Summary
One decade has passed since seminal publications described macrophage infiltration into adipose tissue (AT) as a key contributor to inflammation and obesity-related insulin resistance. Currently, a PubMed search for ‘adipose tissue inflammation’ reveals over 3500 entries since these original reports. We now know that resident macrophages in lean AT are alternatively activated, M2-like, and play a role in AT homeostasis. In contrast, the macrophages in obese AT are dramatically increased in number and are predominantly classically activated, M1-like, and promote inflammation and insulin resistance. Mediators of AT macrophage (ATM) phenotype include adipokines and fatty acids secreted from adipocytes as well as cytokines secreted from other immune cells in AT. There are several mechanisms that could explain the large increase in ATMs in obesity. These include recruitment-dependent mechanisms such as adipocyte death, chemokine release, and lipolysis of fatty acids. Newer evidence also points to recruitment-independent mechanisms such as impaired apoptosis, increased proliferation, and decreased egress. Although less is known about the homeostatic function of M2-like resident ATMs, recent evidence suggests roles in AT expansion, thermoregulation, antigen presentation, and iron homeostasis. The field of immunometabolism has come a long way in the past decade, and many exciting new discoveries are bound to be made in the coming years that will expand our understanding of how AT stands at the junction of immune and metabolic co-regulation.
Keywords: adipose tissue, inflammation, macrophages, insulin resistance
Historical perspective on adipose tissue inflammation
An association between the immune system and metabolism had been appreciated clinically for many decades; however, the impact of immune cell-secreted inflammatory cytokines on adipocyte function was not studied in detail until the mid-1980s. These initial studies showed that endotoxin-treated macrophages secrete products that can promote lipolysis in adipocytes (1) and that a macrophage-secreted factor, cachectin, has the metabolic effect of inducing cachexia (2). Simultaneously, tumor necrosis factor-α (TNF-α) was being studied for its cytotoxic, anti-tumorigenic, and inflammatory properties. It was soon discovered that cachectin and TNF-α are the same protein, which has since been referred to as TNF-α (3, 4). This marks the beginning of our understanding of the effects of inflammatory factors secreted by macrophages on metabolic processes. The mechanism underlying this new concept lies at the intersection of inflammatory and insulin signaling pathways (reviewed in 5, 6). In the mid-1990s, it was discovered that inflammatory mediators, including TNF-α, IL-6, iNOS, and CCL2, are elevated in obese compared to lean AT (7-10). Furthermore, it was discovered that genetic deficiency of TNF-α (11, 12), iNOS (9), and JNK1 (13) improve systemic insulin sensitivity in obese models. These studies gave insight into the possible role of adipose tissue (AT) inflammation in metabolic homeostasis.
Macrophages are the inflammatory source in adipose tissue
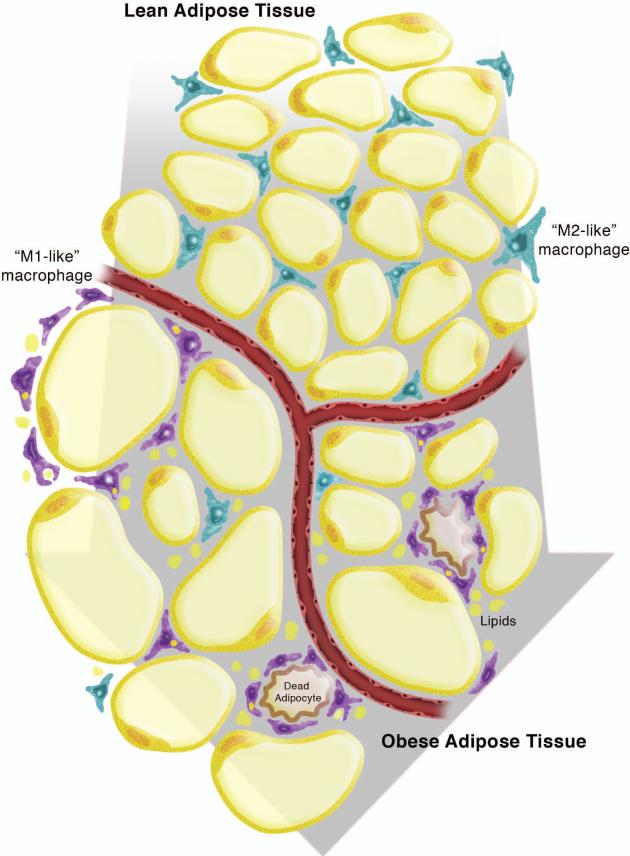
Despite the growing body of evidence linking inflammation and metabolism, the cellular sources of inflammatory mediators in AT were unknown. Localization of macrophages to AT had been mentioned by several groups (14-17); however, the functional contribution of AT macrophages (ATMs) to obesity-related metabolic diseases remained unappreciated. In 2003, two seminal manuscripts were published by Weisberg et al. (18) and Xu et al. (19). These groups used microarray analysis to establish differences in gene expression between AT from lean and obese mice. They found many differences in genes related to macrophages including surface markers and secreted products. Separation of AT into its two primary components, the stromal vascular fraction (SVF) and adipocytes, showed that canonical macrophage inflammatory genes are most highly expressed in the SVF of obese AT. Flow cytometry and immunohistochemical analyses confirmed the increased presence of macrophages in AT of obese mice. Importantly, this increase in ATMs occurs independently of the etiology of obesity: monogenetic forms of obesity and diet-induced obesity both result in increased ATMs (18, 19). Notably, this dramatic accumulation of macrophages was not found in liver, muscle, lung, or spleen (19). In addition, human subjects displayed a similar elevation in macrophages in obese compared to lean individuals (18). Thus, these two groups unequivocally demonstrated that macrophage number and inflammatory potential increase in AT in obesity (Fig. 1). While this review article focuses on macrophages, it should be noted that nearly every type of immune cell is present in AT, with their phenotypes and proportions changing in obesity (reviewed in 20-23).
Fig. 1. Macrophages in lean and obese adipose tissue.
As adipose tissue (AT) transitions from a lean to an obese state, the adipocytes undergo hypertrophy and macrophages numbers increase. The resident macrophages in lean AT are M2-like, interstitially spaced, and likely play key roles in AT homeostasis. The macrophages in obese AT are M1-like, are localized to crown-like structures, and secrete pro-inflammatory cytokines. This leads to local and systemic insulin resistance.
Relevance of ATMs to metabolic disease
As Dixit points out in his commentary (24), 1 g of AT can contain up to 5 million stromal vascular cells, greater than 50% of which are leukocytes. Thus, even in lean individuals, AT cannot be excluded as a major contributor to systemic immune regulation – including immunometabolism. The newly discovered increase in pro-inflammatory macrophages has significant implications for insulin resistance and metabolic disease associated with obesity. In fact, Xu et al. (19) showed that the increased AT inflammatory response in obesity preceded rises in plasma insulin, an indication of insulin resistance. Macrophages can impact AT function (i) by inducing adipocyte insulin resistance leading to dysregulation of basal lipolysis and ectopic lipid storage, (ii) by inducing adipocyte chemokine and cytokine production, or (iii) by impacting AT expansion capacity during obesity. Thus, continued exploration of ATM function in lean and obese conditions enables a better understanding of how AT impacts systemic insulin action and glucose metabolism. Obesity-related accumulation of ATMs in humans is less robust than in mice, but it has been clearly demonstrated by multiple groups (25-28). The majority of human ATMs accumulate in omental rather than subcutaneous depots (27-29). Importantly, omental ATMs have been shown to correlate positively with fasting glucose and insulin levels, suggesting a link between AT inflammation and metabolic disease (27, 30). In addition, it has been demonstrated that weight loss decreases macrophage content in omental AT, improving glucose homeostasis (29). Thus, the preponderance of current literature supports a role for ATMs in metabolic homeostasis in rodents and humans.
AT macrophage heterogeneity
It was noted in publications by Weisberg and Xu (18, 19) that lean AT also contains macrophages, albeit at lower levels than obese AT. Thus, researchers became interested in the phenotypic differences between ATMs in lean and obese AT. Although macrophage phenotypes span a continuum and no single system of nomenclature can provide all of the required definitions, investigators have gravitated to identifying ATMs as either M1-like or M2-like. Regardless of the nomenclature used, pioneering investigators in the AT field have pursued the notion that resident M2-like ATMs have a role in AT homeostasis, while recruited M1-like macrophages contribute to inflammation and insulin resistance. For the purpose of this review, we utilize the M1/M2 nomenclature with the acknowledgement that a multitude of ATM phenotypes exist.
M1 and M2 categorization
M1 or ‘classically activated’ macrophages are produced upon exposure to T-helper 1 (Th1) type cytokines or inflammatory mediators such as IFNγ and LPS. Thereafter, they generate reactive oxygen species and release inflammatory cytokines such as TNF-α or IL-6. M2 or ‘alternatively activated’ macrophages are produced upon exposure to Th2 cytokines such as IL-4 and IL-13 and express factors including IL-10 and arginase (31, 32). Macrophage fuel utilization varies by polarization as M1 macrophages primarily utilize glucose, whereas M2 macrophages utilize fatty acids (33, 34). Throughout the body, M1 macrophages are involved in inflammatory processes (such as combating infectious agents), while M2 macrophages play a role in immunosuppressive activities (such as tissue repair). Polarization of macrophages in AT is thought to confer similar properties. For example resident M2 macrophages likely contribute to AT homeostasis, while M1 macrophages in obese AT likely promote inflammation leading to insulin resistance. These properties are discussed in detail below. Although there is no standard set of markers to identify M1 versus M2 ATMs, we have compiled a list of markers most encountered in the literature (Table 1).
Table 1.
Markers used to define M1 vs. M2 macrophages in AT.
| M1 | M2 | Ref | Notes |
|---|---|---|---|
| Mouse | |||
| Nos2, Itgax, Il6, | Il10, Arg1, Ym1, Mrc2, Clec10a, Mgl2 | (35) | F4/80+CD11b+CD11c+ (FBC) cells were identified as the recruited cells in obese AT |
| Nos2, Il1b, Ccl3, Ccl4, CCR2, TLR4, CD11c | Il10, Arg1, Pgc1b, MGL-1, IL-10 | (39) | M2 macrophages were not repolarized to an M1 phenotype in obesity. |
| CD11c, Il12, Il1β, Nos2, Mcp1, CD86 | MGL-1, Arg1, Il13, Il10, Cd163, Cd206, Il1ra, Stat6, Mmp12, Adam8, Vegf, Clec-7a, Ym1 | (40) | M1 and M2 macrophage markers were elevated after high fat feeding |
| Nos2 | Arg1, Mrc1, Clec7a | (43) | PPARγ deficiency reduced macrophage numbers in AT and M2-related genes expression |
| Itgax, Il18, Il6, Saa3 | Arg1, Il10, Msr1, Clec7a, sIL1ra | (47) | PPARγ activation via rosiglitazone treatment induced the expression of several M2-related genes but did not alter traditional M1-related genes. |
| CD11c | CD209a | (48) | M1 macrophages from obese, ob/ob, mice have increased lipid accumulation. |
| Tnfa, Ccl2, Il6 | Clec10a, Mgl2, IL4r, IL13ra1, Mrc2, Il10 | (49) | Adipocyte derived IL-13 and IL-4 promote M2 polarization or ATMs |
| TNF-α, CCL2, Tnfa, Ccl2, Il6, Nos2 | Arg-1, CD206, Arg1, Clec10a, Il10, Chi3l3 | (51) | Adiponectin promoted an M2 phenotype in macrophages |
| CCL2, TNF-α | CD206, CD163 | (52) | M1 and M2 macrophage markers were elevated after high fat feeding. |
| Arg1, Chi3l3, Pdcd1lg2, Arg-1 | (54) | NKT cells promote M2 polarization via IL-4 and STAT6 signaling | |
| Arginase via the use of reporter mice (YARG) | (55) | Eosinophils produce IL-4 and promote M2 polarization of ATMs | |
| Ccl2, Tnfa, Il1rn | (62) | Dietary fish oil reduces inflammatory gene expression in AT | |
| CD11c, Il6 | MGL-1 | (66) | Treatment of obese, db/db, mice with resolvin D1 reduced CLS formation and increased M2 ATMs |
| Nos2, Tnfa, Ccl2 | MGL-1, Mrc1, Clec10a, Mgl2, Arg1, Cd163 | (76) | TLR4 deficiency promoted an M2-like phenotype in peritoneal and AT macrophages |
| Itgax, Nos2, Ccl2, Tnfa | Mgl2, Argl, Mrc2, Chi3l3 | (79) | Storage of lipid as TG in ATMs protects from inflammation |
| Tnfa, Ccl20, Cxcl11, Nos2, Ifng | IL10, Arg1 | (92) | Nlrp3 deficiency increased expression of M2 markers and decreased expression of M1 markers |
| Itgax, Il1b, Il6, Nos, Tnfa | Arg1, IL10, Clec10a, Mgl2, Mrc1, Mrc2 | (99) | JNK1/2 deficiency resulted in decreased M1 and increased M2 gene expression |
| Itgax, Ccl2, Tnfa | Clec10a, Il10 | (110) | Treatment of HFD-fed mice with a CCR2 antagonist reduced total ATMs and promotes an M2 phenotype |
| Tnfa, Nos2 | Arg1, Clec10a, Mgl2 | (115) | Macrophage leptin receptor deficiency did not change ATM number or polarization |
| Human | |||
| Il1ra, Mcp1, Tnfa | IL-10 | (25) | ATMs expressed M1 and M2 markers in subcutaneous AT after surgical weight loss |
| Il8, Cox2 | CD206, Il10, Tgfb, Ccl18 | (41) | ATMs in lean AT displayed a mixed phenotype while those in obese AT displayed a remodeling phenotype |
| TNF-α, IL-6, IL-1, MCP-1, MIP-1α | CD206, CD163, integrin ανβ5, IL-10, IL1Ra | (42) | M2-like CD206+ cells were capable of secreting inflammatory cytokines |
| CD11c, TLR4, CCR2 | CD206 | (30) | M1-like ATMs are associated with insulin resistance in humans |
| Il10, IL1Ra | (136) | Weight loss induced by very low calorie diet led to increased anti-inflammatory gene expression from stromal vascular cells |
Flow cytometric and immunohistochemical analysis of proteins are displayed in capitalized letters. Gene expression analysis of mRNA transcripts and are displayed in italicized text.
FBs and FBCs categorization
Another system of nomenclature for identifying resident versus recruited ATMs is based on their expression of F4/80, CD11b, and CD11c. Lumeng and colleagues (35) observed that macrophages in lean AT primarily express F4/80 and CD11b, while those in obese AT also expressed CD11c. Thus, although CD11c is traditionally used as a dendritic cell marker, in the context of AT inflammation, it designates the pro-inflammatory ATMs recruited in obesity (35, 36). The resident F4/80+CD11b+ macrophages are termed ‘FBs’ and the recruited F4/80+CD11b+CD11c+ triple positive cells are termed ‘FBCs’. Infiltration of FBCs into AT has been shown to occur as early as 1 week after high fat diet (HFD) feeding (36). Furthermore, Patsouris and colleagues (37) have demonstrated that ablation of FBCs by genetic deletion of CD11c attenuates many of the negative metabolic effects of obesity in mice. Interestingly, after FBC ablation, the FB population is maintained and contributes to increased anti-inflammatory cytokine expression. The FB/FBC nomenclature is methodologically favorable because these specific macrophage subtypes can be distinguished and isolated by FACS. However, for the purpose of this review, we maintain the more commonly accepted M1/M2 nomenclature, which aligns with FBC/FB, respectively.
M1 versus M2 ATM localization and plasticity
Localization of macrophages within AT differs in lean and obese mice, demonstrating yet another difference in ATM subpopulations. While the resident macrophages in lean AT are interstitially spaced, Cinti et al. (38) demonstrated that a preponderance of all macrophages in obese AT are localized in clusters referred to as “crown-like structures (CLSs)” – a term that is now commonly used in the Immunometabolism field. Lumeng and colleagues made the novel observation that ATMs within CLSs express M1 markers such as CCR2 and TLR4, while interstitially spaced ATMs express M2 markers such as Mgl1 and IL-10 (39). Using PKH26 labeling studies, they showed that recruited macrophages primarily localize to CLSs.
Although obesity induces a dramatic increase in M1-like ATMs, M2 macrophages increase in absolute number as well, even if their proportion compared to M1 ATMs decreases (40). Not all of the macrophages within the CLSs are M1 macrophages; and in fact, M2 ATMs are retained in obesity and can be found interstitially spaced and in CLSs (35, 39). In addition, CD11c+ ATMs in obese mice express varying levels of Mgl1, indicating a broad range of phenotypes in AT (40). During AT expansion in obesity, the M2 macrophages may be needed to clear dead adipocytes (38), remodel matrix proteins, and promote angiogenesis. Thus, even in obesity, M2 macrophages may have a unique function with regards to AT homeostasis.
Macrophage polarization in human AT
Many different groups have recapitulated the findings of increased M1 macrophages in obese mouse AT; however, the relevance of macrophage phenotype in humans is somewhat less clear. Early studies of human AT focused on macrophages from subcutaneous depots where Bouloumié and colleagues (41) found mixed expression of both pro- and anti-inflammatory genes. For example, ATMs from obese individuals express high levels of CD206, considered an M2 marker in mice, and low levels of CCR2, an M1 marker in mice. In addition, although iNOS and arginase are common markers of mouse M1/M2 polarization, they are poorly expressed in the human subcutaneous ATMs. Zeyda et al. (42) demonstrated that ATMs from humans display surface markers of M2-like cells and are capable of secreting both pro- and anti-inflammatory cytokines. Wentworth et al. (30) reported that a subset of the CLSs in human subcutaneous and omental fat contain ATMs that are double positive for both M1 marker CD11c and M2 marker CD206; however, their conclusions support the concept that M1 ATMs are associated with insulin resistance in human obesity. Thus, the distinction between M1 and M2 macrophages in human AT are not as well defined as in mouse AT.
Mediators of M2 polarization
M2 macrophages are thought to promote AT homeostasis and to protect against insulin resistance. Through their efforts to elucidate the origin of M2 macrophages in AT, investigators have defined multiple AT-specific mediators of M2 polarization, including transcription factors, adipokines, fatty acids, and other immune cells.
Transcription factors
Peroxisome proliferator-activated receptor γ (PPARγ) has been shown to be one of the primary activators of M2 polarization. The role of PPARγ in driving the M2 phenotype has been studied in various macrophage populations such as blood monocytes, Kupffer cells, and arterial macrophages. In general, these studies have demonstrated that PPARγ is required for efficient alternative activation of macrophages (43) and that PPARγ deficiency results in increased inflammatory potential and decreased M2-like polarization (44, 45). These findings were further refined by Nagy and colleagues (46), who showed that PPARγ is facilitated by IL-4 leading to STAT6 activation, which acts to modulate the number of genes regulated by PPARγ and the degree to which they respond. Their data indicate that responsiveness of M2-like genes to upregulation by PPARγ is highly dependent upon stimulation with IL-4. In contrast, pharmacological activation of PPARγ with rosiglitazone induces the accumulation of M2-like macrophages even in the context of HFD feeding (47) and in ob/ob mice (48). PPARδ has also been shown to play a role in M2 polarization of ATMs (49, 50). A detailed description of transcriptional regulation of M1 and M2 macrophages can be found in a review article by Olefsky and Glass (6).
Adipokines
Adipokines locally secreted from adipocytes in the AT can directly alter macrophage polarization. Adiponectin is an insulin sensitizing adipokine produced by adipocytes in concentrations that are inversely correlated with the degree of adiposity. Walsh and colleagues showed that exposure to recombinant adiponectin resulted in an increase in M2-like phenotype in human and mouse macrophages (51). In fact, Arg1 expression in macrophages treated with adiponectin is elevated to the same degree as in cells treated with IL-4, and the effect of adiponectin and IL-4 co-stimulation on Arg1 is additive. Furthermore, the SVF from adiponectin knockout mice has increased M1 markers and decreased M2 markers. Other groups have also demonstrated a role for adiponectin in macrophage polarization, specifically through a PPARα/STAT6-mediated mechanism (52, 53).
IL-4/IL-13 from adipocytes and other immune cells
Adipose tissue contains many cells capable of producing M2-polarizing cytokines, including adipocytes and other immune cells. Studies by Lee and colleagues demonstrated that adipocytes produce IL-4 and IL-13 that promote an M2 phenotype by inducing the expression of PPARδ (49). With regards to other immune cells, Qi and colleagues demonstrated that natural killer T cells can enhance M2 polarization via IL-4/STAT6 signaling (54). Finally, Wu et al. (55) used IL-4 reporter mice to show that eosinophils are the primary IL-4 expressing cell in AT, thereby playing a role in driving M2 differentiation.
Unsaturated fatty acids
Macrophages in AT are exposed to various types of fatty acids released from adipocytes in both basal and demand lipolysis. The degree of fatty acid saturation greatly impacts macrophage polarization: saturated fatty acids (SFAs) induce an M1-like phenotype while unsaturated fatty acids (UFAs) induce an M2-like phenotype. For example, we have shown that macrophages treated with the UFA oleic acid express increased levels of the M2-markers Clec10a and Cd163 (56). Even more effective at promoting M2 polarization are very long chain omega-3 polyunsaturated fatty acids (PUFAs). The fish oil-derived eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) strongly promote the alternative activation of macrophages. In vitro treatment of macrophages with EPA and DHA results in an M2-like phenotype, exhibiting reduced pro-inflammatory gene expression and increased anti-inflammatory gene expression (57-59). We (60, 61) and others have shown that supplementation of mice with dietary fish oils reduced AT expression of pro-inflammatory genes (62) while increasing adiponectin expression (63, 64).
Beyond direct effects of EPA and DHA, some of their metabolic products have also been shown to promote M2-like polarization. For example, dietary fish oil reduces tissue levels of the pro-inflammatory arachidonic acid metabolite F2-isoprostane and increases tissue levels of the anti-inflammatory EPA and DHA metabolites F3- and F4-isoprostanes (60). It is possible that these EPA and DHA metabolites contributed to the reduced AT inflammation associated with fish oil supplementation. In addition to F3- and F4- isoprostanes, omega-3 PUFAs are metabolized into bioactive lipids such as resolvins, protectins, and maresins [reviewed in (65)]. Spite and colleagues showed that treatment of obese leptin receptor deficient mice with resolvin D1 results in an increase in MGL-1+CD11c− ATMs, implicating that resolvin D1 promotes M2 polarization (66).
Mediators of M1 polarization
Reports from both Weisberg et al. (18) and Xu et al. (19) describe the inflammatory nature of macrophages in obese AT. Based upon the knowledge that inflammatory cytokines can induce insulin resistance in multiple cell types – including adipocytes – subsequent studies focused on blocking inflammatory pathways to ameliorate AT insulin resistance. Extracellular signals that can induce inflammatory signaling pathways include cytokines, lipids, and other molecules that activate pattern recognition receptors in ATMs. With regards to intracellular signaling pathways, the TLR4, NF-κB, and JNK pathways as well as the NLRP3 inflammasome have all been areas of relevance to ATMs, specifically M1 polarization.
Lipid-mediated activation of ATMs
ATMs are exposed to excess lipids via at least three different routes: delivery from dietary chylomicrons, basal and demand lipolysis from adipocytes, and adipocyte cell death. Subsequent to dietary fat ingestion, chylomicrons and chylomicron remnants are routed to AT where lipoprotein lipase facilitates release of fatty acids for uptake and storage in adipocytes. Fatty acids from very low density lipoproteins (VLDLs) can also be delivered to AT for storage. Our laboratory reasoned that in obesity, hyperlipidemia could result in increased exposure of ATMs to lipolyzed fatty acids, thereby promoting inflammation in a paradigm similar to what is known for arterial macrophages in atherosclerotic lesions. In support of this, we have shown that exposure of macrophages to VLDL (67) and SFAs (68) induces secretion of pro-inflammatory cytokines, typical of an M1-like phenotype. We therefore sought to test the hypothesis that delivery of excess lipids to the AT in a hyperlipidemic state would increase ATM numbers and inflammatory potential. We crossed the obesity-prone agouti yellow mice (Ay/a) with hyperlipidemia-prone low density lipoprotein receptor deficient mice to develop an obese hyperlipidemic model (69). Despite the extreme hyperlipidemia, macrophage accumulation in AT and inflammatory status was related only to the obesity of the mice and not the hyperlipidemia. We therefore concluded that plasma lipid quantity does not impact macrophage accrual or inflammation in AT.
It is known that AT takes on the fatty acid composition of the diet (70). Therefore, ATMs are exposed to the fatty acids that have been consumed. This implies that dietary SFAs could promote inflammatory pathways and M1 polarization. SFAs have been implicated in activating transcriptional signaling pathways that lead to M1 polarization via TLR4 (58, 71, 72). Suganami et al. published two reports (73, 74) in which they proposed the idea that macrophages and adipocytes act in a paracrine loop of inflammation and insulin resistance. Their theory holds that inflammatory cytokines released from macrophages, such as TNF-α, induce insulin resistance in adipocytes that results in uncontrolled release of fatty acids. These fatty acids further activate macrophages to release additional inflammatory cytokines. This creates a continuous cycle of cytokine production by macrophages and fatty acid release by adipocytes. Furthermore, their data demonstrated this cycle is interrupted when macrophages cannot activate TLR4 pathways. In vitro studies from our laboratory (68, 75) have shown that SFAs can induce inflammation, ER stress, and apoptosis in primary peritoneal macrophages. Interestingly, in our hands these effects did not require the presence of TLR4 or TLR2, indicating other mechanisms by which SFAs induce inflammatory pathways in macrophages. Regardless of the role of SFAs in macrophage polarization, we have shown that TLR4 deficiency promotes an M2-like phenotype (76). Despite a few inconsistences, the observation that fatty acids can activate inflammatory pathways in macrophages has important relevance to the polarization of ATMs, and to their contribution to metabolic consequences of obesity.
In addition to extracellular exposure to fatty acids, ATMs may also take up and store lipids released from adipocytes. In genetic and diet-induced obesity, pro-inflammatory macrophages have greater lipid content compared to anti-inflammatory macrophages (48). This finding suggests a greater propensity for M1 ATMs to accumulate excess lipid in AT, or conversely, that lipid accumulation promotes the formation of M1 ATMs. In support of this idea, it was shown that foam-like ATMs have increased triglyceride (TG) stores, containing more relatively short-chained saturated lipid species (48). Furthermore, PPARγ agonists reverse the effects of toxic lipid species on ATMs by decreasing total lipid storage, promoting preferential storage of unsaturated long chain containing TGs, and inducing an anti-inflammatory phenotype (48). An alternative view is that SFAs in AT not only induce an inflammatory polarization but also induce lysosome biogenesis associated with lipid catabolism (77).
Adipocyte death may also contribute to lipid-related changes in ATM phenotype. Phagocytic ingestion of dead cells results in lipid droplet accumulation in ATMs (38). In fact, macrophages that surround dead adipocytes display a morphology similar to that of foam cells in atherosclerotic plaques (38, 47, 75, 78), although they most likely contain TG rather than cholesterol (47). Thus, lipid debris from dead adipocytes could also be lipotoxic to the ATMs promoting inflammation and M1 polarization.
While uncontrolled lipid accumulation in macrophages is generally shown to promote inflammation, other studies have demonstrated that proper lipid storage promotes an anti-inflammatory ATM phenotype. Acyl CoA: diacylglycerol acyltransferase (DGAT) is an enzyme involved in TG synthesis. Transgenic overexpression of DGAT in adipocytes and macrophages using the ap2 promoter decreases the number of CLSs and inflammatory gene expression in ATMs (79).
TLR4-mediated activation of ATMs
TLR4 is the established receptor for LPS, and its downstream signaling pathways leading to NFκB activation are well-established (reviewed in 80). The portion of LPS that is thought to bind TLR4 is the polyA tail containing several SFA side chains. Based upon this knowledge, Hwang and colleagues demonstrated that SFAs activate TLR4 signaling pathways in macrophages (72). Furthermore, exchanging SFAs on the polyA tail with PUFAs reduces activation by LPS. Subsequent studies have supported this finding (71, 81), but concerns have been raised as to whether slight LPS contamination of the BSA used as a carrier for the fatty acids was responsible for the effects (82). In fact, it has recently been shown that SFAs do not directly bind TLR4 (83); rather, Fetuin-A is the endogenous TLR4 ligand (84). Pal et al. demonstrated that SFAs bind Fetuin-A rather than TLR4 itself and suggested that a ternary complex between the three is required for activation of the pathway.
Many in vivo studies have focused on the role of TLR4 in obesity-related inflammation and insulin resistance with varying results (Table 2). TLR4-deficient or mutant mice were hypothesized to have reduced overall AT inflammation and improved insulin sensitivity based on the concept that obesity results in dysregulated basal lipolysis leading to exposure of ATMs to elevated levels of SFAs. Many studies substantiated this hypothesis. For example, in 2006, Flier and colleagues (81) demonstrated that TLR4−/− mice (6th backcross into C57BL/6 background) are more insulin sensitive than controls in a lipid infusion model of insulin resistance – an improvement that was noted at the level of muscle and AT. Female TLR4−/− mice gained more weight upon HFD-feeding, but remained more insulin sensitive than controls. This was attributed to a reduction in inflammatory cytokines in AT of the TLR4−/− mice. In contrast, male TLR4−/− mice also showed a reduction in AT inflammation but did not have improved insulin sensitivity, suggesting that the protective effect of TLR4 deficiency cannot be simply explained by changes in AT inflammation. Subsequent studies by Poggi et al. (85), Suganami et al. (86), and Tsukumo et al. (87) showed similar results in C3H/HeJ mice, which have a spontaneous mutation in TLR4. In slight contrast, Davis et al. (70) only detected an effect of TLR4 deficiency in C57BL/10ScN mice fed diets high in SFA but not UFA. In addition, Saberi et al. (88) showed that when TLR4 is exclusively removed from hematopoietic cells, mice become resistant to HFD-induced AT inflammation and systemic insulin resistance.
Table 2.
Impact of TLR4 deficiency on AT macrophages and systemic insulin action.
| Model | Controls | Gender | Diet | Time on diet | Main Findings | Ref |
|---|---|---|---|---|---|---|
| C57BL/10ScN | C57BL/10J | male | 45% unsaturated fat 45% palmitate |
↓ weight gain ↓ AT macrophages* ↓ AT inflammation ↓ plasma insulin* *only in high palmitate diet groups |
(70) | |
| TLR4−/− on C57BL/6 background | littermates | male | Low fat (10%); high fat with MUFA (45%); high fat with SFA (45%) | 16 wk | ↓ food intake ↓ weight gain ↓ plasma adiponectin ↔ in AT inflammatory markers ↑ in AT M2 markers ↓ hepatic steatosis ↔ in systemic insulin sensitivity of global TLR4−/− (clamp) ↓ insulin sensitivity BMT model (ITT, GTT) |
(76) |
|
TLR4−/− transplant into C57BL/6 C57BL/6 transplant into TLR4−/− |
C57BL/6 transplant into C57BL/6 TLR4−/− transplant into TLR4−/− |
male | high fat with SFA (45%) | 16 wk | ↔ body weight ↔ serum insulin |
(76) |
| TLR4−/− | Wild type | female | Lipid infusion; 58% fat | 39 wk | ↑ insulin sensitive in lipid infusion model ↑ food intake ↑ body weight on HFD ↑ insulin sensitive on HFD (ITT) ↓ AT expression of macrophage markers ↓ AT expression of inflammatory genes |
(81) |
|
TLR4 Knockdown in BalB/c
TLR4−/− on C57BL/6 background |
C57BLKS/6J littermates |
female | 32.5% lard and 32.5% corn oil with sucrose | ↑ insulin sensitivity in HFD-fed mice with TLR4 or fetuin A knockdown. | (84) | |
| C3H/HeJ | C3H/HeOuJ and C57BL/6 | male | 42% fat pelleted diet; 35% fat liquid diet | 22 wk | ↓ food intake ↑ AT mass ↓ inflammation ↑ insulin sensitivity in AT ↔ in systemic insulin sensitivity (clamp) ↑ adiponectin ↑ macrophages ↓ AT inflammation ↓ hepatic steatosis |
(85) |
| C3H/HeJ | C3H/HeN | male | HFD (60%) | 16 wk | ↔ in weight gain ↑ AT mass ↔ ATM number ↓ AT Tnfa ↑ AT Adipoq ↑ systemic insulin sensitivity (plasma glucose and insulin measurements) |
(86) |
| C3H/HeJ | C3H/HeN and C57BL/10ScNC | male | 55% fat | 8 months | ↓ weight gain ↓ AT mass ↓ AT macrophages ↑ glucose tolerance ↑ insulin tolerance ↑ AT insulin sensitivity |
(87) |
| TLR4lps-del transplant into C57BL/10 | C57BL/10 transplant into C57BL/10 | 12 wk | ↔ in glucose tolerance (GTT) ↑ insulin tolerance ↑ insulin sensitivity (clamp) ↓ hepatic steatosis ↓ AT macrophages ↓ AT inflammation |
(88) | ||
| macrophage-specific TLR4 knockout | TLR4fl/fl | male | 42% fat from milkfat | 12 wk | ↔ AT inflammation ↔ glucose tolerance ↔ insulin tolerance |
(89) |
Note 1: If columns are left empty it is because it was not clear from the Methods of the cited papers.
Note 2: Metabolic tests for glucose tolerance consist of glucose tolerance tests (GTT), for insulin tolerance consist of insulin tolerance tests (ITT) and for insulin sensitivity consisted of measuring plasma insulin and glucose or hyperinsulinemic-euglycemic clamps as indicated.
Note 3: Adipose tissue specific insulin sensitivity was measured by quantifying phosphorylation of proteins in the insulin signaling pathway.
Despite these reports demonstrating a protective effect of TLR4 deficiency against AT inflammation and systemic insulin resistance, we were not able to recapitulate these observations (76). We fed male TLR4−/− mice (backcrossed 10 times into C57BL/6 background) and their littermate controls, HFD enriched in either monounsaturated fatty acids or SFAs. In contrast to the previous reports, our studies demonstrated TLR4−/− mice had reduced food intake accompanied by reduced weight gain on both HF diets. We detected very little change in inflammatory cytokine expression in AT of TLR4−/− mice, although they did have a significant increase in mRNA and protein levels of M2 markers such as Mgl1, Mgl2, and mannose receptor. We also performed bone marrow transplantation studies to determine the contribution of hematopoietic versus parenchymal TLR4 deficiency on AT and detected only modest increases in M2 markers. Jia et al. also showed that myeloid deficiency of TLR4 has no impact on expression of inflammatory genes in AT; and in fact, resulted in increased circulating inflammatory cytokines (89). Thus, the role of macrophage TLR4 in AT inflammation is still not clear.
Inflammasome-mediated activation of ATMs
ATMs can activate many pathways that promote secretion of pro-inflammatory cytokines in response to pathogens through pattern recognition receptors such as the Toll-like receptors. This can also be achieved through recognition of danger-associated molecular patterns (DAMPs). DAMP signaling results in the activation of the Nlrp3 inflammasome, which involves the formation of a multiprotein scaffold complex, including Nlrp3 and caspase-1. Formation of this complex is required for caspase 1 to obtain full activation allowing for cleavage and release of IL-1β and IL-18 (reviewed in 90). Stienstra et al. (91) have shown that global deficiency of caspase 1 or Nlrp3 results in improved insulin sensitivity, concluded to be due to effects on adipocytes rather than ATMs. In contrast, Dixit and colleagues (92) demonstrated that Nlrp3 co-localized to lipid-engorged ATMs. In their study, expression of IL-1β and Nlrp3 in visceral AT was positively correlated with body weight and adiposity (92), and conversely, chronic caloric restriction reversed these effects and resulted in improved insulin sensitivity in mice and in human subjects. Elimination of Nlrp3 resulted in decreased caspase-1 cleavage, along with reduced IL-18 and IFN-γ expression concomitant with improved insulin sensitivity (92). Importantly, the Nlrp3−/− mice had increased M2-like gene expression along with decreased M1-like gene expression in AT, possibly accounting for the improved insulin sensitivity detected. The Nlrp3 inflammasome has been shown to recognize DAMPS such as ATP, urate, asbestos, β amyloid and SFAs. With relevance to ATMs, Dixit and colleagues (92) demonstrated that the Nlrp3 inflammasome also recognizes ceramides. This new role of the inflammasome in lipid-laden macrophages uncovered a mechanism by which toxic lipid species (SFAs and ceramides) may act as danger signals to ATMs and promote an inflammatory phenotype.
NF-κB-mediated activation of ATMs
NF-κB is a multi-subunit transcription factor composed of Rel subunits such as RelA (p65), RelB, c-Rel, p50, and p52, which form various homo- and hetero-dimers that bind DNA to induce transcription of a plethora of genes. The p65/p50 heterodimer is the most common form of NF-κB and regulates transcription of inflammatory genes in many cell types, including macrophages. NF-κB activation is regulated by its activators I kappa kinases (IKKs) and its inhibitor IκBα. Common obesity-related stimuli such as TNFα, LPS, and other inflammatory cytokines can induce activation of the NF-κB pathway to promote even further inflammation. In this regard, researchers have been interested in ATM NF-κB activation.
Dampening of the NF-κB pathway has been known for some time to improve systemic insulin sensitivity (93); however, AT-specific effects of NF-κB were not discovered until more recently. Using NF-κB reporter mice, Chiang et al. (94) demonstrated HFD-fed mice displayed a twofold increase in luminescence in the AT depots compared to chow-fed controls. Furthermore, they demonstrated that ATMs from obese mice have increased IKK and NF-κB activity compared to ATMs from their lean counterparts. Upon closer examination by confocal microscopy, it was shown that luciferase illumination and nuclear localization of the NF-κB p65 subunit was only enriched in ATMs in HFD-fed mice.
Manipulating upstream IKK activators of NF-κB has been a major focus in understanding the role of NF-κB-induced activation of inflammatory pathways in ATMs. For example, mice lacking IKK-β in myeloid cells retain insulin sensitivity; however, whether this protection is due to reduced ATM inflammation was not determined (95). Another IKK, IKKε, was shown to be significantly upregulated in pro-inflammatory ATMs of HFD-fed mice compared to controls. Furthermore, IKKε deficiency attenuated inflammation and insulin resistance in HFD-fed mice (94).
With regards to lipid-mediated activation of ATMs, SFAs have been suggested as being a modulator of NF-κB activation in ATMs. As noted above, exposure to SFAs and PUFAs resulting in inflammatory or anti-inflammatory polarization of ATMs has been extensively studied. Although the mechanism remains to be determined, many studies suggest that SFAs activate NF-κB pathways in macrophages promoting an inflammatory phenotype. Reports from Suganami and colleagues (74) have shown that treatment of macrophages with the SFA palmitate significantly induces NF-κB activation. This activation was followed by a significant increase in expression of NF-κB regulated inflammatory molecules TNF-α and CCL2. Thus, NF-κB activation is a likely player in driving the M1 phenotype in ATMs; however, more studies are needed to address the role of NF-κB in ATM homeostasis.
JNK-mediated activation of ATMs
C-Jun NH2-terminal kinases JNK1 and JNK2 are considered to be key players in ATM inflammation. Macrophages activate JNK in response to stress and inflammatory stimuli. JNK is also known to be elevated in obesity, while global deficiency of JNK preserves insulin signaling and improves insulin sensitivity (13). Because JNK itself phosphorylates insulin receptor substrates, it can directly impair insulin signaling pathways and induce insulin resistance. Therefore, investigators hypothesized that pharmacologic or genetic deletion of this pathway in macrophages would reduce inflammation and improve insulin sensitivity. Indeed, an early report showed that the JNK1 deficiency in hematopoietic cells protected against obesity-induced insulin resistance (96). This was concomitant with a reduced inflammatory response of JNK1−/− macrophages to SFA stimulation. However, Hotamisligil's group used similar bone marrow transplantation studies and showed that parenchymal – but not hematopoietic – deficiency of JNK1 is responsible for changes in ATM numbers and improvements in insulin sensitivity. Although, the recipients of JNK1−/− marrow had reduced AT expression of some inflammatory genes (97). These findings were recapitulated by Davis and colleagues (98), who showed that myeloid-specific deficiency of JNK1 did not impact systemic glucose and insulin tolerance. Differences between the studies may have been due to the more severe obesity in the animals studied by Solinas et al. (96). A more recent study by Davis and colleagues (99) utilized macrophage-specific JNK1 and JNK2 double knockout mice and demonstrated that mice deficient for JNK1 and JNK2 in their macrophages are protected from macrophage infiltration into AT and systemic insulin resistance. Their results suggest that JNK1 and JNK2 have some redundancy and that deficiency of both is required to see beneficial changes in ATM inflammation and in systemic insulin resistance.
Mechanisms for macrophage accrual in AT
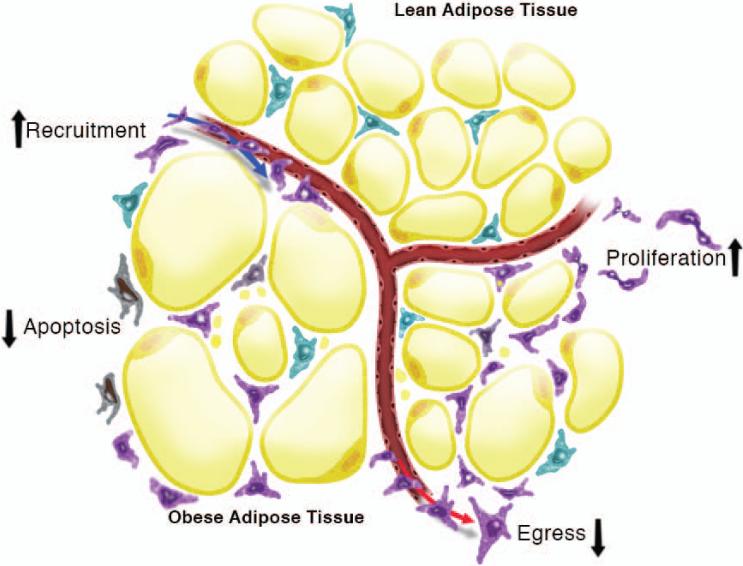
The number of cells that accumulate in any given tissue can be theoretically attributed to fluxes in at least four different mechanisms: recruitment, egress, proliferation, or cell death (Fig. 2). Although the overwhelming number of studies in AT have focused on the recruitment side of the equation, there is evidence in the literature for all four of these mechanisms contributing to macrophage accumulation in AT. We have divided our discussion of this topic into recruitment-dependent and recruitment-independent mechanisms.
Fig. 2. Multiple mechanisms exist for macrophage recruitment to adipose tissue.
Many publications have focused on recruitment-independent mechanisms that can account for macrophage recruitment to adipose tissue. In addition, there is recent evidence for recruitment-independent mechanism such as apoptosis, proliferation, and egress to also contribute to total adipose tissue macrophage numbers.
Recruitment-dependent pathways of macrophage accrual in AT
After the discovery of increased macrophages in obese AT, Weisberg et al. (18) performed traceable bone marrow transplants in newly obese mice to determine the origin of ATMs with obesity. These studies demonstrated that the majority of the macrophages in the obese AT are bone marrow derived, and thus, recruited. Since this landmark publication, many studies have aimed to identify the major factors responsible for monocyte recruitment, underlying ATM accumulation during AT hypertrophy. These factors include adipocyte death, chemokines, adipokines, and lipids.
Adipocyte death
Hypoxia can occur in AT when adipocytes expand in excess of microvasculature growth, or when adipocyte size exceeds the diffusion of nutrients, leading to adipocyte cell death (reviewed in 100). Similar to macrophage functions in other tissues, it has been hypothesized that ATMs are recruited to phagocytose cellular debris following adipocyte apoptosis. This can be visualized in the CLS in obese AT. The clearance of dead adipocytes is driven by inflammatory cytokines and classically activated macrophages, and the presence of these macrophages may contribute to collagen deposition and AT fibrosis in mice (101).
Chemokines /chemokine receptors
In humans and mice, expression of many different chemokines and chemokine receptors is elevated in obese compared to lean AT (18, 19, 102). The MCP1 (CCL2)/CCR2 chemokine/chemokine receptor axis is one of the most potent for monocyte recruitment in inflammatory settings. Further support for a potential role of CCL2/CCR2 in ATM recruitment stems from the fact that AT gene expression of CCR2 and its ligands (CCL2, CCL7, and CCL8) is increased 2-7-fold in obese compared to lean mice (103). Thus, several groups have assessed CCL2 and CCR2-deficient mice to determine whether ATM recruitment is reduced.
Kanda et al. (104) showed increased levels of CCL2 both in AT and plasma of obese mice corresponding with increased AT macrophage content, and identified adipocytes as one source of CCL2. Transgenic AT-specific overexpression of CCL2 increases AT macrophage infiltration, insulin resistance, fasting blood glucose, serum free fatty acid (FFA), and hepatic steatosis, even in the lean state. From the other end of the spectrum, CCL2−/− mice in their studies had reduced HFD-induced ATM accumulation, associated with decreased insulin resistance, serum FFA, and hepatic steatosis. In stark contrast to this, studies by Inouye et al. (105) and Kirk et al. (106) saw no reduction in ATM accumulation in CCL2−/− mice challenged with short-term or long-term HFD. In both of these studies, the CCL2−/− mice gained more weight and had slightly worsened insulin resistance compared to controls (105, 106). Thus, although the published literature is mixed, there is more support for an absence of effect of CCL2 on macrophage recruitment to AT.
Because CCR2 is a receptor for several chemokines in addition to CCL2, and CCR2 deficiency results in a near absence of circulating Ly6Chi inflammatory monocyte precursors (107), it is plausible that CCR2 deficiency could have a greater impact than CCL2 deficiency on macrophage recruitment to AT. Weisberg et al. compared weight-matched CCR2−/− and wild type mice and found that CCR2−/− mice fed HFD for 24 weeks display reduced ATMs concomitant with lower fasting blood glucose and insulin levels as well as higher plasma adiponectin (103). This finding was reproduced by Sullivan et al. (108) in mice fed HFD for 20 weeks and by Lumeng et al. (39)who detected reduced recruitment of ATMs to CLSs in CCR2−/− mice. We (109) performed a time course study of HFD-feeding in CCR2−/− mice and were only able to detect a reduction in ATMs after 20 weeks. Thus, the age of mice and time on HFD may be important to detecting effects of CCR2 deficiency. We have also found that CCR2−/− mice have increases in F4/80+CD11b+ eosinophils in their AT early after HFD-feeding (109). The contribution of these unique CCR2 deficiency-related eosinophils to AT inflammation is not known.
Several groups have also used antagonists of CCR2 and quantified effects on ATM numbers. Weisberg et al. (103) showed that two weeks of CCR2 antagonism with the small-molecule INCB3344 is sufficient to reduce ATM content and to improve insulin sensitivity. Another CCR2 antagonist, CCX417 has also been shown to ameliorate CD11c+ macrophage recruitment to AT and to improve systemic insulin action (108). Finally, a third CCR2 antagonist propagermanium has been shown to reduce macrophage numbers and to shift the polarization of the remaining ATMs from M1 to M2 (110).
Many other chemokines and their receptors have also been studied with regards to their role in macrophage recruitment to AT. Similar to the findings with CCL2 and CCR2, the results have been mixed. For example, CCR5 is the receptor for CCL3 (macrophage inflammatory 1α), CCL4 (macrophage inflammatory 1β), and CCL5 (RANTES). Our laboratory (111, 112) has shown no effect of CCL3 or CCR5 deficiency on macrophage recruitment to AT. However, Kitade et al. (113) reported that CCR5 deficient mice have reduced ATM numbers and inflammatory gene expression, resulting in improved insulin sensitivity.
Leptin
Like adiponectin, leptin is secreted primarily from adipocytes, although its secretion is in proportion to AT mass. Furthermore, while adiponectin is an insulin-sensitizing adipokine that has been implicated in M2 polarization as previously discussed, leptin is generally considered to be pro-inflammatory. Furthermore, ATM numbers correlate with plasma leptin concentrations (69). Thus, we and others considered whether leptin could mediate the recruitment and inflammatory status of ATMs. Curat et al. (26) demonstrated that leptin activation of endothelial cells induces monocyte transmigration. Using in vitro migration assays, we demonstrated that at physiological concentrations, leptin acts as a potent monocyte chemoattractant, requiring the presence of leptin receptors on the migrating cells (114). We hypothesized that an inability of macrophages to respond to obesity-related changes in leptin gradients would prevent macrophage accrual in AT during HFD feeding. To test this hypothesis, we reconstituted wild type mice with leptin receptor (LepR−/−) marrow and placed them on a HFD (115). However, we detected no differences in the number or phenotype of their ATMs. We also used LepR−/−CCR2−/− double knockout bone marrow donors to amplify the potential impact of LepR deficiency. However, LepR−/−CCR2−/− reconstituted mice also had no significant changes in ATM presence or phenotype following HFD feeding. In contrast, Dib et al. (116) recently reported that mice reconstituted with LepR−/− marrow have reduced weight gain and ATM accrual. A thorough discussion of the differences between these leptin studies is provided in a published commentary (117).
Lipids
As previously discussed, it is known that various fatty acids can alter the inflammatory potential of ATMs. Furthermore, ATMs in expanding AT form multinucleated syncytia filled with large lipid droplets (38) and increased expression of genes associated with lipid metabolism (39). In fact, lipolysis (pharmacologically induced or through short-term fasting) is associated with increased macrophage recruitment to AT (78). The levels of FFAs in circulation are also positively associated with increased AT chemokine expression and lipid uptake by ATMs. Inversely, reduced lipolysis (through genetic or dietary means) leads to reduced accumulation of macrophages in AT. Furthermore, blocking lipolysis prevents macrophage influx into AT. These findings suggest that as ATMs accumulate lipids they take on a foam-like state so that they can function to buffer local increases in lipid concentrations.
Summary of recruitment-dependent mechanisms for macrophage recruitment to AT Some of the studies discussed above demonstrate that the absence of a single chemokine or chemokine-like molecule can substantially impact macrophage accumulation in AT; however, results are not consistent from laboratory to laboratory. In the case of chemokine-mediated recruitment of macrophages to AT, it is likely that chemokines have redundant roles and can compensate for one another. Ultimately, it appears that no single chemokine or factor is single-handedly responsible for the recruitment of circulating monocytes to AT. Likewise, recruitment may not be the only explanation for M1 ATM accumulation with increased adiposity; in some situations, recruitment-independent mechanisms may prevail.
Recruitment-independent pathways of macrophage accrual in AT
While recruitment of circulating macrophages to AT was the focus of many early experiments, several of these published studies unexpectedly revealed the likelihood of recruitment-independent mechanisms for ATM accrual. For example, in CCR2−/− and MGL1−/− mice, there are significantly lower levels of circulating Ly6Chi monocytes (107, 118). If circulating Ly6Chi monocytes are a primary driver of macrophage accrual in AT, it would be expected that the mice would have a near absence of macrophage recruitment in obesity. Interestingly, HFD-fed MGL1−/− mice have only a 30% reduction CD11b+ macrophages and no significant differences in AT expression of F4/80 compared to controls (118). Furthermore, in CCR2−/− mice, a difference in number of ATMs is only detected after prolonged periods of HFD feeding (103, 109). If circulating Ly6Chi monocytes are the cells recruited to AT in obesity, CCR2−/− mice would be expected to have dramatic reductions in ATMs even early after HFD-feeding. Thus, recruitment-independent mechanisms for macrophage accrual in AT have been the topic of recent publications. These studies demonstrate a role for apoptosis, proliferation, and retention in driving ATM accumulation in obese AT (Fig. 2).
Apoptosis
Apoptosis is a common mechanism for cell turnover in many tissues. The possibility of ATM apoptosis being important for maintenance of AT homeostasis is suggested by two lines of evidence. First, clodronate liposome-mediated depletion of ATMs from obese mice reduces AT inflammation and improves insulin sensitivity (119, 120). These data indicate that loss of M1 inflammatory macrophages can improve AT function. In support of the relevance of this observation to humans, Kern and colleagues (121) have shown that treatment of humans with the insulin sensitizing drug pioglitazone reduces the number of macrophages in AT via apoptotic mechanisms. Whether apoptosis of resident macrophages in lean AT controls their turnover is yet to be determined.
Proliferation
Proliferation has only very recently been shown to contribute to increased ATM content in obese AT. Two groups published the novel finding that macrophages in obese AT proliferate at higher rates than those in lean AT (122, 123). Using Ki67 and EdU staining coupled with immunofluorescence and flow cytometric assays, Amano and colleagues (122) demonstrated that 10-17% of ATMs are proliferating in obese ob/ob or diet-induced obese mice. This process is not impacted by the presence or absence of circulating monocytes and does not occur in other organs such as liver, spleen, or blood. CCL2 was determined to be a likely AT-specific macrophage proliferation cue. A concurrent report by Hasse et al. (123) similarly demonstrated that macrophages in AT proliferate at higher rates in obese compared to lean mice. In both studies, the proliferation was shown to occur mostly in the CLS-localized ATMs. However, Hasse et al. (123) uniquely observed that the proliferating ATMs expressed markers of M2 rather than M1 polarization. With only two major studies addressing ATM proliferation, many questions remain regarding the contribution of proliferation to ATM numbers and will certainly be the topic of future investigation in the field.
Retention
Moore and colleagues (124) have given the first insight into the role of retention in the accumulation of macrophage in obese AT. Their studies focus on the neuronal molecule Netrin-1 and its target receptor Unc5b. Activation of Unc5b by Netrin-1 results in chemorepulsive signaling that decreases cell migration out of tissues. Interestingly, they reported an increased expression of Netrin-1 and Unc5b in the AT of mice fed HFD compared to chow-fed controls. This expression was localized to CLSs in the AT. Interestingly, this localized expression was also seen in AT from obese humans. Importantly, they showed that hematopoietic Netrin-1 deficiency facilitates macrophage egress from AT. The model outlined by the Moore group suggests that Netrin-1 promotes defective ATM migration and accumulation in AT by blocking chemokine induced migration. Egress of macrophages from other sites such as atherosclerotic plaques has also been studied by several groups, but controversy remains as to the contribution of egression to lesional macrophage content during progression and regression of atherosclerosis (reviewed in 125). Because resident macrophages poorly egress from most tissues, it will be important for future studies to address the degree to which ATM egression impacts their total numbers and inflammatory potential in AT and whether signals other than Netrin-1 and Unc5b are involved. Overall, this is the first study of its kind, and brings an innovative idea of involvement of egress signaling in ATM accumulation.
Summary of recruitment-independent mechanisms for macrophage recruitment to AT In the past couple of years, Immunometabolism investigators have expanded their thinking and experimental design to embrace the idea that recruitment-independent mechanisms may also play a role in macrophage accrual in AT during obesity. The relative contribution of all of these mechanisms is likely context dependent, overlapping, and complicated to decipher. It will be interesting to see what other molecules contribute to apoptosis, proliferation, and retention based on new exploration in coming years.
Role of resident macrophages in lean AT homeostasis
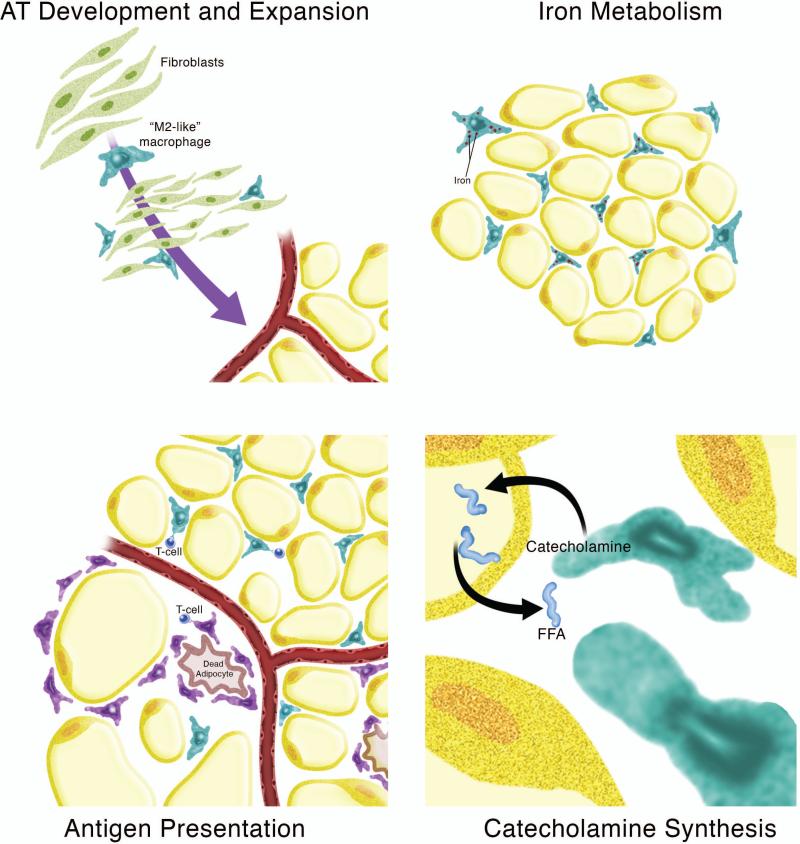
Since the discovery of obesity-associated increases in ATMs, most published work has focused on the contribution of these inflammatory CLS-associated macrophages to metabolic diseases. In contrast, the contribution of resident M2-like macrophages to AT homeostasis has been relatively unexplored. A simplistic view holds that resident ATMs maintain an anti-inflammatory state in the AT, and thus protect against tissue insulin resistance. However, more complex roles have been suggested. Hypothesized roles for resident ATM include AT development and expansion, iron metabolism, antigen presentation, and catecholamine synthesis (Fig. 3). It is likely that not only the increase in inflammatory M1 macrophages but also the decreased homeostatic function of the resident M2 macrophages contributes to dysregulation of AT homeostasis in obesity.
Fig. 3. Roles for resident macrophages in adipose tissue homeostasis.
Potential roles for resident M2-like macrophages in lean adipose tissue (AT) include AT development and expansion, iron metabolism, antigen presentation, and catecholamine synthesis.
AT development and expansion
ATMs may contribute to AT development and expansion by influencing angiogenesis and adipogenesis. In vivo, confocal imaging has shown that lectin-binding CD68+F4/80+CD34+ macrophage-like cells are present in adipogenic clusters in the developing fat pads of young mice (126). Importantly, depletion of macrophages impairs angiogenesis and adipogenesis, implicating an important role for resident macrophages in AT development. In adult mouse epididymal AT, the fat pad tip is the primary site of AT hyperplasia and contains a dense vascular network. Macrophages are recruited to this area in a CCL2/CCR2-independent manner (127), and are required for angiogenesis and outgrowth of the AT. Together, these data implicate a role for resident ATMs in AT development, expansion, and possibly even remodeling.
Iron metabolism
An emerging function of M2 macrophages is in iron handling. Although M1 macrophages are known to sequester iron as a bacteriostatic mechanism, M2 macrophages have recently been shown to have increased capacity to import and export iron, implicating a role in iron recycling (128, 129). This has in vivo relevance to hemorrhagic atherosclerotic plaque rupture, where macrophages take up hemoglobin-haptoglobin complexes via CD163, the hemoglobin-haptoglobin scavenger receptor (130, 131). These specialized macrophages are protected from oxidative stress and are anti-inflammatory, despite being iron-loaded. CD163-expressing macrophages also upregulate cholesterol efflux genes. They do this presumably to protect themselves from lipid overload, thereby preventing lipid peroxidation in the face of iron-overload (131, 132).
In concordance with a role for macrophages in iron handling, we have shown that 25% of ATMs in lean mice have a twofold increase in iron content, making them ferromagnetic (56). These ATMs, which we call ‘MFehi’ also have increased expression of iron handling genes such as CD163, transferrin receptor, hemoxygenase, ferritin heavy and light chains, and the iron exporter ferroportin. MFehi cells are also highly M2-polarized as evidenced by increased expression of Mgl1 and IL-10 and decreased expression of CD11c and CCR7. After 16 weeks of HFD feeding, MFehi ATMs have lower iron content and increased inflammatory gene expression. Interestingly, this is concurrent with an increase in adipocyte iron content. Thus, we have hypothseized that this subpopulation of resident M2 ATMs plays a ‘ferrostatic’ role in maintaining AT iron homeostasis; MFehi ATMs may supply iron to adipocytes during adipogenesis, or, they may sequester iron from adipocytes to protect against oxidative stress and insulin resistance.
Antigen presentation
Although not a focus of this review article, T cells play a role in AT homeostasis and in the inflammation associated with obesity. It has been shown that ATMs present MHC-II restricted antigens to T cells and can promote the proliferation of IFN-γ-producing CD4+ T cells in AT (133). Antigen presentation appears to be a function of both M2-like and M1-like ATMs. In obesity, most of the MHC-II expression was in ATMs in CLSs. In addition, ATMs from human AT have been shown to express the lipid antigen-presenting molecules Cd1b and Cd1c (42).
Catecholamine synthesis
Thermoregulation is another unique function of resident ATMs. Chawla and colleagues (134) demonstrated that cold exposure promotes the alternative activation of macrophages in brown and white AT. Catecholamine synthesis by these M2 ATMs induces lipolysis in white AT and thermogenic gene expression in brown AT. The cumulative effect is that brown AT then has a fuel source and machinery in place to generate heat. Loss of IL-4/IL-13 signaling by the M2-like macrophages abrogates their ability to upregulate catecholamine synthesis in response to cold exposure. These results demonstrate that resident macrophages may play a key role in response to cold stress.
Weight loss and weight cycling
While lipolysis induced by fasting or caloric restriction initially promotes macrophage recruitment to AT, within a few weeks of weight loss in mice, ATMs become less inflammatory and their numbers remain unchanged (135). Ultimately, prolonged weight loss in mice results in reduced ATMs (78). Weight loss in humans has also been shown to reduce the number of macrophages in AT. Following bariatric surgery, there is a greater than 10% reduction in ATMs in subcutaneous AT (25). Furthermore, the remaining ATMs are less frequently found in CLSs and they start to express the anti-inflammatory cytokine IL-10. In addition, there is a reduction in 30-50% of the inflammatory genes expressed in the SVF (25, 136).
Alternating periods of weight loss and weight gain (weight cycling) is common in humans and has been shown to increase the risk of insulin resistance, diabetes, and cardiovascular disease (137-139). We hypothesized that ATM number and/or polarization could contribute to this association (140). Surprisingly, we found that macrophage number and phenotype were not different in weight-cycled mice compared with those that were equally obese but had not weight-cycled. Instead, CD4+ and CD8+ T cells were increased along with elevated expression of Th1 cytokines. These data demonstrate the complex nature of innate and adaptive immune responses in AT.
Common caveats
The question arises then as to why there are so many disparate results from seemingly identical studies. There are many different elements in the study design that could impact the metabolic outcomes of these immunometabolic studies. There are some obvious differences between some of the studies mentioned above that make the data sets difficult to compare with one another. These include gender of the mice, percent of fat in the diet, type of fat in the diet, source of the carbohydrate in the diet, and length of the HFD feeding. There are also some differences that may influence results in more subtle but nonetheless important ways. First, the genetic background of the mice is critical. The most commonly used strain for obesity studies, C57BL/6, are obesity and insulin resistance susceptible. In contrast, the 129 strain is obesity resistant (141). Because the 129 strain is often used to develop knockout models, if mice are not completely backcrossed into the C57BL/6 background, individual mouse susceptibilities to diet-induced obesity can influence their metabolic results. Second, the use of non-littermate controls can impact metabolic results in two ways: (i) there could be slight strain differences because of genetic drift if the wildtype controls are purchased or have been bred separately within the same facility and (ii) the microbiome of mice from different sources could be markedly different. Although incompletely understood, the gut microbiota is currently in the spotlight as a strong contributor to metabolic phenotypes in obesity studies (142). Other seemingly minor details that should be considered are whether mice are housed singly or in groups, whether mice were from primiparous or multiparous dams (143), and other events that can impact weight gain of all mice in a particular cage. We suggest that whenever possible, fully backcrossed mice with co-housed littermate controls should be used in immunometabolic studies.
There are also other variables within the metabolic analyses that should be carefully considered. First, baseline body weight of the control and experimental mice should be carefully matched, as slight differences can impact their growth curve trajectories independent of their genotype. Second, the length of fast before studies are performed is critical. Ferrante and colleagues (78) have shown that lipolysis induced by caloric restriction or overnight fast results in a transient increase in ATMs. Thus, all studies should be performed with the same length of fast before analyses. Third, ideally, the amount of glucose and insulin used in glucose and insulin tolerance tests should be matched to lean body mass rather than total body weight. Because obesity can alter the ratio of lean to fat mass and lean mass is the major contributor to glucose uptake, results of studies dosed according to body weight can be easily misinterpreted if one genotype has greater adiposity than the other.
Conclusions
This decade-old field of immunometabolism is indeed flourishing and exciting. Despite some conflicting data, much progress has been made in understanding the role of macrophages in AT and other metabolic organs. Overall, the published studies support the concept that resident macrophages help maintain homeostasis of lean AT, while inflammatory macrophages contribute to insulin resistance in obese AT. Yet, there are still many unanswered questions. What is the primary etiology underlying ATM accumulation in obesity, recruitment-dependent or recruitment-independent mechanisms? Which is more important in ATM contribution to insulin resistance in obesity, the influx of inflammatory macrophages or reduced capacity of resident macrophages to maintain AT homeostasis? Which inflammatory pathways are most relevant to ATMs? How do macrophages interact with other immune cells in the AT? Perhaps most importantly, how can ATMs be modulated to protect against the metabolic effects of obesity? These questions and many others will be the focus of further interest and investigation in the coming years.
Acknowledgements
AH Hasty is supported by an American Heart Association Established Investigator Award (12EIA827) and by NIH R21DK095456. AA Hill is supported by an individual NRSA (F31DK100144). WR Bolus is supported by the Molecular Endocrinology Training Program (DK07563). The authors thank the other members of their laboratory for their careful reading and critique of this article.
Footnotes
The authors have no conflicts of interest to declare.
References
- 1.Pekala PH, Price SR, Horn CA, Hom BE, Moss J, Cerami A. Model for cachexia in chronic disease: secretory products of endotoxin-stimulated macrophages induce a catabolic state in 3T3-L1 adipocytes. Transactions of the Association of American Physicians. 1984;97:251–259. [PubMed] [Google Scholar]
- 2.Torti FM, Dieckmann B, Beutler B, Cerami A, Ringold GM. A macrophage factor inhibits adipocyte gene expression: an in vitro model of cachexia. Science. 1985;229:867–869. doi: 10.1126/science.3839597. [DOI] [PubMed] [Google Scholar]
- 3.Beutler B, et al. Identity of tumour necrosis factor and the macrophage-secreted factor cachectin. Nature. 1985;316:552–554. doi: 10.1038/316552a0. [DOI] [PubMed] [Google Scholar]
- 4.Price SR, Olivecrona T, Pekala PH. Regulation of lipoprotein lipase synthesis in 3T3-L1 adipocytes by cachectin. Further proof for identity with tumour necrosis factor. The Biochemical journal. 1986;240:601–604. doi: 10.1042/bj2400601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nature reviews Molecular cell biology. 2006;7:85–96. doi: 10.1038/nrm1837. [DOI] [PubMed] [Google Scholar]
- 6.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annual review of physiology. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 7.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 8.Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. The Journal of clinical endocrinology and metabolism. 1998;83:847–850. doi: 10.1210/jcem.83.3.4660. [DOI] [PubMed] [Google Scholar]
- 9.Perreault M, Marette A. Targeted disruption of inducible nitric oxide synthase protects against obesity-linked insulin resistance in muscle. Nature medicine. 2001;7:1138–1143. doi: 10.1038/nm1001-1138. [DOI] [PubMed] [Google Scholar]
- 10.Sartipy P, Loskutoff DJ. Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:7265–7270. doi: 10.1073/pnas.1133870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature. 1997;389:610–614. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- 12.Ventre J, et al. Targeted disruption of the tumor necrosis factor-alpha gene: metabolic consequences in obese and nonobese mice. Diabetes. 1997;46:1526–1531. doi: 10.2337/diab.46.9.1526. [DOI] [PubMed] [Google Scholar]
- 13.Hirosumi J, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 14.Soukas A, Cohen P, Socci ND, Friedman JM. Leptin-specific patterns of gene expression in white adipose tissue. Genes & development. 2000;14:963–980. [PMC free article] [PubMed] [Google Scholar]
- 15.Cousin B, Andre M, Casteilla L, Penicaud L. Altered macrophage-like functions of preadipocytes in inflammation and genetic obesity. Journal of cellular physiology. 2001;186:380–386. doi: 10.1002/1097-4652(2001)9999:9999<000::AID-JCP1038>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 16.Ochi M, Sawada T, Kusunoki T, Hattori T. Morphology and cell dynamics of adipose tissue in hypothalamic obese mice. The American journal of physiology. 1988;254:R740–745. doi: 10.1152/ajpregu.1988.254.5.R740. [DOI] [PubMed] [Google Scholar]
- 17.Kahaly G, Hansen C, Felke B, Dienes HP. Immunohistochemical staining of retrobulbar adipose tissue in Graves' ophthalmopathy. Clinical immunology and immunopathology. 1994;73:53–62. doi: 10.1006/clin.1994.1169. [DOI] [PubMed] [Google Scholar]
- 18.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. The Journal of clinical investigation. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu H, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. The Journal of clinical investigation. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mathis D, Shoelson SE. Immunometabolism: an emerging frontier. Nature reviews Immunology. 2011;11:81. doi: 10.1038/nri2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lumeng CN, Maillard I, Saltiel AR. T-ing up inflammation in fat. Nature medicine. 2009;15:846–847. doi: 10.1038/nm0809-846. [DOI] [PubMed] [Google Scholar]
- 22.Winer S, Winer DA. The adaptive immune system as a fundamental regulator of adipose tissue inflammation and insulin resistance. Immunology and cell biology. 2012;90:755–762. doi: 10.1038/icb.2011.110. [DOI] [PubMed] [Google Scholar]
- 23.Kanneganti TD, Dixit VD. Immunological complications of obesity. Nature immunology. 2012;13:707–712. doi: 10.1038/ni.2343. [DOI] [PubMed] [Google Scholar]
- 24.Dixit VD. Adipose tissue macrophages are innate to the immunological awareness of adipose tissue. Diabetes. 2013;62:2656–2658. doi: 10.2337/db13-0608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cancello R, et al. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes. 2005;54:2277–2286. doi: 10.2337/diabetes.54.8.2277. [DOI] [PubMed] [Google Scholar]
- 26.Curat CA, et al. From blood monocytes to adipose tissue-resident macrophages: induction of diapedesis by human mature adipocytes. Diabetes. 2004;53:1285–1292. doi: 10.2337/diabetes.53.5.1285. [DOI] [PubMed] [Google Scholar]
- 27.Cancello R, et al. Increased infiltration of macrophages in omental adipose tissue is associated with marked hepatic lesions in morbid human obesity. Diabetes. 2006;55:1554–1561. doi: 10.2337/db06-0133. [DOI] [PubMed] [Google Scholar]
- 28.Shapiro H, et al. Adipose tissue foam cells are present in human obesity. The Journal of clinical endocrinology and metabolism. 2013;98:1173–1181. doi: 10.1210/jc.2012-2745. [DOI] [PubMed] [Google Scholar]
- 29.Aron-Wisnewsky J, et al. Human adipose tissue macrophages: m1 and m2 cell surface markers in subcutaneous and omental depots and after weight loss. The Journal of clinical endocrinology and metabolism. 2009;94:4619–4623. doi: 10.1210/jc.2009-0925. [DOI] [PubMed] [Google Scholar]
- 30.Wentworth JM, et al. Pro-inflammatory CD11c+CD206+ adipose tissue macrophages are associated with insulin resistance in human obesity. Diabetes. 2010;59:1648–1656. doi: 10.2337/db09-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gordon S. Alternative activation of macrophages. Nature reviews Immunology. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 32.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nature reviews Immunology. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 33.Vats D, et al. Oxidative metabolism and PGC-1beta attenuate macrophage-mediated inflammation. Cell metabolism. 2006;4:13–24. doi: 10.1016/j.cmet.2006.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Freemerman AJ, et al. Metabolic Reprogramming of Macrophages: GLUCOSE TRANSPORTER 1 (GLUT1)-MEDIATED GLUCOSE METABOLISM DRIVES A PROINFLAMMATORY PHENOTYPE. The Journal of biological chemistry. 2014;289:7884–7896. doi: 10.1074/jbc.M113.522037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. The Journal of clinical investigation. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nguyen MT, et al. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. The Journal of biological chemistry. 2007;282:35279–35292. doi: 10.1074/jbc.M706762200. [DOI] [PubMed] [Google Scholar]
- 37.Patsouris D, Li PP, Thapar D, Chapman J, Olefsky JM, Neels JG. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell metabolism. 2008;8:301–309. doi: 10.1016/j.cmet.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cinti S, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. Journal of lipid research. 2005;46:2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 39.Lumeng CN, DelProposto JB, Westcott DJ, Saltiel AR. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes. 2008;57:3239–3246. doi: 10.2337/db08-0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shaul ME, Bennett G, Strissel KJ, Greenberg AS, Obin MS. Dynamic, M2-like remodeling phenotypes of CD11c+ adipose tissue macrophages during high-fat diet--induced obesity in mice. Diabetes. 2010;59:1171–1181. doi: 10.2337/db09-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bourlier V, et al. Remodeling phenotype of human subcutaneous adipose tissue macrophages. Circulation. 2008;117:806–815. doi: 10.1161/CIRCULATIONAHA.107.724096. [DOI] [PubMed] [Google Scholar]
- 42.Zeyda M, et al. Human adipose tissue macrophages are of an anti-inflammatory phenotype but capable of excessive pro-inflammatory mediator production. Int J Obes (Lond) 2007;31:1420–1428. doi: 10.1038/sj.ijo.0803632. [DOI] [PubMed] [Google Scholar]
- 43.Odegaard JI, et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hevener AL, et al. Macrophage PPAR gamma is required for normal skeletal muscle and hepatic insulin sensitivity and full antidiabetic effects of thiazolidinediones. The Journal of clinical investigation. 2007;117:1658–1669. doi: 10.1172/JCI31561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bouhlel MA, et al. PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell metabolism. 2007;6:137–143. doi: 10.1016/j.cmet.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 46.Szanto A, et al. STAT6 transcription factor is a facilitator of the nuclear receptor PPARgamma-regulated gene expression in macrophages and dendritic cells. Immunity. 2010;33:699–712. doi: 10.1016/j.immuni.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stienstra R, Duval C, Keshtkar S, van der Laak J, Kersten S, Muller M. Peroxisome proliferator-activated receptor gamma activation promotes infiltration of alternatively activated macrophages into adipose tissue. The Journal of biological chemistry. 2008;283:22620–22627. doi: 10.1074/jbc.M710314200. [DOI] [PubMed] [Google Scholar]
- 48.Prieur X, et al. Differential lipid partitioning between adipocytes and tissue macrophages modulates macrophage lipotoxicity and M2/M1 polarization in obese mice. Diabetes. 2011;60:797–809. doi: 10.2337/db10-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kang K, et al. Adipocyte-derived Th2 cytokines and myeloid PPARdelta regulate macrophage polarization and insulin sensitivity. Cell metabolism. 2008;7:485–495. doi: 10.1016/j.cmet.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Odegaard JI, et al. Alternative M2 activation of Kupffer cells by PPARdelta ameliorates obesity-induced insulin resistance. Cell metabolism. 2008;7:496–507. doi: 10.1016/j.cmet.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ohashi K, et al. Adiponectin promotes macrophage polarization toward an anti-inflammatory phenotype. The Journal of biological chemistry. 2010;285:6153–6160. doi: 10.1074/jbc.M109.088708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lovren F, et al. Adiponectin primes human monocytes into alternative anti-inflammatory M2 macrophages. American journal of physiology Heart and circulatory physiology. 2010;299:H656–663. doi: 10.1152/ajpheart.00115.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mandal P, Pratt BT, Barnes M, McMullen MR, Nagy LE. Molecular mechanism for adiponectin-dependent M2 macrophage polarization: link between the metabolic and innate immune activity of full-length adiponectin. The Journal of biological chemistry. 2011;286:13460–13469. doi: 10.1074/jbc.M110.204644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ji Y, et al. Activation of natural killer T cells promotes M2 Macrophage polarization in adipose tissue and improves systemic glucose tolerance via interleukin-4 (IL-4)/STAT6 protein signaling axis in obesity. The Journal of biological chemistry. 2012;287:13561–13571. doi: 10.1074/jbc.M112.350066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu D, et al. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332:243–247. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Orr JS, et al. Obesity alters adipose tissue macrophage iron content and tissue iron distribution. Diabetes. 2014;63:421–432. doi: 10.2337/db13-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.De Boer AA, Monk JM, Robinson LE. Docosahexaenoic acid decreases pro-inflammatory mediators in an in vitro murine adipocyte macrophage co-culture model. PloS one. 2014;9:e85037. doi: 10.1371/journal.pone.0085037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee JY, Sohn KH, Rhee SH, Hwang D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. The Journal of biological chemistry. 2001;276:16683–16689. doi: 10.1074/jbc.M011695200. [DOI] [PubMed] [Google Scholar]
- 59.Jung UJ, Torrejon C, Chang CL, Hamai H, Worgall TS, Deckelbaum RJ. Fatty acids regulate endothelial lipase and inflammatory markers in macrophages and in mouse aorta: a role for PPARgamma. Arteriosclerosis, thrombosis, and vascular biology. 2012;32:2929–2937. doi: 10.1161/ATVBAHA.112.300188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saraswathi V, Morrow JD, Hasty AH. Dietary fish oil exerts hypolipidemic effects in lean and insulin sensitizing effects in obese LDLR-/- mice. The Journal of nutrition. 2009;139:2380–2386. doi: 10.3945/jn.109.111567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saraswathi V, Gao L, Morrow JD, Chait A, Niswender KD, Hasty AH. Fish oil increases cholesterol storage in white adipose tissue with concomitant decreases in inflammation, hepatic steatosis, and atherosclerosis in mice. The Journal of nutrition. 2007;137:1776–1782. doi: 10.1093/jn/137.7.1776. [DOI] [PubMed] [Google Scholar]
- 62.Todoric J, et al. Adipose tissue inflammation induced by high-fat diet in obese diabetic mice is prevented by n-3 polyunsaturated fatty acids. Diabetologia. 2006;49:2109–2119. doi: 10.1007/s00125-006-0300-x. [DOI] [PubMed] [Google Scholar]
- 63.Rossi AS, et al. Dietary fish oil positively regulates plasma leptin and adiponectin levels in sucrosefed, insulin-resistant rats. American journal of physiology Regulatory, integrative and comparative physiology. 2005;289:R486–R494. doi: 10.1152/ajpregu.00846.2004. [DOI] [PubMed] [Google Scholar]
- 64.Neschen S, et al. Fish oil regulates adiponectin secretion by a peroxisome proliferator-activated receptor-gamma-dependent mechanism in mice. Diabetes. 2006;55:924–928. doi: 10.2337/diabetes.55.04.06.db05-0985. [DOI] [PubMed] [Google Scholar]
- 65.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nature reviews Immunology. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hellmann J, Tang Y, Kosuri M, Bhatnagar A, Spite M. Resolvin D1 decreases adipose tissue macrophage accumulation and improves insulin sensitivity in obese-diabetic mice. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2011;25:2399–2407. doi: 10.1096/fj.10-178657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Saraswathi V, Hasty AH. The role of lipolysis in mediating the proinflammatory effects of very low density lipoproteins in mouse peritoneal macrophages. Journal of lipid research. 2006;47:1406–1415. doi: 10.1194/jlr.M600159-JLR200. [DOI] [PubMed] [Google Scholar]
- 68.Anderson EK, Hill AA, Hasty AH. Stearic acid accumulation in macrophages induces toll-like receptor 4/2-independent inflammation leading to endoplasmic reticulum stress-mediated apoptosis. Arteriosclerosis, thrombosis, and vascular biology. 2012;32:1687–1695. doi: 10.1161/ATVBAHA.112.250142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Coenen KR, Gruen ML, Chait A, Hasty AH. Diet-induced increases in adiposity, but not plasma lipids, promote macrophage infiltration into white adipose tissue. Diabetes. 2007;56:564–573. doi: 10.2337/db06-1375. [DOI] [PubMed] [Google Scholar]
- 70.Davis JE, Gabler NK, Walker-Daniels J, Spurlock ME. Tlr-4 deficiency selectively protects against obesity induced by diets high in saturated fat. Obesity (Silver Spring) 2008;16:1248–1255. doi: 10.1038/oby.2008.210. [DOI] [PubMed] [Google Scholar]
- 71.Lee JY, et al. Reciprocal modulation of Toll-like receptor-4 signaling pathways involving MyD88 and phosphatidylinositol 3-kinase/AKT by saturated and polyunsaturated fatty acids. The Journal of biological chemistry. 2003;278:37041–37051. doi: 10.1074/jbc.M305213200. [DOI] [PubMed] [Google Scholar]
- 72.Hwang D. Modulation of the expression of cyclooxygenase-2 by fatty acids mediated through toll-like receptor 4-derived signaling pathways. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2001;15:2556–2564. doi: 10.1096/fj.01-0432com. [DOI] [PubMed] [Google Scholar]
- 73.Suganami T, Nishida J, Ogawa Y. A paracrine loop between adipocytes and macrophages aggravates inflammatory changes: role of free fatty acids and tumor necrosis factor alpha. Arteriosclerosis, thrombosis, and vascular biology. 2005;25:2062–2068. doi: 10.1161/01.ATV.0000183883.72263.13. [DOI] [PubMed] [Google Scholar]
- 74.Suganami T, et al. Role of the Toll-like receptor 4/NF-kappaB pathway in saturated fatty acid-induced inflammatory changes in the interaction between adipocytes and macrophages. Arteriosclerosis, thrombosis, and vascular biology. 2007;27:84–91. doi: 10.1161/01.ATV.0000251608.09329.9a. [DOI] [PubMed] [Google Scholar]
- 75.Saraswathi V, Hasty AH. Inhibition of long-chain acyl coenzyme A synthetases during fatty acid loading induces lipotoxicity in macrophages. Arteriosclerosis, thrombosis, and vascular biology. 2009;29:1937–1943. doi: 10.1161/ATVBAHA.109.195362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Orr JS, Puglisi MJ, Ellacott KL, Lumeng CN, Wasserman DH, Hasty AH. Toll-like receptor 4 deficiency promotes the alternative activation of adipose tissue macrophages. Diabetes. 2012;61:2718–2727. doi: 10.2337/db11-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xu X, Grijalva A, Skowronski A, van Eijk M, Serlie MJ, Ferrante AW., Jr Obesity activates a program of lysosomal-dependent lipid metabolism in adipose tissue macrophages independently of classic activation. Cell metabolism. 2013;18:816–830. doi: 10.1016/j.cmet.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kosteli A, et al. Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. The Journal of clinical investigation. 2010;120:3466–3479. doi: 10.1172/JCI42845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Koliwad SK, et al. DGAT1-dependent triacylglycerol storage by macrophages protects mice from diet-induced insulin resistance and inflammation. The Journal of clinical investigation. 2010;120:756–767. doi: 10.1172/JCI36066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42:145–151. doi: 10.1016/j.cyto.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 81.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. The Journal of clinical investigation. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Erridge C, Samani NJ. Saturated fatty acids do not directly stimulate Toll-like receptor signaling. Arteriosclerosis, thrombosis, and vascular biology. 2009;29:1944–1949. doi: 10.1161/ATVBAHA.109.194050. [DOI] [PubMed] [Google Scholar]
- 83.Schaeffler A, et al. Fatty acid-induced induction of Toll-like receptor-4/nuclear factor-kappaB pathway in adipocytes links nutritional signalling with innate immunity. Immunology. 2009;126:233–245. doi: 10.1111/j.1365-2567.2008.02892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pal D, et al. Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nature medicine. 2012;18:1279–1285. doi: 10.1038/nm.2851. [DOI] [PubMed] [Google Scholar]
- 85.Poggi M, et al. C3H/HeJ mice carrying a toll-like receptor 4 mutation are protected against the development of insulin resistance in white adipose tissue in response to a high-fat diet. Diabetologia. 2007;50:1267–1276. doi: 10.1007/s00125-007-0654-8. [DOI] [PubMed] [Google Scholar]
- 86.Suganami T, Mieda T, Itoh M, Shimoda Y, Kamei Y, Ogawa Y. Attenuation of obesity-induced adipose tissue inflammation in C3H/HeJ mice carrying a Toll-like receptor 4 mutation. Biochemical and biophysical research communications. 2007;354:45–49. doi: 10.1016/j.bbrc.2006.12.190. [DOI] [PubMed] [Google Scholar]
- 87.Tsukumo DM, et al. Loss-of-function mutation in Toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes. 2007;56:1986–1998. doi: 10.2337/db06-1595. [DOI] [PubMed] [Google Scholar]
- 88.Saberi M, et al. Hematopoietic cell-specific deletion of toll-like receptor 4 ameliorates hepatic and adipose tissue insulin resistance in high-fat-fed mice. Cell metabolism. 2009;10:419–429. doi: 10.1016/j.cmet.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jia L, et al. Hepatocyte Toll-like receptor 4 regulates obesity-induced inflammation and insulin resistance. Nature communications. 2014;5:3878. doi: 10.1038/ncomms4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lamkanfi M, Dixit VM. Inflammasomes: guardians of cytosolic sanctity. Immunological reviews. 2009;227:95–105. doi: 10.1111/j.1600-065X.2008.00730.x. [DOI] [PubMed] [Google Scholar]
- 91.Stienstra R, et al. The inflammasome-mediated caspase-1 activation controls adipocyte differentiation and insulin sensitivity. Cell metabolism. 2010;12:593–605. doi: 10.1016/j.cmet.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vandanmagsar B, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nature medicine. 2011;17:179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yuan M, et al. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science. 2001;293:1673–1677. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- 94.Chiang SH, et al. The protein kinase IKKepsilon regulates energy balance in obese mice. Cell. 2009;138:961–975. doi: 10.1016/j.cell.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Arkan MC, et al. IKK-beta links inflammation to obesity-induced insulin resistance. Nature medicine. 2005;11:191–198. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- 96.Solinas G, et al. JNK1 in hematopoietically derived cells contributes to diet-induced inflammation and insulin resistance without affecting obesity. Cell metabolism. 2007;6:386–397. doi: 10.1016/j.cmet.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 97.Vallerie SN, Furuhashi M, Fucho R, Hotamisligil GS. A predominant role for parenchymal c-Jun amino terminal kinase (JNK) in the regulation of systemic insulin sensitivity. PloS one. 2008;3:e3151. doi: 10.1371/journal.pone.0003151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sabio G, et al. A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science. 2008;322:1539–1543. doi: 10.1126/science.1160794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Han MS, et al. JNK expression by macrophages promotes obesity-induced insulin resistance and inflammation. Science. 2013;339:218–222. doi: 10.1126/science.1227568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Trayhurn P, Wang B, Wood IS. Hypoxia in adipose tissue: a basis for the dysregulation of tissue function in obesity? The British journal of nutrition. 2008;100:227–235. doi: 10.1017/S0007114508971282. [DOI] [PubMed] [Google Scholar]
- 101.Strissel KJ, et al. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes. 2007;56:2910–2918. doi: 10.2337/db07-0767. [DOI] [PubMed] [Google Scholar]
- 102.Huber J, et al. CC chemokine and CC chemokine receptor profiles in visceral and subcutaneous adipose tissue are altered in human obesity. The Journal of clinical endocrinology and metabolism. 2008;93:3215–3221. doi: 10.1210/jc.2007-2630. [DOI] [PubMed] [Google Scholar]
- 103.Weisberg SP, et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. The Journal of clinical investigation. 2006;116:115–124. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kanda H, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. The Journal of clinical investigation. 2006;116:1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Inouye KE, et al. Absence of CC chemokine ligand 2 does not limit obesity-associated infiltration of macrophages into adipose tissue. Diabetes. 2007;56:2242–2250. doi: 10.2337/db07-0425. [DOI] [PubMed] [Google Scholar]
- 106.Kirk EA, Sagawa ZK, McDonald TO, O'Brien KD, Heinecke JW. Monocyte chemoattractant protein deficiency fails to restrain macrophage infiltration into adipose tissue [corrected]. Diabetes. 2008;57:1254–1261. doi: 10.2337/db07-1061. [DOI] [PubMed] [Google Scholar]
- 107.Tsou CL, et al. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. The Journal of clinical investigation. 2007;117:902–909. doi: 10.1172/JCI29919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sullivan TJ, et al. Experimental evidence for the use of CCR2 antagonists in the treatment of type 2 diabetes. Metabolism: clinical and experimental. 2013;62:1623–1632. doi: 10.1016/j.metabol.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 109.Gutierrez DA, et al. Aberrant accumulation of undifferentiated myeloid cells in the adipose tissue of CCR2-deficient mice delays improvements in insulin sensitivity. Diabetes. 2011;60:2820–2829. doi: 10.2337/db11-0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tamura Y, et al. C-C chemokine receptor 2 inhibitor improves diet-induced development of insulin resistance and hepatic steatosis in mice. Journal of atherosclerosis and thrombosis. 2010;17:219–228. doi: 10.5551/jat.3368. [DOI] [PubMed] [Google Scholar]
- 111.Surmi BK, Webb CD, Ristau AC, Hasty AH. Absence of macrophage inflammatory protein-1{alpha} does not impact macrophage accumulation in adipose tissue of diet-induced obese mice. American journal of physiology Endocrinology and metabolism. 2010;299:E437–445. doi: 10.1152/ajpendo.00050.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kennedy A, Webb CD, Hill AA, Gruen ML, Jackson LG, Hasty AH. Loss of CCR5 results in glucose intolerance in diet-induced obese mice. American journal of physiology Endocrinology and metabolism. 2013;305:E897–906. doi: 10.1152/ajpendo.00177.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kitade H, et al. CCR5 plays a critical role in obesity-induced adipose tissue inflammation and insulin resistance by regulating both macrophage recruitment and M1/M2 status. Diabetes. 2012;61:1680–1690. doi: 10.2337/db11-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gruen ML, Hao M, Piston DW, Hasty AH. Leptin requires canonical migratory signaling pathways for induction of monocyte and macrophage chemotaxis. American journal of physiology Cell physiology. 2007;293:C1481–1488. doi: 10.1152/ajpcell.00062.2007. [DOI] [PubMed] [Google Scholar]
- 115.Gutierrez DA, Hasty AH. Haematopoietic leptin receptor deficiency does not affect macrophage accumulation in adipose tissue or systemic insulin sensitivity. The Journal of endocrinology. 2012;212:343–351. doi: 10.1530/JOE-11-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dib LH, Ortega MT, Fleming SD, Chapes SK, Melgarejo T. Bone marrow leptin signaling mediates obesity-associated adipose tissue inflammation in male mice. Endocrinology. 2014;155:40–46. doi: 10.1210/en.2013-1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hasty AH, Gutierrez DA. What have we really learned about macrophage recruitment to adipose tissue? Endocrinology. 2014;155:12–14. doi: 10.1210/en.2013-2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Westcott DJ, et al. MGL1 promotes adipose tissue inflammation and insulin resistance by regulating 7/4hi monocytes in obesity. The Journal of experimental medicine. 2009;206:3143–3156. doi: 10.1084/jem.20091333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Feng B, et al. Clodronate liposomes improve metabolic profile and reduce visceral adipose macrophage content in diet-induced obese mice. PloS one. 2011;6:e24358. doi: 10.1371/journal.pone.0024358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bu L, Gao M, Qu S, Liu D. Intraperitoneal injection of clodronate liposomes eliminates visceral adipose macrophages and blocks high-fat diet-induced weight gain and development of insulin resistance. The AAPS journal. 2013;15:1001–1011. doi: 10.1208/s12248-013-9501-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bodles AM, et al. Pioglitazone induces apoptosis of macrophages in human adipose tissue. Journal of lipid research. 2006;47:2080–2088. doi: 10.1194/jlr.M600235-JLR200. [DOI] [PubMed] [Google Scholar]
- 122.Amano SU, et al. Local proliferation of macrophages contributes to obesity-associated adipose tissue inflammation. Cell metabolism. 2014;19:162–171. doi: 10.1016/j.cmet.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Haase J, et al. Local proliferation of macrophages in adipose tissue during obesity-induced inflammation. Diabetologia. 2014;57:562–571. doi: 10.1007/s00125-013-3139-y. [DOI] [PubMed] [Google Scholar]
- 124.Ramkhelawon B, et al. Netrin-1 promotes adipose tissue macrophage retention and insulin resistance in obesity. Nature medicine. 2014;20:377–384. doi: 10.1038/nm.3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Randolph GJ. Mechanisms that regulate macrophage burden in atherosclerosis. Circulation research. 2014;114:1757–1771. doi: 10.1161/CIRCRESAHA.114.301174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nishimura S, et al. Adipogenesis in obesity requires close interplay between differentiating adipocytes, stromal cells, and blood vessels. Diabetes. 2007;56:1517–1526. doi: 10.2337/db06-1749. [DOI] [PubMed] [Google Scholar]
- 127.Cho CH, et al. Angiogenic role of LYVE-1-positive macrophages in adipose tissue. Circulation research. 2007;100:e47–57. doi: 10.1161/01.RES.0000259564.92792.93. [DOI] [PubMed] [Google Scholar]
- 128.Recalcati S, et al. Differential regulation of iron homeostasis during human macrophage polarized activation. European journal of immunology. 2010;40:824–835. doi: 10.1002/eji.200939889. [DOI] [PubMed] [Google Scholar]
- 129.Corna G, et al. Polarization dictates iron handling by inflammatory and alternatively activated macrophages. Haematologica. 2010;95:1814–1822. doi: 10.3324/haematol.2010.023879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Boyle JJ, et al. Coronary intraplaque hemorrhage evokes a novel atheroprotective macrophage phenotype. The American journal of pathology. 2009;174:1097–1108. doi: 10.2353/ajpath.2009.080431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Finn AV, et al. Hemoglobin directs macrophage differentiation and prevents foam cell formation in human atherosclerotic plaques. Journal of the American College of Cardiology. 2012;59:166–177. doi: 10.1016/j.jacc.2011.10.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Boyle JJ, et al. Activating transcription factor 1 directs Mhem atheroprotective macrophages through coordinated iron handling and foam cell protection. Circulation research. 2012;110:20–33. doi: 10.1161/CIRCRESAHA.111.247577. [DOI] [PubMed] [Google Scholar]
- 133.Morris DL, et al. Adipose tissue macrophages function as antigen-presenting cells and regulate adipose tissue CD4+ T cells in mice. Diabetes. 2013;62:2762–2772. doi: 10.2337/db12-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nguyen KD, et al. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011;480:104–108. doi: 10.1038/nature10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Li P, et al. Functional heterogeneity of CD11c-positive adipose tissue macrophages in diet-induced obese mice. The Journal of biological chemistry. 2010;285:15333–15345. doi: 10.1074/jbc.M110.100263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Clement K, et al. Weight loss regulates inflammation-related genes in white adipose tissue of obese subjects. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2004;18:1657–1669. doi: 10.1096/fj.04-2204com. [DOI] [PubMed] [Google Scholar]
- 137.Hamm P, Shekelle RB, Stamler J. Large fluctuations in body weight during young adulthood and twenty-five-year risk of coronary death in men. Am J Epidemiol. 1989;129:312–318. doi: 10.1093/oxfordjournals.aje.a115135. [DOI] [PubMed] [Google Scholar]
- 138.Olson MB, et al. Weight cycling and high-density lipoprotein cholesterol in women: evidence of an adverse effect: a report from the NHLBI-sponsored WISE study. Women's Ischemia Syndrome Evaluation Study Group. Journal of the American College of Cardiology. 2000;36:1565–1571. doi: 10.1016/s0735-1097(00)00901-3. [DOI] [PubMed] [Google Scholar]
- 139.Waring ME, Eaton CB, Lasater TM, Lapane KL. Incident diabetes in relation to weight patterns during middle age. Am J Epidemiol. 2010;171:550–556. doi: 10.1093/aje/kwp433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Anderson EK, Gutierrez DA, Kennedy A, Hasty AH. Weight cycling increases T-cell accumulation in adipose tissue and impairs systemic glucose tolerance. Diabetes. 2013;62:3180–3188. doi: 10.2337/db12-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Almind K, Kahn CR. Genetic determinants of energy expenditure and insulin resistance in diet-induced obesity in mice. Diabetes. 2004;53:3274–3285. doi: 10.2337/diabetes.53.12.3274. [DOI] [PubMed] [Google Scholar]
- 142.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 143.Rebholz SL, et al. Multiparity leads to obesity and inflammation in mothers and obesity in male offspring. American journal of physiology Endocrinology and metabolism. 2012;302:E449–457. doi: 10.1152/ajpendo.00487.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]