Abstract

5-Chloro-3-ethyl-N-(4-(piperidin-1-yl)phenethyl)-1H-indole-2-carboxamide (1; ORG27569) is a prototypical allosteric modulator for the cannabinoid type 1 receptor (CB1). Here, we reveal key structural requirements of indole-2-carboxamides for allosteric modulation of CB1: a critical chain length at the C3-position, an electron withdrawing group at the C5-position, the length of the linker between the amide bond and the phenyl ring B, and the amino substituent on the phenyl ring B. These significantly impact the binding affinity (KB) and the binding cooperativity (α). A potent CB1 allosteric modulator 5-chloro-N-(4-(dimethylamino)phenethyl)-3-propyl-1H-indole-2-carboxamide (12d) was identified. It exhibited a KB of 259.3 nM with a strikingly high binding α of 24.5. We also identified 5-chloro-N-(4-(dimethylamino)phenethyl)-3-hexyl-1H-indole-2-carboxamide (12f) with a KB of 89.1 nM, which is among the lowest KB values obtained for any allosteric modulator of CB1. These positive allosteric modulators of orthosteric agonist binding nonetheless antagonized the agonist-induced G-protein coupling to the CB1 receptor, yet induced β-arrestin mediated ERK1/2 phosphorylation.
Introduction
The cannabinoid type 1 (CB1) receptor is the most abundant G-protein coupled receptor (GPCR) expressed in the central nervous system (CNS), where it attenuates the release of excitatory and inhibitory neurotransmitters.1−3 The CB1 receptor is also present in lower concentrations in a variety of peripheral tissues, including, spleen, tonsil, gastrointestinal tract, liver, kidney, and heart.4−6 It regulates a variety of physiological functions including neuronal development, neuromodulatory processes, metabolism, nociception, and cardiovascular as well as reproductive functions.1,7,8 While CB1 preferentially couples to Gi/o type G proteins, it can interact with Gs9 or Gq10 under some conditions. The CB1 receptor also modulates the activation of mitogen-activated protein kinases (MAPKs),11 inhibits N- and P/Q-type voltage-gated Ca2+ channels, and activates A-type and inwardly rectifying K+ channels.12 Moreover, the CB1 receptor can interact with non-G protein partners such as β-arrestins, adaptor protein AP-3, GPCR-associated sorting protein 1 (GASP1), and the adaptor protein FAN to control receptor signaling or trafficking.13,14 The complex signaling network of the CB1 receptor suggests the existence of finely controlled modulatory mechanisms of receptor functions.
Traditionally, the functions of the CB1 receptor is regulated through various agonists, partial agonists, antagonists, and inverse agonists,15 which bind to the orthosteric site where the endogenous cannabinoids bind. Recently, several allosteric modulators of the CB1 receptor have been identified, which bind to sites that are topologically distinct from the orthosteric binding site. These include 5-chloro-3-ethyl-N-(4-(piperidin-1-yl)phenethyl)-1H-indole-2-carboxamide (1, ORG27569),16 1-(4-chlorophenyl)-3-(3-(6-(pyrrolidin-1-yl)pyridin-2-yl)phenyl)urea (PSNCBAM-1),17 3-(4-chlorophenyl)-5-(8-methyl-3-p-tolyl-8-azabicyclo[3.2.1]octan-2-yl)isoxazole (RTI-371),18 and the endogenous ligand (5S,6R,7E,9E,11Z,13E,15S)-5,6,15-trihydroxyicosa-7,9,11,13-tetraenoic acid (lipoxin A4).19 Allosteric modulators typically work cooperatively with orthosteric ligands and stabilize the receptor in various biological conformations that may be difficult to achieve by the orthosteric ligands.20 This increases the possibility of regulating receptor activities in more sophisticated ways than with orthosteric ligands. Thus, allosteric modulation can significantly expand the pharmacological repertoire for a given receptor.21,22 Additionally, allosteric sites are less structurally conserved than the corresponding orthosteric site and thus provide new opportunities for the development of more selective therapeutics.8,23 The discovery of CB1 allosteric modulators lays the foundation for receptor-selective and signaling-pathway-selective therapies.
Compound 1 was the first allosteric modulator identified for the CB1 receptor.16 It augments specific binding of the CB1 agonist 2-[(1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl) cyclohexyl]-5-(2-methyloctan-2-yl)phenol ([3H]CP55,940) but decreases the binding of the inverse agonist 5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-N-(piperidin-1-yl)-1H-pyrazole-3-carboxamide ([3H]SR141716A) in membranes from cells expressing the CB1 receptor.16,24 Despite acting as an enhancer of agonist binding, it antagonizes agonist-induced G-protein coupling to the receptor.16,24 It was further demonstrated that 1 in the absence of any orthosteric agonist can induce cellular internalization of the CB1 receptor and downstream activation of ERK signaling mediated by β-arrestins24−26 as a consequence of CB1 receptor activation. This indicates that allosteric modulators of the CB1 receptor offer the potential to develop drugs capable of generating therapeutic effects via ligand-biased signaling pathways.
Following the discovery of 1,16 structure–activity relationship (SAR) studies have revealed that the indole-2-carboxamide scaffold is a viable template for developing CB1 allosteric modulators.25,27,28 The general structure of this class of compounds can be divided into two moieties comprising the bicyclic aryl fragment and the amide fragment (Figure 1).
Figure 1.

Compound 1 and general structure of indole-2-carboxamides.
Earlier results from us25,28 and others27 have identified several key SARs within this class of compounds (I). These include (1) the indole ring of I (Figure 1) impacts the ligand’s capability to bind to the allosteric site more than the ligand’s capability to modulate the orthosteric site;28 (2) the presence of a linear alkyl group at the C3 position of the indole ring is instrumental and its length has a profound influence on the allosteric modulation of the orthosteric binding site;25,28 (3) the amide functionality at the C2 position of the indole ring is critical for the allosteric effects on the orthosteric site;27 (4) shortening the linker between the amide bond and the phenyl ring B abolished the allosteric modulation of the orthosteric binding site;28 (5) replacing the piperidinyl group of 1 with various functional groups27,28 generally led to reduced allosteric modulation except for the methylamino27 and dimethylamino groups.27,28 Along with 1, a few other indole-2-carboxamides16,25,27 have also shown allosteric modulation of the CB1 receptor. These molecules, 5-chloro-3-pentyl-N-(4-(piperidin-1-yl)phenethyl)-1H-indole-2-carboxamide (2, ICAM-b),25N-(4-(dimethylamino)phenethyl)-3-ethyl-5-fluoro-1H-indole-2-carboxamide (3, ORG27759),16 5-chloro-N-(4-(dimethylamino)phenethyl)-3-ethyl-1H-indole-2-carboxamide (4),27 and 5-chloro-N-(4-(dimethylamino)phenethyl)-3-pentyl-1H-indole-2-carboxamide (5),28 are shown in Figure 2.
Figure 2.
Representative indole-2-carboxamides showing CB1 allostery.
In this context, we expanded our SAR studies of indole-2-carboxamides. Our efforts include elongation of the linker between the amide bond and the phenyl ring B, further investigation of the requirement of the C3 alkyl group (i.e., R1) and modification of the substitutions (i.e., R2) on the phenyl ring A of the bicyclic aryl fragment. To date, most of the reported indole-2-caboxamides showing CB1 allostery were developed from a 5-chloro-indole-2-carboxamide template16,25,27,28 except 3.16 Hence, we investigated the impact of different substitutions of the phenyl ring A (Figure 1) on the allosteric effects. This effort led to the compounds 21a–d (Table 2). The allosteric effects of these compounds were evaluated by two essential parameters:23,29 the equilibrium dissociation constant (KB), which reflects the binding affinity of the ligands to the allosteric site, and the binding cooperativity factor (α) that denotes the allosteric interaction between the orthosteric and allosteric ligands when they both occupy the receptor, i.e., it quantifies the direction of and magnitude by which the affinity of one ligand is changed by the other ligand when both are bound to the receptor to form the ternary complex.30 When α is 1.0, the test modulator does not alter orthosteric ligand binding. If α is less than 1.0, the test modulator reduces orthosteric ligand binding (negative allosteric modulation of orthosteric ligand binding). If α is greater than 1.0, the modulator increases orthosteric ligand binding (positive allosteric modulation of orthosteric ligand binding).23 The α and KB values were analyzed according to the allosteric ternary complex model.16 Selected allosteric modulators were assessed for their effects on agonist-induced G-protein coupling activity and β-arrestin mediated ERK1/2 phosphorylation.
Table 2. Allostery of Indole-2-carboxamides 21a–d and 26.

| entry | compd | R1 | R2 | n | KB (nM)a | αb |
|---|---|---|---|---|---|---|
| 13 | 4c | Cl | H | 1 | 207.4 (155.9–2759) | 19.7 |
| 14 | 21a | H | Cl | 1 | 3673 (1048–12880) | 16.0 |
| 15 | 21b | H | F | 1 | 1580 (328.7–7599) | 22.9 |
| 16 | 21c | OCH3 | H | 1 | 2708 (973.4–7535) | 6.2 |
| 17 | 21d | H | OCH3 | 1 | 4084 (1213–13750) | 11.9 |
| 18 | 26 | Cl | H | 2 | NDd | NDd |
KB: equilibrium dissociation constant of a potential allosteric ligand.
α: binding cooperativity factor for the tested allosteric modulator. Both parameters were tested using [3H]CP55,940 as the orthosteric ligand.
Data cited for 4 are from our earlier report28 and are given for comparison.
ND: no detectable modulation of [3H]CP55,940 binding using up to 32 μM of test compound.
Chemistry
The syntheses of C3-alkylated indole-2-carboxamides (12a–f) were achieved through the methods illustrated in Scheme 1. The C3 substituents were introduced through Friedel–Crafts acylation of the ethyl 5-chloroindole-2-carboxylate (6), which is commercially available. Acylation of 6 with various selected acyl chlorides (7a–c) provided the desired 3-acyl-5-chloroindole-2-carboxylates (8a–c). Reduction of their ketone groups by triethylsilane generated the C3 alkylated 5-chloroindole-2-carboxylates (9a–c), which were then hydrolyzed in basic conditions to yield the key intermediate indole-2-carboxylic acids (10a–c). The final compounds (12a–f) were prepared by coupling commercially available amines (11a,b) with the acids (10a–c) individually in the presence of BOP and diisopropylethyl amine (DIPEA) in anhydrous DMF at room temperature.
Scheme 1. Synthesis of 3-Alkyl-5-chloroindole-2-carboxamides 12a–f.
Reagents and conditions: (i) AlCl3, 1,2-dichloromethane, reflux, 2–3 h; (ii) (Et)3SiH, CF3COOH, 0 °C–rt, 4–12 h; (iii) 3N NaOH, EtOH, reflux, 2 h; (iv) BOP, DIPEA, DMF, rt, 4–12 h.
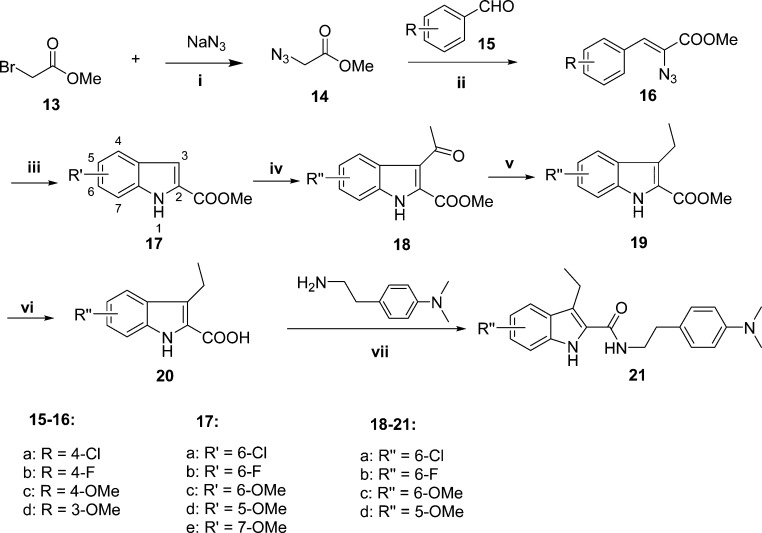
To access the indole-2-carboxamides with different substituents on the phenyl ring A (21a–d), we employed Hemetsberger–Knittel indole synthesis,31 by which the required indole-2-carboxylate 17 can be obtained through Knoevenagel condensation of methyl 2-azidoacetate 14 with the substituted benzaldehyde 15 followed by a thermolysis of the azide of the resultant methyl-2-azidocinnamate 16 and an electrophilic cyclization. The synthesis of indole-2-carboxylate 17 through Hemetsberger–Knittel reaction depends on the reaction conditions, which include the reaction temperature and stoichiometry of reactants in the Knoevenagel condensation (step ii, Scheme 2) and the concentration of reactant in the subsequent thermolytic cyclization (step iii, Scheme 2). We optimized the reaction conditions to obtain 16 and 17 in good yield to proceed with the synthesis (see the discussion in Experimental Section). The cyclization of 16d provided two regioisomers, the 5- and 7-substituted indole-2-carboxylates (17d and 17e), with the 5-regioisomer (17d) being slightly favored over the 7-regioisomer (17e). The structures of the two regioisomers were assigned by comparison of their 1H NMR with the reported data.32,33 Acylation of 17a–d by Friedel–Crafts reaction led to the major product as the desired 3-acyl-indol-2-carboxylates 18a–d. However, acylation of 17e generated the 4-acyl-indole-2-carboxamide as the major product, which is not suitable for SAR study in this series; hence, the 4-acylated product was not further pursued in the synthesis of corresponding indole-2-carboxamide 21. Following the preparation of 3-acylated indole-2-carboxylates 18a–d, we synthesized the corresponding final products 21a–d by following Scheme 1 (steps i–iv).
Scheme 2. Synthesis of 3-Ethyl Indole-2-carboxamides 21a–d.
Reagents and conditions: (i) NaN3, DMF, rt, 1.5 h; (ii) NaOCH3/CH3OH, −20 °C, 5 h; (iii) xylene, reflux, 3 h; (iv) AlCl3, 1,2-dichloromethane, reflux, 2.5 h; (v) (Et)3SiH, CF3COOH, 0 °C–rt, 4 h; (vi) 3 N NaOH, EtOH, reflux, 2 h; (vii) BOP, DIPEA, DMF, rt, 4–12 h.
The indole-2-carboxamide 26 was synthesized according to the route illustrated in Scheme 3. It was prepared through coupling of 4-(3-aminopropyl)-N,N-dimethyl aniline 24 with 5-chloro-3-ethyl-1H-indole-2-carboxylic acid 25, which was synthesized according to the reported method.28 For the synthesis of 24, 4-dimethylaminobenzaldehyde 22 was condensed with acetonitrile through aldol condensation in strong basic conditions to yield a mixture of (E)- and (Z)-3-(4-(dimethylamino)phenyl)acrylonitrile 23, which was reduced to yield amine 24.
Scheme 3. Synthesis of Indole-2-carboxamide 26.
Reagents and conditions: (i) CH3CN, KOH, reflux, 10 min; (ii) THF, LiAlH4, AlCl3, reflux, 1 h; (iii) BOP, DIPEA, DMF, rt, 4–12 h.
Results and Discussion
Our previous investigations revealed that structural variation at the C3 position of indole-2-carboxamide has a profound influence on CB1 allostery. We found that the C3 position prefers a linear alkyl group.28 When the C3 ethyl group of 1 was replaced with a n-pentyl group (2), it led to the enhancement of the allosteric effect, which is reflected by an improvement in the cooperativity factor α from 6.9 (1) to 17.6 (2).25 However, further increasing the length of the C3 alkyl to n-heptyl (12g) and n-nonyl (12h) groups did not improve the allosteric effects on CB1.28 Here, we elaborated our investigation of the C3 position with variations of the linear alkyl groups such as n-propyl, n-butyl, and n-hexyl groups. The allosteric parameters of the analogues are presented in Table 1. The results reflect that a specific length of the linear alkyl group is required at the C3 position. Increasing the length of the C3 alkyl group of 1 to n-propyl (12a), n-butyl (12b), and n-pentyl (2)25 led to the significant enhancement of binding cooperativity (α). When the length was further elongated to n-hexyl (12c), n-heptyl (12g),28 and n-nonyl (12h),28 the binding cooperativity (α) decreased to a level comparable to 1. Notably, the significant increase of the binding cooperativity factor (α) of 12a and 12b was accompanied by reduced binding affinities to the allosteric site. This reflects that an allosteric modulator can induce a receptor conformation that enhances orthosteric ligand binding despite having a relatively low affinity for the allosteric site. As has been shown previously, the affinity of an allosteric modulator (KB) and the allostery (α) it exhibits for the orthosteric compound are not necessarily correlated.34,35 In this series of modifications (entries 1–7, Table 1), the C3 n-propyl provided markedly enhanced allosteric modulation of the orthosteric site (binding cooperativity factor α = 26.7). We further assessed the effects of the length of C3 alkyl chain on allosteric properties using 4 as a scaffold because replacing the N-piperidinyl group of 1 with a dimethylamino group resulted in improvement of the allosteric effects of indole-2-carboxamides.27,28 This effort led to the analogues 12d–f with improved allosteric parameters. The results suggest that the dimethylamino group on the phenyl ring B is superior to the N-piperidinyl group of the indole-2-carboxamides, yielding modulators with higher binding affinity for the allosteric site and greater cooperativity to the orthosteric site (e.g., 12d vs 12a, 12e vs 12b, 5 vs 2, and 4 vs 1, Table 1). Strikingly, the n-hexyl substituent (12f) improved the equilibrium dissociation constant (KB) to 89.1 nM with an α comparable to 1.
Table 1. Allostery of Indole-2-carboxamides 12a–f and Some Referenced Compounds.

| entry | compd | R1 | R2 | KB (nM)a | αb |
|---|---|---|---|---|---|
| 1 | 1c | C2H5 | N-piperidinyl | 217.3 (170.3–277.2) | 6.9 |
| 2 | 12a | n-C3H7 | N-piperidinyl | 1746 (377.8–8065) | 26.7 |
| 3 | 12b | n-C4H9 | N-piperidinyl | 1985 (775.1–5082) | 17.7 |
| 4 | 2d | n-C5H11 | N-piperidinyl | 469.9 (126.2–1750) | 17.6 |
| 5 | 12c | n-C6H13 | N-piperidinyl | 310.6 (110.5–873.2) | 4.6 |
| 6 | 12ge | n-C7H15 | N-piperidinyl | 651.2 (81.51–5203) | 7.4 |
| 7 | 12he | n-C9H19 | N-piperidinyl | 259.7 (87.56–770) | 6.8 |
| 8 | 4e | C2H5 | N(CH3)2 | 207.4 (155.9–2759) | 19.7 |
| 9 | 12d | n-C3H7 | N(CH3)2 | 259.3 (19.8–3365) | 24.5 |
| 10 | 12e | n-C4H9 | N(CH3)2 | 209.0 (62.7–696.7) | 12.8 |
| 11 | 5e | n-C5H11 | N(CH3)2 | 167.3 (23.39–1197) | 16.5 |
| 12 | 12f | n-C6H13 | N(CH3)2 | 89.1 (47.08–168.4) | 5.1 |
KB: equilibrium dissociation constant of a potential allosteric ligand.
α: binding cooperativity factor for the tested allosteric modulator. Both parameters were tested using [3H]CP55,940 as the orthosteric ligand.
Data cited for 1 are from our earlier report24 and are given for comparison.
Data cited for 2 are from our earlier report25 and are given for comparison.
Data cited for compounds 4, 5, 12g, and 12h are from our earlier report28 and are given for comparison.
The indole-2-carboxamide 4(27,28) was used as a reference compound to vary the substitutions (Table 2) on the phenyl ring A (Figure 1). The C5-chloro group of 4 is an electron withdrawing group (EWG) inductively and is electron donating by resonance. To evaluate the impact of the substitution position, we moved the C5-chloro group of 4 to the C6 position. This modification (21a) drastically reduced the binding affinity to the allosteric site (KB = 3673 nM) but not the allosteric modulation of the orthosteric ligand binding (α = 16.0). Because a fluoro group has a greater electron-withdrawing inductive effect than a chloro group, we replaced the C6-chloro group with a C6-fluoro group (21b), and this modification did not improve the binding affinity to the allosteric site (KB = 1580 nM) while the allosteric modulation on orthosteric ligand binding is well preserved (α = 22.9) in comparison with 4 (α = 19.7). The result from 21b along with an earlier result of 3, which is a 5-fluoro-indole-2-carboxamide,16 suggested that fluoro as a substituent on ring A is more suboptimal than a chloro group. Taken the fact that chloro group is an EWG inductively and is electron donating group (EDG) by resonance, we replaced it with a methoxy group, which is a stronger EDG. This modification led to 21c, which exhibited a significantly decreased cooperativity factor (α = 6.2) and binding affinity (KB = 2708 nM) in comparison with 4 (α = 19.7, KB = 207.4 nM). Moving the methoxy group to the C6-position (21d) also reduced the allosteric effect on the orthosteric site and the binding affinity to the allosteric site. This series of compounds (entries 13–17) suggested that the nature and the position of the substituent on the phenyl ring A are critical for both the binding affinity (KB) to the allosteric site and the binding cooperativity with the orthosteric site.
In line with our earlier finding that the one carbon linker between the amide bond and the phenyl ring B (Figure 1) abolished the allostery of this class of compounds on CB1 receptor,28 the loss of allosteric modulation of orthosteric agonist CP55,940 binding with 26 indicated the critical role of the 2-carbon linker between the amide bond and the phenyl ring B of the amide fragment within the structure of indole-2-carboxamides (I, Figure 1).
The two robust allosteric modulators 12d and 12f were further tested for their effect on CP55,940-induced G-protein coupling activity. It was found that both the compounds showed a concentration-dependent inhibition of agonist-induced GTPγS binding as shown in Figure 3.
Figure 3.
Dose–response curves for CP55,940-induced [35S]GTPγS binding to HEK293 cell membranes expressing the CB1 receptor in the absence and presence of compounds 12d (A) and 12f (B) at the indicated concentrations. Nonspecific binding was determined in the presence of 10 μM unlabeled GTPγS. Data is presented as specific binding of GTPγS (fmol/mg) to the membranes. Each data point represents the mean ± SE (error bars) of at least three independent experiments performed in duplicate.
We previously demonstrated that ERK1/2 can be activated via CB1 in a G-protein-dependent manner by CP55,940 alone and a G-protein-independent manner in the presence of 1 or 2 and CP55,940.24,25,36 In agreement, CP55,940-induced ERK1/2 phosphorylation was substantially attenuated by pertussis toxin (PTX) but not by β-arrestin knockdown indicating alone it is Gi-protein mediated (Figure 4A). In contrast, yet in line with the observed inhibition of G protein coupling activity (Figure 3), ERK1/2 phosphorylation due to 12f treatment was PTX insensitive (Figure 4A), suggesting utilization of a β-arrestin mediated pathway. Furthermore, Figure 4B–D show that cotreatment of 2, 12d, or 12f and CP55,940 induce concentration-dependent ERK1/2 phosphorylation that is β-arrestin 1 sensitive; the β-arrestin 1 knockdown resulted in substantial inhibition of ERK1/2 phosphorylation induced by 2, 12d, and 12f. These results indicate that 2, 12d, and 12f are functionally positive allosteric modulators, at least for ERK1/2 phosphorylation. Interestingly, 12d and 12f reached a plateau at 5 μM of the modulator whereas 2 required 10 μM to achieve comparable levels of ERK1/2 phosphorylation (Figure 4E). This may reflect the higher binding affinity of 12d and 12f relative to that of 2 (Table 1).
Figure 4.
Effect of 12d and 12f on ERK1/2 phosphorylation. (A) Mock-transfected and treatment conditions for CP55,940 (0.2 μM), PTX, and siRNA knockdown of HEK293 cells expressing CB1 are indicated and shown for comparison. (B–D) HEK293 cells expressing CB1 receptors were exposed to 0, 0.05, 0.1, 0.5, 1, 5, and 10 μM of 2 (B), of 12d (C), or of 12f (D) in the presence of 0.2 μM CP55,940 for 5 min with PTX pretreatment for 16 h. Cell lysates were separated on SDS-PAGE and analyzed by Western blots probed with phospho-ERK1/2 (p-ERK1/2). The total level of ERK1/2 was detected for comparison. Note that the two bands correspond to the predominant isoforms, p42 (ERK2) and p44 (ERK1), for ERK1/2. (E) Graphs provide the quantified ERK1/2 phosphorylation levels induced by each compound for 5 min. Data represent the mean ± SE and are expressed as a percent of the level of CP55,940-induced ERK1/2 phosphorylation.
Conclusion
The results from the newly synthesized indole-2-carboxamides along with our earlier findings28 elucidated key structural requirements of indole-2-carboxamides for allosteric modulation of the CB1 receptor. The critical structural factors include: (1) the chain length of the C3-alkyl group is critical with n-propyl being preferred for allosteric modulation of orthosteric ligand binding and n-hexyl being preferred for enhancing affinity of the allosteric modulator to the CB1 receptor, (2) an electron withdrawing group needs to reside at the C5 position of the indole ring, (3) the linker between the amide bond and the phenyl ring B must be an ethylene group whereas shortening or elongating the linker abolishes allosteric effects, and (4) the substituent on the phenyl ring B explicitly influences both the binding to the allosteric site and the binding cooperativity with the orthosteric ligand, with the N,N-dimethyl amino group being preferred over the piperidinyl functionality of the prototypical CB1 allosteric modulator 1. These SARs will guide the future design and synthesis of more potent CB1 allosteric modulators based on the indole-2-carboxamide scaffold. The therapeutic usefulness of CB1 allosteric modulators is becoming evident. For instance, the CB1 allosteric modulator PSNCBAM-1 exhibits acute hypophagic effects17 and antagonism of neuronal excitability,37 which have the potential for the treatment of obesity and some CNS disorders. The endogenous CB1 allosteric modulator Lipoxin A4 is capable of protecting neuronal cells from β-amyloid-induced neurotoxicity19 that has been implied in the etiology of Alzheimer’s disease. Given the nature of biased signaling of some CB1 allosteric modulators from the indole-2-carboxamide class, selective regulation of signaling-pathway-specific functions of the CB1 receptor is possible and this may be therapeutically beneficial. The angiotensin II receptor, for example, may exhibit G-protein dependent or β-arrestin-dependent signaling. Biased agonism of this receptor to promote β-arrestin mediated effects only may provide a beneficial cytoprotective response and obviate a deleterious increase in blood pressure that is observed in a G-protein manner.38 This offers tremendous opportunities for developing drugs for many disorders that have been linked to the CB1 receptor in the CNS and periphery.
Experimental Section
Compounds
Tested compounds (12a–f and 21a–d) were synthesized for this study except 1, which was purchased from Tocris Bioscience (Minneapolis, MN). Compounds 2(25) and 12g, 12h, and 5 have previously been reported by us28 and were cited in this report for comparison. Compound 4 was first reported by Piscitelli et al.27 and was resynthesized and tested in our laboratories for comparative purposes.
CB1 Expression and Membrane Preparation
HEK 293 cells were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum and 3.5% mg/mL glucose at 37 °C in 5% CO2. One day prior to transfection, cells were seeded at approximately 900000 cells/100 mm dishes. The cells were transiently transfected by the calcium phosphate precipitation method.39 At 24 h post transfection, the cells were harvested and washed twice with phosphate buffered saline (PBS). The cells were resuspended in PBS solution containing mammalian protease inhibitor cocktail ((4-2-aminoethyl) benzene-sulfonyl fluoride, pepstatin A, E-64, bestatin, leupeptin, and aprotinin) (Sigma-Aldrich, St. Louis, MO) and lysed by nitrogen cavitation at 750 psi for 5 min using a Parr cell disruption bomb. The lysate was spun at 500g for 10 min at 4 °C, and the supernatant was subsequently spun at 100000g for 45 min at 4 °C. The membrane-containing pellet was resuspended in TME buffer (25 mM Tris-HCl, 5 mM MgCl2, and 1 mM EDTA, pH 7.4) containing 7% w/v sucrose. For immunoblotting studies, HEK 293 cells were transfected using lipofectamine (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. Then 24 h post-transfection, cells were washed and incubated for an additional 18 h in serum-free growth media. siRNA transfection was carried out as previously described.36 Briefly, HEK293 cells in a 6-well plate were transfected with the plasmid encoding CB1 and 2.6 μg of siRNA (Qiagen, Valencia, CA) targeting β-arrestin 1 or nonsilencing RNA duplex for control.
Radioligand Binding Assay
Ligand binding assays were performed as previously described to determine the cooperativity between the orthosteric and allosteric ligands.24 Briefly, 6 μg of membrane preparation was incubated for 60 min with a fixed concentration of tracer [3H]CP55940 (141 Ci/mmol, PerkinElmer Life Sciences (Boston, MA)), typically at its Kd, which was determined from a saturation binding isotherm in a total volume of 200 μL of TME buffer containing 0.1% fatty acid-free BSA. Ligand depletion was avoided by adjusting the amount of membrane sample and total assay volume to keep the bound ligand less than 10% of the total. At least nine concentrations of unlabeled test compound (ranging between 100 pM and 100 μM) were used for the binding assays as described previously.24 Nonspecific binding was determined in the presence of unlabeled CP55,940 (1 μM). The reaction was terminated by addition of 250 μL of TME buffer containing 5% BSA followed by filtration with a Brandell cell harvester through Whatman GF/C filter paper followed by washing with ice-cold TME buffer. Radioactivity was measured using liquid scintillation counting.
GTPγS Binding Assay
GTPγS binding assays were performed as described previously.24 Briefly, 7.5 μg of membranes were incubated for 60 min at 30 °C in a total volume of 200 μL of GTPγS binding assay buffer (50 mM Tris-HCl, pH 7.4, 3 mM MgCl2, 0.2 mM EGTA, and 100 mM NaCl) with unlabeled CP55,940 (at least nine different concentrations were used ranging between 100 pM and 100 μM), 0.1 nM [35S]GTPγS (1250 Ci/mmol; PerkinElmer Life Sciences, Boston, MA), 10 μM GDP (Sigma, St. Louis, MO), and 0.1% (w/v) BSA in the absence and presence of varying concentrations of the allosteric compounds as indicated. Nonspecific binding was determined with 10 μM unlabeled GTPγS (Sigma, St. Louis, MO). The reaction was terminated by rapid filtration through Whatman GF/C filters. The radioactivity trapped in the filters was determined by liquid scintillation counting.
Ligand and GTPγS Binding Data Analysis
All ligand binding assays were carried out in duplicate. Data are presented as the mean ± SE or the mean with the corresponding 95% confidence limits from at least three independent experiments. The interactions between the orthosteric radiolabeled agonist [3H]CP55,940 and the test modulators were analyzed by nonlinear regression using Prism 6.0 (Graphpad Software Inc., San Diego, CA) as previously described.24
Immunoblotting Studies
Cells expressing the CB1 receptor and siRNA targeting β-arrestin 1 or nonsilencing RNA duplex were washed twice with PBS and exposed to varying concentrations of allosteric modulators (2, 12d, or 12f) in the presence of 0.2 μM CP55,940 for 5 min. To observe the effect of the modulators on pertussis toxin (PTX)-insensitive ERK1/2 phosphorylation, cells were treated with 5 ng/mL PTX for 16 h at 37 °C prior to compound treatment. The media were aspirated, and the cells were washed with ice-cold PBS and lysed in ice-cold lysis buffer (150 mM NaCl, 1.0% IGEPAL CA-630, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris, pH 7.5) and a protease inhibitor cocktail (4-(2-aminoethyl)benzenesulfonyl fluoride (AEBSF), pepstatin A, E-64, bestatin, leupeptin, and aprotinin; Sigma, St Louis, MO). Solubilized cell extracts were centrifuged at 18500g for 15 min at 4 °C, and the supernatant was transferred to a new tube and heated at 95 °C for 3 min. Then 12 μg of total protein was resolved by 10% SDS-PAGE and transferred to polyvinylidene fluoride (PVDF) membrane. After washing with blocking reagent (Fisher Scientific, Pittsburgh, PA), the membrane was incubated for 1h at rt with the primary antibody (1:3000 phospho-p44/42 and p44/42 antibodies; Cell Signaling Technology, Danvers, MA). After washing with PBS, the membrane was incubated with antirabbit peroxidase-conjugated secondary antibody (1:5000; Cell Signaling Technology, Danvers, MA) for 60 min at rt. Immunoreactivity was visualized and quantified as reported earlier.36
Synthesis
All chemical reagents and solvents were purchased from Sigma-Aldrich Chemical Co. unless specified otherwise and used without further purification. All anhydrous reactions were performed under a static argon atmosphere in dried glassware using anhydrous solvents. Organic phases in the work up were dried over anhydrous Na2SO4 and removed by evaporation under reduced pressure. The crude compounds were purified by a Combiflash Rf chromatography system (Teledyne Technologies, Inc., Thousand Oaks, CA) unless specified otherwise. Purities of the intermediates were established by thin-layer chromatography (TLC), melting point, 1H NMR, and mass spectrometry. Analytical thin-layer chromatography (TLC) was run on precoated silica gel TLC aluminum plates (Whatman, UV254, layer thickness 250 μm), and the chromatograms were visualized under ultraviolet (UV) light. Melting points were determined on a capillary Electrothermal melting point apparatus and are uncorrected. 1H NMR spectra of intermediates were recorded on a Bruker Avance DPX-300 spectrometer operating at 300 MHz. 1H NMR spectra of the final compounds were recorded on a Bruker AV-500 spectrometer operating at 500 MHz. All NMR spectra were recorded using CDCl3 or DMSO-d6 as solvent unless otherwise stated, and chemical shifts are reported in ppm (parts per million) relative to tetramethylsilane (TMS) as an internal standard. Multiplicities are indicated as br (broadened), s (singlet), d (doublet), t (triplet), q (quartet), m (multiplet), and bs (broadened singlet), and coupling constants (J) are reported in hertz (Hz). Low resolution mass spectra were performed at the School of Chemical Sciences, University of Illinois at Urbana–Champaign. The purity of each tested compound was analyzed by combustion elemental analysis and was confirmed to be greater than 99%. (Roberson Microlit laboratories, Madison, NJ).
The Hemesberger–Knittel Reaction
The Hemesberger–Knittel reaction (steps ii and iii, Scheme 2) is postulated to proceed via a highly electrophilic singlet nitrene species, which then inserts into the phenyl ring to form the indole derivatives.31,40,41 In some circumstances, the Knoevenagel condensation (step ii, Scheme 2) requires highly excessive quantities of the reactant azidoacetate and the catalytic base (e.g., benzaldehyde:azidoacetate:methoxide = 1:10:10 molar ratio) to achieve high yields.42 We tried different stoichiometries of the reactants in the condensation of 14 with 15 and found when the molar ratio of reactants (benzaldehyde:azidoacetate:methoxide) is 1:3:3, it provided an acceptable yield ranging from 58% to 74% for the products 16a–d. When the molar ratio of reactants used was benzaldehyde:azidoacetate:methoxide = 1:10:10, the yields for 16a and 16b were increased from 59% and 58% to 93% (16a) and 69.6% (16b), respectively. Additionally, the yield of the 2-azidocinnamate from the Knoevenagel condensation also depends on the reaction temperature.43 We found that optimal yields can be obtained when the reaction was first carried out at −20 °C for 30 min and then at −5 to 0 °C for 6–19 h (depending on the benzaldehyde employed). To obtain the indole-2-carboxylates 17 via thermolytic cyclization of 16 (step iii, Scheme 2), there are many thermolysis conditions reported, which include carrying the reaction with microwave and flow chemistry facilitated thermolytic cyclization41,44,45 and employment of various catalysts to facilitate the indole ring formation.46,47 We tried various conditions including carrying the thermolytic reaction in different solvents such as regular xylene, anhydrous xylene, anhydrous toluene, and anhydrous THF heated in a pressure tube, as well as using iron(II) triflate to catalyze the reaction in THF.46 We found that carrying the reaction in dilute and freshly prepared anhydrous xylene solution (i.e., 1 g of azidocinnamate 16 in ∼100 mL of xylene, approximately 40 mM) for 30 min generally led to good yield of the products (88.6–94.6%) except for 17b (53%).
General Procedure A: Preparation of Ethyl 3-Acyl-5-chloro-1H-indole-2-carboxylates (8a–8c)
To the solution of ethyl 5-chloro-1H-indole-2-carboxylate (6; 10 mmol) in anhydrous 1,2-dichloroethane (25 mL) was added anhydrous aluminum chloride (10 mmol) powder and the selected acyl chloride liquid (7; 11.5 mmol) dropwise at room temperature. The reaction mixture was stirred and refluxed under argon atmosphere for 2–3 h. The completion of the reaction was monitored by TLC (25–33% ethyl acetate in hexane). Upon completion, the reaction mixture was poured into ice-cold water (150 mL) then treated with 4N HCl to pH 2. The mixture was extracted with ethyl acetate twice. The combined organic phase was washed with water and brine successively. The organic layer was separated and dried over anhydrous sodium sulfate. Filtration and removal of the solvent in vacuo provided the corresponding crude product, which was purified by Combiflash chromatography (0–40% of ethyl acetate in hexane) which yielded the desired products (8a–8c).
General Procedure B: Preparation of Ethyl 3-Alkyl-5-chloro-1H-indole-2-carboxylates (9a–c)
Liquid triethylsilane (1.18 mL, 7.40 mmol) was added dropwise to the solution of ethyl 3-acyl-5-chloro-1H-indole-2-carboxylate 8 (3.76 mmol) in trifluoroacetic acid (2.88 mL) at 0 °C. The reaction mixture was then gradually warmed up to room temperature and stirred for 4–12 h. The reaction is monitored by TLC (30% of ethyl acetate in hexane). Upon completion of the reaction, the mixture was poured onto ice and treated with saturated sodium carbonate aqueous solution to pH 7 and then extracted with ethyl acetate. The combined organic phase was washed with water and brine and dried over anhydrous sodium sulfate. Filtration and removal of solvent provided the crude product, which was purified by Combiflash silica gel column chromatography (0–30% of ethyl acetate in hexane), which yielded the corresponding products (9a–c).
General Procedure C: Preparation of 3-Alkyl-5-chloro-1H-indole-2-carboxylic Acids (10a–c)
To the solution of ethyl 5-chloro-3-alkyl-1H-indole-2-carboxylate 9 (1.9 mmol) in anhydrous ethanol (20 mL) was added 3 N sodium hydroxide aqueous solution (3.8 mL, 11.44 mmol). The reaction mixture was stirred and refluxed for 2 h. Upon completion of the hydrolysis monitored by TLC (30% ethyl acetate in hexane), it was cooled to room temperature and treated with 0.1 N HCl to pH 2. The white solid precipitate was filtered. The cake was washed with a minimal amount of cold water and hexane (2 mL) and then dried in a vacuum oven overnight to provide the white solid products (10a–c).
General Procedure D: Preparation of 5-Chloroindole-2-carboxamides (12a–f and 21a–d)
The solution of an appropriate amine 11 (0.82 mmol) in 1 mL of anhydrous DMF was added to the solution of 5-chloroindole-2-carboxylic acid (10 or 20; 0.68 mmol), BOP (502 mg, 1.02 mmol), and N,N-diisopropylethylamine (0.71 mL, 4.08 mmol) in anhydrous DMF (4 mL). The reaction mixture was stirred at room temperature for 4–12 h. Upon completion of the coupling reaction, which was monitored by TLC (30% ethyl acetate in hexane), the mixture was poured into cold water (40 mL) and extracted with ethyl acetate twice. The organic layer was separated and washed with water and brine and dried over anhydrous sodium sulfate. Filtration and removal of solvent in vacuo provided the crude product, which was then purified either by trituration followed by recrystallization in the solvent as specified or by flash chromatography to generate the final compounds (12a–f) or (21a–d), respectively.
Ethyl 5-Chloro-3-propionyl-1H-indole-2-carboxylate (8a)
The title compound was prepared according to the general procedure A by acylation of ethyl 5-chloro-1H-indole-2-carboxylate (6, 3.0 g, 13.41 mmol) with propionyl chloride 7a (1.17 mL, 13.41 mmol). The crude product was purified by Combiflash chromatography (0–20% ethyl acetate in hexane) to yield 1.28 g (34%) of white solid product; mp 120–122 °C. 1H NMR (300 MHz, chloroform-d): δ 9.14 (bs, 1H), 7.99 (s, 1H), 7.40–7.30 (m, 2H), 4.47 (q, J = 7.3 Hz, 2H), 3.10 (q, J = 7.5 Hz, 2H), 1.45 (t, J = 7.3 Hz, 3H), 1.29–1.21 (m, 3H). MS (EI) m/z = 278.9 (M+).
Ethyl 3-Butyryl-5-chloro-1H-indole-2-carboxylate (8b)
The title compound was prepared by acylation of ethyl 5-chloro-1H-indole-2-carboxylate 6 (3.0 g, 13.41 mmol) with butyryl chloride 7b (1.39 mL, 13.41 mmol) according to the general procedure A. The crude product was purified by Combiflash chromatography (0–20% ethyl acetate in hexane), which provided 1.70 g (43.3%) of white solid product; mp 119–120 °C. 1H NMR (300 MHz, chloroform-d): δ 9.22 (bs, 1H), 7.99 (s, 1H), 7.40–7.30 (m, 2H), 4.48 (q, J = 7.1 Hz, 2H), 3.06 (t, J = 7.4 Hz, 2H), 1.78 (h, J = 7.4 Hz, 2H), 1.45 (t, J = 7.1 Hz, 3H), 1.00 (t, J = 7.4 Hz, 3H). MS (EI) m/z = 293.2 (M+).
Ethyl 5-Chloro-3-hexanoyl-1H-indole-2-carboxylate (8c)
The title compound was prepared by acylation of ethyl 5-chloro-1H-indole-2-carboxylate 6 (3.0 g, 13.41 mmol) with hexanoyl chloride 7c (1.87 mL, 13.41 mmol) according to the general procedure A. The crude product was purified by Combiflash chromatography (0–20% ethyl acetate in hexane), which yielded 1.89 g (43.8%) of white solid product; mp 119–121 °C. 1H NMR (300 MHz, chloroform-d): δ 9.17 (bs, 1H), 7.98 (s, 1H), 7.40–7.29 (m, 2H), 4.48 (q, J = 7.1 Hz, 2H), 3.08 (t, J = 7.4 Hz, 2H), 1.75 (p, J = 7.4 Hz, 2H), 1.45 (t, J = 7.1 Hz, 3H), 1.41–1.28 (m, 4H), 0.97–0.86 (m, 3H). MS (EI) m/z = 321.1 (M+).
Ethyl 5-Chloro-3-propyl-1H-indole-2-carboxylate (9a)
The title compound was prepared according to general procedure B from ethyl 5-chloro-3-propionyl-1H-indole-2-carboxylate 8a (1.25 g, 4.47 mmol). The crude product was purified by Combiflash chromatography (0–20% ethyl acetate in hexane), which provided 925 mg (77.9%) of white solid product; mp 142–144 °C. 1H NMR (300 MHz, chloroform-d): δ 8.71 (bs, 1H), 7.66 (s, 1H), 7.34–7.24 (m, 2H), 4.43 (q, J = 7.1 Hz, 2H), 3.04 (t, J = 7.5 Hz, 2H), 1.71 (h, J = 7.5 Hz, 2H), 1.44 (t, J = 7.1 Hz, 3H), 0.99 (t, J = 7.5 Hz, 3H). MS (EI) m/z = 265.0 (M+).
Ethyl 3-Butyl-5-chloro-1H-indole-2-carboxylate (9b)
The title compound was prepared from ethyl 5-chloro-3-butyryl-1H-indole-2-carboxylate 8b (1.67 g, 5.70 mmol) according to the general procedure B. The crude product was purified by Combiflash chromatography (0–20% ethyl acetate in hexane), which provided 1.34 g (84.0%) of white solid product; mp 122–125 °C. 1H NMR (300 MHz, chloroform-d): δ 8.74 (bs, 1H), 7.66 (s, 1H), 7.35–7.24 (m, 2H), 4.43 (q, J = 7.2 Hz, 2H), 3.06 (t, J = 7.6 Hz, 2H), 1.67 (p, J = 7.6 Hz, 2H), 1.51–1.34 (m, 5H), 0.96 (t, J = 7.6 Hz, 3H). MS (EI) m/z = 279.1 (M+).
Ethyl 5-Chloro-3-hexyl-1H-indole-2-carboxylate (9c)
The title compound was prepared from ethyl 5-chloro-3-hexanoyl-1H-indole-2-carboxylate 8c (1.86 g, 5.78 mmol) according to the general procedure B. The crude product was purified by Combiflash chromatography (0–20% ethyl acetate in hexane), which provided 1.58 g (88.8%) of white solid product; mp 103–105 °C. 1H NMR (300 MHz, chloroform-d): δ 9.17 (bs, 1H), 7.98 (s, 1H), 7.40–7.29 (m, 2H), 4.48 (q, J = 7.1 Hz, 2H), 3.08 (t, J = 7.4 Hz, 2H), 1.75–1.73 (m, 2H), 1.45 (t, J = 7.1 Hz, 3H), 1.41–1.28 (m, 6H), 0.97–0.86 (m, 3H). MS (EI) m/z = 306.9 (M+).
5-Chloro-3-propyl-1H-indole-2-carboxylic Acid (10a)
The title compound was prepared by hydrolysis of ethyl 5-chloro-3-propyl-1H-indole-2-carboxylate 9a (900 mg, 3.39 mmol) in ethanolic NaOH solution according to the general procedure C, which provided 625 mg (77.6%) of white solid; mp 166–168 °C. 1H NMR (300 MHz, DMSO-d6): δ 13.03 (bs, 1H), 11.58 (s, 1H), 7.69 (s, 1H), 7.39 (d, J = 8.8 Hz, 1H), 7.21 (d, J = 8.8 Hz, 1H), 2.99 (t, J = 7.4 Hz, 2H), 1.59 (h, J = 7.4 Hz, 2H), 0.89 (t, J = 7.4 Hz, 3H). MS (EI) m/z = 237.0 (M+).
3-Butyl-5-chloro-1H-indole-2-carboxylic Acid (10b)
The title compound was prepared by hydrolysis of ethyl 5-chloro-3-butyl-1H-indole-2-carboxylate 9b (1.28 g, 4.58 mmol) in ethanolic NaOH solution according to the general procedure C to provide 1.03 g (89.3%) of white solid product; mp 158–160 °C. 1H NMR (300 MHz, chloroform-d): δ 8.80 (s, 1H), 7.69 (s, 1H), 7.42–7.26 (m, 2H), 3.13 (t, J = 7.4 Hz, 2H), 1.69 (p, J = 7.4 Hz, 2H), 1.45 (h, J = 7.4 Hz, 2H), 0.98 (t, J = 7.4 Hz, 3H). MS (EI) m/z = 250.9 (M+).
5-Chloro-3-hexyl-1H-indole-2-carboxylic Acid (10c)
The title compound was prepared by hydrolysis of ethyl 5-chloro-3-hexyl-1H-indole-2-carboxylate 9c (1.55 g, 5.04 mmol) in ethanolic NaOH solution according to the general procedure C to provide 965 mg (68.4%) of white solid product; mp 138–140 °C. 1H NMR (300 MHz, chloroform-d): δ 8.78 (s, 1H), 7.69 (s, 1H), 7.39–7.31 (m, 2H), 3.11 (t, J = 7.6 Hz, 2H), 1.70 (p, J = 7.2 Hz, 2H), 1.50–1.25 (m, 6H), 0.96–0.83 (m, 3H). MS (EI) m/z = 278.9 (M+).
5-Chloro-N-(4-(piperidin-1-yl)phenethyl)-3-propyl-1H-indole-2-carboxamide (12a)
The title compound was prepared from 5-chloro-3-propyl-1H-indole-2-carboxylic acid 10a (216 mg, 0.92 mmol) and 2-(4-piperidin-1-ylphenyl)-ethylamine 11a (224 mg, 1.1 mmol) according to the general procedure D. The crude product was purified by Combiflash chromatography (30% ethyl acetate in hexane), which yielded 142 mg (36.6%) of white solid product; mp 190–192 °C. 1H NMR (500 MHz, chloroform-d): δ 9.13 (bs, 1H), 7.53 (bs, 1H), 7.29 (d, J = 8.6 Hz, 1H), 7.20 (d, J = 8.6 Hz, 1H), 7.13 (d, J = 8.3 Hz, 2H), 6.91 (d, J = 8.3 Hz, 2H), 6.00 (s, 1H), 3.78 (q, J = 6.2 Hz, 2H), 3.12 (t, J = 5.3 Hz, 4H), 2.87 (t, J = 6.2 Hz, 2H), 2.66 (t, J = 7.6 Hz, 2H), 1.79–1.65 (m, 4H), 1.59–1.45 (m, 2H), 1.37–1.20 (m, 2H), 0.87 (t, J = 6.9 Hz, 3H). MS (EI) m/z = 423.2 (M+). Anal. Calcd for (C25H30ClN3O): C, 70.82; H, 7.13; N, 9.91. Found: C, 71.09; H, 7.24; N, 9.64.
5-Chloro-3-butyl-N-(4-piperidin-1-yl)phenethyl)-1H-indole-2-carboxamide (12b)
The title compound was prepared from 5-chloro-3-butyl-1H-indole-2-carboxylic acid 10a (200 mg, 0.79 mmol) and 2-(4-piperidin-1-ylphenyl)-ethylamine 11a (194 mg, 0.95 mmol) according to the general procedure D. The crude product was purified by Combiflash chromatography (30% ethyl acetate in hexane), which provided 111 mg (31.9%) of white solid product; mp: 218–220 °C. 1H NMR (500 MHz, chloroform-d): δ 9.19 (bs, 1H), 7.53 (bs, 1H), 7.30 (d, J = 8.6 Hz, 1H), 7.20 (d, J = 8.6 Hz, 1H), 7.12 (d, J = 8.2 Hz, 2H), 6.91 (d, J = 8.2 Hz, 2H), 6.02 (bs, 1H), 3.78 (q, J = 6.3 Hz, 2H), 3.13 (t, J = 5.4 Hz, 4H), 2.87 (t, J = 6.3 Hz, 2H), 2.69 (t, J = 7.7 Hz, 2H), 1.75–1.66 (m, 2H), 1.60–1.53 (m, 6H), 0.86 (t, J = 7.7 Hz, 3H). MS (EI) m/z = 437.2 (M+). Anal. Calcd for (C26H32ClN3O): C, 70.82; H, 7.13; N, 9.91. Found: C, 71.06; H, 7.42; N, 9.49.
5-Chloro-3-hexyl-N-(4-piperidin-1-yl)phenethyl)-1H-indole-2-carboxamide (12c)
The title compound was prepared from 5-chloro-3-hexyl-1H-indole-2-carboxylic acid 10c (200 mg, 0.71 mmol) and 2-(4-piperidin-1-ylphenyl)-ethylamine 11a (175 mg, 0.86 mmol) according to the general procedure D. The crude product was purified by Combiflash chromatography (0–30% ethyl acetate in hexane), which provided 174 mg (52.6%) of white solid product; mp 160–162 °C. 1H NMR (500 MHz, chloroform-d): δ 9.14 (s, 1H), 7.52 (s, 1H), 7.29 (d, J = 8.7 Hz, 1H), 7.20 (dd, J = 8.7 Hz, 1.8 Hz, 1H), 7.12 (d, J = 8.1 Hz, 2H), 6.71 (d, J = 8.1 Hz, 2H), 6.02 (s, 1H), 3.78 (q, J = 6.3 Hz, 2H), 2.93 (s, 6H), 2.86 (t, J = 6.3 Hz, 2H), 2.69 (t, J = 6.9 Hz, 2H), 1.44 (q, J = 6.9 Hz, 2H), 1.33–1.13 (m, 6H), 0.87 (t, J = 6.9 Hz, 3H). MS (EI) m/z = 465.2 (M+). Anal. Calcd for (C28H36ClN3O): C, 72.16; H, 7.79; N, 9.02. Found: C, 72.24; H, 7.65; N, 9.01.
5-Chloro-N-(4-(dimethylamino)phenethyl)-3-propyl-1H-indole-2-carboxamide (12d)
The title compound was prepared from 5-chloro-3-propyl-1H-indole-2-carboxylic acid 10a (200 mg, 0.84 mmol) and 4-(2-aminoethyl)-N,N-dimethyl aniline 11b (166 mg,1.01 mmol) according to the general procedure D. The crude product was purified by Combiflash chromatography (30% ethyl acetate in hexane), which yielded 51 mg (15.8%) of white solid product; mp 112–114 °C. 1H NMR (500 MHz, chloroform-d): δ 9.20 (s, 1H), 7.53 (s, 1H), 7.29 (d, J = 8.5 Hz, 1H), 7.20 (d, J = 8.5 Hz, 1H), 7.13 (d, J = 8.2 Hz, 2H), 6.73 (d, J = 8.2 Hz, 2H), 6.02 (s, 1H), 3.77 (q, J = 6.3 Hz, 2H), 2.93 (s, 6H), 2.87 (t, J = 6.3 Hz, 2H), 2.66 (t, J = 7.5 Hz, 2H), 1.49 (h, J = 7.5 Hz, 2H), 0.83 (t, J = 7.2 Hz, 3H). MS (EI) m/z = 384.5 (M+ + 1). Anal. Calcd for (C22H26ClN3O): C, 68.83; H, 6.83; N, 10.95. Found: C, 68.56; H, 6.63; N, 10.68.
3-Butyl-5-chloro-N-(4-(dimethylamino)phenethyl)-1H-indole-2-carboxamide (12e)
The title compound was prepared from 3-butyl-5-chloro-1H-indole-2-carboxylic acid 10b (200 mg, 0.79 mmol) and 4-(2-aminoethyl)-N,N-dimethyl aniline 11b (156 mg, 0.95 mmol) according to the general procedure D. The crude product was purified by Combiflash chromatography (30% ethyl acetate in hexane), which provided 80 mg (25.0%) of white solid product; mp 198–200 °C. 1H NMR (500 MHz, chloroform-d): δ 9.19 (s, 1H), 7.52 (s, 1H), 7.29 (d, J = 8.5 Hz, 1H), 7.20 (d, J = 8.5 Hz, 1H), 7.12 (d, J = 7.9 Hz, 2H), 6.72 (d, J = 8.2 Hz, 2H), 6.03 (s, 1H), 3.78 (q, J = 6.3 Hz, 2H), 2.93 (s, 6H), 2.87 (t, J = 6.3 Hz, 2H), 2.69 (t, J = 7.6 Hz, 2H), 1.43 (p, J = 7.5 Hz, 2H), 1.27–1.16 (m, 3H), 0.84 (t, J = 7.2 Hz, 3H). MS (EI) m/z = 398.5 (M+ + 1). Anal. Calcd for (C23H28ClN3O): C, 69.42; H, 7.09; N, 10.56. Found: C, 69.38; H, 7.22; N, 10.28.
5-Chloro-N-(4-(dimethylamino)phenethyl)-3-hexyl-1H-indole-2-carboxamide (12f)
The title compound was prepared from 5-chloro-3-hexyl-1H-indole-2-carboxylic acid 10c (200 mg, 0.71 mmol) and 4-(2-aminoethyl)-N,N-dimethyl aniline 11b (140 mg, 0.85 mmol) according to the general procedure D. The crude product was purified by Combiflash chromatography (30% ethyl acetate in hexane), which yielded 150 mg (49.6%) of white solid product; mp 158–160 °C. 1H NMR (500 MHz, chloroform-d): δ 9.14 (s, 1H), 7.52 (s, 1H), 7.29 (d, J = 8.7 Hz, 1H), 7.20 (d, J = 8.6 Hz, 1H), 7.12 (d, J = 8.2 Hz, 2H), 6.71 (d, J = 8.1 Hz, 2H), 6.02 (bs, 1H), 3.78 (q, J = 6.3 Hz, 2H), 2.93 (s, 6H), 2.86 (t, J = 6.6 Hz, 2H), 2.69 (t, J = 7.9 Hz, 2H), 1.50–1.40 (m, 2H), 1.33–1.13 (m, 6H), 0.87 (t, J = 6.9 Hz, 3H). MS (EI) m/z = 426.6 (M+ + 1). Anal. Calcd for (C25H32ClN3O): C, 70.49; H, 7.57; N, 9.86. Found: C, 70.38; H, 7.63; N, 9.79.
Methyl 2-Azidoacetate (14)
Methylbromoacetate 13 (40 g, 263.0 mmol) was added dropwise to the suspension of sodium azide (19.0 g, 292.3 mmol) in anhydrous DMF (72 mL) at room temperature. The reaction mixture was stirred at room temperature for 2 h. During the reaction, sodium bromide was formed which precipitated out. Water was added until the NaBr solid was dissolved and then the reaction mixture was extracted three times with diethyl ether (3 × 50 mL). The combined organic layers were washed six times with water (total 360 mL) and then separated and washed with brine and dried over magnesium sulfate. Removal of the solvent in vacuo yielded 25 g (83.3%) of colorless oil. 1H NMR (300 MHz, chloroform-d): δ 3.90 (s, 2H), 3.81 (s, 3H). The NMR result is in agreement with published data.41 Its purity matches with the commercially available methyl-2-azidoacetate (Sigma-Aldrich), therefore 14 was used without further purification.
(Z)-Methyl 2-Azido-3-(4-chlorophenyl)acrylate (16a)
To the solution of freshly prepared sodium methoxide (480 mg, 8.89 mmol) in anhydrous methanol (12 mL) cooled to −20 °C was added the solution of 4-chlorobenzaldehyde (500 mg, 3.56 mmol) in anhydrous methanol (2.5 mL) in one portion. The temperature was maintained at −20 °C, and liquid methyl 2-azidoacetate (1.02 g, 8.89 mmol) was added dropwise to the reaction mixture during 30 min. The mixture was then allowed to warm to −5–0 °C and stirred at the same temperature until reaction completion (7 h), which was monitored by TLC. Then ice–water was added to the reaction mixture, which led to the precipitation of a white solid, which was filtered off and then washed with ice water and dried in vacuum oven to give 499 mg (59%) of the product 16a. Alternatively, 16a can be prepared by condensation of 4-chlorobenzaldehyde 15a (3 g, 21.34 mmol) and methyl-2-azidoacetate 14 (24.6 g, 213.4 mmol) in similar conditions as above to afford 4.72 g (93%) of white solid product; mp 35–37 °C. 1H NMR (300 MHz, chloroform-d): δ 7.77 (d, J = 8.6 Hz, 2H), 7.37 (d, J = 8.6 Hz, 2H), 6.78 (s, 1H), 3.93 (s, 3H). MS (EI) m/z = 237.1 (M+).
(Z)-Methyl 2-Azido-3-(4-fluorophenyl)acrylate (16b)
To a freshly prepared sodium methoxide (432 mg, 8.00 mmol) in anhydrous methanol (12 mL) cooled to −20 °C was added 4-fluorobenzaldehyde 15b (500 mg, 4.03 mmol) in anhydrous methanol (2.5 mL). The temperature was maintained at −20 °C and liquid methyl 2-azidoacetate 14 (1.40 g, 12.09 mmol) was added dropwise to the reaction mixture during 30 min. The mixture was allowed to warm to −5 to 0 °C and kept at this temperature for 19 h until the reaction was complete. Then ice–water was added to the reaction mixture, which led to the precipitation of a white solid, which was filtered off and then washed with ice–water and dried in vacuum oven to give 516 mg (58%) of the product. Alternatively, 16b can be prepared by condensation of 4-fluorobenzaldehyde 15b (5 g, 40.29 mmol) and methyl-2-azidoacetate 14 (46.4 g, 402.9 mmol) in similar conditions as above to yield 6.2 g (69.6%) of 16b; mp 33–34 °C. 1H NMR (300 MHz, chloroform-d): δ 7.84 (dd, J = 8.6 Hz, 5.9 Hz, 2H), 7.09 (t, J = 8.6 Hz, 2H), 6.89 (s, 1H), 3.93 (s, 3H). MS (EI) m/z = 221.1 (M+).
(Z)-Methyl 2-Azido-3-(4-methoxyphenyl)acrylate (16c)
To a freshly prepared solution of sodium methoxide (496 mg, 9.18 mmol) in anhydrous methanol (12 mL) cooled to −20 °C was added 4-methoxybenzaldehyde 15c (500 mg, 3.67 mmol) in anhydrous methanol (2.5 mL). The temperature was maintained at −20 °C, and liquid methyl 2-azidoacetate 14 (1.06 g, 9.18 mmol) was added dropwise to the reaction mixture during 30 min. The mixture was allowed to warm to −5 to 0 °C and kept at this temperature for 6 h until the reaction was complete. Then ice–water was added to the reaction mixture, which led to the precipitation of a white solid, which was filtered off and then washed with ice-cooled water and dried in vacuum oven to give 637 mg (74%) of white solid product; mp 70–72 °C. 1H NMR (300 MHz, chloroform-d): δ 7.81 (d, J = 8.9 Hz, 2H), 6.93 (d, J = 9.4 Hz, 3H), 3.91 (s, 3H), 3.86 (s, 3H). MS (EI) m/z = 233.1 (M+). Alternatively, 16c can be prepared by condensation of 4-methoxybenzaldehyde 15c (5 g, 36.72 mmol) and methyl-2-azidoacetate 7 (21.1 g, 183.6 mmol) in the solution of sodium methoxide (322.29 mmol) in anhydrous methanol (130 mL) in similar conditions as above to yield 4.3 g (50.2%) of white solid.
(Z)-Methyl 2-Azido-3-(3-methoxyphenyl)acrylate (16d)
To a freshly prepared sodium methoxide (417 mg, 7.71 mmol) solution in anhydrous methanol (12 mL) cooled to −20 °C was added 3-methoxybenzaldehyde 15d (500 mg, 3.67 mmol) in anhydrous methanol (2.5 mL). The temperature was maintained at −20 °C, and liquid methyl 2-azidoacetate 14 (846 mg, 7.34 mmol) was added dropwise to the reaction mixture during 30 min. The mixture was allowed to warm to −10 °C and stirred at the same temperature for 5 h until the reaction was complete. Then ice-cooled water was added to the reaction mixture, which led to the precipitation of a white solid, which was filtered off and then washed with ice-cooled water and dried in a vacuum oven to give 580 mg (68%) of white solid product; mp 163–165 °C. 1H NMR (300 MHz, chloroform-d): δ 7.77 (d, J = 8.6 Hz, 2H), 7.37 (d, J = 8.6 Hz, 2H), 6.78 (s, 1H), 3.93 (s, 3H). MS (EI) m/z = 233.1 (M+).
Methyl 6-Chloro-1H-indole-2-carboxylate (17a)
The solution of (Z)-methyl 2-azido-3-(4-chlorophenyl)acrylate 16a (1.1 g, 4.63 mmol) in freshly prepared anhydrous xylene (110 mL) was bubbled through with argon flow for 3 min. The reaction mixture was refluxed for 30 min in argon atmosphere and then cooled to room temperature. The solvent was evaporated in vacuo, and the residue was purified by Combiflash chromatography (0–20% ethyl acetate in hexane) to yield 858 mg (88.6%) of a white solid product; mp 178–180 °C. 1H NMR (300 MHz, chloroform-d): δ 9.02 (bs, 1H), 7.62 (d, J = 8.6 Hz, 1H), 7.44 (s, 1H), 7.21 (s, 1H), 7.15 (d, J = 8.6 Hz, 1H), 3.92 (s, 3H). MS (EI) m/z = 209.0 (M+).
Methyl 6-Fluoro-1H-indole-2-carboxylate (17b)
The title compound was prepared from (Z)-methyl 2-azido-3-(4-fluorophenyl)acrylate 16b (500 mg, 2.26 mmol) in anhydrous xylene (60 mL) according to the procedure described for 17a. The crude product was purified by Combiflash chromatography (0–20% ethyl acetate in hexane) to yield 231 mg (53%) of white solid product; mp 149–151 °C. 1H NMR (300 MHz, chloroform-d): δ 9.01 (bs, 1H), 7.64 (dd, J = 8.6 Hz, 5.5 Hz, 1H), 7.22 (s, 1H), 7.11 (d, J = 9.3 Hz, 1H), 6.95 (t, J = 9.3 Hz, 1H), 3.96 (s, 3H). MS (EI) m/z = 193.0 (M+).
Methyl 6-Methoxy-1H-indole-2-carboxylate (17c)
The title compound was prepared from (Z)-methyl 2-azido-3-(4-methoxyphenyl)acrylate 16c (500 mg, 3.67 mmol) in anhydrous xylene (55 mL) according to the procedure described for 17a. The crude product was purified by Combiflash chromatography (0–20% ethyl acetate in hexane) to give 390 mg (87%) of a white solid product; mp 110–112 °C. 1H NMR (300 MHz, chloroform-d): δ 8.91 (bs, 1H), 7.57 (d, J = 9.5 Hz, 1H), 7.18 (s, 1H), 6.89–6.81 (m, 2H) 3.95 (s, 3H), 3.87 (s, 3H). MS (EI) m/z = 205.1 (M+).
Methyl 5-Methoxy-1H-indole-2-carboxylate (17d) and Methyl 7-Methoxy-1H-indole-2-carboxylate (17e)
The solution of (Z)-methyl 2-azido-3-(3-methoxyphenyl) acrylate 16d (2.7 g, 4.63 mmol) in freshly prepared anhydrous xylene (110 mL) was bubbled through with argon flow for 3 min. The reaction mixture was refluxed for 30 min in argon atmosphere and then cooled to room temperature. The solvent was evaporated in vacuo, and the residue was purified by Combiflash chromatography (0–20% ethyl acetate in hexane) to give 1.3 g (55%) of 17d as a white solid product and 940 mg (39.6%) of 17e as a white solid product. 17d: mp 175–177 °C. 1H NMR (300 MHz, chloroform-d) δ 8.91 (s, 1H), 7.33 (d, J = 8.9 Hz, 1H), 7.16 (s, 1H), 7.10 (s, 1H), 7.02 (d, J = 9.8 Hz, 1H), 3.96 (s, 3H), 3.87 (s, 3H). MS (EI) m/z = 205.0 (M+). The 1H NMR chemical shifts of 17d are in agreement with the published results.3217e: mp 115–117 °C. 1H NMR (300 MHz, chloroform-d): δ 9.11 (s, 1H), 7.30 (d, J = 8.3 Hz, 1H), 7.22 (bs, 1H), 7.08 (t, J = 7.9 Hz, 1H), 6.74 (d, J = 7.7 Hz, 1H), 3.99 (s, 3H), 3.96 (s, 3H). MS (EI) m/z = 205.0 (M+). The 1H NMR chemical shifts of 17e are also in agreement with the published data.33
Methyl 3-Acetyl-6-chloro-1H-indole-2-carboxylate (18a)
The title compound was prepared by acylation of methyl 6-chloro-1H-indole-2-carboxylate 17a (1.7 g, 8.11 mmol) with acetyl chloride (0.72 mL, 10.13 mmol) according to the general procedure A. The crude product was purified by Combiflash chromatography (0–20% ethyl acetate in hexane), which provided 1.2 g (58.8%) of yellow solid product; mp 220–222 °C. 1H NMR (300 MHz, chloroform-d): δ 12.61 (s, 1H), 7.94 (d, J = 8.7 Hz, 1H), 7.52 (s, 1H), 7.23 (d, J = 8.7 Hz, 1H), 3.95 (s, 2H), 2.96 (s, 3H). MS (EI) m/z = 251.1 (M+).
Methyl 3-Acetyl-6-fluoro-1H-indole-2-carboxylate (18b)
The title compound was prepared by acylation of methyl 6-fluoro-1H-indole-2-carboxylate 17b (1.5 g, 7.77 mmol) with acetyl chloride (0.72 mL, 10.09 mmol) according the general procedure A. The crude product was purified by Combiflash chromatography (0–20% ethyl acetate in hexane), which provided 846 mg (46.3%) of white solid product; mp 198–200 °C. 1H NMR (300 MHz, DMSO-d6): δ 12.54 (s, 1H), 7.95 (dd, J = 8.8 Hz, 5.7 Hz, 1H), 7.23 (d, J = 9.3 Hz, 1H), 7.09 (t, J = 9.3 Hz, 1H), 3.94 (s, 3H), 2.60 (s, 3H). MS (EI) m/z = 235.1 (M+).
Methyl 3-Acetyl-6-methoxy-1H-indole-2-carboxylate (18c)
The title compound was prepared by acylation of methyl 6-methoxy-1H-indole-2-carboxylate 17c (1.5 g, 7.31 mmol) with acetyl chloride (0.67 mL, 9.50 mmol) according to the general procedure A. The crude product was purified by Combiflash chromatography (0–20% ethyl acetate in hexane) to provide 1.05 g (58.1%) of white solid product; mp 128–130 °C. 1H NMR (300 MHz, chloroform-d): δ 11.03 (bs, 1H), 7.84 (d, J = 8.8 Hz, 1H), 7.20 (d, J = 2.3 Hz, 1H), 6.92 (d, J = 8.8 Hz, 1H), 4.04 (s, 3H), 3.95 (s, 3H), 2.74 (s, 3H). MS (EI) m/z = 247.1 (M+).
Methyl 3-Acetyl-5-methoxy-1H-indole-2-carboxylate (18d)
The title compound was prepared by acylation of methyl 5-methoxy-1H-indole-2-carboxylate 17d (1.2 g, 5.84 mmol) with acetyl chloride (0.44 mL, 6.13 mmol) according to the general procedure A. The crude product was purified by Combiflash chromatography (0–20% ethyl acetate in hexane), which provided 1.0 g (69.3%) of white solid product; mp 187–189 °C. 1H NMR (300 MHz, acetone-d6): δ 11.33 (bs, 1H), 7.58 (d, J = 2.4 Hz, 1H), 7.48 (d, J = 9.0 Hz, 1H), 7.01 (dd, J = 9.0 Hz, 2.4 Hz, 1H), 3.98 (s, 3H), 3.84 (s, 3H), 2.66 (s, 3H). MS (EI) m/z = 247.0 (M+).
Methyl 3-Ethyl-6-chloro-1H-indole-2-carboxylate (19a)
The title compound was prepared by reduction of methyl 3-acetyl-6-chloro-1H-indole-2-carboxylate 18a (500 mg, 1.99 mmol) with triethylsilane (0.57 mL, 4.96 mmol) in trifluoroacetic acid (1.52 mL) according to the general procedure B. The crude product was purified by Combiflash chromatography (0–30% ethyl acetate in hexane) to yield 460 mg (97.3%) of white solid product; mp 163–165 °C. 1H NMR (300 MHz, chloroform-d): δ 8.72 (bs, 1H), 7.62 (d, J = 8.6 Hz, 1H), 7.38 (s, 1H), 7.12 (d, J = 8.6 Hz, 1H), 3.98 (s, 3H), 3.11 (q, J = 7.5 Hz, 2H), 1.28 (t, J = 7.5 Hz, 3H). MS (EI) m/z = 237.0 (M+).
Methyl 3-Ethyl-6-fluoro-1H-indole-2-carboxylate (19b)
The title compound was prepared by reduction of methyl 3-acetyl-6-fluoro-1H-indole-2-carboxylate 18b (500 mg, 2.13 mmol) with triethylsilane (0.84 mL, 5.31 mmol) in trifluoroacetic acid (2.42 mL) according to the general procedure B. The crude product was purified by Combiflash chromatography (0–30% ethyl acetate in hexane) to yield 446 mg (94.6%) of white solid product; mp 131–133 °C. 1H NMR (300 MHz, DMSO-d6): δ 8.75 (s, 1H), 7.63 (dd, J = 8.3 Hz, 5.5 Hz, 1H), 7.05 (d, J = 9.2 Hz, 1H), 6.93 (t, J = 9.2 Hz, 1H), 3.97 (s, 3H), 3.11 (q, J = 7.5 Hz, 2H), 1.29 (t, J = 7.5 Hz, 3H). MS (EI) m/z = 221.0 (M+).
Methyl 3-Ethyl-6-methoxy-1H-indole-2-carboxylate (19c)
The title compound was prepared by reduction of methyl 3-acetyl-6-methoxy-1H-indole-2-carboxylate 18c (1.0 g, 4.04 mmol) with triethylsilane (1.29 mL, 8.08 mmol) in trifluoroacetic acid (3 mL) according to the general procedure B. The crude product was purified by Combiflash chromatography (0–30% ethyl acetate in hexane), which provided 900 mg (95.5%) of white solid product; mp 150–152 °C. 1H NMR (300 MHz, chloroform-d): δ 8.66 (s, 1H), 7.50 (d, J = 8.8 Hz, 1H), 7.19 (s, 1H), 6.91 (d, J = 8.8 Hz, 1H), 3.95 (s, 3H), 3.92 (s, 3H), 2.87 (q, J = 7.5 Hz, 2H), 1.28 (t, J = 7.5 Hz, 3H). MS (EI) m/z = 233.1 (M+).
Methyl 3-Ethyl-5-methoxy-1H-indole-2-carboxylate (19d)
The title compound was prepared by reduction of methyl 3-acetyl-5-methoxy-1H-indole-2-carboxylate 18d (600 mg, 2.43 mmol) with triethylsilane (0.95 mL, 6.06 mmol) in trifluoroacetic acid (1.86 mL) according to the general procedure B. The crude product was purified by Combiflash chromatography (0–30% ethyl acetate in hexane), which yielded 450 mg (79.4%) of white solid product; mp 125–127 °C. 1H NMR (300 MHz, chloroform-d): δ 8.66 (s, 1H), 7.27 (d, J = 8.7, 1H), 7.08 (d, J = 8.2 Hz, 1H), 7.02 (d, J = 8.5 Hz, 1H), 3.96 (s, 3H), 3.89 (s, 3H), 3.11 (q, J = 7.6 Hz, 2H), 1.28 (t, J = 7.6 Hz, 3H). MS (EI) m/z = 233.1 (M+).
3-Ethyl-6-chloro-1H-indole-2-carboxylic Acid (20a)
The title compound was prepared by hydrolysis of methyl 3-ethyl-6-chloro-1H-indole-2-carboxylate 19a (400 mg, 1.68 mmol) according to the general procedure C to yield 300 mg (79.8%) of white solid product; mp 193–195 °C. 1H NMR (300 MHz, DMSO-d6): δ 13.03 (bs, 1H), 11.50 (s, 1H), 7.67 (d, J = 8.6 Hz, 1H), 7.39 (s, 1H), 7.05 (d, J = 8.6 Hz, 1H), 3.03 (q, J = 7.4 Hz, 2H), 1.17 (t, J = 7.4 Hz, 3H). MS (EI) m/z = 223.1 (M+).
3-Ethyl-6-fluoro-1H-indole-2-carboxylic Acid (20b)
The title compound was prepared by hydrolysis of methyl 3-ethyl-6-fluoro-1H-indole-2-carboxylate 19b (400 mg, 1.81 mmol) according to the general procedure C to yield 300 mg (80.0%) of white solid product; mp 192–194 °C. 1H NMR (300 MHz, DMSO-d6): δ 12.92 (s, 1H), 11.44 (s, 1H), 7.67 (dd, J = 8.6 Hz, 5.7 Hz, 1H), 6.91 (d, J = 9.9 Hz, 1H), 6.91 (t, J = 9.3 Hz, 1H), 3.03 (q, J = 7.4 Hz, 2H), 1.17 (t, J = 7.4 Hz, 3H). MS (EI) m/z = 207.1 (M+).
3-Ethyl-6-methoxy-1H-indole-2-carboxylic Acid (20c)
The title compound was prepared by hydrolysis of methyl 3-ethyl-6-methoxy-1H-indole-2-carboxylate 19c (400 mg, 1.71 mmol) according to the general procedure C to provide 300 mg (80.0%) of white solid product; mp 193–195 °C. 1H NMR (300 MHz, chloroform-d): δ 8.71 (s, 1H), 7.54 (d, J = 8.8 Hz, 1H), 7.36 (s, 1H), 6.94 (d, J = 8.8 Hz, 1H), 3.93 (s, 3H), 2.84 (q, J = 7.5 Hz, 2H), 1.27 (t, J = 7.5 Hz, 3H). MS (EI) m/z = 219.1 (M+).
3-Ethyl-5-methoxy-1H-indole-2-carboxylic Acid (20d)
The title compound was prepared by hydrolysis of methyl 3-ethyl-5-methoxy-1H-indole-2-carboxylate 19d (389 mg, 1.68 mmol) according to the general procedure C to provide 318 mg (86.3%) of white solid product; mp 193–195 °C. 1H NMR (300 MHz, acetone-d6): δ 10.38 (s, 1H), 7.40 (d, J = 8.8 Hz, 1H), 7.15 (s, 1H), 6.96 (d, J = 8.8 Hz, 1H), 3.85 (s, 1H), 3.14 (q, J = 7.4 Hz, 2H), 1.17 (t, J = 7.4 Hz, 3H). MS (EI) m/z = 219.0 (M+).
N-(4-(Dimethylamino)phenethyl)-3-ethyl-6-chloro-1H-indole-2-carboxamide (21a)
The title compound was prepared from 3-ethyl-6-chloro-1H-indole-2-carboxylic acid 20a (200 mg, 0.89 mmol) and 4-(2-aminoethyl)-N,N-dimethylaniline 11b (176 mg, 1.07 mmol) according to the general procedure D. The crude product was purified by Combiflash chromatography (0–20% ethyl acetate in hexane), which yielded 130 mg (39.5%) of white solid product; mp 166–168 °C. 1H NMR (500 MHz, chloroform-d): δ 9.29 (bs, 1H), 7.48 (d, J = 8.6 Hz, 1H), 7.37 (d, J = 1.8 Hz, 1H), 7.16–7.12 (m, 2H), 7.06 (dd, J = 8.6 Hz, 1.8 Hz, 1H), 6.73 (d, J = 8.7 Hz, 2H), 6.00 (bs, 1H), 3.78 (q, J = 6.4 Hz, 2H), 2.93 (s, 6H), 2.88 (t, J = 6.4 Hz, 2H), 2.73 (q, J = 7.7 Hz, 2H), 1.09 (t, J = 7.7 Hz, 3H). MS (EI) m/z = 369.2 (M+). Anal. Calcd for (C21H24ClN3O): C, 68.19; H, 6.54; N, 11.36. Found: C, 68.29; H, 6.60; N, 11.26.
N-(4-(Dimethylamino)phenethyl)-3-ethyl-6-fluoro-1H-indole-2-carboxamide (21b)
The title compound was prepared from 3-ethyl-6-fluoro-1H-indole-2-carboxylic acid 20b (200 mg, 0.97 mmol) and 4-(2-aminoethyl)-N,N-dimethylaniline 11b (190 mg, 1.16 mmol) according to the general procedure D. The crude product was purified by Combiflash chromatography (0–20% ethyl acetate in hexane), which yielded 136 mg (39.7%) of white solid product; mp 160–162 °C. 1H NMR (500 MHz, chloroform-d): δ 9.20 (bs, 1H), 7.49 (dd, J = 8.8 Hz, 5.3 Hz, 1H), 7.13 (d, J = 8.5 Hz, 2H), 7.04 (dd, J = 9.5 Hz, 2.2 Hz, 1H), 6.87 (td, J = 9.1 Hz, 2.2 Hz, 1H), 6.73 (d, J = 8.7 Hz, 2H), 5.96 (bs, 1H), 3.77 (q, J = 6.6 Hz, 2H), 2.93 (s, 6H), 2.88 (t, J = 6.6 Hz, 2H), 2.74 (q, J = 7.7 Hz, 2H), 1.10 (t, J = 7.7 Hz, 3H). MS (EI) m/z = 353.2 (M+). Anal. Calcd for (C21H24FN3O): C, 71.36; H, 6.84; N, 11.89. Found: C, 71.45; H, 6.95; N, 11.62.
N-(4-(Dimethylamino)phenethyl)-3-ethyl-6-methoxy-1H-indole-2-carboxamide (21c)
The title compound was prepared from 3-ethyl-6-methoxy-1H-carboxylic acid 20c (200 mg, 0.91 mmol) and 4-(2-aminoethyl)-N,N-dimethylaniline 11b (180 mg, 1.10 mmol) according to the general procedure D. The crude product was purified by Combiflash chromatography (0–30% ethyl acetate in hexane), which provided 131 mg (39.4%) of white solid product; mp 158–161 °C. 1H NMR (500 MHz, chloroform-d): δ 8.99 (bs, 1H), 7.42 (d, J = 8.8 Hz, 1H), 7.13 (d, J = 8.7 Hz, 2H), 6.88 (d, J = 8.8 Hz, 1H), 6.75 (d, J = 8.7 Hz, 2H), 6.66 (d, J = 2.4 Hz, 1H), 6.14 (bs, 1H), 3.90 (s, 3H), 3.70 (q, J = 6.8 Hz, 2H), 2.95 (bs, 6H), 2.87–2.81 (m, 4H), 1.23 (t, J = 7.6 Hz, 3H). MS (EI) m/z = 365.3 (M+). Anal. Calcd for (C22H27N3O2·1/10H2O): C, 71.95; H, 7.46; N, 11.44. Found: C, 71.88; H, 7.63; N, 11.09.
N-(4-(Dimethylamino)phenethyl)-3-ethyl-5-methoxy-1H-indole-2-carboxamide (21d)
The title compound was prepared from 3-ethyl-5-methoxy-1H-carboxylic acid 20d (224 mg, 1.02 mmol) and 4-(2-aminoethyl)-N,N-dimethylaniline 11b (201 mg, 1.22 mmol) according to the general procedure D. The crude product was purified by Combiflash chromatography (0–30% ethyl acetate in hexane), which yielded 125 mg (33.5%) of white solid product; mp 183–185 °C. 1H NMR (500 MHz, chloroform-d): δ 9.01 (bs, 1H), 7.28 (d, J = 10.0 Hz, 1H), 7.14 (d, J = 7.3 Hz, 2H), 6.97 (s, 1H), 6.95 (d, J = 10.0 Hz, 1H), 6.74 (d, J = 7.3 Hz, 2H), 6.01 (bs, 1H), 3.86 (s, 3H), 3.87 (q, J = 6.6 Hz, 2H), 2.94 (s, 6H), 2.88 (t, J = 6.6 Hz, 2H), 2.75 (q, J = 7.6 Hz, 2H), 1.12 (t, J = 7.6 Hz, 3H). MS (EI) m/z = 365.2 (M+). Anal. Calcd for (C22H27N3O2): C, 72.30; H, 7.45; N, 11.50. Found: C, 72.04; H, 7.20; N, 11.42.
(E)- and (Z)-3-(4-(Dimethylamino)phenyl)acrylonitrile (23a and 23b)
The title compound was prepared according to a reported procedure with modifications.48 A 100 mL 3-necked round bottomed flask equipped with a pressure equalization addition funnel and a reflux condenser was charged with powdered KOH (2.26 g, 40.22 mmol) and anhydrous acetonitrile (50 mL) under argon atmosphere. The mixture was heated to reflux and the solution of 4-dimethylaminobenzaldehyde (5.0 g, 33.5 mmol) was added to 20 mL of acetonitrile in a stream. After the addition, stirring was continued for 10 min and the hot solution was poured onto cracked ice. The mixture was extracted three times with CH2Cl2 (3 × 75 mL). The organic layer was separated and washed with brine and dried over anhydrous Na2SO4. After filtration, the filtrate was condensed in vacuo in the water bath maintained at 30 °C to minimize decomposition. The crude product was purified by Combiflash chromatography (0–10% ethyl acetate in hexane), which yielded 3.15 g (54.8%) of the mixture of the E- and Z-isomers as light-yellow powder. The mixture gives an E/Z ratio of 4:1; mp 149–151 °C. 1H NMR (300 MHz, chloroform-d) Z-isomer: δ 7.76 (d, J = 8.9 Hz, 2H), 6.96 (d, J = 12.1 Hz, 1H), 6.73 (d, J = 8.9 Hz, 2H), 5.1 (d, J = 12.1 Hz, 1H), 3.05 (s, 6H). E-isomer: 7.34 (d, J = 8.8 Hz, 2H), 7.27 (d, J = 16.4 Hz, 1H), 6.67 (d, J = 8.5 Hz, 2H), 5.59 (d, J = 16.4 Hz, 1H), 3.05 (s, 6H). MS (EI) m/z = 172.1 (M+).
4-(3-Aminopropyl)-N,N-dimethylaniline (24)
The title compound was prepared according to a reported procedure with modifications.48 The 3-(4-(dimethylamino)phenyl)acrylonitrile 23 (450 mg, 2.61 mmol) was dissolved in anhydrous THF (20 mL) and added dropwise to a freshly prepared mixture suspension of AlCl3 (1.22 g, 9.144 mmol) and LiAlH4 (347 mg, 9.144 mmol) in anhydrous THF (20 mL) under argon atmosphere. The reaction mixture was stirred and refluxed for 1 h and then cooled to room temperature. Then 10 mL of water was carefully added to the reaction mixture. Then 0.5 N NaOH (100 mL) was cautiously added to the reaction mixture, which was subsequently extracted with ethyl acetate (3 × 25 mL). The organic layers were separated and combined and washed with brine and dried over sodium sulfate. Filtration and removal of solvent yielded 300 mg (64.4%) of the desired amine as viscous oil which was used directly without further purification. 1H NMR (300 MHz, DMSO-d6): δ 6.99 (d, J = 7.8 Hz, 2H), 6.65 (d, J = 7.8 Hz, 2H), 2.95–2.86 (m, 4H), 2.83 (s, 6H), 2.59–2.42 (m, 2H). MS (EI) m/z = 178.1 (M+).
5-Chloro-N-(3-(4-(dimethylamino)phenyl)propyl)-3-ethyl-1H-indole-2-carboxamide (26)
The title compound was prepared from 5-chloro-3-ethyl-1H-indole-2-carboxylic acid 25 (200 mg, 0.89 mmol) and 4-(3-aminopropyl)-N,N-dimethyl aniline 24 (192 mg, 1.08 mmol) according to the general procedure D. The crude product was purified by Combiflash chromatography (0–30% ethyl acetate in hexane), which yielded 69 mg (20.2%) of white solid product; mp 164–166 °C. 1H NMR (500 MHz, chloroform-d) δ 9.01 (s, 1H), 7.59 (s, 1H), 7.30 (d, J = 8.8 Hz, 1H), 7.22 (d, J = 8.8 Hz, 1H), 7.10 (d, J = 8.3 Hz, 2H), 6.71 (d, J = 8.3 Hz, 2H), 5.98 (bs, 1H), 3.55 (q, J = 7.3 Hz, 2H), 2.92 (s, 6H), 2.89 (q, J = 7.8 Hz, 2H), 2.67 (t, J = 7.3 Hz, 2H), 1.97 (p, J = 7.3 Hz, 2H), 1.32 (t, J = 7.8 Hz, 3H). MS (EI) m/z = 383.2 (M+). Anal. Calcd for (C22H26ClN3O): C, 68.83; H, 6.83; N, 10.95. Found: C, 68.93; H, 6.83; N, 10.68.
Acknowledgments
This work was supported in part by National Institutes of Health grant DA020763 (to D.A.K.) and Faculty Development Fund by Texas A&M Health Sciences Center (to D.L.).
Glossary
Abbreviations Used
- CB1
cannabinoid receptor type 1
- CB2
cannabinoid receptor type 2
- SAR
structure–activity relationship
- [35S]GTPγS
guanosine 5′-O-(3-[35S]thio)triphosphate
- ERK
extracellular signal-regulated kinases
- cAMP
3′-5′-cyclic adenosine monophosphate
- BOP
(benzotriazol-1-yloxy) tris (dimethylamino)phosphonium hexafluorophosphate
- DIPEA
diisopropylethyl amine
- DMF
dimethylformamide
- rt
room temperature
- HEK293
human embryonic kidney 293 cells
- BSA
bovine serum albumin
- TME
Tris-Mg2+-EDTA
- EDTA
ethylene diamine tetraacetate
- EGTA
ethylene glycol tetraacetic acid
- TLC
thin-layer chromatography
- MS
mass spectrometry
- PTX
pertussis toxin
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
References
- Howlett A. C.; Breivogel C. S.; Childers S. R.; Deadwyler S. A.; Hampson R. E.; Porrino L. J. Cannabinoid physiology and pharmacology: 30 years of progress. Neuropharmacology 2004, 47Suppl 1345–358. [DOI] [PubMed] [Google Scholar]
- Mackie K. Cannabinoid receptors: where they are and what they do. J. Neuroendocrinol. 2008, 20Suppl 110–14. [DOI] [PubMed] [Google Scholar]
- Mackie K. Signaling via CNS cannabinoid receptors. Mol. Cell. Endocrinol. 2008, 286, S60–S65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerard C. M.; Mollereau C.; Vassart G.; Parmentier M. Molecular cloning of a human cannabinoid receptor which is also expressed in testis. Biochem. J. 1991, 279, 129–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straiker A. J.; Maguire G.; Mackie K.; Lindsey J. Localization of cannabinoid CB1 receptors in the human anterior eye and retina. Invest. Ophthalmol. Vis. Sci. 1999, 40, 2442–2448. [PubMed] [Google Scholar]
- Galiegue S.; Mary S.; Marchand J.; Dussossoy D.; Carriere D.; Carayon P.; Bouaboula M.; Shire D.; Le Fur G.; Casellas P. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur. J. Biochem. 1995, 232, 54–61. [DOI] [PubMed] [Google Scholar]
- Pertwee R. G. Cannabinoid pharmacology: the first 66 years. Br. J. Pharmacol. 2006, 147Suppl 1S163–S171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie K. Cannabinoid receptors as therapeutic targets. Annu. Rev. Pharmacol. Toxicol. 2006, 46, 101–122. [DOI] [PubMed] [Google Scholar]
- Glass M.; Felder C. C. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors augments cAMP accumulation in striatal neurons: evidence for a Gs linkage to the CB1 receptor. J. Neurosci. 1997, 17, 5327–5333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauckner J. E.; Hille B.; Mackie K. The cannabinoid agonist WIN55,212-2 increases intracellular calcium via CB1 receptor coupling to Gq/11 G proteins. Proc. Natl. Acad. Sci. U. S. A. 2005, 102, 19144–19149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turu G.; Hunyady L. Signal transduction of the CB1 cannabinoid receptor. J. Mol. Endocrinol. 2010, 44, 75–85. [DOI] [PubMed] [Google Scholar]
- Howlett A. C. Cannabinoid receptor signaling. Handb. Exp. Pharmacol. 2005, 53–79. [DOI] [PubMed] [Google Scholar]
- Howlett A. C.; Blume L. C.; Dalton G. D. CB(1) cannabinoid receptors and their associated proteins. Curr. Med. Chem. 2010, 17, 1382–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T. H.; Sim-Selley L. J.; Selley D. E. Cannabinoid CB1 receptor-interacting proteins: novel targets for central nervous system drug discovery?. Br. J. Pharmacol. 2010, 160, 454–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee R. G. The pharmacology of cannabinoid receptors and their ligands: an overview. Int. J. Obes. 2006, 30Suppl 1S13–S18. [DOI] [PubMed] [Google Scholar]
- Price M. R.; Baillie G. L.; Thomas A.; Stevenson L. A.; Easson M.; Goodwin R.; McLean A.; McIntosh L.; Goodwin G.; Walker G.; Westwood P.; Marrs J.; Thomson F.; Cowley P.; Christopoulos A.; Pertwee R. G.; Ross R. A. Allosteric modulation of the cannabinoid CB1 receptor. Mol. Pharmacol. 2005, 68, 1484–1495. [DOI] [PubMed] [Google Scholar]
- Horswill J. G.; Bali U.; Shaaban S.; Keily J. F.; Jeevaratnam P.; Babbs A. J.; Reynet C.; Wong Kai In P. PSNCBAM-1, a novel allosteric antagonist at cannabinoid CB1 receptors with hypophagic effects in rats. Br. J. Pharmacol. 2007, 152, 805–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro H. A.; Howard J. L.; Pollard G. T.; Carroll F. I. Positive allosteric modulation of the human cannabinoid (CB) receptor by RTI-371, a selective inhibitor of the dopamine transporter. Br. J. Pharmacol. 2009, 156, 1178–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamplona F. A.; Ferreira J.; Menezes de Lima O. Jr.; Duarte F. S.; Bento A. F.; Forner S.; Villarinho J. G.; Bellochio L.; Wotjak C. T.; Lerner R.; Monory K.; Lutz B.; Canetti C.; Matias I.; Calixto J. B.; Marsicano G.; Guimaraes M. Z.; Takahashi R. N. Anti-inflammatory lipoxin A4 is an endogenous allosteric enhancer of CB1 cannabinoid receptor. Proc. Natl. Acad. Sci. U. S. A. 2012, 109, 21134–21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. I.; Lewis R. J. Emerging opportunities for allosteric modulation of G-protein coupled receptors. Biochem. Pharmacol. 2013, 85, 153–162. [DOI] [PubMed] [Google Scholar]
- Wootten D.; Christopoulos A.; Sexton P. M. Emerging paradigms in GPCR allostery: implications for drug discovery. Nature Rev. Drug Discovery 2013, 12, 630–644. [DOI] [PubMed] [Google Scholar]
- Gao Z. G.; Jacobson K. A. Allosteric modulation and functional selectivity of G protein-coupled receptors. Drug Discovery Today Technol. 2013, 10, e237–e243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopoulos A.; Kenakin T. G protein-coupled receptor allosterism and complexing. Pharmacol. Rev. 2002, 54, 323–374. [DOI] [PubMed] [Google Scholar]
- Ahn K. H.; Mahmoud M. M.; Kendall D. A. Allosteric modulator ORG27569 induces CB1 cannabinoid receptor high affinity agonist binding state, receptor internalization, and Gi protein-independent ERK1/2 kinase activation. J. Biol. Chem. 2012, 287, 12070–12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn K. H.; Mahmoud M. M.; Samala S.; Lu D.; Kendall D. A.. Profiling two indole-2-carboxamides for allosteric modulation of the CB1 receptor. J. Neurochem. 2013, 124, 584–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baillie G. L.; Horswill J. G.; Anavi-Goffer S.; Reggio P. H.; Bolognini D.; Abood M. E.; McAllister S.; Strange P. G.; Stephens G. J.; Pertwee R. G.; Ross R. A. CB(1) receptor allosteric modulators display both agonist and signaling pathway specificity. Mol. Pharmacol. 2013, 83, 322–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piscitelli F.; Ligresti A.; La Regina G.; Coluccia A.; Morera L.; Allara M.; Novellino E.; Di Marzo V.; Silvestri R. Indole-2-carboxamides as allosteric modulators of the cannabinoid CB(1) receptor. J. Med. Chem. 2012, 55, 5627–5631. [DOI] [PubMed] [Google Scholar]
- Mahmoud M. M.; Ali H. I.; Ahn K. H.; Damaraju A.; Samala S.; Pulipati V. K.; Kolluru S.; Kendall D. A.; Lu D. Structure–activity relationship study of indole-2-carboxamides identifies a potent allosteric modulator for the cannabinoid receptor 1 (CB1). J. Med. Chem. 2013, 56, 7965–7975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May L. T.; Leach K.; Sexton P. M.; Christopoulos A. Allosteric modulation of G protein-coupled receptors. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 1–51. [DOI] [PubMed] [Google Scholar]
- Christopoulos A.; May L. T.; Avlani V. A.; Sexton P. M. G-Protein-coupled receptor allosterism: the promise and the problem(s). Biochem. Soc. Trans. 2004, 32, 873–877. [DOI] [PubMed] [Google Scholar]
- Hemetsberger H.; Knittel D. Synthese und Thermolyse von α-Azidoacrylestern. Monatsh. Chem. 1972, 103, 194–204. [Google Scholar]
- Yamazaki K.; Nakamura Y.; Kondo Y. Solid phase synthesis of indolecarboxylate using palladium-catalyzed reactions. J. Org. Chem. 2003, 68, 6011–6019. [DOI] [PubMed] [Google Scholar]
- Vieira T. O.; Meaney L. A.; Shi Y. L.; Alper H. Tandem palladium-catalyzed N,C-coupling/carbonylation sequence for the synthesis of 2-carboxyindoles. Org. Lett. 2008, 10, 4899–4901. [DOI] [PubMed] [Google Scholar]
- Christopoulos A.; Sorman J. L.; Mitchelson F.; El-Fakahany E. E. Characterization of the subtype selectivity of the allosteric modulator heptane-1,7-bis-(dimethyl-3′-phthalimidopropyl) ammonium bromide (C7/3-phth) at cloned muscarinic acetylcholine receptors. Biochem. Pharmacol. 1999, 57, 171–179. [DOI] [PubMed] [Google Scholar]
- May L. T.; Lin Y.; Sexton P. M.; Christopoulos A. Regulation of M2 muscarinic acetylcholine receptor expression and signaling by prolonged exposure to allosteric modulators. J. Pharmacol. Exp. Ther. 2005, 312, 382–390. [DOI] [PubMed] [Google Scholar]
- Ahn K. H.; Mahmoud M. M.; Shim J. Y.; Kendall D. A. Distinct roles of beta-arrestin 1 and beta-arrestin 2 in ORG27569-induced biased signaling and internalization of the cannabinoid receptor 1 (CB1). J. Biol. Chem. 2013, 288, 9790–9800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.; Horswill J. G.; Whalley B. J.; Stephens G. J. Effects of the allosteric antagonist 1-(4-chlorophenyl)-3-[3-(6-pyrrolidin-1-ylpyridin-2-yl)phenyl]urea (PSNCBAM-1) on CB1 receptor modulation in the cerebellum. Mol. Pharmacol. 2011, 79, 758–767. [DOI] [PubMed] [Google Scholar]
- Kenakin T.; Miller L. J. Seven transmembrane receptors as shapeshifting proteins: the impact of allosteric modulation and functional selectivity on new drug discovery. Pharmacol. Rev. 2010, 62, 265–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.; Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol. Cell. Biol. 1987, 7, 2745–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemetsberger H.; Knittel D.; Weldmann H. Synthese von α-Azidozimtsaureestern. Monatsh. Chem. 1969, 100, 1599–1603. [Google Scholar]
- O’Brien A. G.; Lévesque F.; Seeberger P. H. Continuous flow thermolysis of azidoacrylates for the synthesis of heterocycles and pharmaceutical intermediates. Chem. Commun. 2011, 47, 2688–2690. [DOI] [PubMed] [Google Scholar]
- Condie G. C.; Channon M. F.; Ivory A. I.; Kumar N.; Black D. S. Regioselective reactivity of some 5,7-dimethoxylindoles. Tetrahedron 2005, 61, 4989–5004. [Google Scholar]
- Murakami Y.; Watanabe T.; Suzuki H.; Kotake N.; Takahashi T.; Toyonari K.; Ohno M.; Takase K.; Suzuki T.; Kondo K. New findings on the Hemetsberger–Knittel reaction (synthetic studies on indoles and related compounds. XLIII). Chem. Pharm. Bull. 1997, 45, 1739–1744. [Google Scholar]
- Ranasinghe N.; Jones G. B. Extending the versatility of the Hemetsberger–Knittel indole synthesis through microwave and flow chemistry. Bioorg. Med. Chem. Lett. 2013, 23, 1740–1742. [DOI] [PubMed] [Google Scholar]
- Lehmanna F.; Holma M.; Laufer S. Rapid and easy access to indoles via microwave-assisted Hemetsberger–Knittel synthesis. Tetrahedron Lett. 2009, 50, 1708–1709. [Google Scholar]
- Bonnamour J.; Bolm C. Iron(II) Triflate as a catalyst for the Synthesis of Indole by Intramolecular C–H animation. Org. Lett. 2011, 13, 2012–2014. [DOI] [PubMed] [Google Scholar]
- Stokes B. J.; Dong H.; Leslie B. E.; Pumphrey A. L.; Driver T. G. Intramolecular C–H amination reactions: exploitation of the Rh(2)(II)-catalyzed decomposition of azidoacrylates. J. Am. Chem. Soc. 2007, 129, 7500–7501. [DOI] [PubMed] [Google Scholar]
- Vermeulen E. S.; van Smeden M.; Schmidt A. W.; Sprouse J. S.; Wikstrom H. V.; Grol C. J. Novel 5-HT7 receptor inverse agonists. Synthesis and molecular modeling of arylpiperazine- and 1,2,3,4-tetrahydroisoquinoline-based arylsulfonamides. J. Med. Chem. 2004, 47, 5451–5466. [DOI] [PubMed] [Google Scholar]