Abstract
Originally shown to promote the growth and activation of B cells, thymic stromal lymphopoietin (TSLP) is now known to have wide-ranging effects on both hematopoietic and non-hematopoietic cell lineages. These include dendritic cells (DCs), basophils, mast cells, CD4+, CD8+ and natural killer (NK) T cells, B cells and epithelial cells. While TSLP’s role in the promotion of TH2 responses has been extensively studied in the context of lung- and skin-specific allergic disorders, it is becoming increasingly clear that TSLP may impact multiple disease states within multiple organ systems. This review will highlight recent advances in the understanding of the surprising role of TSLP in the control of a variety of cancers, both solid tumors and leukemia, where the TSLP/TSLPR axis has been shown to be an important regulator.
TSLP Biology
Thymic stromal lymphopoietin (TSLP) is a member of the 4-helix bundle cytokine family, and a distant paralog of IL-7(1). As the name suggests, TSLP was first identified as an activity in the supernatants of a mouse thymic stromal cell line that was capable of supporting immature B cell proliferation and development(2-4). In addition, TSLP could act as a co-stimulator for thymocyte proliferation, suggesting that it acted as a lymphopoietin(1). A TSLP homolog was subsequently identified in humans using in silico methods(5,6). Similarly, several groups isolated a TSLP-binding protein in both humans and mice (referred to as TSLP receptor (TSLPR)), which bound TSLP with low affinity(7-10). Sequence analysis found that TSLPR was most closely related to the common gamma chain (γc; (7)). It is now known that the functional, high affinity, TSLPR complex is a heterodimer of TSLPR and interleukin 7 receptor alpha (IL-7Rα)(7,8). Cross-species homology for both the cytokine and its receptor is relatively low (~40% for each), although, as described below, functionally they appear to be quite similar. Thus, the role of this cytokine axis is conserved between man and mouse in spite of a loss of sequence identity.
The similarity of TSLP to interleukin (IL)-7, and the homology of TSLPR to γc suggested that TSLP may play a role in regulating lymphocyte development and/or function. Indeed, early studies did show that TSLP was capable of influencing both T and B cell development and proliferation, both in vitro and in vivo(1,4,11). However, the effects were modest suggesting that the influence of TSLP on lymphocyte development is redundant.
Analysis of the expression profiles of the two receptor subunits in human cell populations provided important insights into the primary biological role of TSLP. The cell population with the highest known coexpression of TSLPR and IL-7Rα were myeloid dendritic cells (DC) (5). Confirming the expression data, TSLP treatment of human DCs induced several phenotypic changes, including increased survival, upregulation of major histocompatibility complex class II (MHCII) and co-stimulatory molecules (CD86 and CD40), and production of a variety of chemokines, most notably the CCR4 ligands CCL17 and CCL22(5,12). Murine bone marrow-derived DCs acquired a similar activated phenotype following TSLP stimulation(13).
Little is known as to the signaling pathways that are activated following engagement of the TSLP receptor complex. Initial studies in the mouse showed that signal transducers and activators of transcription (STAT)5 was activated, but in the absence of detectable JAK activation(2), making TSLPR unique among members of the hematopoietic receptor family. However, two subsequent papers have demonstrated robust and sustained activation of JAK-1 and -2 following TSLP signaling in primary human dendritic cells and primary human and mouse CD4+ T cells(5,14). Surprisingly, unlike IL-7Rα and γc in IL-7 signaling, which utilize JAK-1 and -3, the TSLPR subunit bound and utilized JAK-2 in concert with IL-7Rα-associated JAK-1. These findings resolve a long-standing question about the mode of TSLP signaling, and show that TSLP-induced JAK activation precedes the activation of STAT proteins. In the human, studies have shown that, in addition to STAT5, TSLP stimulation activated STAT 1,3,4, and 6, as well as JAKs 1 and 2(15). One possible explanation for the discrepancy in the data between species is that the mouse signaling work primarily used a pre-B cell line, while the human studies were largely in primary dendritic cells. Consistent with this explanation, our lab has shown that TSLP-treated mouse DCs activate Jak1 and Jak2, as well as STATs 1, 3, and 5 although only Stat5-deficient DCs fail to induce TSLP-specific genes(16). In addition, studies using non-hematopoietic cells (airway smooth muscle cells) have shown that TSLP signals through Stat3(17). Taken together, these data demonstrate that TSLP is capable of activating multiple STAT proteins. Whether TSLP utilizes similar signaling pathways in other cell lineages and how each STAT molecule contributes has yet to be elucidated.
As mentioned above, several cell types have been shown to respond to TSLP. TSLP was originally isolated and characterized as a lymphocyte growth factor(1,2,4), and subsequent studies have shown that TSLP can promote T cell proliferation and differentiation both in vivo and in vitro(18-20). Finally, as will be detailed below, TSLP responsiveness of CD4 T cells is a critical feature of the challenge phase of allergic inflammation(21,22).
It has now become apparent that a major TSLP-responsive cellular subset in both humans and mice are myeloid-derived dendritic cells (mDCs)(5,13). Co-culture of TSLP-activated DCs with naïve syngeneic CD4+ T cells led to T cell proliferation but no differentiation, suggesting a role for TSLP in CD4+ T cell homeostasis(23). However, when TSLP-stimulated DCs primed CD4+ T cells in an antigen-specific manner (e.g., in an allogeneic culture), the resulting T cells display characteristic features of Th2 differentiated cells (production of IL-4, IL-5, IL-13, and TNFα), with the exception that IL-10 production was not evident(12). These data suggest that TSLP-activated DCs primed for inflammatory Th2 cell differentiation. Interestingly, TSLP, in the absence of IL-12, induced OX40L expression on DCs, and OX40-OX40L interactions were critical for the ability of the DCs to drive Th2 cell differentiation(24). Consistent with a role in regulating Th2 cytokine responses, TSLP-activated DCs were also capable of supporting the maintenance and further polarization of Th2 effector memory cells(25). TSLP-conditioned DCs also augmented intestinal epithelial cell-mediated IgA2 class switching through the induction of APRIL (21). Finally, some in vitro studies have suggested a role for TSLP in the generation of tolerogenic DCs that can drive the differentiation of regulatory T cells (Tregs) (26-28), although other studies have indicated that TSLP may hinder the production and/or maintenance of FOXP3+ Tregs in vivo in certain disease processes (29).
Finally, several innate immune cells express the TSLPR and respond to TSLP. For example, TSLP can enhance cytokine production from mast cells, NKT cells and eosinophils (30-32). Recent work has highlighted direct effects of TSLP on basophils during TH2 cytokine-associated inflammatory diseases, including promotion of basophil hematopoiesis from the bone marrow in an IL-3-independent manner (33).
Taken as a whole, the plethora of cell types that can respond to TSLP demonstrate the important role of this cytokine in orchestrating the initial response to an epithelial insult (Figure 1). While the normal function of TSLP is likely the maintenance of Th2-type homeostasis at barrier surfaces(14), dysregulated TSLP expression can result in the development of type 2 inflammatory responses leading to allergic disease.
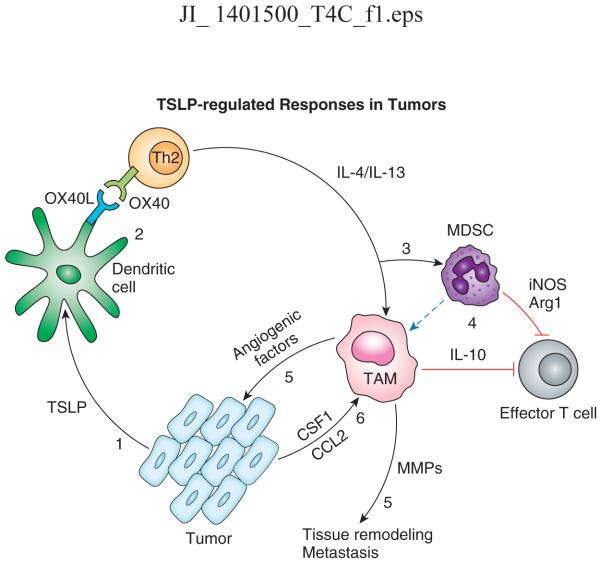
Figure 1. Schematic of TSLP-regulated responses in tumors.
(1) Tumor or tumor-associated stromal cells produce TSLP, which promotes the maturation of resident dendritic cells through upregulation of costimulatory molecules, including OX40L; DCs drive the differentiation of Th2 cells through OX40/OX40L interactions (2); Th2 cells secrete IL4 and IL-13, leading to the recruitment and activation of MDSCs and tumor-associated macrophages (TAM;3), both of which are capable of effector T cell responses against the tumor (4); TAMs also produce factors that promote angiogenesis and matrix remodeling (5), while the tumor and associated stromal cells produce chemotactic and survival factors for the TAMs (6). Dotted line: proposed differentiation path.
Recently a new and unexpected function for TSLP has been found for the induction and regulation of a variety of tumors. TSLP has been found to both promote and suppress solid tumor growth, and somatic mutations and chromosomal translocations in genes encoding members of the TSLP receptor complex have been found in a subset of pediatric patients with B cell acute lymphocytic leukemia (B-ALL). The remainder of this review will discuss this aspect of TSLP biology, along with the potential for therapeutic intervention through modulation of the TSLP pathway.
Role for TSLP in growth and metastasis of solid tumors
It has been shown that for many different types of cancers, a Th2 response is dominant over cytotoxicity induced by CD8 T cells and T-helper 1 (Th1) response(34). Tumors with this type of phenotype generally have a worse prognosis relative to tumors where Th1-type responses predominate(35,36). However, the mechanism by which Th2-biased immune responses are initiated in tumors remains largely unknown. However, two recent studies in humans demonstrated a role for TSLP in promoting a Th2-like environment in the tumor through expression of the cytokine in the tumor microenvironment (Fig. 1). In the first study, De Monte et al.(36), studying pancreatic cancer where a GATA3+ Th2 cellular infiltrate is dominant, showed that cancer associated fibroblasts (CAFs) can produce TSLP. In vitro, supernatants from CAFs were capable of activating DCs to drive Th2 differentiation. Importantly, they found that tumors and tumor-draining lymph nodes contained TSLPR+ DCs, while non-draining lymph nodes did not(36). Finally, using a completely in vitro system, TSLP was shown to be released by human cervical carcinoma cells(37). These authors suggested that this tumor-derived TSLP can act on TSLPR+ endothelial cells to promote angiogenesis in cervical cancer. These data suggest that there is cross-talk between hematopoetic cells that infiltrate the tumor and stromal elements associated with the tumor that can promote a microenvironment favorable to the tumor itself.
In the second study Pedroza-Gonzalez et al.(38) investigated the factors that drive a Th2 microenvironment in breast tumors. They demonstrated that TSLP is produced directly by tumor cells in breast cancer patients. They found that supernatants from explanted tumors were capable of inducing OX40L on DCs in a TSLP-dependent manner, and that these DCs could then promote Th2 differentiation of naïve CD4 T cells. In addition, OX40L+ DCs were found in breast tumors. Interestingly, they used a xeno transfer model to show that blockade of either TSLP or OX40L could reduce tumor growth and IL-13 production(38). Taken together, these 2 papers suggest that TSLP is an important player in promoting tumor survival through manipulation of the immune response in the tumor itself, and that TSLP blockade could be an important therapy for these cancers.
The role of TSLP in tumor growth and metastasis is supported by work using an orthotopic model of metastatic breast cancer in the mouse(39). This model uses a cell line (4T1) derived from a Balb/c breast ductal carcinoma, which, when transplanted into a mammary gland leads to growth of primary tumor with metastases to several organs, including lung(40). This group showed that 4T1 cells produce TSLP, and that the level of TSLP expression is correlated with tumor growth and metastasis. They also found that primary tumor growth was delayed when transplanted into TSLPR-deficient hosts. Unlike the studies in human tumors, they found that the deficit in these mice was not due to lack of TSLP responses in DCs, but rather it was CD4+ T cells that required TSLP responses. Our lab has found that transplantation of 4T1 cells into a TSLP-deficient host results in strikingly reduced growth of the primary tumor and a complete inhibition of lung metastasis (Kuan and Ziegler, in preparation). While the underlying mechanism is not yet clear, we have found that TSLP can functionally activate monocytic lineage myeloid-derived suppressor cells, and that they are lacking in the tumor bearing TSLP-deficit hosts.
In contrast to these studies that suggest a tumor-promoting role in TSLP, two independent groups demonstrated a tumor-suppressing role in TSLP in murine model of skin cancer(41,42). Both papers used keratinocyte-specific ablation of Notch signaling, which has been shown to lead to skin barrier defects and TSLP-dependent dermatitis(43). Demehri et al. showed that mice with inactivation of Notch signaling through deletion of RBPj in keratinocytes failed to develop skin tumors, even following a chemical carcinogenesis regimen that lead to tumor formation in wild-type mice. They found that blocking TSLP signaling in these mice reduced dermal inflammation and allowed for tumor formation, and that induction of TSLP in the skin of wild-type mice inhibited tumor formation. Using a variety of techniques they found that TSLP-responsive CD4 T cells were both necessary and sufficient for the effects of TSLP in this model.
Using a similar strategy, De Piazza et al.(41) found that induced deletion of Notch1 and Notch2 in keratinocytes leads to development of dermatitis and hair follicle associated cysts. Deletion of TSLP signaling in these animals led to overt tumor formation. Unlike the previous study, they showed CD8+ T cells, but not CD4+ T cells, NK cells, B cells, or DCs, are more important for TSLP suppression of tumor formation(41). This group also indicated TSLPR signaling may function differently in distinct cell types. For example, TSLP signaling in CD11b+Gr1+ cells, which are generally viewed as granulocytic myeloid-derived suppressor cells(44), in this model are tumor-promoting instead of tumor-suppressing. A better understanding of the complexity of TSLP-responsive cell subsets in the context of tumors is clearly required to sort out these data. Another interesting concept in the Demehri et al. paper is that the temporal and magnitude of TSLP expression is critical(42). Interestingly, a recent study using the 4T1 transplant breast tumor model showed that transplanted TSLPR deficient mice displayed greater metastasis to the brain but lower in lung(45). This paradoxical result, they claimed, may be because the blood brain barrier keeps tumor cells out. But enhanced systemic Th1 responses they observed in TSLPR deficient tumor bearing mice may open this gate for tumor cell entry. TSLP, although not detected in normal skin, is expressed in glandular breast epithelial cells in non-tumor normal donors.
TSLP receptor complex and Pediatric B-ALL
Pediatric acute lymphocytic leukemia (ALL) is a very heterogeneous disease that is associated with a variety of genetic lesions, including recurring chromosomal translocations, deletions and amplifications(46). It is the most common childhood tumor, and while 80% of affected children are successfully treated, it remains a leading cause of childhood morbidity and mortality(47,48). Recent advances in molecular genetic profiling of B-ALL have uncovered the nature of many of these genetic abnormalities. They include chromosomal translocations (e.g., ETV6-RUNX1, BCR-ABL and TCF3-PBX1) and mutations in genes known to be involved in B cell development (e.g., PAX5, EBF1 and IKZF1)(46). The IKZF1 mutations are especially interesting in that they are a hallmark of Philadelphia chromosome (Ph+) B-ALL (with BCR-ABL translocations) with poor outcomes, but are also seen in Ph− cases that resemble Ph+ patients (referred to as Ph-like ALL)(49,50). These Ph-like cases encompass approximately 15% of B-ALL, and have a higher risk of relapse when compared to Ph+ cases .
Using a variety of methods several groups simultaneously found that mutations in the TSLP signaling pathway correlated with a significant number of B-ALL cases (Fig. 2). For example, ~50% of the Ph-like patients were found to have chromosomal rearrangements involving the TSLPR gene (also referred to as CRLF2)(51). These rearrangements include deletions that join TSLPR and P2RY8 (a gene closely linked to TSLPR) and translocations between TSLPR and the IGH locus(51-53). These alterations lead to increased expression of the TSLPR by coupling its expression to the promoter/enhancer of the translocation partner. In addition, these translocations were also seen in approximately 60 percent of acute lymphoblastic leukemia cases in children with Down’s Syndrome(52-54). Interestingly, these mutations were highly correlated with the presence of JAK2 mutations and were associated with a poor prognosis(55-57). Thus, genetic alterations in TSLPR gene expression are associated with a form of B-ALL with poor prognosis.
Figure 2. Schematic of TSLPR-IL7Rα mutations in B-ALL. L-R.
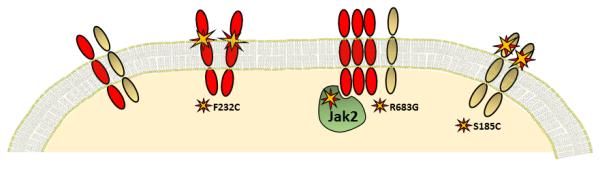
TSLPR (red) and IL-7Rα (grey)-Wild type receptor complex; TSLPR homo-dimer with F232C mutation; Increased TSLPR expression and R683G JAK2 mutation; IL-7Rα homo-dimer with S185C mutation.
In addition to the chromosomal rearrangements, an activating mutation in the TSLPR gene has also been found in Ph-like B-ALL. This mutation changes a Phenylalanine residue in the extracellular domain of the TSLPR adjacent to the transmembrane domain to a Cysteine (F232C)(58). This leads to a gain-of-function as the resulting TSLPR is able to constitutively homo-dimerize and signal. Interestingly, a similar mutation in the IL7Rα, which forms a heterodimer with TSLPR to generate a functional TSLP receptor complex, has been found in B-ALL (S185C)(59). In addition, insertions and deletions within the transmembrane domain of the IL-7Rα, all of which resulted in the presence of a de novo Cysteine residue, were found in several patients(59). Finally, activating mutation in JAK2 have been associated with elevated TSLPR expression in B-ALL(60). These data make a compelling case for enhanced TSLP receptor signaling and the development of B-ALL.
The predominant method that has been used to assess the functional consequences of mutations in TSLPR/CRLF2, IL-7Ra and JAK2 has been expression in factor-dependent cell lines. Retroviral transduction of the mutant genes into the factor dependent cell line BaF3 showed that their expression rendered the cell line growth factor independent(51,58,59,61). While these experiments provided insights into the consequences of these mutations on signaling from the receptor, they did not address the nature of cell affected in vivo or the effect on overall B cell development driven by these mutations.
In an attempt to determine the in vivo function of the TSLPR/CRLF2 F232C mutation, bone marrow of wild-type mice was transduced with retroviruses cDNA clones expressing the human mutant, followed by transplantation into lethally-irradiated hosts. From these studies one animal displayed splenomengaly and indications of increased myeloproliferation in the blood, and elevated number of immature granulocytes and megakaryocytes in the bone marrow(58). While this study demonstrates that TSLPR F232C is an activated allele, there are important issues with these studies. First, the authors stated that the transduced bone marrow progenitors did not contribute to the lymphoid compartment in an appreciable manner, thus limiting the usefulness of this approach for studying B-ALL. Second, the method used to introduce the mutant receptor allows its expression in all hematopoietic lineages. This may allow for off-target effects as other studies suggest that the mutations occur somatically(51,59). Finally, and possibly most important, using the human TSLPR for these studies precludes the ability to determine whether the mutated receptor requires interaction with IL-7Rα and subsequent binding to TSLP as the human and mouse cytokines are species specific in their ability to bind the TSLP receptor complex(7). While these studies are important in that they demonstrate altered functionality of the mutant protein, they have limitations.
Mice with systemic over-expression of TSLP may provide a model for understanding the signaling mechanisms involved. Interestingly, over-expression of TSLP early in the postnatal period was sufficient to drive a B cell lymphoproliferative disorder, but administration or induction of TSLP after postnatal day 14 was not, although other studies have shown expansion of B cell compartments following TSLP expression in adult mice(62). Importantly, in these studies the target of TSLP in the bone marrow were late pro-B cells, similar to the phenotype seen in pediatric B-ALL(62). One possibility is that the acquisition of mutations targeted to the TSLP signaling pathway leads to an unregulated expansion of this population of B cell progenitors, allowing for subsequent neoplastic transformation. The development of appropriate animal models is required to properly test whether this is the case.
Conclusions
A role for TSLP in type-2 inflammatory responses, especially those at barrier surfaces, is now accepted. In the past 4-5 years a new role for TSLP in tumor immunology has emerged. Interstingly, these studies have found a rather complicated role for TSLP, with being tumor-promoting in some instances and tumor-inhibiting in others. Furthermore, enhanced activation of the TSLP signaling pathway can lead to neoplastic transformation of B cell progenitors. Therefore, the decision how to manipulate TSLP or its signaling pathway is dependent on the tumor type. Defining more specific target(s) underling TSLPR signaling that regulate tumor-suppressive or tumor-promoting functions in different cell types will be important to study and is a future direction for cancer therapy.
Acknowledgements
The authors thank Michael Stolley for generation of Figure 2.
This work was partially supported by NIH grants AI68731, AR56113, HL098067, and CA182783
References
- 1.Sims JE, Williams DE, Morrissey PJ, Garka K, Foxworthe D, Price V, Friend SL, Farr A, Bedell MA, Jenkins NA, Copeland NG, Grabstein K, Paxton RJ. Molecular cloning and biological characterization of a novel murine lymphoid growth factor. J Exp Med. 2000;192:671–680. doi: 10.1084/jem.192.5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levin SD, Koelling RM, Friend SL, Isaksen DE, Ziegler SF, Perlmutter RM, Farr AG. Thymic stromal lymphopoietin: a cytokine that promotes the development of IgM+ B cells in vitro and signals via a novel mechanism. J. Immunol. 1999;162:677–683. [PubMed] [Google Scholar]
- 3.Friend SL, Hosier S, Nelson A, Foxworthe D, Williams DE, Farr A. A thymic stromal cell line supports in vitro development of surface IgM+ B cells and produces a novel growth factor affecting B and T lineage cells. Exp. Hematol. 1994;22:321–328. [PubMed] [Google Scholar]
- 4.Ray RJ, Furlonger C, Williams DE, Paige CJ. Characterization of thymic stromal-derived lymphopoietin (TSLP) in murine B cell development in vitro. Eur J Immunol. 1996;26:10–16. doi: 10.1002/eji.1830260103. [DOI] [PubMed] [Google Scholar]
- 5.Reche PA, Soumelis V, Gorman DM, Clifford T, Liu M, Travis M, Zurawski SM, Johnston J, J. Liu Y, Spits H, Waal Malefyt R, Kastelein RA, Bazan JF. Human thymic stromal lymphopoietin preferentially stimulates myeloid cells. J Immunol. 2001;167:336–343. doi: 10.4049/jimmunol.167.1.336. [DOI] [PubMed] [Google Scholar]
- 6.Quentmeier H, Drexler HG, Fleckenstein D, Zaborski M, Armstrong A, Sims JE, Lyman SD. Cloning of human thymic stromal lymphopoietin (TSLP) and signaling mechanisms leading to proliferation. Leukemia. 2001;15:1286–1292. doi: 10.1038/sj.leu.2402175. [DOI] [PubMed] [Google Scholar]
- 7.Park LS, Martin U, Garka K, Gliniak B, Di Santo JP, Muller W, Largaespada DA, Copeland NG, Jenkins NA, Farr AG, Ziegler SF, Morrissey PJ, Paxton R, Sims JE. Cloning of the murine thymic stromal lymphopoietin (TSLP) receptor: Formation of a functional heteromeric complex requires interleukin 7 receptor. J. Exp. Med. 2000;192:659–670. doi: 10.1084/jem.192.5.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pandey A, Ozaki K, Baumann H, Levin SD, Puel A, Farr AG, Ziegler SF, Leonard WJ, Lodish HF. Cloning of a receptor subunit required for signaling by thymic stromal lymphopoietin. Nat. Immunol. 2000;1:59–64. doi: 10.1038/76923. [DOI] [PubMed] [Google Scholar]
- 9.Fujio K, Mosaka T, Kojima T, Kawashima T, Yahata T, Copeland NG, Gilbert DJ, Jenkins NA, Yamamoto K, Mishimura T, Kitamura T. Molecular cloning of a novel type 1 cytokine receptor similar to the common gamma chain. Blood. 2000;95:2210. [PubMed] [Google Scholar]
- 10.Tonozuka Y, Fujio K, Sugiyama T, Nosaka T, Hirai M, Kitamura T. Molecular cloning of a human novel type I cytokine receptor related to delta1/TSLPR. Cytogenet Cell Genet. 2001;93:23–25. doi: 10.1159/000056941. [DOI] [PubMed] [Google Scholar]
- 11.Al Shami A, Spolski R, Kelly J, Fry T, Schwartzberg PL, Pandey A, Mackall CL, Leonard WJ. A role for thymic stromal lymphopoietin in CD4(+) T cell development. J. Exp. Med. 2004;200:159–168. doi: 10.1084/jem.20031975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, Gilliet M, Ho S, Antonenko S, Lauerma A, Smith K, Gorman D, Zurawski S, Abrams J, Menon S, McClanahan T, Waal-Malefyt RR, Bazan F, Kastelein RA, Liu YJ. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat. Immunol. 2002;3:673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 13.Zhou B, Comeau MR, De Smedt T, Liggitt HD, Dahl ME, Lewis DB, Gyarmati D, Aye T, Campbell DJ, Ziegler SF. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat. Immunol. 2005;6:1047–1053. doi: 10.1038/ni1247. [DOI] [PubMed] [Google Scholar]
- 14.Rochman Y, Kashyap M, Robinson GW, Sakamoto K, Gomez-Rodriguez J, Wagner KU, Leonard WJ. Thymic stromal lymphopoietin-mediated STAT5 phosphorylation via kinases JAK1 and JAK2 reveals a key difference from IL-7-induced signaling. Proc. Natl. Acad. Sci. U. S. A. 2010;107:19455–19460. doi: 10.1073/pnas.1008271107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arima K, Watanabe N, Hanabuchi S, Chang M, Sun SC, Liu YJ. Distinct signal codes generate dendritic cell functional plasticity. Sci. Signal. 2010;3:ra4. doi: 10.1126/scisignal.2000567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bell BD, Kitajima M, Larson RP, Stoklasek TA, Dang K, Sakamoto K, Wagner KU, Kaplan DH, Reizis B, Hennighausen L, Ziegler SF. The transcription factor STAT5 is critical in dendritic cells for the development of T(H)2 but not T(H)1 responses. Nat Immunol. 2013;14:364–371. doi: 10.1038/ni.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shan L, Redhu NS, Saleh A, Halayko AJ, Chakir J, Gounni AS. Thymic stromal lymphopoietin receptor-mediated IL-6 and CC/CXC chemokines expression in human airway smooth muscle cells: role of MAPKs (ERK1/2, p38, and JNK) and STAT3 pathways. J Immunol. 2010;184:7134–7143. doi: 10.4049/jimmunol.0902515. [DOI] [PubMed] [Google Scholar]
- 18.Omori M, Ziegler S. Induction of IL-4 expression in CD4(+) T cells by thymic stromal lymphopoietin. J Immunol. 2007;178:1396–1404. doi: 10.4049/jimmunol.178.3.1396. [DOI] [PubMed] [Google Scholar]
- 19.Rochman I, Watanabe N, Arima K, Liu YJ, Leonard WJ. Cutting edge: direct action of thymic stromal lymphopoietin on activated human CD4+ T cells. J Immunol. 2007;178:6720–6724. doi: 10.4049/jimmunol.178.11.6720. [DOI] [PubMed] [Google Scholar]
- 20.Kitajima M, Lee HC, Nakayama T, Ziegler SF. TSLP enhances the function of helper type 2 cells. Eur. J. Immunol. 2011;41:1862–1871. doi: 10.1002/eji.201041195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He R, Oyoshi MK, Garibyan L, Kumar L, Ziegler SF, Geha RS. TSLP acts on infiltrating effector T cells to drive allergic skin inflammation. Proc. Natl. Acad. Sci. U. S. A. 2008;105:11875–11880. doi: 10.1073/pnas.0801532105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al Shami A, Spolski R, Kelly J, Keane-Myers A, Leonard WJ. A role for TSLP in the development of inflammation in an asthma model. J. Exp. Med. 2005;202:829–839. doi: 10.1084/jem.20050199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watanabe N, Hanabuchi S, Soumelis V, Yuan W, Ho S, Waal-Malefyt R, Liu YJ. Human thymic stromal lymphopoietin promotes dendritic cell-mediated CD4+ T cell homeostatic expansion. Nat Immunol. 2004;5:426–434. doi: 10.1038/ni1048. [DOI] [PubMed] [Google Scholar]
- 24.Ito T, Wang YH, Duramad O, Hori T, Delespesse GJ, Watanabe N, Qin FX, Yao Z, Cao W, Liu YJ. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J. Exp. Med. 2005;202:1213–1223. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang YH, Ito T, Wang YH, Homey B, Watanabe N, Martin R, Barnes CJ, McIntyre BW, Gilliet M, Kumar R, Yao Z, Liu YJ. Maintenance and polarization of human TH2 central memory T cells by thymic stromal lymphopoietin-activated dendritic cells. Immunity. 2006;24:827–838. doi: 10.1016/j.immuni.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 26.Watanabe N, Wang YH, Lee HK, Ito T, Wang YH, Cao W, Liu YJ. Hassall's corpuscles instruct dendritic cells to induce CD4+CD25+ regulatory T cells in human thymus. Nature. 2005;436:1181–1185. doi: 10.1038/nature03886. [DOI] [PubMed] [Google Scholar]
- 27.Besin G, Gaudreau S, Ménard M, Guindi C, Dupuis G, Amrani A. Thymic stromal lymphopoietin and thymic stromal lymphopoietin-conditioned dendritic cells induce regulatory T-cell differentiation and protection of NOD mice against diabetes. Diabetes. 2008;57:2107–2117. doi: 10.2337/db08-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iliev ID, Spandoni I, Mileti E, Matteoli G, Sonzogni A, Sampietro GM, Foschi D, Viale G, Rescigno M. Human intestinal epithelial cells promote the differentiation of tolerogenic dendritic cells. Gut. 2009;58:1481–1489. doi: 10.1136/gut.2008.175166. [DOI] [PubMed] [Google Scholar]
- 29.Lei L, Zhang Y, Yao W, Kaplan MH, Zhou B. Thymic stromal lymphopoietin interferes with airway tolerance by suppressing the generation of antigen-specific regulatory T cells. J Immunol. 2011;186:2254–2261. doi: 10.4049/jimmunol.1002503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagata Y, Kamijuku H, Taniguchi M, Ziegler S, Seino K. Differential role of thymic stromal lymphopoietin in the induction of airway hyperreactivity and Th2 immune response in antigen-induced asthma with respect to natural killer T cell function. Int. Arch. Allergy Immunol. 2007;144:305–314. doi: 10.1159/000106319. [DOI] [PubMed] [Google Scholar]
- 31.Allakhverdi Z, Comeau MR, Jessup HK, Yoon BR, Brewer A, Chartier S, Paquette N, Ziegler SF, Sarfati M, Delespesse G. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J. Exp. Med. 2007;204:253–258. doi: 10.1084/jem.20062211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong CK, Hu S, Cheung PF, Lam CW. TSLP Induces Chemotactic and Pro-survival Effects in Eosinophils: Implications in Allergic Inflammation. Am J Respir Cell Mol Biol. 2009 doi: 10.1165/rcmb.2009-0168OC. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 33.Siracusa MC, Saenz SA, Hill DA, Kim BS, Headley MB, Doering TA, Wherry EJ, Jessup HK, Siegel LA, Kambayashi T, Dudek EC, Kubo M, Cianferoni A, Spergel JM, Ziegler SF, Comeau MR, Artis D. TSLP promotes interleukin-3-independent basophil haematopoiesis and type 2 inflammation. Nature. 2011;477:229–233. doi: 10.1038/nature10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loose D, Van de Wiele C. The immune system and cancer. Cancer Biother. Radiopharm. 2009;24:369–376. doi: 10.1089/cbr.2008.0593. [DOI] [PubMed] [Google Scholar]
- 35.Aspord C, Pedroza-Gonzalez A, Gallegos M, Tindle S, Burton EC, Su D, Marches F, Banchereau J, Palucka AK. Breast cancer instructs dendritic cells to prime interleukin 13-secreting CD4+ T cells that facilitate tumor development. J Exp Med. 2007;204:1037–1047. doi: 10.1084/jem.20061120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Monte L, Reni M, Tassi E. l. C. D., Papa I, Recalde H, Braga M, Di C, Doglioni C, Protti MP. Intratumor T helper type 2 cell infiltrate correlates with cancer-associated fibroblast thymic stromal lymphpoietin production and reduced survival in pancreatic cancer. J Exp Med. 2011;208:469–478. doi: 10.1084/jem.20101876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xie F, Meng YH, Liu LB, Chang KK, LI H, Q. Li M, Li DJ. Cervical carcinoma cells stimulate the angiogenesis through TSLP promoting growth and activation of vascular endothelial cells. Am J Reprod. Immunol. 2013;70:69–79. doi: 10.1111/aji.12104. [DOI] [PubMed] [Google Scholar]
- 38.Pedroza-Gonzalez A, Xu K, Wu TC, Aspord C, Tindle S, Marches F, Gallegos M, Bruton EC, Savino D, Hori T, Tanaka Y, Zurawski S, Bover L, Liu YJ, Banchereau J, Palucka AK. Thymic stromal lymphopoietin fosters human breast tumor growth by promoting type 2 inflammation. J Exp Med. 2011;208:479–490. doi: 10.1084/jem.20102131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olkhanud PB, Rochman Y, Bodogai M, Malchinkhuu E, Wejksza K, Xu M, Gress RE, Hesdorffer C, Leonard WJ, Biragyn A. Thymic stromal lymphopoietin is a key mediator of breast cancer progression. J. Immunol. 2011;186:5656–5662. doi: 10.4049/jimmunol.1100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aslakson CJ, Miller FR. Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumor. Cancer Res. 1992;52:1399–1405. [PubMed] [Google Scholar]
- 41.Di Piazza M, Nowell CS, Koch U, Durham AD, Radtke F. Loss of cutaneous TSLP-dependent immune responses skews the balance of inflammation from tumor protective to tumor promoting. Cancer Cell. 2012;22:479–493. doi: 10.1016/j.ccr.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 42.Demehri S, Turkoz A, Manivasagam S, Yockey LJ, Turkoz M, Kopan R. Elevated epidermal thymic stromal lymphopoietin levels establish an antitumor environment in the skin. Cancer Cell. 2012;22:494–505. doi: 10.1016/j.ccr.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Demehri S, Liu Z, Lee J, Lin MH, Crosby SD, Roberts CJ, Grigsby PW, Miner JH, Farr AG, Kopan R. Notch-deficient skin induces a lethal systemic B-lymphoproliferative disorder by secreting TSLP, a sentinel for epidermal integrity. PLoS. Biol. 2008;6:e123. doi: 10.1371/journal.pbio.0060123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gabrilovich DI, Nagaraj S. Myeloid-derived suuppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Erdmann RB, Gartner JG, Leonard WJ, Ellison CA. Lack of functional TSLP receptors mitigates Th2 polarization and the establishment and growth of 4T1 primary breast tumours but has different effects on tumour quantities in the lung and brain. Scand. J. Immunol. 2013;78:408–418. doi: 10.1111/sji.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mullighan CG. The molecular genetic makeup of acute lymphoblastic leukemia. Hematology. Am. Soc. Hematol. Educ. Program. 2012;2012:389–396. doi: 10.1182/asheducation-2012.1.389. [DOI] [PubMed] [Google Scholar]
- 47.Pui CH, Robison LL, Look DC. Acute lymphoblastic leukaemia. Lancet. 2008;371:1030–1043. doi: 10.1016/S0140-6736(08)60457-2. [DOI] [PubMed] [Google Scholar]
- 48.Cobaleda C, Sánchez-García I. B-cell acute lymphblastic leukaemia: towartd understanding its cellular origin. Bioessays. 2009;31:600–609. doi: 10.1002/bies.200800234. [DOI] [PubMed] [Google Scholar]
- 49.Roberts KG, Morin RD, Zhang J, Hirst M, Zhao Y, Su X, Chen SC, Payne-Turner D, Churchman ML, Harvey RC, Chen X, Kasap C, Yan C, Becksfort J, Finney RP, Teachey DT, Maude SL, Tse K, Moore R, Jones S, Mungall K, Birol I, Edmonson MN, Hu Y, Buetow KE, Chen IM, Carroll WL, Wei L, Ma J, Kleppe M, Levine RL, Garcia-Manero G, Larsen E, Shah NP, Devidas M, Reaman G, Smith M, Paugh SW, Evans WE, Grupp SA, Jeha S, Pui CH, Gerhard DS, Downing JR, Willman CL, Loh M, Hunger SP, Marra MA, Mullighan CG. Genetic alterations activating kinase and cytokine receptor signaling in high-risk acute lymphoblastic leukemia. Cancer Cell. 2012;22:153–166. doi: 10.1016/j.ccr.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pui CH, Relling MV, Downing JR. Acute lymphoblastic leukemia. N Eng J Med. 2004;350:1535–1548. doi: 10.1056/NEJMra023001. [DOI] [PubMed] [Google Scholar]
- 51.Yoda A, Yoda Y, Chiaretti S, Bar-Natan M, Mani K, Rodig SJ, West N, Xiao Y, Brown JR, Mitsiades C, Sattler M, Kutok JL, DeAngelo DJ, Wadleigh M, Piciocchi A, Dal Cin P, Bradner JE, Griffin JD, Anderson KC, Stone RM, Ritz J, Foà R, Aster JC, Frank DA, Weinstock DM. Functional screening identifies CRLF2 in precursor B-cell acute lymphoblastic leukemia. Proc Natl Acad Sci U S A. 2010;107:252–257. doi: 10.1073/pnas.0911726107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Russell LJ, Capasso M, Vater I, Akasaka T, Bernard OA, Calasanz MJ, Chandrasekaran T, Chapiro E, Gesk S, Griffiths M, Guttery DS, Haferlach C, Harder L, Heidenriech O, Irving J, Kearney L, Nguyen-Khac F, Machado L, Minto L, Majid A, Moorman AV, Morrison H, Rand V, Strefford JC, Schwab C, Tönnies H, Dyer MJ, Siebert R, Harrison CJ. Deregulated expression of cytokine receptor gene, CRLF2, is involved in lymphoid transformation in B-cell precursor acute lymphoblastic leukemia. Blood. 2009;114:2688–2698. doi: 10.1182/blood-2009-03-208397. [DOI] [PubMed] [Google Scholar]
- 53.Mullighan CG, Collins-Underwood JR, Phillips LA, Loudin MG, Liu W, Zhang J, Ma J, Coustan-Smith E, Harvey RC, Willman CL, Mikhail FM, Meyer J, Carroll AJ, Williams RT, Cheng J, Heerema NA, Basso G, Pession A, Pui CH, Raimondi SC, Hunger SP, Downing JR, Carroll WL, Rabin KR. Rearrangement of CRLF2 in B-progenitor- and Down syndrome-associated acute lymphoblastic leukemia. Nat. Genet. 2009;41:1243–1246. doi: 10.1038/ng.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ensor HM, Schwab C, Russell LJ, Richards SM, Morrison H, Masic D, Jones L, Kinsey SE, Vora AJ, Mitchell CD, Harrison CJ, Moorman AV. Demographic, clinical, and outcome features of children with acute lymphoblastic leukemia and CRLF2 deregulation: results from the MRC ALL97 clinical trial. Blood. 2011;117:2129–2136. doi: 10.1182/blood-2010-07-297135. [DOI] [PubMed] [Google Scholar]
- 55.Cario G, Zimmermann M, Roney R, Gesk S, Vater I, Harbott J, Schrauder A, Moericke A, Izraeli S, Akasaka T, Dyer MJ, Siebert R, Schrappe M, Stanulla M. Presence of the P2RY8-CRLF2 rearrangement is associated with a poor prognosis in non-high-risk precursor B-cell acute lymphoblastic leukemia in children treated according to the ALL-BFM 2000 protocol. Blood. 2010;115:5393–5397. doi: 10.1182/blood-2009-11-256131. [DOI] [PubMed] [Google Scholar]
- 56.Harvey RC, Mullighan CG, Chen IM, Wharton W, Mikhail FM, Carroll AJ, Kang H, Liu W, Dobbin KK, Smith MA, Carroll WL, Devidas M, Bowman WP, Camitta BM, Reaman GH, Hunger SP, Downing JR, Willman CL. Rearrangement of CRLF2 is associated with mutation of JAK kinases, alteration of IKZF1, Hispanic/Latino ethnicity, and a poor outcome in pediatric B-progenitor acute lymphoblastic leukemia. Blood. 2010;115:5312–5321. doi: 10.1182/blood-2009-09-245944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hertzberg L, Vendramini E, Ganmore I, Cazzaniga, Schmitz M, Chalker J, Shiloh R, Iacobucci I, Shochat C, Zeligson S, Cario G, Stanulla M, Strehl S, Russell LJ, Harrison CJ, Bornhauser B, Yoda A, Rechavi G, Bercovich D, Borkhardt A, Kempski H, te KG, Bourquin J-P, Domany E, Izraeli S. Down syndrome acute lymphoblastic leukemia, a highly heterogeneous disease in which aberrant expression of CRLF2 is associated with mutated JAK2: a report from the International BFM Study Group. Blood. 2010;115:1006–1017. doi: 10.1182/blood-2009-08-235408. [DOI] [PubMed] [Google Scholar]
- 58.Chapiro E, Russell L, Lainey E, Kaltenbach S, Ragu C, Della-Valle V, Hanssens K, Macintyre EA, Radford-Weiss I, Delabesse E, Cavé H, Mercher T, Harrison CJ, Nguyen-Khac F, Dubreuil P, Bernard OA. Activating mutation in the TSLPR gene in B-cell precursor lymphoblastic leukemia. Leukemia. 2010;24:642–645. doi: 10.1038/leu.2009.231. [DOI] [PubMed] [Google Scholar]
- 59.Shochat C, Tal N, Bandapalli OR, Palmi C, Ganmore I, te KG, Cario G, Cazzaniga G, Kulozik AE, Stanulla M, Schrappe M, Biondi A, Basso G, Bercovich D, Muckenthaler MU, Izraeli S. Gain-of-function mutations in interleukin-7 receptor-alpha (IL7R) in childhood acute lymphoblastic leukemias. J. Exp. Med. 2011;208:901–908. doi: 10.1084/jem.20110580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roll JD, Reuther GW. CRLF2 and JAK2 in B-progenitor acute lymphoblastic leukemia: a novel association in oncogenesis. Cancer Res. 2010;70:7347–7352. doi: 10.1158/0008-5472.CAN-10-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Malinge S, Ben-Abdelali R, Settegrana C, Radford-Weiss I, Debre M, Beldjord K, Macintyre EA, Villeval JL, Vainchenker W, Berger R, Bernard OA, Delabesse E, Penard-Lacronique V. Novel activating JAK2 mutation in a patient with Down syndrome and B-cell precursor acute lymphoblastic leukemia. Blood. 2007;109:2202–2204. doi: 10.1182/blood-2006-09-045963. [DOI] [PubMed] [Google Scholar]
- 62.Astrakhan A, Omori M, Nguyen T, Becker-Herman S, Iseki M, Aye T, Hudkins K, Dooley J, Farr A, Alpers CE, Ziegler SF, Rawlings DJ. Local increase in thymic stromal lymphopoietin induces systemic alterations in B cell development. Nat Immunol. 2007;8:522–531. doi: 10.1038/ni1452. [DOI] [PubMed] [Google Scholar]