Abstract
For decades, mouse xenografts utilizing well-established human tumor cell lines have been the most common models used to study human cancers. More recently, “fresh” human tumors implanted directly into immunodeficient mice have become increasingly popular as evidence accrues that they more accurately recapitulate features of original patient tumors. Here we describe our methods for the orthotopic and heterotopic implantation of pancreatic cancer cell lines and freshly isolated patient tumors into immunodeficient mice. In addition, we describe procedures for the digestion of tumor into single cell suspensions for the isolation of sub-populations of tumor cells. Orthotopic or heterotopic placement of established cell lines requires 1–2 hours, with 1cm diameter tumors arising after 2–5 weeks. Direct engraftment of freshly harvested tumor samples takes approximately 2 hours and growth of palpable tumor requires an average of 14 weeks. Once established, direct xenograft tumor requires 1 and 3 hours for heterotopic and orthotopic passage, respectively, and an average of 5 weeks for palpable tumor growth.
Introduction
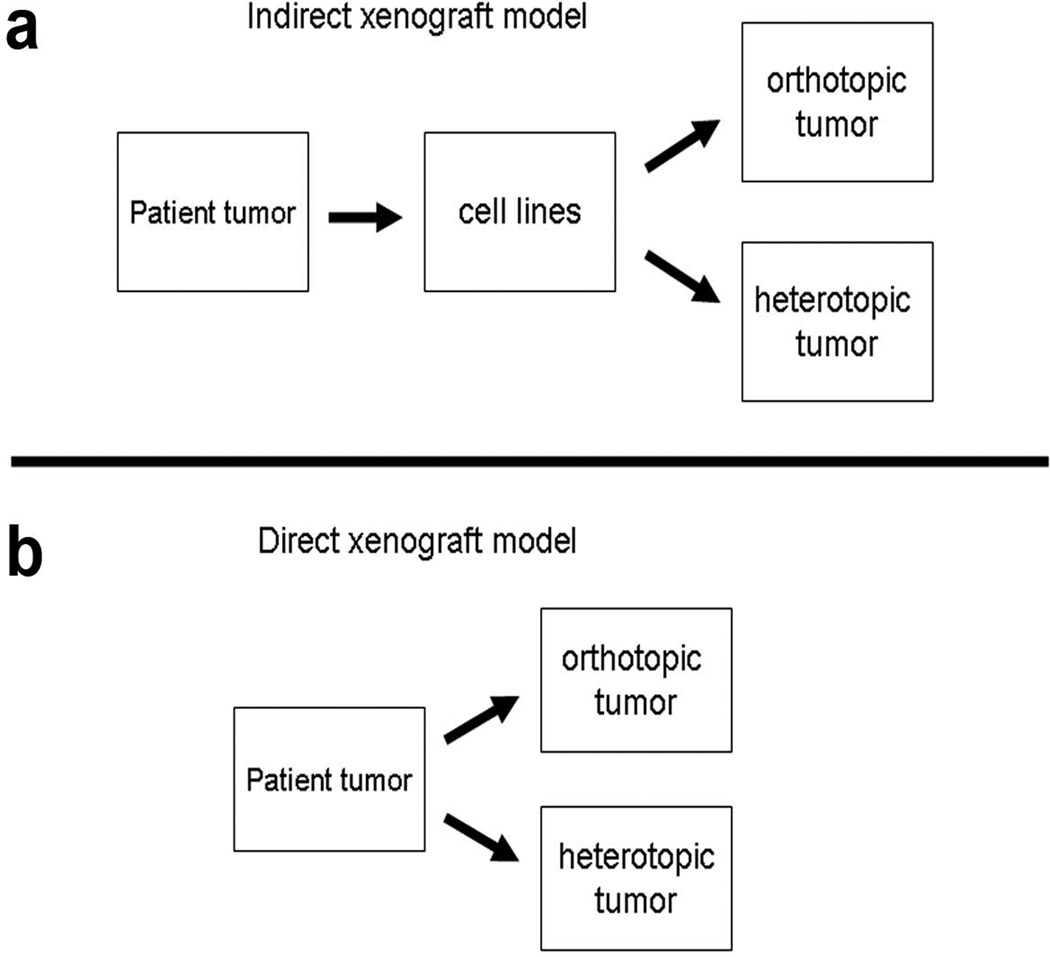
Numerous genetic alterations are associated with the development and progression of pancreatic cancer1. However, the spectrum of genetic and epigenetic changes and the roles of tumor microenvironment in this process remain poorly understood. Lack of this understanding stems, in part, from deficiencies in model systems used to study the disease. An increasing awareness has therefore developed regarding the necessity for new models of pancreatic cancer that better incorporate the complex factors that promote tumor progression and metastasis. One popular strategy in this regard is the development of transgenic mouse models of pancreatic cancer. Such genetic models hold much promise both for studying early disease progression and for screening of new therapeutics, especially potential chemopreventive agents2,3, 4. However, to date, the utility of transgenic mouse models is limited for the study of late-stage disease due to their inability to faithfully recapitulate the complete cascade of events in tumor cell invasion and metastasis to distant organs5. Another strategy gaining recent interest involves the direct implantation of primary tumor samples from patients at the time of surgical resection into immunodeficient mice6–8. Models resulting from this approach utilize human tumor and recapitulate many tumor/microenvironment interactions, retain important genetic features and heterogeneity, and often develop distant metastases 6–8. Here, we focus on of the requirements to optimize heterotopic and orthotopic tumor development by this latter strategy, as well as from traditional cell lines which are still employed by many investigators (Figure 1).
Figure 1.
Comparison of current xenograft models. Indirect xenografts are created from established from cell lines and may undergo heterotopic or orthotopic implantation. In contrast, direct xenografts are created from original patient tumor explants without a cell line intermediary.
Several classes of immunodeficient mice have been utilized as biologic platforms upon which to grow xenograft tumors. Athymic nude mice (T-cell deficient) have proven useful for the establishment of tumors both from patients’ tumor samples and from established from human cancer cell lines 9. Reduced cost, widespread availability, and the absence of fur have offered investigators convenient access to these useful in vivo tumor model systems of human cancers. However, a relatively intact humoral immunity in nude mice likely results in reduced efficiency of tumor formation relative to mice with severe combined immunodeficiencies (SCID mice-lack functional T- and B-cells), particularly in cases of low cell inoculum10. Further still, NOD/SCID mice harboring additional deficiencies in natural killer cells (NOD/SCID Il2rg−/−) have permitted tumor establishment from even fewer numbers of tumor cells than previously seen in NOD/SCID and nude mice11, 12. Additional cell selection almost certainly occurs against xenografts in nude mice relative to SCID mice, further reducing tumor heterogeneity and the biologic complexity present in original human tumor. Nevertheless, both athymic nude and NOD/SCID mice continue to be used widely for molecular and translational studies of human cancers.
Differences in mouse hosts aside, established cancer cell lines are easily implanted into mouse tissues at heterotopic or orthotopic sites and can be passaged serially into congenic mouse strains with excellent efficiency. Such tumors now are frequently termed “indirect xenografts”, due to long periods of cancer cell growth (often decades) between acquisition from patients and animal implantation. Indirect xenografts continue to be extensively utilized and offer convenience in cell manipulation and require limited technical skill for successful engraftment. However, indirect xenograft models inconsistently predict efficacy of novel therapeutics in select human tumors, including pancreatic cancer13, 14. For example, the anti-tumor effects of specific therapeutics in indirect xenograft test models, such as farnesyltransferase inhibitors in pancreatic cancer, have correlated poorly with patient response15, 16. To some degree, this lack of predictability stems from poor experimental design, e.g. the initiation of therapeutic treatment within a few days of tumor cell inoculation. Additional problems that lead to the poor predictive capability of indirect xenografts likely include long-term selection for growth in culture media rather than in a host environment, and a relative paucity of peri-tumor stroma. Further, most tumors are generated from large initial inocula of tumor cells, subverting the natural processes of genetic and epigenetic progression that occur from small, clonal tumors.
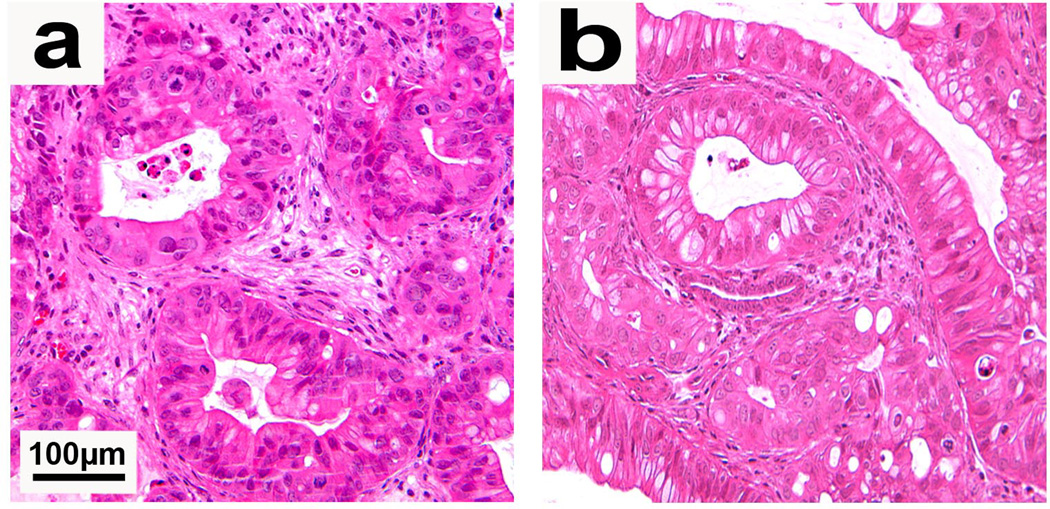
In response to these and other concerns, models initiated by direct heterotopic and/or orthotopic engraftment of primary human tumor samples into immunodeficient mice have received increasing interest. Such direct xenograft models, generated directly from tumor samples of patients without a cell line intermediary, have been termed “tumorgrafts” for this distinction17. This approach may limit clonal selection and the ex vivo manipulation inherent in the culturing of cancer cell lines. Most direct xenografts grow with considerable stromal elements and recapitulate the histologic appearance of the original patient tumor over multiple passages in mice (Fig. 2) 7, 8, 18. Published data indicate that when utilized appropriately, direct xenograft models reliably predict clinical response in pediatric malignancies19, 20. Likewise, recent studies indicate that direct pancreatic cancer xenografts may be utilized to effectively screen therapeutic agents when heterotopically implanted in nude mice7. Direct xenografts have also been used to identify and enrich for distinct subpopulations of cells, such as tumor initiating cells (cancer stem cells), in a variety of solid organ systems21–23.
Figure 2.
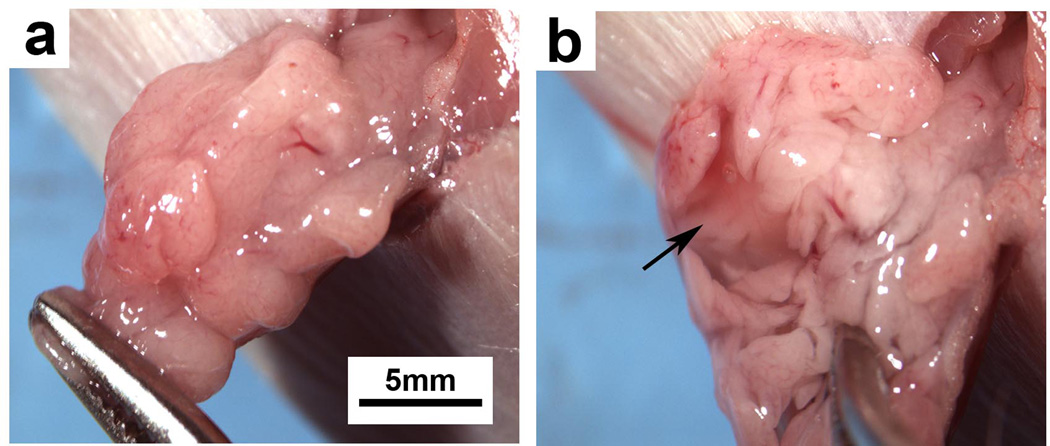
(a) Direct xenograft tumor established in a heterotopic site (subcutaneous space) of a NOD/SCID mouse. (b) Direct xenograft tumor established from injection of digested tumor (single cells) into the pancreas of a NOD/SCID mouse. Both heterotopic and orthotopic tumors show tumor gland formation and the presence of peri-tumoral stroma. Permission from the M.D. Anderson Cancer Center (MDACC) Institutional Regulatory Board was obtained for this study.
We detail below our methods for the orthotopic and heterotopic implantation of primary patient tumor samples, direct pancreatic cancer xenografts, and established pancreatic cancer cell lines. Further, due to widespread emerging interest in the isolation of subpopulations of cancer cells, we describe our method for the processing of bulk tumor samples into single cell suspensions for analysis by flow cytometry and cell sorting.
MATERIALS
REAGENTS
-
-
Sterile RPMI-1640 (HyClone, cat.# SH30027.02)
-
-
Antiobiotic-antimycotic 100X (Gibco, cat.#15240)
-
-
70% ethanol
-
-
NOD.SCID mice, female (NCI, 4–6 weeks)
-
-
Ketamine HCl (100mg/ml) (Fort Dodge Animal Health, NADA 45–290)
-
-
Xylazine hydrochloride (Sigma, cat.# X1251-5G)
-
-
Collagenase type IV (Worthington, 4210)
-
-
RBC lysis buffer (eBioscience, cat.#00-4333-57)
EQUIPMENT
-
-
Microscissors (Henry Schein, cat.# 100–8820)
-
-
Curved forceps (Henry Schein, cat.# 100–0673)
-
-
Splinter forceps (Henry Schein, cat.# 100–2111)
-
-
25G needle
-
-
1cc tuberculin syringe
-
-
50ml centrifuge tubes (Corning, cat.#430828)
-
-
Scale
-
-
Electric clippers
-
-
Sterile petri dishes
-
-
Disposable, sterile 21-blade scalpels (Feather)
-
-
Silk suture 3-0 (Ethicon)
-
-
Sterile syringe filters (Corning, cat.# 431229)
-
-
Hanks’ Balanced Salt Solution (HBSS,1X) (cellgro, cat.#21-021-CV)
-
-
Gauze
-
-
25ml pipette (costar, cat.#4489)
-
-
10ml pipette (costar, cat.#4488)
-
-
70µm cell strainer (BD Falcon, cat.#352350)
-
-
40µm cell strainer (BD Falcon, cat.#352340)
-
-
Hemacytometer or cell counter (Beckman Coulter, Vi-cell XR)
-
-
Microscope
-
-
PBS
-
-
10X Trypsin-EDTA, 0.5% trypsin-EDTA (Gibco, cat.# 15400)
-
-
Incubator (37°C, 5% CO2)
-
-
Growth factor reduced Matrigel (BD Biosciences, cat.# 356230)
-
-
Countertop Vortex (Fisher)
-
-
Antibiotic ointment
-
-
Centrifuge capable of accepting 15 and 50mL centrifuge tubes.
-
-
Euthanasia chamber (CO2)
Procedures
*Ensure that all cell lines are mycoplasma-free and free of gross contamination that could result in animal sickness.
*All animals should be monitored daily for health and maintained under pathogen-free conditions. Palpable lesions and tumors should be recorded for monitoring of tumor progression and growth.
Primary tumor processing and heterotopic implantation TIMING 1h
Sterilize bioguard safety hood with application of copious amounts of 70% ethanol. Sterilize all surgical instruments in an autoclave and allow cooling to room temperature.
-
Transfer tumor specimen into a sterile 50mL centrifuge tube containing 30mLs of sterile, serum-free RPMI supplemented with 1X antibiotic/antimycotic (antibiotic media) chilled to 4°C.
! CAUTION All surgical specimens must be assumed to be bio-hazardous and handled with extreme caution using universal precautions. Acquire excess tumor in accordance with institutional review board guidelines and regulations only after adequate tissue diagnosis and evaluation of tumor margins have been performed by a staff pathologist.
CRITICAL STEP The time interval between tumor resection to implantation in mice must be kept to a minimum. Ideally, this should occur in less than one hour after resection with tumor maintained in serum-free antibiotic media, on ice.
Wash tumor sample twice with 30ml of serum-free antibiotic media chilled to 4°C to reduce contaminant load.
-
Weigh NOD/SCID mice and anesthetize using ketamine/xylazine cocktail at ratio of 100mg/kg: 20mg/kg.
! CAUTION All animal experiments require approval by institutional review board and animal use and care committees and must be conducted in accordance with institutional and national regulations.
Once anesthetized, shave left abdominal/flank region of NOD/SCID mouse with electric clippers and place mouse on its right side. Paint left side of mouse from base of neck to tail with gauze soaked in 70% ethanol solution.
-
In a sterile, ventilated hood, place tumor specimen on a sterile petri dish and cover with antibiotic media. Mince tumor with a sterile 21 blade until 1–2 mm3 tumor fragments are obtained.
CRITICAL STEP Do not allow tumor to dry; keep immersed in antibiotic media at all times.
Incise skin with microscissors and implant tumor fragments into subcutaneous space (see Box 1).
Close skin with interrupted 3-0 silk sutures.
Recover mice from the effects of anesthetic.
Box 1 Implantation of tumor fragments.
-
Picking up skin with forceps, make a ~1.2cm incision in the skin at superior aspect of flank with sterile, curved micro-scissors. Develop subcutaneous pocket through insertion of scissor tips into the subcutaneous space and a gentle spreading motion.
! CAUTION Incision should involve only skin layers and not deep muscle layers. The skin is highly mobile and identified when grasped and lifted from deep tissue by forceps.
While holding incision site open with forceps, gently lay five 1–2mm3 tumor fragments onto underlying tissue. Tumor fragments should individually remain adherent to underlying tissue within formed subcutaneous tunnel.
Close skin incision with interrupted stitches (3-0 silk suture).
Heterotopic passage of xenograft tumor TIMING 1.5h
Mice should be sacrificed and tumor harvested once tumor sizes reaches 1.2–1.5cm in largest diameter or upon ulceration of skin.
-
Weigh tumor-bearing and recipient NOD/SCID mice and anesthetize using ketamine/xylazine cocktail at ratio of 100mg/kg: 20mg/kg.
! CAUTION All animal experiments must be approved by institutional animal use and care committees and conducted in accordance with institutional and national regulations.
Once anesthetized, shave tumor-bearing region of mice with electric clippers and repeat at desired site of tumor implantation in recipient NOD/SCID mice. Paint tumor region or recipient site of mice from base of neck to tail with gauze soaked in 70% ethanol solution.
Dissect xenograft tumor from adherent subcutaneous tissue using sterile forceps and sterile, curved microscissors. Start by incising skin at the base of the xenograft tumor. Insert miroscissor tips into incision and gently spread, slowly developing a dissection plane between skin and underlying tumor. Extend incision as needed to completely uncover tumor and dissect the tumor from underlying deep tissue.
Once tumor is free, place in sterile, serum-free RPMI supplemented with 1X antibiotic/antifungal (antibiotic media) chilled to 4°C. Wash specimen twice with 30 mL volumes of serum-free antibiotic media.
Mince tumor with a sterile 21 blade until 1mm3 fragments are obtained and proceed as described above for the implantation of original patient tumors into recipient NOD/SCID mice (Box 1).
Recover mice from the effects of anesthetic.
Processing of xenograft tumor into a single cell suspension TIMING 3h
-
1
Prepare collagenase IV solution (200units/mL) and filter sterilize.
-
2
Euthanize mouse in CO2 chamber and shave tumor site with electric clippers. Paint tumor site with 70% ethanol and harvest tumor with sterile instruments in a sterile hood. Wash tumor twice with 30mLs of serum-free antibiotic media. Place tumor on petri dish and cover with 2mLs of collagenase IV solution.
CRITICAL STEP Do not allow tumor to dry; keep immersed in collagenase IV solution at all times.
-
3
Using a sterile 21 blade scalpel, mince tumor into smallest possible fragments (1.0mm3) and place 0.2g of tumor (paste-like consistency) into each well of a 6-well plate. Place 5mls of collagenase IV solution into each well and pipette with 25ml pipette. Place 6-well plate(s) into 37°C incubator for 2–2.5 hours, mixing with a 10ml pipette every 20 minutes to augment digestion. Continue to mince tumor fragments to smallest possible pieces prior to pipetting.
-
4
After incubation, fill each well with 8ml of sterile, serum-free RPMI and mix suspension with a 25ml pipette.
-
5
Strain digested tumor solution through sterile 70µM cell strainer, collecting cell solution in a 50ml centrifuge tube. Discard filter and associated tissue fragments.
-
8
Centrifuge cell solution at 1200rpm for 5 minutes and remove supernatant. Add RBC cell lysis buffer 1:10 volume pellet:buffer and re-suspend. Place on ice for 10 minutes.
-
9
Centrifuge solution at 1200rpm for 5 minutes and remove buffer. Re-suspend in serum-free RPMI and pass cell solution through 40µM cell strainer. Count cells using hemacytometer or cell counter.
-
10
Proceed to orthotopic or heterotopic injection into immunodeficient mice (see below) or antibody and/or dye staining for flow cytometry.
Preparation of pancreatic cancer cell lines into single cell suspensions TIMING 45m
*Pre-warm PBS and culture media to 37°C prior to addition to cell culture. Allow trypsin to warm to room temperature.
Remove media from plate containing adherent pancreatic cancer cells.
Rinse cell culture plate twice with sterile PBS.
Add 2ml of 1X trypsin-EDTA (0.05%) to plate or enough to just cover surface area of cells. Place back in 37°C incubator.
Three minutes after the addition of trypsin, examine cell adherence to surface of cell culture plate or flask. Lightly tap the cell culture plate or flask if majority of cells have been released. If many cells remain adherent, place back in 37°C incubator for an additional 3 minutes, lightly tapping cell container as needed.
Once most cells (>98%) have detached from the plate, add sterile culture media 5:1 to volume of trypsin added to culture flask. Gently pass cell suspension through 10ml pipette 5 times to reduce cell clumping and to remove remaining adherent cells.
Collect cells into 15ml or 50ml centrifuge tube, depending on the volume of cell solution collected. Proceed to centrifuge cells at 1200rpm for 5 minutes, resulting in the formation of a cell pellet. Wash cell pellet once with sterile PBS and re-suspend pellet in sterile culture media.
Quantitate cells using a hemacytometer or cell counter. Adjust cell concentration to 1 million cells/ml with sterile culture media.
-
Divide into aliquots of 10 × 106 cells (10ml) and again centrifuge at 1200rpm for 5 minutes. Thoroughly re-suspend pellet in 20mL of sterile Hank’s balanced salt solution (HBSS) containing 1% serum-free matrigel by volume (4°C), bringing cell concentration to 500,000cells/100ul (final cell inoculum).
! CAUTION The concentration of cells in the prepared cell solution may be altered depending on the desired cell inoculum, site of injection, and specific experimental objectives. Regardless, due to technical considerations, one should prepare at least twice the volume of cell solution required for experiments.
-
Keeping cell solution on ice, aspirate into an open 1.0 ml syringe and cap with a 25 gauge needle. Repeat as needed with additional syringes and immediately place on ice.
! CAUTION Avoid aspirating cell solution THROUGH the 25 gauge needle as this may shear cells and reduce cell inoculum.
-
Proceed to heterotopic or orthotopic cell injection.
! CAUTION Maintain 1% matrigel-cell solution on ice at all times to prevent premature gel formation.
Orthotopic implantation of pancreatic cancer cells TIMING 30m/mouse
Prepare pancreatic cancer cell solution (from cell lines or tumor) to a concentration of 500,000 cells/100µl in Hank’s balanced salt solution containing 1% matrigel. Leave on ice with occasional vortex agitation.
Anesthetize recipient nude or NOD/SCID mice using ketamine/xylazine cocktail. Weigh mice and administer ketamine:xylazine cocktail at ratio of 100mg/kg: 20mg/kg.
Shave left abdominal/flank region of mice with clippers and place mice on their right side. Paint left sides of mice from the base of the neck to tail with a 70% ethanol solution.
Proceed with animal surgery and cell implantation as described in Box 2.
Box 2 Orthotopic injection of pancreatic cancer cells.
Identify silhouette of spleen through intact, shaved skin. Pick up skin with forceps and make a 1.2cm incision with sterile micro-scissors slightly medial to splenic silhouette (see picture).
Grasp underlying muscle with forceps and lift to enter the abdominal cavity without injury to underlying organs. Extend muscle incision with micro-scissors to 0.75cm.
Using a pair of blunt-nose forceps, gently grasp the tip of the pancreatic tail and externalize pancreas/spleen in a lateral fashion, exposing the entire pancreatic body and spleen.
Remove syringe containing cell suspension (cell line or xenograft-derived) from ice and vortex agitate to disrupt cell clumps/cluster.
-
While gently retracting pancreas laterally, insert needle into the tail of pancreas and pass into the pancreatic head region. Slowly inject 100µl of cell solution while withdrawing needle to mid-body of pancreas. Remove needle from pancreas and observe needle tract for leak/bleeding.
CRITICAL STEP Make sure the needle is passed parallel to any visible vessels (arrows) and along the length of the pancreas. Oblique passage of the needle will likely injure vessels and result in hemorrhage.
Leave pancreas externalized and untouched for 2 minutes for matrigel to solidify while inspecting pancreas for vascular injury and/or leakage of cell solution. Gently internalize pancreas/spleen with blunt forceps and close abdominal muscle layer with interrupted stitches (3-0 silk suture). Close overlying skin with a second set of interrupted stitches (3-0 silk suture) and apply antibiotic ointment.
Recover animals from anesthetic.
Heterotopic implantation of pancreatic cancer cells TIMING 15m/mouse
Anesthetize nude or NOD/SCID mice using ketamine/xylazine cocktail (as described above).
If required, shave desired injection site and place mouse on its contralateral side. Depending on the location of cell implantation, paint corresponding side of mouse with 70% ethanol solution.
Gently agitate syringe containing cell suspension to disrupt cell clumps/clusters.
Identify desired location of cell implantation. While gently grasping skin with forceps for counter-traction, penetrate skin with the needle tip approximately a needle’s length from desired injection site. Direct needle to desired implantation site while passing the needle parallel to animal body.
Once the needle is confirmed to be in the subcutaneous space, slowly inject 150µl. Confirm successful cell injections by presence of bullae.
Withdraw needle slowly from tract and monitor for 2 minutes for gross leakage.
Recover mice from the effects of anesthetic.
Anticipated results
Palpable growth of patient tumor in mice may take 4–30 weeks with an average time 14 weeks. However, once passaged into subsequent generations of mice, maximum time until tumor formation should be less than 10 weeks. Orthotopic tumors derived from xenograft tumors will take 10–15 weeks to reach tumor sizes palpable through the abdominal wall. It is common for us to implant xenograft tumor at heterotopic and orthotopic sites in the same mouse. However, we discourage this practice if sizeable pancreatic tumors are required for experimental purposes as animals will likely require euthanasia for heterotopic tumor size prior to the development of substantial pancreatic tumors. Tumors derived from established cell lines (heterotopic or orthotopic) form palpable tumors between 2–5 weeks post-implantation.
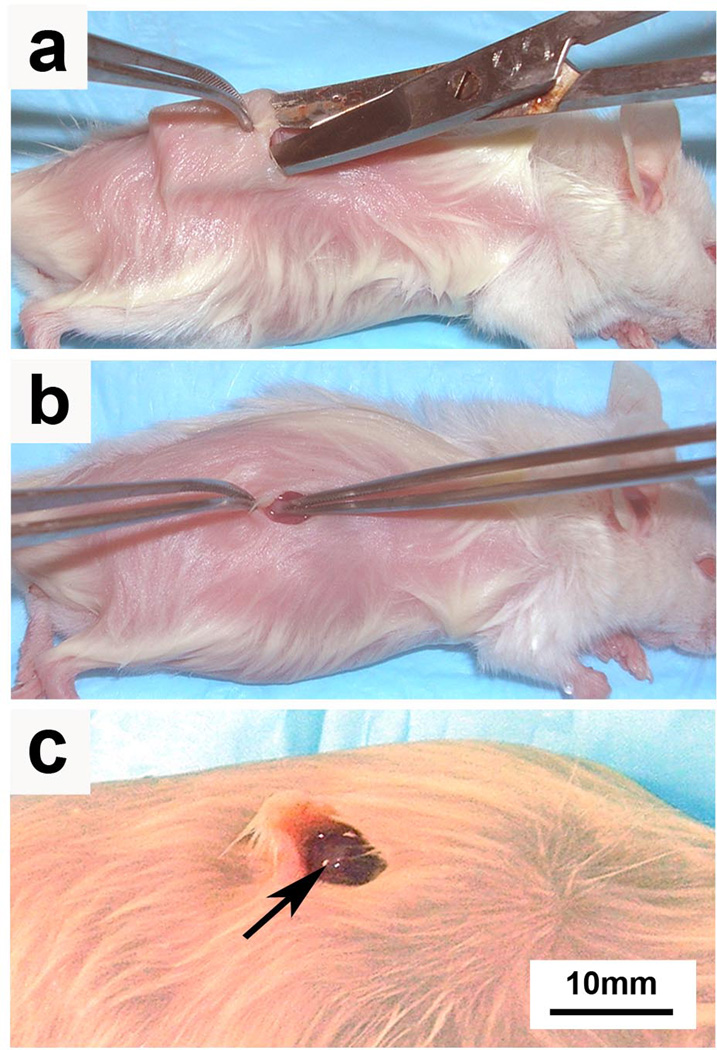
Figure 3.
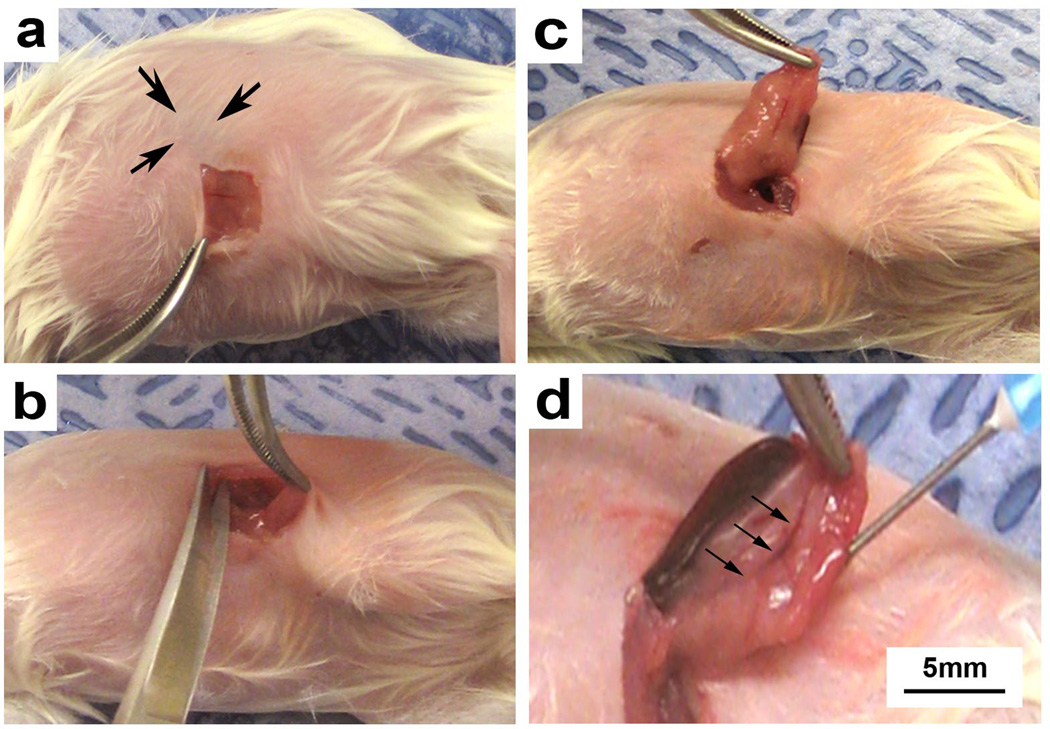
Tumor fragments isolated directly from patient tumors or from direct xenograft tumors are placed in the subcutaneous space of immunodeficient mice. (a) A subcutaneous pocket is developed with microscissors and (b) minced tumor fragments are implanted on the top of deep muscle tissue. (c) A single tumor fragment is shown loosely adherent to underlying mouse muscle (arrow). Permission from the M.D. Anderson Cancer Center (MDACC) Institutional Regulatory Board was obtained for this study.
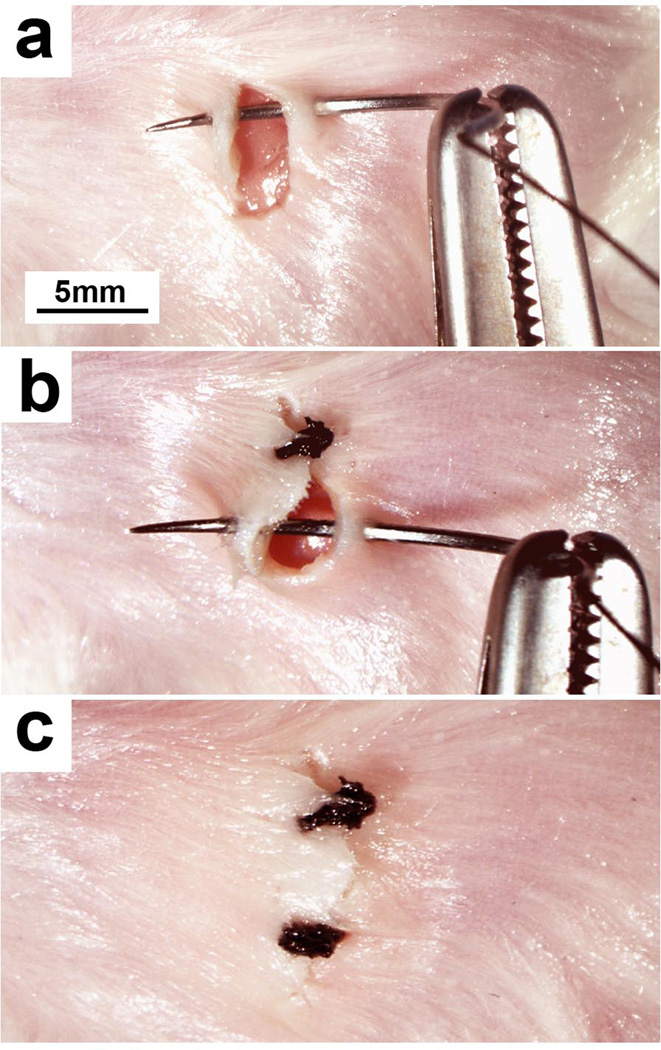
Figure 4.
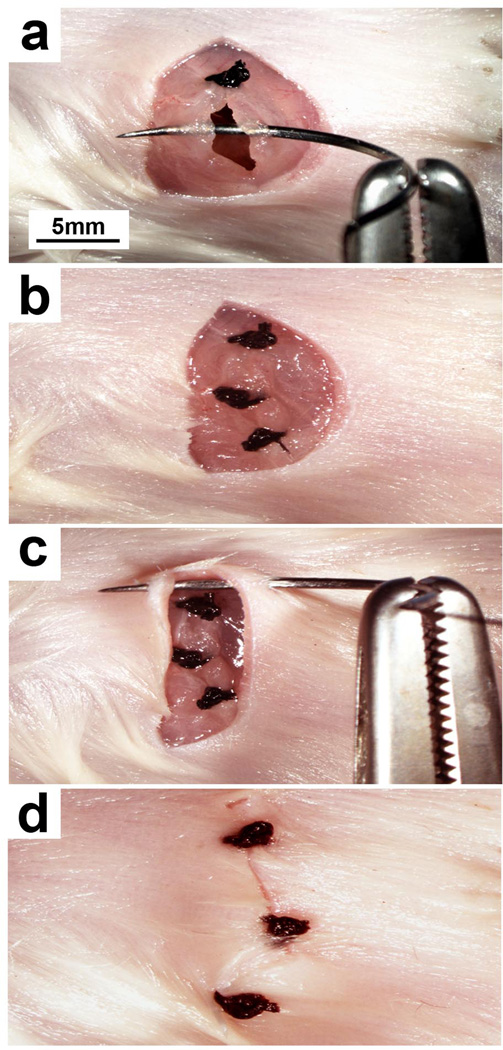
(a) The needle of a 3-0 silk suture is passed through the skin on each side of the incision and the ends tied. (b) Excess suture is cut at the knot and additional sutures placed along the entire length of the incision. (c) Once complete, the incision edges should be well approximated and without any large gaps. Permission from the M.D. Anderson Cancer Center (MDACC) Institutional Regulatory Board was obtained for this study.
Figure 5.
Established pancreatic cancer cell lines or digested patient/xenograft tumor may be injected into the mouse pancreatic parenchyma for tumor growth. (a) The mouse skin layer is incised (approx1 cm) and the underlying muscle layer identified. (b) The muscle layer is incised (approx1 cm) while lifting it away from the intra-abdominal contents. (c) The pancreas is identified and laterally externalized to expose its entire length. (d) A needle is passed parallel to the length of the pancreas into the pancreatic head region and pancreatic cancer cells are injected.
Figure 6.
(a) Normal mouse pancreas externalized through a left subcostal incision. (b) Appearance of mouse pancreas after injection of cell solution (100 mul). A small fluid filled sac should immediately appear upon injection (arrow). Once the needle is withdrawn from the pancreas, no gross leakage or hemorrhage should be evident.
Figure 7.
(a) The needle of a 3-0 silk suture is passed through the muscular portion of the abdominal wall on each side of the deep incision. Using the suture ends, a knot is tied and excess suture cut at the level of the knot. (b) Additional sutures are placed along the entire length of the deep incision, effectively closing the abdominal cavity. (c) The skin incision may then be closed with interrupted 3-0 silk sutures starting from one end of the incision. (d) Sutures should approximate skin incision edges so that no large gaps are evident.
Figure 8.
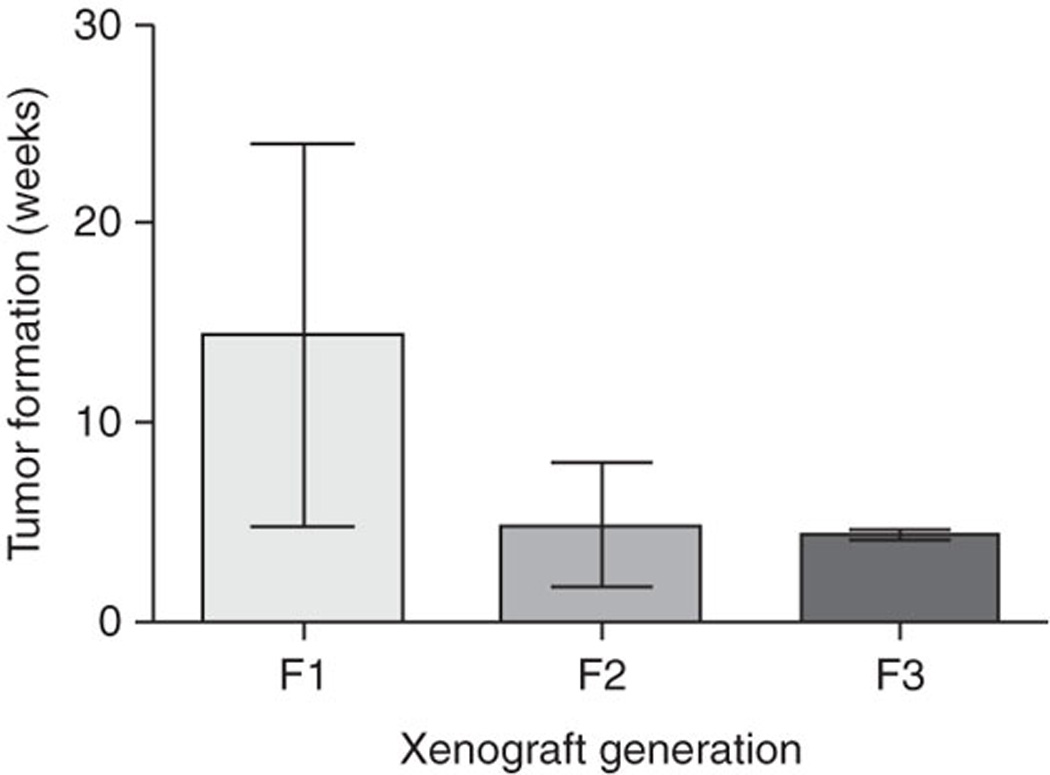
Patient tumor directly engrafted into immunodeficient mice initially takes an average of 14.4 weeks (s.d. plusminus 9.5) to form a palpable tumor. Second- (F2) and third-generation (F3) direct xenograft tumors require an average of 4.9 weeks (s.d. plusminus 3.1) and 4.4 weeks (s.d. plusminus 0.3) for palpable tumor growth, respectively. Permission from the M.D. Anderson Cancer Center (MDACC) Institutional Regulatory Board was obtained for this study.
Table 1.
Troubleshooting table
| Step | Problem | Possible reason | Possible solution |
|---|---|---|---|
| 1B(viii) | Persistent cell clumping | Incomplete incubation with trypsin–EDTA |
Vortex-agitate cell solution, followed by vigorous pipette agitation |
| 10 | Inadequate pipette agitation during resuspension |
||
| 1B(xv) | Bulla fail to form after injection of cell suspension |
Needle has been passed into deep subcutaneous layers/muscle |
Insert needle into skin at an angle more parallel to the mouse body |
| Confirm needle location by lifting the needle during passage into skin |
|||
| 1B(xvi) | Cell suspension leaks from injection site |
Volume of injected cell solution is too large |
Reduce injection volume by increasing concentration of cell solution |
| Needle tract too short | Pass entire length of needle through mouse skin prior to injection |
||
| 10 | Poor cell yield | Inadequate enzymatic or mechanical tumor digestion |
Extend incubation time in collagenase IV solution |
| Increase frequency and/or intensity of pipette agitation | |||
| Cells adherent to precipitated DNA |
Add DNase to collagenase IV solution26. Pass cell solution through additional nylon filters to disrupt cell clusters/clumps |
||
| Inappropriate cell death | Reduce the concentration of collagenase IV solution | ||
| 19 | Cell suspension leaks from the pancreatic injection site |
Volume of injected cell solution is too large |
Reduce injection volume to 50 µl. Concentration of final cell suspension should therefore be 500,000 cells per 50 µl |
| Repeated penetration of the pancreas by needle at multiple sites |
Ensure that the needle only penetrates the mouse pancreas upon insertion and does not exit through another site in the pancreas |
||
| Minimize repeated insertions of the needle into the mouse pancreas |
References
- 1.Jones S, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–1806. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharpless NE, Depinho RA. The mighty mouse: genetically engineered mouse models in cancer drug development. Nat Rev Drug Discov. 2006;5:741–754. doi: 10.1038/nrd2110. [DOI] [PubMed] [Google Scholar]
- 3.Frese KK, Tuveson DA. Maximizing mouse cancer models. Nat Rev Cancer. 2007;7:645–658. doi: 10.1038/nrc2192. [DOI] [PubMed] [Google Scholar]
- 4.Olive KP, Tuveson DA. The use of targeted mouse models for preclinical testing of novel cancer therapeutics. Clin Cancer Res. 2006;12:5277–5287. doi: 10.1158/1078-0432.CCR-06-0436. [DOI] [PubMed] [Google Scholar]
- 5.Rangarajan A, Weinberg RA. Opinion: Comparative biology of mouse versus human cells: modelling human cancer in mice. Nat Rev Cancer. 2003;3:952–959. doi: 10.1038/nrc1235. [DOI] [PubMed] [Google Scholar]
- 6.Loukopoulos P, et al. Orthotopic transplantation models of pancreatic adenocarcinoma derived from cell lines and primary tumors and displaying varying metastatic activity. Pancreas. 2004;29:193–203. doi: 10.1097/00006676-200410000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Rubio-Viqueira B, et al. An in vivo platform for translational drug development in pancreatic cancer. Clin Cancer Res. 2006;12:4652–4661. doi: 10.1158/1078-0432.CCR-06-0113. [DOI] [PubMed] [Google Scholar]
- 8.Rubio-Viqueira B, Hidalgo M. Direct in vivo xenograft tumor model for predicting chemotherapeutic drug response in cancer patients. Clin Pharmacol Ther. 2009;85:217–221. doi: 10.1038/clpt.2008.200. [DOI] [PubMed] [Google Scholar]
- 9.Flanagan SP. 'Nude', a new hairless gene with pleiotropic effects in the mouse. Genet Res. 1966;8:295–309. doi: 10.1017/s0016672300010168. [DOI] [PubMed] [Google Scholar]
- 10.Bosma GC, Custer RP, Bosma MJ. A severe combined immunodeficiency mutation in the mouse. Nature. 1983;301:527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- 11.Ito M, et al. NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002;100:3175–3182. doi: 10.1182/blood-2001-12-0207. [DOI] [PubMed] [Google Scholar]
- 12.Quintana E, et al. Efficient tumour formation by single human melanoma cells. Nature. 2008;456:593–598. doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Voskoglou-Nomikos T, Pater JL, Seymour L. Clinical predictive value of the in vitro cell line, human xenograft, and mouse allograft preclinical cancer models. Clin Cancer Res. 2003;9:4227–4239. [PubMed] [Google Scholar]
- 14.Johnson JI, et al. Relationships between drug activity in NCI preclinical in vitro and in vivo models and early clinical trials. Br J Cancer. 2001;84:1424–1431. doi: 10.1054/bjoc.2001.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.End DW, et al. Characterization of the antitumor effects of the selective farnesyl protein transferase inhibitor R115777 in vivo and in vitro. Cancer Res. 2001;61:131–137. [PubMed] [Google Scholar]
- 16.Van Cutsem E, et al. Phase III trial of gemcitabine plus tipifarnib compared with gemcitabine plus placebo in advanced pancreatic cancer. J Clin Oncol. 2004;22:1430–1438. doi: 10.1200/JCO.2004.10.112. [DOI] [PubMed] [Google Scholar]
- 17.Garber K. From human to mouse and back: 'tumorgraft' models surge in popularity. J Natl Cancer Inst. 2009;101:6–8. doi: 10.1093/jnci/djn481. [DOI] [PubMed] [Google Scholar]
- 18.Fichtner I, et al. Establishment of patient-derived non-small cell lung cancer xenografts as models for the identification of predictive biomarkers. Clin Cancer Res. 2008;14:6456–6468. doi: 10.1158/1078-0432.CCR-08-0138. [DOI] [PubMed] [Google Scholar]
- 19.Houghton PJ, et al. The pediatric preclinical testing program: description of models and early testing results. Pediatr Blood Cancer. 2007;49:928–940. doi: 10.1002/pbc.21078. [DOI] [PubMed] [Google Scholar]
- 20.Peterson JK, Houghton PJ. Integrating pharmacology and in vivo cancer models in preclinical and clinical drug development. Eur J Cancer. 2004;40:837–844. doi: 10.1016/j.ejca.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Li C, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 22.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]