Zhou QY, Bunzow JR, Akil H, Civelli O, Watson SJ., Jr Comparison of the distributions of D1 and D2 dopamine receptor mRNAs in rat brain. Neuropsychopharmacology. 1991;5:231–242. [PubMed] [Google Scholar]
- Meguid MM, Yang ZJ, Koseki M. Eating induced rise in LHA-dopamine correlates with meal size in normal and bulbectomized rats. Brain Res Bull. 1995;36:487–490. doi: 10.1016/0361-9230(95)92128-3. [DOI] [PubMed] [Google Scholar]
- Morganstern I, Barson JR, Leibowitz SF. Regulation of drug and palatable food overconsumption by similar peptide systems. Curr Drug Abuse Rev. 2011;4:163–173. doi: 10.2174/1874473711104030163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morganstern I, Chang GQ, Barson JR, Ye Z, Karatayev O, Leibowitz SF. Differential effects of acute and chronic ethanol exposure on orexin expression in the perifornical lateral hypothalamus. Alcohol Clin Exp Res. 2010a;34:886–896. doi: 10.1111/j.1530-0277.2010.01161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morganstern I, Chang GQ, Chen YW, Barson JR, Zhiyu Y, Hoebel BG, Leibowitz SF. Role of melanin-concentrating hormone in the control of ethanol consumption: Region-specific effects revealed by expression and injection studies. Physiol Behav. 2010b;101:428–437. doi: 10.1016/j.physbeh.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morutto SL, Phillips GD. Interactions between sulpiride infusions within the perifornical region of the lateral hypothalamus and the nucleus accumbens on measures of locomotor activity and conditioned place preference. Behav Pharmacol. 1998;9:345–355. [PubMed] [Google Scholar]
- Morutto SL, Phillips GD. Post-session intra-perifornical region quinpirole infusions retard the consolidation of an appetitive differential conditioning task. Behav Pharmacol. 1999;10:113–118. doi: 10.1097/00008877-199902000-00011. [DOI] [PubMed] [Google Scholar]
- Nowend KL, Arizzi M, Carlson BB, Salamone JD. D1 or D2 antagonism in nucleus accumbens core or dorsomedial shell suppresses lever pressing for food but leads to compensatory increases in chow consumption. Pharmacol Biochem Behav. 2001;69:373–382. doi: 10.1016/s0091-3057(01)00524-x. [DOI] [PubMed] [Google Scholar]
- Parada MA, Hernandez L, Hoebel BG. Sulpiride injections in the lateral hypothalamus induce feeding and drinking in rats. Pharmacol Biochem Behav. 1988;30:917–923. doi: 10.1016/0091-3057(88)90120-7. [DOI] [PubMed] [Google Scholar]
- Parada MA, Hernandez L, Puig de Parada M, Paez X, Hoebel BG. Dopamine in the lateral hypothalamus may be involved in the inhibition of locomotion related to food and water seeking. Brain Res Bull. 1990;25:961–968. doi: 10.1016/0361-9230(90)90195-6. [DOI] [PubMed] [Google Scholar]
- Ray LA, Chin PF, Heydari A, Miotto K. A human laboratory study of the effects of quetiapine on subjective intoxication and alcohol craving. Psychopharmacology (Berl) 2011;217:341–351. doi: 10.1007/s00213-011-2287-3. [DOI] [PubMed] [Google Scholar]
- Rusk IN, Cooper SJ. Parametric studies of selective D1 or D2 antagonists: effects on appetitive and feeding behaviour. Behav Pharmacol. 1994;5:615–622. doi: 10.1097/00008877-199410000-00007. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Schneider ER, Rada P, Darby RD, Leibowitz SF, Hoebel BG. Orexigenic peptides and alcohol intake: differential effects of orexin, galanin, and ghrelin. Alcohol Clin Exp Res. 2007;31:1858–1865. doi: 10.1111/j.1530-0277.2007.00510.x. [DOI] [PubMed] [Google Scholar]
- Smith DG, Tzavara ET, Shaw J, Luecke S, Wade M, Davis R, Salhoff C, Nomikos GG, Gehlert DR. Mesolimbic dopamine super-sensitivity in melanin-concentrating hormone-1 receptor-deficient mice. J Neurosci. 2005;25:914–922. doi: 10.1523/JNEUROSCI.4079-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R, Weiss F. The dopamine hypothesis of reward: past and current status. Trends Neurosci. 1999;22:521–527. doi: 10.1016/s0166-2236(99)01447-2. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Katana JM, Ashby CR, Jr, Michaelides M, Gardner EL, Heidbreder CA, Volkow ND. The selective dopamine D3 receptor antagonist SB-277011-A attenuates ethanol consumption in ethanol preferring (P) and non-preferring (NP) rats. Pharmacol Biochem Behav. 2005;81:190–197. doi: 10.1016/j.pbb.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Taintor NB, Rivera SN, Umegaki H, Ikari H, Roth G, Ingram DK, Hitzemann R, Fowler JS, Gatley SJ, Wang GJ, Volkow ND. DRD2 gene transfer into the nucleus accumbens core of the alcohol preferring and nonpreferring rats attenuates alcohol drinking. Alcohol Clin Exp Res. 2004;28:720–728. doi: 10.1097/01.alc.0000125270.30501.08. [DOI] [PubMed] [Google Scholar]
- Touzani K, Bodnar RJ, Sclafani A. Lateral hypothalamus dopamine D1-like receptors and glucose-conditioned flavor preferences in rats. Neurobiol Learn Mem. 2009;92:464–467. doi: 10.1016/j.nlm.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupala E, Hall H, Bergstrom K, Mantere T, Rasanen P, Sarkioja T, Tiihonen J. Dopamine D2 receptors and transporters in type 1 and 2 alcoholics measured with human whole hemisphere autoradiography. Hum Brain Mapp. 2003;20:91–102. doi: 10.1002/hbm.10129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Hitzemann R, Ding YS, Pappas N, Shea C, Piscani K. Decreases in dopamine receptors but not in dopamine transporters in alcoholics. Alcohol Clin Exp Res. 1996;20:1594–1598. doi: 10.1111/j.1530-0277.1996.tb05936.x. [DOI] [PubMed] [Google Scholar]
- Wamsley JK, Gehlert DR, Filloux FM, Dawson TM. Comparison of the distribution of D-1 and D-2 dopamine receptors in the rat brain. J Chem Neuroanat. 1989;2:119–137. [PubMed] [Google Scholar]
- Wellman PJ. Modulation of eating by central catecholamine systems. Curr Drug Targets. 2005;6:191–199. doi: 10.2174/1389450053174532. [DOI] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Wortley KE, Chang GQ, Davydova Z, Leibowitz SF. Peptides that regulate food intake: orexin gene expression is increased during states of hypertriglyceridemia. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1454–R1465. doi: 10.1152/ajpregu.00286.2002. [DOI] [PubMed] [Google Scholar]
- Yamanaka A, Muraki Y, Tsujino N, Goto K, Sakurai T. Regulation of orexin neurons by the monoaminergic and cholinergic systems. Biochem Biophys Res Commun. 2003;303:120–129. doi: 10.1016/s0006-291x(03)00299-7. [DOI] [PubMed] [Google Scholar]
- Yan QS, Zheng SZ, Feng MJ, Yan SE. Involvement of 5-HT1B receptors within the ventral tegmental area in ethanol-induced increases in mesolimbic dopaminergic transmission. Brain Res. 2005;1060:126–137. doi: 10.1016/j.brainres.2005.08.051. [DOI] [PubMed] [Google Scholar]
- Zarrindast MR, Owji AA, Hosseini-Nia T. Evaluation of dopamine receptor involvement in rat feeding behaviour. Gen Pharmacol. 1991;22:1011–1016. doi: 10.1016/0306-3623(91)90570-v. [DOI] [PubMed] [Google Scholar]
- Zippel U, Plagemann A, Davidowa H. Altered action of dopamine and cholecystokinin on lateral hypothalamic neurons in rats raised under different feeding conditions. Behav Brain Res. 2003;147:89–94. doi: 10.1016/s0166-4328(03)00140-2. [DOI] [PubMed] [Google Scholar]