Abstract
Nitrite-treated meat is a reported risk factor for colon cancer. Mice that ingested sodium nitrite (NaNO2) or hot dogs (a nitrite-treated product) showed increased fecal excretion of apparent N-nitroso compounds (ANC). Here, we investigated for the first time whether rats excrete increased amounts of ANC in their urine after they are fed NaNO2 and/or hot dogs. Rats were treated for 7 days with NaNO2 in drinking water or were fed hot dogs. Their 24 h urine samples were analyzed for ANC by thermal energy analysis on days 1–4 after nitrite or hot dog treatment was stopped. For two rats fed 480 mg NaNO2/L drinking water, mean urinary ANC excretion on days 1–4 was 30, 5.2, 2.5, and 0.8 nmol/day, respectively. For two to eight rats/dose given varied NaNO2 doses, mean urinary ANC output on day 1 increased from 0.9 (for no nitrite) to 37 (for 1000 mg NaNO2/L drinking water) nmol ANC/day. Urine samples of four rats fed 40–60% hot dogs contained 12–13 nmol ANC on day 1. Linear regression analysis showed highly significant correlations between urinary ANC excretion on day 1 after stopping treatment and varied (a) NaNO2 level in drinking water for rats fed semipurified or commercials diet and (b) hot dog levels in the diet. Some correlations remained significant up to 4 days after nitrite treatment was stopped. Urinary output of ANC precursors (compounds that yield ANC after mild nitrosation) for rats fed semipurified or commercial diet was 11–17 or 23–48 μmol/day, respectively. Nitrosothiols and iron nitrosyls were not detected in urinary ANC and ANCP. Excretion of urinary ANC was about 60% of fecal ANC excretion for 1 to 2 days after NaNO2 was fed. Administered NaNO2 was not excreted unchanged in rat urine. We conclude that urinary ANC excretion in humans could usefully be surveyed to indicate exposure to N-nitroso compounds.
1. Introduction
We report here studies on the urinary excretion by rats of total apparent N-nitroso compounds (ANC) formed in vivo from ingested sodium nitrite (NaNO2) or hot dogs. This appears to be the first published report on the urinary excretion of ANC by rodents. A significant proportion of ingested N-nitroso compounds (NOC) appears to arise from processed nitrite-treated meat.1 Most of the ANC in nitrite-treated meat products appears to consist of NOC.2 Fresh and, especially, processed (mostly nitrite-treated) red meat products, including hot dogs, are reported risk factors for the etiology of colon cancer.3−6 In 1991, Rowland et al.7 proposed that NOC in red meat could induce colon cancer if these NOC reached the colon. In fact, ANC were detected in the feces of humans and mice after they ingested fresh or processed red meat.8−10
Processed meat is also a reported risk factor for the etiology of human cancer of several organs in addition to the colon.11 These organs include the prostate,12−14 pancreas,15,16 breast,17 esophagus,11 and brain.11 NOC exposure in these organs could be detected by measuring urinary rather than fecal excretion of NOC. Linkages with cancer of the gastrointestinal (GI) tract18 could occur if GI tissues directly absorbed carcinogenic NOC from the GI lumen.
Santorelli et al.19 reported experiments indicating that (a) ANC and oxidized lipids are the agents in processed meat that induce colon cancer and (b) these effects were exacerbated by also feeding hemoglobin. In later studies,18,20 rats were injected with 1,2-dimethylhydrazine (a colon carcinogen) and then fed hot dogs. They showed more colonic mucin-depleted foci (a precursor lesion for colon cancer) and higher levels of fecal ANC than did rats receiving only dimethylhydrazine. When other similarly treated rats were also fed calcium carbonate or α-tocopherol, these effects were significantly reduced18,20
Here, we investigated the effect of feeding NaNO2 on urinary ANC excretion by rats because NaNO2 is the major additive in processed meat and feeding only 32 mg NaNO2/L significantly increased fecal ANC excretion in mice.21 This effect was attributed largely to acid-catalyzed gastric nitrosation of NOC precursors (NOCP, usually measured as apparent NOCP (ANCP)). Nitrosated ANCP that were partially purified from hot dogs were directly mutagenic in the Ames test on bacteria22 (a property well correlated with rodent carcinogenicity23) and induced colonic aberrant crypts in mice.24 (This is another putative precursor of colon cancer.) These findings support the hypothesis that NOC present in or derived from NOCP in processed meat are a cause of colon cancer.
In addition to NOC, ANC can include nitrosothiols (RSNO) and nitrosyl iron compounds (RFeNO),2 which are probably not carcinogenic because they are unlikely to alkylate DNA bases. Therefore, we analyzed some urine samples for RSNO and RFeNO in addition to total ANC. We studied rats here, rather than mice as we had done before,9,21,24 because the larger urine volume for rats made it easier to collect their urine. The rats were mostly fed semipurified diet. Commercial diet was also fed in some tests because it is similar to most human diets.
2. Methods
2.1. Treatment of Rats and Collection of Urine and Feces
Animal experiments were approved by the Institutional Animal Care and Use Committee of the University of Nebraska Medical Center. The study animals were male Sprague–Dawley rats (Charles Rivers, Wilmington, MA) aged 8–12 weeks and housed two/cage. They weighed 250–350 g and excreted 6–10 mL urine/day. The rats were fed tap water for drinking (distilled water sometimes contained low levels of nitrite, probably derived from atmospheric nitrogen oxides) or tap water containing 60, 120, 240, 480, or 1000 mg/L of NaNO2 (Sigma-Aldrich Reagent Plus grade). The diet was pelleted AIN93G semipurified diet (TD-94245) or pelleted commercial diet (TD-98186), both from Harlan Teklad (Madison, WI).
In other experiments, 12 rats were fed Bar-S hot dogs (smoked sausages, Bar-S Foods, Phoenix, AZ) containing, in order of abundance according to their label, chicken, beef, pork, and nonmeat constituents. Another four rats were fed “beef brat” hot dogs (Johnsonville Sausages, LLC Dist., Sheboygan Falls, WI). Both hot dog brands were manufactured with the addition of NaNO2. The remainder of these diets was the semipurified diet. In the store, the hot dogs were kept refrigerated but not frozen because of the antibacterial action of the nitrite. After purchase, the hot dogs were stored at −30 °C until they were fed at levels of 18, 40, or 60% by weight of the diet. Hot dogs were mixed with semipurified diet in a commercial blender. The mixture was stored at 4 °C for up to 7 days and then fed in stainless steel feeders.
All treatments were given for 7 days, after which the rats were fed tap water and the same diet as before, but without NaNO2 or hot dogs. Urine and, in one experiment, feces were collected daily on days 1–4. Day 1 was the 24 h period starting as soon as nitrite or hot dog feeding had been stopped. Days 2–4 were the subsequent 24 h periods. For collecting the urine and feces, we used a rack of metabolic cages. Urine passed through wire mesh and a funnel into heavy test tubes, which were kept on ice until the urine was collected after 24 h. Feces was collected daily from the wire mesh. Urine and feces were collected on days 1–4, starting as soon as the treatments were stopped. Before each collection, the cages were washed with water but without soap. The urine was estimated to have been in contact with only 20% of the feces. The feces and diets appeared dry and were analyzed without first drying them. Urine and fecal samples were stored at −30 °C until they were analyzed.
2.2. Determination of ANC in Urine
ANC were determined using a thermal energy analysis (TEA) instrument (Advanced Chromatographic Systems, Charleston, SC).1,9,21 A mixture of 700 μL of urine, 100 μL of water, 100 μL of 0.1 N HCl, and 100 μL of a freshly prepared saturated solution of sulfamic acid (SA) in water (SA reagent) was kept for up to 4 h on ice, and 100 μL samples of this mixture were injected into the TEA reaction vessel and analyzed for ANC.1,9 The TEA output was integrated with a model 202 chromatograph data system (Peak Simple, Torrance, CA) and expressed as nmol ANC/day. Standards with 0.1 nmol of N-nitrosoproline were analyzed 3 times daily.
2.3. Determination of RSNO and RFeNO in Urinary ANC
This followed the method of Kuhnle et al.2 We first determined ANC in urine (Methods, Section 2.2). In other tubes, the 100 μL water added in the ANC method was replaced by 100 μL of 50 mM mercuric chloride in water for RSNO analysis or 100 μL of 50 mM potassium ferricyanide in water for RFeNO analysis. The resulting solutions were reacted for 30 min at room temperature, mixed with SA reagent and HCl, and analyzed for ANC. The results were subtracted from those for total ANC to give RSNO and RFeNO levels.
2.4. Determination of Urinary ANCP and Precursors of RSNO and RFeNO
To prepare nitrosated ANCP, a mixture of 200 μL of urine, 110 μL of 2 N HCl, 110 μL of 2 M NaNO2, and 1.58 mL of water was incubated for 1 h at 37 °C, mixed with 300 μL of SA reagent, kept for at least 5 min, diluted 10 or 100 times with tap water, and analyzed for ANC. To determine RSNO and RFeNO in the urinary ANCP, a solution of nitrosated urinary ANCP was diluted 10 or 100 times with water, brought to pH 7 with sodium carbonate, and analyzed for total ANC, RSNO, and RFeNO.
2.5. Determination of ANC in Feces and Diet
Fecal and diet samples were not dried, unlike our previous practice,21 because ANC results were expressed as amount/day. Samples (500 mg) of feces or diet were soaked in 4 mL of water for 18 h at room temperature, vortexed four times for 30 s each, and centrifuged for 10 min at 9000g. Of the supernatant, 800 μL was mixed with 100 μL of 0.1 N HCl and 100 μL of SA reagent. After the mixtures were kept for 15 min at room temperature and up to 4 h on ice, 100 μL samples were analyzed for ANC by TEA.
2.6. Measuring ANC Formation in Rat Urine after Incubation with NaNO2
Urine was collected for 24 h from two rats maintained on semipurified diet. Rat 1 was untreated. Rat 2 was fed 1.0 g NaNO2/L water for 7 days before its urine was collected. Urine samples were incubated with water or a NaNO2 solution (final nitrite level, 1.8 mM) for 7 days at 4 °C and were then analyzed for ANC.
2.7. Determination of Nitrite in Urine
Nitrite was determined by the Griess colorimetric method.25 One milliliter of rat urine, urine–water mixtures, or (in standards) a solution of 6.9 μg NaNO2/mL water was mixed with 2.5 mL of Griess reagent and kept for 20 min at room temperature. The resulting purple color was measured as A540 – A620. A620 was included to correct for turbidity. In a test with 6.9 μg of NaNO2 in 1 mL of water, A540 was 0.47 and A620 was 0.02.
3. Results
3.1. Subtracting Blank Values
All NOC, RSNO, and RFeNO results in the feeding experiments are listed without first subtracting blank values measured when NaNO2 or hot dogs were administered. Blank values were generally less than 10% of those when NaNO2 or hot dogs were fed.
3.2. Urinary ANC Formation Due to Contamination of Urine with NaNO2
We initially collected urine for ANC analysis from rats while they were fed NaNO2 in their drinking water. However, we wondered whether drinking water containing NaNO2 would spill from the feeding bottles into the urine and react there with urinary ANCP to form artifactual ANC. In fact, storage of 1.8 mM NaNO2 in two rat urine samples for 7 days at 4 °C led to the formation of 4.3 and 20.6 μM ANC. Hence, NaNO2 can react with urinary ANCP to generate ANC. In subsequent experiments, urine was collected only after nitrite treatment was stopped.
3.3. Urinary ANC in Rats Fed NaNO2 and/or Hot Dogs
Table 1 shows urinary ANC output/day for 4 days (called day 1, day 2, etc.) after the NaNO2 feeding was stopped. Urine collection was started immediately after NaNO2 had been fed for 7 days. The rats were maintained on semipurified or commercial diet. Urine volume varied from 5 to 20 mL/day; the higher values are attributed to spilled drinking water (which, at this stage, did not contain nitrite) in the urine collection tubes. When increasing doses of NaNO2 were administered to rats fed semipurified diet, urinary ANC excretion on day 1 rose from 0.9 nmol/day for untreated rats to a mean of 37 nmol/day for a dose of 1000 mg NaNO2/L water. For the latter dose of NaNO2 given with semipurified diet, ANC output on day 4 fell to 2.8 nmol (still 3.1 times the control value of 0.9 nmol/day; Table 1). Urinary ANC levels were elevated for all five nitrite doses on days 1 and 2, but only for 1000 mg NaNO2/L on day 3.
Table 1. Effect of Varied Doses of Sodium Nitrite on Urinary ANC Excretion on Days 1–4 after Nitrite Treatment Was Stoppeda.
| urinary
ANC (nmol/day) |
||||||
|---|---|---|---|---|---|---|
| NaNO2 (mg/L water) | diet | no. of rats | day 1 | day 2 | day 3 | day 4 |
| 0 | semipurified | 8 | 0.9 ± 0.3 | |||
| 60 | semipurified | 2 | 4.2 (3.6, 4.8) | 2.0 (1.3, 2.6) | 2.1 (1.7, 2.5) | 2.0 (1.4, 2.5) |
| 120 | semipurified | 2 | 5.8 (5.6, 6.1) | 2.4 (1.8, 3.0) | 1.7 (1.4, 2.0) | 0.9 (0.8, 1.0) |
| 240 | semipurified | 4 | 10 ± 3 | 2.8 ± 0.3 | 2.2 ± 0.7 | 1.7 ± 0.5 |
| 480 | semipurified | 2 | 30 (28, 32) | 5.2 (3.2, 7.2) | 2.5 (1.4, 3.6) | 0.8 (0.6, 0.9) |
| 1000 | semipurified | 4 | 37 ± 2 | 19 ± 5 | 6.3 ± 1.2 | 2.8 ± 1.4 |
| 0 | commercial | 6 | 12 ± 4 | |||
| 120 | commercial | 2 | 15 (11, 19) | 10 (8, 13) | 9.2 (8.8, 9.6) | 8.7 (5.7, 11.7) |
| 240 | commercial | 2 | 23 (16, 30) | 13 (10, 16) | 14 (14, 14) | 11 (8, 15) |
When commercial diet was fed without nitrite, urinary ANC output was 12 nmol/day, 13 times the value for rats fed semipurified diet without nitrite (Table 1). This increased urinary ANC excretion may have been due to ANC in the commercial diet, which contained 0.93 nmol ANC/g diet compared to 0.14 nmol ANC/g semipurified diet.9 When 120 or 240 mg NaNO2/L water was fed with commercial diet, the ANC results on day 1 were 2.3–2.6 times those for the same NaNO2 dose given with semipurified diet.
When Bar-S hot dogs were fed to rats as mixtures of 18, 40, or 60% hot dogs in semipurified diet, the mean urinary ANC output increased on day 1 from 0.9 nmol/day in the absence of hot dogs to 13 nmol/day for 60% hot dogs (Table 2). When 40% hot dogs were fed together with 240 mg NaNO2/L, the mean nanomoles of urinary ANC on day 1 was 25 nmol/day compared to 12 nmol/day for hot dogs alone (Table 2), 10 nmol/day for 240 mg NaNO2 alone (Table 1), and 0.9 nmol/day for no treatment (Table 2). This indicates a simple additive effect for the two test materials with no indication that nitrite had produced ANC from ANCP in the hot dogs. Urinary ANC output on day 3/urinary ANC output on day 1 was 9% for 40% hot dogs fed with semipurified diet (Table 2), 22% for 240 mg NaNO2/L fed with semipurified diet (Table 1), and 61% for 240 mg NaNO2/L fed with commercial diet (Table 1). Hence, it appears that nitrite or the resulting ANC were cleared from the body more slowly when commercial diet was fed than when semipurified diet was fed. Feeding Johnsonville hot dogs yielded urinary ANC levels not significantly different from those for Bar-S hot dogs (Table 2).
Table 2. Effect of Feeding Hot Dogs Mixed with Semipurified Diet (with or without NaNO2 in Drinking Water) on Urinary Excretion of ANC 1–4 Days after Nitrite Treatment Was Stopped.
| hot
dogs |
urinary
ANC (nmol/day) |
||||||
|---|---|---|---|---|---|---|---|
| brand | percent of diet | NaNO2 (mg/L water) | no. of rats | day 1 | day 2 | day 3 | day 4 |
| 0 | 0 | 8 | 0.9 ± 0.3 | a | |||
| Bar-S | 18 | 0 | 4 | 4.0 ± 1.6 | 2.3 (1.8, 2.8) | 1.8 (1.7, 1.9) | 1.0 (0.8, 1.1) |
| Bar-S | 40 | 0 | 2 | 12 (10, 14) | 4.2 (3.8, 4.5) | 1.1 (1.1, 1.2) | |
| Bar-S | 60 | 0 | 2 | 13 (10, 17) | 4.8 (2.5, 7.2) | 1.5 (1.4, 1.5) | 1.9 (1.6, 2.1) |
| Bar-S | 40 | 240 | 4 | 25 ± 4 | 6.4 ± 1.7 | ||
| Johnsonville | 18 | 0 | 4 | 2.8 ± 1.4 | 0.9 ± 0.6 | 1.1 ± 0.7 | |
Empty cells indicate that samples were not collected or were lost.
3.4. Statistical Analysis of Urinary ANC Results
Table 3 shows linear regression analyses (a) for the correlations between urinary ANC excretion measured as nmol ANC/day for rats eating semipurified or commercial diet with varied concentrations of NaNO2 in drinking water and (b) for rats fed various proportions of hot dogs in the diet. Results are shown for days 1–4. PC SAS version 9.3 was used for all summaries and analyses.32 Parameter estimates for the intercept and regression coefficients are presented in addition to the R2 and p values.
Table 3. Significance of Correlations of Urinary ANC Output with NaNO2 Dose or Percent Hot Dogs in the Diet 1–4 Days after Treatments Were Stopped.
| material fed | diet | day | regression coefficient | R2 | p value |
|---|---|---|---|---|---|
| NaNO2 | semipurified | 1 | 0.0375 | 0.918 | <0.01 |
| 2 | 0.0177 | 0.886 | <0.01 | ||
| 3 | 0.0050 | 0.853 | <0.01 | ||
| 4 | 0.0016 | 0.367 | 0.003 | ||
| NaNO2 | commercial | 1 | 0.038 | 0.666 | <0.01 |
| 2 | 0.0073 | 0.116 | 0.28 | ||
| 3 | 0.004 | 0.055 | 0.46 | ||
| 4 | 0.003 | 0.025 | 0.62 | ||
| hot dogs | semipurified | 1 | 0.226 | 0.870 | <0.01 |
| 2 | 0.0692 | 0.720 | <0.01 | ||
| 3 | 0.0081 | 0.161 | 0.123 | ||
| 4 | 0.0146 | 0.521 | 0.004 |
The p values were <0.01 for the effect of varied NaNO2 levels on all 4 days (days 1–4) when NaNO2 was administered with semipurified diet, were significant only on day 1 when NaNO2 was administered with commercial diet, and was <0.01 for days 1, 2, and 4 when varied proportions of hot dogs in the diet were fed.
3.5. Comparison between Urinary and Fecal ANC Outputs after Rats Were Fed NaNO2
Urinary and fecal ANC excretions in the same rats and at the same time were compared 1–4 days after four rats were fed 1000 mg NaNO2/L water for 7 days (Table 4). The 20 fecal collections on day 1 weighed 1.2 ± 0.5 g/sample (mean ± SD). On days 1 and 2 after nitrite treatment, urinary ANC output was about 60% of that of fecal ANC output.
Table 4. Comparison between Urinary and Fecal Excretions of ANC 1–4 Days after Four Rats That Were Maintained on Semipurified Diet Had Been Treated for 7 Days with 1000 mg NaNO2/L Water.
| test material | day | ANC in urine (nmol/day)a | ANC in feces (nmol/day) | urinary ANC/fecal ANCb |
|---|---|---|---|---|
| none | 0.9 ± 0.3 | 0.6 ± 0.2 | 0.9 ± 0.4 | |
| NaNO2 | 1 | 37 ± 2 | 115 ± 93 | 0.6 ± 0.4 |
| NaNO2 | 2 | 19 ± 5 | 49 ± 30 | 0.6 ± 0.5 |
| NaNO2 | 3 | 6 ± 1 | 29 ± 11 | 0.2 ± 0.1 |
| NaNO2 | 4 | 2.8 ± 1.4 | 6 ± 3 | 0.5 ± 0 |
The urine results are also listed in Table 1.
Mean values for urinary ANC/fecal ANC ratios were calculated from the individual ratios for each rat and are not the same as mean urinary ANC/mean fecal ANC for all rats.
3.6. RSNO and RFeNO as Possible Components of the Urinary ANC and ANCP
Because ANC can include RSNO and RFeNO, we determined RSNO and RFeNO in the ANC of urine samples (with 1 sample/rat) collected from 16 rats. The results were expressed as percentages of RSNO or RFeNO in the total ANC. The analyzed samples included (a) urine from rats that were fed 0, 240, or 480 mg NaNO2/L water (10 samples) or fed 18, 40, or 60% hot dogs (6 samples), (b) urine that was analyzed for RSNO (10 samples) or RFeNO (6 samples), (c) urine from rats fed semipurified diet (12 samples) or commercial diet (4 samples), and (d) urine analyzed for ANC (12 samples) or ANCP (4 samples). Some urine samples were included in more than one category. The 16 urine samples showed RSNO + RFeNO levels that were 1 ± 4% (mean ± SD) of the total ANC in each sample. Hence, it appears that all of the urinary ANC were NOC and all the urinary ANCP were NOCP.
3.7. Urinary Excretion of ANCP
ANCP output on day 1 was measured in the urine of two rats/condition that were fed NaNO2 and/or hot dogs or that were untreated (Table 5). The urine samples contained mean values of 11–18 (for semipurified diet) or 23–48 (for commercial diet) μmol ANCP/day. For rats fed 240 mg NaNO2/L water with semipurified diet, urinary ANCP excretion on day 1 was 18 μmol/day (Table 5), compared to 10 nmol/day for ANC (Table 1). Note that ANCP are expressed as μmol/day, compared to nmol/day for ANC.
Table 5. ANCP Levels in Rat Urine Collected on Day 1 after NaNO2 or Hot Dogs Had Been Fed for 7 Days.
| NaNO2 in water (mg/L) | hot dogs (percent of diet) | diet | urinary ANCP (μmol/day) |
|---|---|---|---|
| 0 | 0 | semipurified | 12 (10, 14) |
| 240 | 0 | semipurified | 18 (17, 18) |
| 480 | 0 | semipurified | 11 (10, 11) |
| 0 | 18 | semipurified | 17 (15, 18) |
| 0 | 60 | semipurified | 12 (10, 14) |
| 240 | 0 | commercial | 48 (44, 51) |
| 480 | 0 | commercial | 23 (20, 25) |
3.8. Search for Nitrite in Rat Urine
In view of the finding that NaNO2 reacts with urinary ANCP to form ANC, we wondered whether some of the administered nitrite had been excreted in the urine and had reacted there to form the urinary ANC listed in Tables 1 and 4. This, rather than the urinary excretion of preformed ANC as assumed up to this point, could have been the origin of the urinary ANC found when NaNO2 or hot dogs were fed (Tables 1, 2, and 4). To check this possibility, we used the Griess colorimetric method, based on the formation from nitrite of a red dye,25 to determine nitrite in the rat urine.
3.8.1. Stability of Nitrite on Storage of Urine Containing Nitrite
Nitrite in urine might have decomposed when the urine was stored at −30 °C so that nitrite might have been present only in freshly excreted urine. To test this view, 500 μL samples of three rat urine samples (pH 7.1–8.3) with added NaNO2 (6.0 μg/mL urine) were kept for 7 days at 4, −20, or −80 °C. Analysis of these samples for nitrite by the Griess test showed A540 – A620 values of 0.30–0.32. Hence, nitrite in urine was stable even after storage for 7 days at 4 °C.
3.8.2. Search for Nitrite in 16 Urine Samples
Rats were treated for 7 days with 480 or 1000 mg NaNO2/L drinking water (four rats/concentration). The rats were fed semipurified diet (10 rats) or commercial diet (6 rats). The urine samples were stored for 2–6 weeks at −30 °C and then analyzed for nitrite by the Griess reaction. The pH of six stored urine samples was 7.7 ± 0.5 (mean ± SD). The urine samples showed <0.5 μg NaNO2/mL in 15 samples and 2 μg NaNO2/mL in one sample. A540 was 0.08–0.20. A620 was 0.07–0.16. Hence, ingested NaNO2 is not excreted unchanged in the urine of rats.
4. Discussion
Feeding either NaNO2 or hot dogs to rats produced large amounts of ANC in the urine for 1–4 days after the feeding stopped (Tables 1, 2, and 4). A total dose of 63 g NaNO2/kg body weight was reported to not induce colon cancer in rats.31,11 Nevertheless, four lines of evidence suggest that NaNO2 could induce colon cancer via the in vivo formation of carcinogenic NOC: (a) The consumption of nitrite-treated processed meat has been linked to the occurrence of colon cancer (Introduction, paragraph 1). (b) Ingested NaNO2 increased the fecal excretion of ANC in mice21 and the urinary and fecal excretion of ANC in rats (Tables 1 and 4). (c) ANC that was obtained by nitrosation of hot-dog-derived ANCP induced colonic aberrant crypt foci (a putative precursor for colon cancer) in mice.24 (d) The Corpet group has convincing evidence linking hot dog-derived ANC with the induction of preneoplastic lesions in the rat colon (Introduction, paragraph 3).
A colorimetric assay for nitrite demonstrated that ingested NaNO2 is not excreted unchanged in the urine of rats (Section 3.8.2). Our failure to detect nitrite in rat urine is consistent with a report that humans who ingested nitrate did not excrete nitrite in their urine,26 even though in humans (but not in rats) 5% of ingested nitrate is reduced to nitrite, mostly in the oral cavity.27
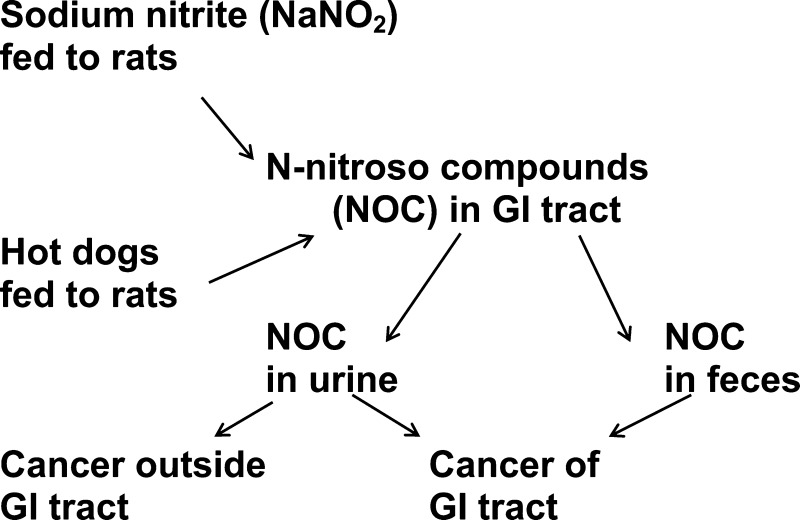
When Bar-S hot dogs were fed to rats as 40–60% of a semipurified diet, mean urinary excretion of ANC was 12–13 nmol on day 1 and 4.2–4.8 nmol on day 2 (Table 2). Presumably, when hot dogs were fed, urinary ANC in excess of the background level of 0.9 nmol/day arose from ANC ingested in the hot dogs and, perhaps, by in vivo nitrosation of ANCP in the hot dogs. The presence of ANC in the urine suggests that, in the reported experiment, most tissues had been exposed to ANC, whereas the presence of ANC in the feces may signify only that the GI tract had been exposed to ANC. Accordingly, urinary ANC could be preferentially associated with cancer outside the GI tract.
Future epidemiological studies could investigate whether human urinary ANC excretion is correlated with (a) nitrate intake in drinking water and (b) cancer incidence. Studies on urinary ANC would be much easier to perform than those on fecal ANC. Because most NOC are carcinogens11,27 and ANC in urine appears to consist entirely of NOC, such exposure to ANC could be a cause of cancers in organs (in addition to the colon) that have been linked with processed meat (Introduction, paragraph 2). Also, drinking water with high levels of nitrate in Iowa was linked28 to an increased incidence of colon cancer, provided that ascorbate intake was low and processed meat intake was high.28
Mice fed NaNO2 showed significantly increased fecal ANC outputs down to a dose of 32 mg NaNO2/L drinking water.21 In a human study, we found significantly increased urinary excretion of N-nitrosoproline after giving single doses of 100–400 mg nitrate as sodium nitrate.29 The observed in vivo nitrosation after human ingestion of nitrate probably occurred because 5% of ingested nitrate is reduced to nitrite, which nitrosated proline to form N-nitrosoproline.27
We could not find previous reports on urinary ANC excretion in rodents. In the only report on urinary ANC excretion in humans, Lin et al. in 200230 assayed urinary ANC levels for male subjects from two areas in Southern China: one (the island of Nan’ao) with a high incidence of esophageal cancer and the other (Lufeng, on the mainland) with a low incidence of esophageal cancer. Mean dietary ANC intake by men was 4.2 ± 0.8 μmol/day in Nan’ao and 0.3 ± 0.1 μmol/day in Lufeng. Twelve-hour urine samples contained 0.04 ± 0.01 nmol ANC in Nan’ao and 0.02 ± 0.01 nmol ANC in Lufeng (mean ± SE). This was a significant difference with p < 0.01. The high ANC urinary output in Nan’ao was attributed to consumption of a diet containing relatively large concentrations of ANC.
Urinary excretion of ANC increased on days 1 and 2 when NaNO2 or hot dogs was fed and was about 60% of fecal ANC excretion when NaNO2 was fed (Tables 1, 2, and 4). This helps to explain how processed meat could be a cause of cancers in organs outside the GI tract, type 2 diabetes, and coronary heart disease (Introduction, paragraph 2). In conclusion, future epidemiological studies could survey ANC excretion in the urine of people showing elevated risks for colon cancer and other diseases that have been linked to the consumption of processed meat.
Acknowledgments
We thank Mary H. Ward and Amanda J. Cross (Division of Cancer Epidemiology and Genetics, National Cancer Institute) for suggesting that we investigate the urinary excretion of dietary ANC in rats. We also thank Darcy Jackson for secretarial assistance.
Glossary
Abbreviations
- ANC
apparent N-nitroso compounds
- ANCP
ANC precursors
- GI
gastrointestinal
- NOC
N-nitroso compounds
- NOCP
NOC precursors
- RFeNO
nitrosyl iron compounds
- RSNO
nitrosothiols
- SA
sulfamic acid
- TEA
thermal energy analysis
This study was supported by grant RO1-CA-143460 and University of Nebraska Medical Center Eppley Cancer Center Support Grant 2P30CA036727, both from the National Institutes of Health.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
References
- Haorah J.; Zhou L.; Wang X.; Xu G.; Mirvish S. S. (2001) Determination of total N-nitroso compounds and their precursors in frankfurters, fresh meat, dried salted fish, sauces, tobacco and tobacco smoke particulates. J. Agric. Food Chem. 49, 6068–6078. [DOI] [PubMed] [Google Scholar]
- Kuhnle G. G.; Story G. W.; Reda T.; Mani A. R.; Moore K. P.; Lunn J. C.; Bingham S. A. (2007) Diet-induced endogenous formation of nitroso compounds in the GI tract. Free Radical Biol. Med. 43, 1040–1047. [DOI] [PubMed] [Google Scholar]
- Ward M. H.; Cross A. J.; Divan H.; Kulldorff M.; Nowell-Kadlubar S.; Kadlubar F. F.; Sinha R. (2007) Processed meat intake, CYP2A6 activity and risk of colorectal adenoma. Carcinogenesis 28, 1210–1216. [DOI] [PubMed] [Google Scholar]
- Santarelli R. L.; Pierre F.; Corpet D. E. (2008) Processed meat and colorectal cancer: a review of epidemiologic and experimental evidence. Nutr. Cancer 60, 131–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross A. J.; Ferrucci L. M.; Risch A.; Graubard B. I.; Ward M. H.; Park Y.; Hollenbeck A. R.; Schatzkin A.; Sinha R. (2010) A large prospective study of meat consumption and colorectal cancer risk: an investigation of potential mechanisms underlying this association. Cancer Res. 70, 2406–2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan D. S.; Lau R.; Aune D.; Vieira R.; Greenwood D. C.; Kampman E.; Norat T. (2011) Red and processed meat and colorectal cancer incidence: meta-analysis of prospective studies. PLoS One 6, e20456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland I. R.; Granli T.; Bockman O. C.; Key P. E.; Massey R. C. (1991) Endogenous N-nitrosation in man assessed by measurement of apparent total N-nitroso compounds in faeces. Carcinogenesis 12, 1395–1401. [DOI] [PubMed] [Google Scholar]
- Hughes R.; Cross A. J.; Pollock J. R. A.; Bingham S. (2001) Dose-dependent effect of dietary meat on endogenous colonic N-nitrosation. Carcinogenesis 22, 199–202. [DOI] [PubMed] [Google Scholar]
- Mirvish S. S.; Haorah J.; Zhou L.; Hartman M.; Morris C. R.; Clapper M. L. (2003) N-Nitroso compounds in the gastrointestinal tract of rats and in the feces of mice with induced colitis or fed hot dogs or beef. Carcinogenesis 24, 595–603. [DOI] [PubMed] [Google Scholar]
- Lunn J. C.; Kuhnle G.; Mai V.; Frankenfeld C.; Shuker D. E.; Glen R. C.; Goodman J. M.; Pollock J. R.; Bingham S. A. (2007) The effect of haem in red and processed meat on the endogenous formation of N-nitroso compounds in the upper gastrointestinal tract. Carcinogenesis 28, 685–690. [DOI] [PubMed] [Google Scholar]
- (2010) Ingested nitrate and nitrite. IARC Monogr. Eval. Carcinog. Risks Hum. 94, 1–412. [PMC free article] [PubMed] [Google Scholar]
- Sinha R.; Park Y.; Graubard B. I.; Leitzmann M. F.; Hollenbeck A.; Schatzkin A.; Cross A. J. (2009) Meat and meat-related compounds and risk of prostate cancer in a large prospective cohort study in the United States. Am. J. Epidemiol. 170, 1165–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez C. A.; Riboli E. (2006) Diet and cancer prevention: where we are, where we are going. Nutr. Cancer 56, 225–231. [DOI] [PubMed] [Google Scholar]
- Rohrmann S.; Platz E. A.; Kavanaugh C. J.; Thuita L.; Hoffman S. C.; Helzlsouer K. J. (2007) Meat and dairy consumption and subsequent risk of prostate cancer in a US cohort study. Cancer, Causes Control 18, 41–50. [DOI] [PubMed] [Google Scholar]
- Risch H. A. (2003) Etiology of pancreatic cancer, with a hypothesis concerning the role of N-nitroso compounds and excess gastric acidity. J. Natl. Cancer Inst. 95, 948–960. [DOI] [PubMed] [Google Scholar]
- Chan J. M.; Wang F.; Holly E. A. (2007) Pancreatic cancer, animal protein and dietary fat in a population-based study, San Francisco Bay Area, California. Cancer, Causes Control 18, 1153–1167. [DOI] [PubMed] [Google Scholar]
- Taylor E. F.; Burley V. J.; Greenwood D. C.; Cade J. E. (2007) Meat consumption and risk of breast cancer in the UK Women’s Cohort Study. Br. J. Cancer 96, 1139–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli R. L.; Naud N.; Tache S.; Gueraud F.; Vendeuvre J. L.; Zhou L.; Anwar M. M.; Mirvish S. S.; Corpet D. E.; Pierre F. H. (2013) Calcium inhibits promotion by hot dog of 1,2-dimethylhydrazine-induced mucin-depleted foci in rat colon. Int. J. Cancer 133, 2533–2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli R. L.; Vendeuvre J. L.; Naud N.; Tache S.; Gueraud F.; Viau M.; Genot C.; Corpet D. E.; Pierre F. H. (2010) Meat processing and colon carcinogenesis: cooked, nitrite-treated, and oxidized high-heme cured meat promotes mucin-depleted foci in rats. Cancer Prev. Res. 3, 852–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierre F. H.; Martin O. C.; Santarelli R. L.; Tache S.; Naud N.; Gueraud F.; Audebert M.; Dupuy J.; Meunier N.; Attaix D.; Vendeuvre J. L.; Mirvish S. S.; Kuhnle G. C.; Cano N.; Corpet D. E. (2013) Calcium and alpha-tocopherol suppress cured-meat promotion of chemically induced colon carcinogenesis in rats and reduce associated biomarkers in human volunteers. Am. J. Clin. Nutr. 98, 1255–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirvish S. S.; Davis M. E.; Lisowyj M. P.; Gaikwad N. W. (2008) Effect of feeding nitrite, ascorbate, hemin, and omeprazole on excretion of fecal total apparent N-nitroso compounds in mice. Chem. Res. Toxicol. 21, 2344–2351. [DOI] [PubMed] [Google Scholar]
- Zhou L.; Haorah J.; Perini F.; Carmella S. G.; Shibamoto T.; Mirvish S. S. (2006) Partial purification from hot dogs of N-nitroso compound precursors and their mutagenicity after nitrosation. J. Agric. Food Chem. 54, 5679–5687. [DOI] [PubMed] [Google Scholar]
- Benigni R. (2011) Alternative approaches for the identification of carcinogens are closer than usually thought, but the present strategies and regulations need to be updated. AltTox.org. http://alttox.org/ttrc/toxicity-tests/carcinogenicity/way-forward/benigni/.
- Davis M. E.; Lisowyj M. P.; Zhou L.; Wisecarver J. L.; Gulizia J. M.; Shostrom V. K.; Naud N.; Corpet D. E.; Mirvish S. S. (2012) Induction of colonic aberrant crypts in mice by feeding apparent N-nitroso compounds derived from hot dogs. Nutr. Cancer 64, 342–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirvish S. S.; Reimers K. J.; Kutler B.; Chen S. C.; Haorah J.; Morris C. R.; Grandjean A. C.; Lyden E. R. (2000) Nitrate and nitrite concentrations in human saliva for men and women at different ages and times of the day and their consistency over time. Eur. J. Cancer Prev. 9, 335–342. [DOI] [PubMed] [Google Scholar]
- Florin T. H. J.; Neale G.; Cummings J. H. (1990) The effect of dietary nitrate on nitrate and nitrite excretion in man. Br. J. Nutr. 64, 387–397. [DOI] [PubMed] [Google Scholar]
- (1981) The Health Effects of Nitrate, Nitrite, and N-Nitroso Compounds, National Academy Press, Washington, DC. [Google Scholar]
- De Roos A. J.; Ward M. H.; Lynch C. F.; Cantor K. P. (2003) Nitrate in public water supplies and the risk of colon and rectum cancers. Epidemiology 14, 640–649. [DOI] [PubMed] [Google Scholar]
- Mirvish S. S.; Gandjean A. C.; Reimers K. J.; Connelly B. J.; Chen S. C.; Gallagher J.; Rosinsky S.; Nie G.; Tuatoo H.; Payne S.; Hinman C.; Ruby E. I. (1995) Dosing time with ascorbic acid and nitrate, gum and tobacco chewing, fasting, and other factors affecting N-nitrosoproline formation in healthy subjects taking proline with a standard meal. Cancer Epidemiol., Biomarkers Prev. 4, 775–782. [PubMed] [Google Scholar]
- Lin K.; Shen W.; Shen Z.; Wu Y.; Lu S. (2002) Dietary exposure and urinary excretion of total N-nitroso compounds, nitrosamino acids and volatile nitrosamine in inhabitants of high- and low-risk areas for esophageal cancer in southern China. Int. J. Cancer 102, 207–211. [DOI] [PubMed] [Google Scholar]
- Mirvish S. S.; Bulay O.; Runge R. G.; Patil K. (1980) Study of the arcinogenicity of large doses of dimethylinitramine, N-nitroso-l-proline, and sodium nitirte adinistered in drinking water to rats. J. Natl. Cancer Inst. 64, 1435–1442. [DOI] [PubMed] [Google Scholar]
- Steel G. D., and Torrie J. H. (1980) Principles and Procedures of Statistics: A Biometrial Approach, McGraw-Hill, New York. [Google Scholar]