Abstract
Previously published reports indicate that serum copper levels are elevated in prostate cancer (PCa) patients and that increased copper uptake can be used as a means to image prostate tumors. It is unclear, however, to what extent copper is required for PCa cell function as we observed only modest effects of chelation strategies on the growth of these cells in vitro. With the goal of exploiting PCa cell proclivity for copper uptake, we developed a “conditional lethal” screen to identify compounds whose cytotoxic actions were manifest in a copper-dependent manner. Emerging from this screen were a series of dithiocarbamates, which when complexed with copper, induced Reactive Oxygen Species (ROS)-dependent apoptosis of malignant, but not normal, prostate cells. One of the dithiocarbamates identified, Disulfiram (DSF), is an FDA approved drug that has previously yielded disappointing results in clinical trials in patients with recurrent prostate cancer. Similarly, in our studies DSF alone had a minimal effect on the growth of PCa tumors when propagated as xenografts. However, when DSF was coadministered with copper a very dramatic inhibition of tumor growth in models of hormone sensitive and of castrate resistant disease was observed. Furthermore, we determined that prostate cancer (PCa) cells express high levels of CTR1, the primary copper transporter, and additional chaperones that are required to maintain intracellular copper homeostasis. The expression levels of most of these proteins are increased further upon treatment of AR-positive PCa cell lines with androgens. Not surprisingly robust CTR1-dependent uptake of copper into PCa cells was observed; an activity that was accentuated by activation of androgen receptor (AR). Given these data linking AR to intracellular copper uptake, we believe that dithiocarbamate/copper complexes are likely to be effective for the treatment of PCa patients whose disease is resistant to classical androgen ablation therapies.
INTRODUCTION
Patients with prostate cancer (PCa) typically undergo a regimen of endocrine treatments designed to block the transcriptional activity of the androgen receptor (AR) by either decreasing the production of endogenous androgens (e.g. GnRH agonists) or competitively inhibiting the activities of androgenic ligands (e.g. bicalutamide) [1]. Unfortunately, these therapies are not curative, and most patients experience relapse of their disease to a hormone-refractory or castration-resistant state. In castration-resistant prostate cancer (CRPC), the AR signaling axis remains intact, and, thus, most cancers are initially responsive to second line endocrine therapies such as CYP17 inhibitors (Abiraterone) and next-generation AR antagonists (Enzalutamide) [2–5]. The therapeutic lifespan of these drugs is relatively short, and there is a growing sentiment in the field that the resolution of the problem of resistance does not lie in the development of better AR antagonists/steroid synthesis inhibitors. This position is supported by a large amount of data indicating that the current approaches to achieve androgen ablation can interrupt regulatory feedback loops leading to (a) upregulation of steroidogenesis [6, 7], (b) increased receptor and coregulator expression [8, 9], (c) neuroendocrine differentiation of PCa cells [10, 11], and (d) the selection for gene translocations/RNA splicing events or receptor mutations that result in the production of constitutively active AR variants [12–14]. These activities have been confirmed in human tumors and hence are likely to contribute in a significant manner to treatment failure [15]. Thus, although tremendous progress has been made of late in treating prostate cancer, there is clearly a need for agents that target this disease in a unique manner.
Whereas most attention has focused on AR as a therapeutic target, it is clear that there are other useful targets, the pharmaceutical exploitation of which are likely to impact PCa progression. Notable in this regard are clinical studies that have highlighted the potential utility of targeting MET, SRC, AKT and several different growth factor receptors [16]. To complement this approach we mined the current literature on PCa and available gene expression data to identify pathways and processes whose activity was restricted or particularly active in PCa. In this manner we determined that all of the major components of the processes involved in uptake and trafficking of copper were upregulated in PCa. Interestingly, there is considerable data indicating that both circulating and intratumoral copper levels are elevated in PCa patients [17–20]. It is not clear why PCa has such a proclivity for copper uptake although copper has been suggested to play a role in RAS/MAP kinase pathway activation, a pathway that has been shown to have a role in cancer pathogenesis [21]. Whereas copper is an essential trace element required for the activity of a large number of enzymes and structural proteins, the concentrations within PCa clearly exceed that required to support viability. Herein we describe our efforts to define the mechanisms by which PCa cells regulate copper homeostasis and how this can be exploited in the development of therapeutic agents that disrupt these activities.
MATERIALS AND METHODS
Chemicals
Methyltrienolone (R1881) was purchased from PerkinElmer (Waltham, MA) and dissolved in DMSO. TTM, DSF, PDTC, BCS, CuSO4, NAC and LGR were obtained from Sigma (St Louis, MO). TTM, DSF and PDTC were dissolved in DMSO while BCS, CuSO4, NAC and LGR in water. EUK118 was from Cayman Chemical (Ann Arbor, MI). Copper-64 was prepared at Washington University; St. Louis, MO.
Cell culture
VCaP, LNCaP, 22RV1, PC3 and RWPE-1 parental cell lines were obtained from the American Type Culture Collection (ATCC, Manassas, VA); which authenticates cell lines by short tandem repeat profiling and passaged for less than 6 months. VCaP cells were cultured in DMEM while LNCaP, 22RV1 and PC3 cells in RPMI 1640 (Life Technologies™, Grand Island, NY). All medias were supplemented with 8% fetal bovine serum (Sigma), 1 mM sodium pyruvate and 0.1 mM non-essential amino acids (Life Technologies™) and cells were maintained in a 37°C incubator with 5% CO2. For androgen treatment experiments, cells were plated in the same media lacking phenol red and supplemented with 8% charcoal-stripped fetal bovine serum.
Proliferation assay
VCaP (10,000 cells/well) or LNCaP, 22RV1, PC3, RWPE-1 (5,000 cells/well) were seeded in 96-well plates containing regular FBS containing media. The following day, the cells were treated as indicated for an additional 72 hr. Cell proliferation was measured using a fluorometric resazurin reduction method (Cell Titer-Blue, Promega, Madison, WI). Each sample was performed in triplicate, and the results from a representative experiment are shown. Results are expressed as relative fluorescence ± SE (n=3).
Apoptosis assay
VCaP cells were collected and double stained with Alexa Fluor 488 Annexin V and Sytox (Invitrogen) according to the manufacturer’s instruction. Annexin V-positive cells were considered apoptotic, and their percentage of the total number of cells was calculated. Ten thousand events were collected for each sample using a BD Accuri C6 flow cytometer (BD San Jose, CA) and data were analyzed using the CFlow Plus program software (BD San Jose, CA).
ROS generation assay
Treated cells were stained with the oxidation-sensitive dye H2DCFDA (5 µmol/L) for 60 min at 37°C and the treatment was terminated by ice-cold PBS. ROS generation was determined by FACS and quantified as the geometric mean fluorescence of a representative experiment. The increase in fluorescence of treated versus untreated samples is shown.
Xenograft study
Five-week-old NOD.SCID.gamma male mice were obtained from the Cancer Center Isolation Facility (Duke Cancer Institute, Durham, NC). All experiments were performed according to local animal experimental ethics committee guidelines, and were approved by an institutional review committee (IACUC). VCaP (2 × 106) Cells in 50% matrigel (BD Matrigel Matrix, BD Biosciences, San Jose, CA) were injected subcutaneously into the mouse right flank. Tumor size was measured with calipers and tumor volume was calculated using the formula volume = width2 × length / 2. When tumor size reached 200 mm3, mice bearing tumors were randomized into 4 groups (n=12). Each group received two separate daily i.p. injections of the following treatments: (1) Control group (vehicle, vehicle) received 100 µl DMSO-Corn oil and 100 µl of physiological saline (0.9%). (2) The copper treated group received 100 µl (DMSO-Corn oil) and 100 µl of CuSO4 in physiological saline (0.5mg/kg). (3) The DSF treated group received 100 µl of DSF (30mg/kg in DMSO-Corn oil) and 100 µl physiological saline. (4) The DSF/Copper group received 100 µl of DSF (30 mg/kg in DMSO-Corn oil) and 100 µl of CuSO4 (0.5mg/kg) physiological saline. For 22RV1 xenograft study, 1 × 106 cells were injected subcutaneously into the mouse right flank. When tumor size reached approximately 150 mm3, mice bearing tumors were randomized into 2 groups (n=5) to receive daily i.p. injections of the control (100 µl DMSO-Corn oil and 100 µl of physiological saline (0.9%) or DSF/Copper (100 µl of DSF (30 mg/kg in DMSO-Corn oil) and 100 µl of CuSO4 (0.5mg/kg)). The study was terminated and mice were humanely euthanized when tumor volume reached ~1,000 mm3 (VCAP) or after 16 days of treatment (22RV1). Tumor growth up to day 16 was analyzed by two-way ANOVA followed by Bonferroni’s multiple comparison test.
Gene expression analysis
Total RNA was isolated using the Aurum™ Total RNA Mini-Kit according to the manufacturer’s instructions (Bio-Rad, Hercules, CA). Total RNA (1µg) was reverse-transcribed to cDNA using iScript™ cDNA synthesis Kit (Bio-Rad). The resulting cDNA was diluted 1:20 with water to use in qPCR analysis. qPCR was performed using the Bio-Rad SYBR green supermix with 0.2 M of each forward and reverse primer and 2.5 l or 1.25 l of diluted cDNA in a total reaction volume of 6.5 l or 3.25 l. PCR amplification was carried out using the Bio-Rad iQ4 or the CFX384 qPCR system. All primers used in the study were tested to have PCR efficiency between 100+/−10% and span intron/exon boundaries when possible. Fold induction was calculated using the 2−ΔΔCt method and GAPDH was used as the normalization control. Data shown are representative of at least three independent experiments. Gene-specific primers were purchased from Integrated DNA Technologies (Coralville, IA).
Immunoblot analysis
Whole-cell extracts were prepared using RIPA buffer [50 mmol/L Tris-HCl (pH 7.5), 150 mmol/L NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.05% SDS, 1 mmol/L EDTA, protease inhibitors (Sigma)]. Lysates were separated on SDS-PAGE (7% polyacrylamide gels), transferred onto nitrocellulose membranes, and detected using the following antibodies: CTR1 [22] or glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Santa Cruz Biotechnology, Dallas, TX).
RNA silencing: (Small Interfering RNA Transfection)
Synthetic small interfering RNA (siRNA) to knock down endogenous AR, CTR1, and nonspecific siRNA-control (siCTRL) were synthesized by Invitrogen, (Life Technologies™, Grand Island, NY). VCaP cells were plated at equal densities in six-well plates (3.3×105 per well) overnight. Subsequently, cells were transfected with 50nmol/L siCTRL or siRNA against AR or CTR1 using DharmaFECT 1 transfection reagent following the manufacturer’s protocol (Dharmacon, Lafayette, CO). 48 hr. post-transfection, the cells were treated overnight with 10nM R1881 and harvested for qPCR analysis or copper uptake studies.
64Cu uptake assay
VCaP cells were treated with 64Cu (5µCi/ml as CuCl2) at 37°C in serum free medium and incubated for different time periods. After each incubation, cells were washed three times with cold PBS, treated with Cell Lysis Reagent (Promega) and the resulting cell lysates were transferred to separate micro-centrifuge tubes for gamma counting. The radioactivity in cell lysates as well as corresponding supernatants was determined using a gamma counter (Wallac Wizard 1480, Perkin-Elmer) and the values were normalized to the total protein content of cells for each well as determined by the Bradford assay.
RESULTS
Disruption of intracellular copper homeostasis as a means to inhibit prostate cancer cell growth
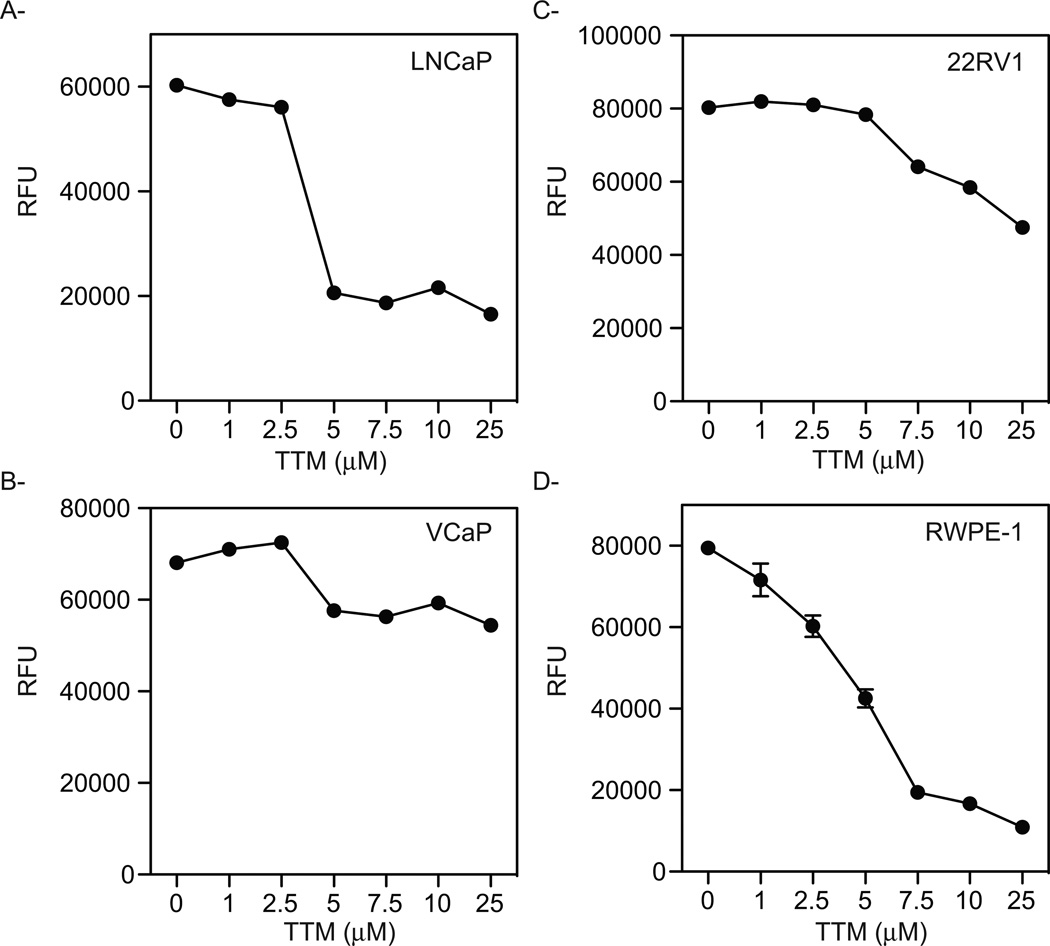
Previous studies have shown that human PCa xenografts propagated in mice have the ability to take up copper [23, 24]. However, the utility of manipulating copper uptake or intracellular copper biology has not been explored as a therapeutic modality in PCa. Thus, as a first step we undertook a study to evaluate the impact of copper chelation on PCa cell proliferation in vitro. As shown in Figure 1A, treatment with the copper chelator tetrathiomolybdate (TTM) efficiently inhibited the growth of AR-expressing LNCaP cells. Further, TTM also inhibited the growth of two different cellular models of CRPC: VCaP cells in which AR is overexpressed (Fig. 1B) and 22RV1 cells which express a constitutively active AR-splice variant (Fig. 1C). However, the effects of TTM in these cell lines were not as dramatic as that observed in LNCaP cells and could only be achieved when high concentrations of the chelator were used. The most disappointing finding, however, was that TTM treatment also inhibited the growth of transformed normal epithelial RWPE-1 cells (Fig. 1D), suggesting that the therapeutic window for copper chelation therapy was extremely small. It was concluded therefore that copper chelation in and of itself was not likely to be a viable therapeutic option for PCa.
Figure 1. Effect of copper chelation on the growth of prostate cancer cells.
LNCaP (A), VCaP (B), and 22RV1 (C) malignant prostate cells as well as the normal epithelial RWPE-1 cell line (D) were treated for 72 hr. with increasing concentrations of TTM. Cell viability was measured using a fluorometric resazurin reduction method and results are expressed as relative fluorescence ± SE (n=3). Representative experiments are shown.
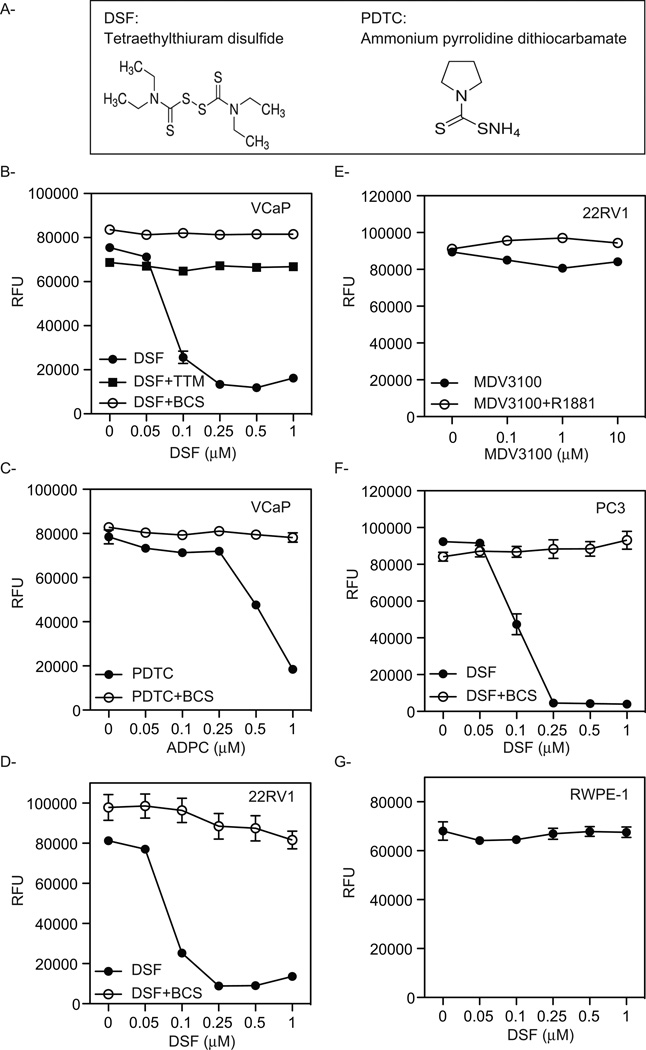
The uptake and regulation of intracellular copper levels are tightly regulated reflecting the balance between the requirement for copper as a cofactor for many enzymes and structural proteins and the need to prevent the oxidative damage that is associated with the redox cycling of elemental copper within cells [25]. We reasoned, therefore, that it might be possible to employ a synthetic lethality approach to identify drugs whose cytostatic/cytocidal activity would capitalize on the ability of PCa cells to take up significant amounts of copper. Thus, we screened for small molecules that would inhibit the growth or induce apoptosis of VCaP cells and whose activity was attenuated by the co-administration of a sub-toxic dose of the copper chelator TTM. Using this approach we identified two chemically distinct drugs that are members of the dithiocarbamate family of metal binding compounds (Fig. 2A). One of the compounds identified, DSF (Disulfiram, tetraethylthiuram disulfide), has previously been identified in high-content screens for PCa therapeutics [26]. It is an orally administered drug that is approved for use in alcohol aversion therapy [27, 28]. The second drug identified, ammonium pyrrolidine dithiocarbamate (PDTC), is a known copper binding compound and has only been tested in vitro for anticancer activity [29]. Importantly, follow-up studies confirmed that when tested over a broad range of concentrations the growth inhibitory activity of these drugs in VCaP cells could be reversed by co-administration of a sub-toxic dose of the copper chelators TTM or Bathocuproine disulfonate (BCS) (Fig. 2B and C). Similarly, DSF dramatically inhibits the growth of 22RV1 cells and this activity can be reversed with BCS (Fig. 2D). This result is particularly significant as this cell line expresses a truncated constitutively active AR variant that renders the cells resistant to all of the currently available androgen synthesis inhibitors and antiandrogens (Fig. 2E) and is a well-validated model of advanced disease. DSF also has a profound copper-dependent effect on the growth of the AR-negative PC3 PCa cell line (Fig. 2F). Thus, the cytotoxic effect of DSF is not restricted to AR-positive prostate cancer cells. Finally, it was shown that DSF had no effect on the growth of normal RWPE-1 cells at the concentrations tested (Fig. 2G). We conclude from these studies that DSF and PDTC inhibit PCa cell growth in a manner that likely capitalizes on the ability of these cells to accumulate copper. Defining the specific role for copper in dithiocarbamate activity was the goal of our next series of studies.
Figure 2. Identification of compounds that rely on copper to inhibit the growth of prostate cancer cells.
(A) Chemical structure of the compounds identified. VCaP cells were treated for 72 hr. with increasing concentrations of DSF alone or in combination with sub toxic doses of the copper chelators TTM or BCS (B) or with PDTC alone or in combination BCS (C). D, 22RV1 cells were treated for 72 hr. with increasing concentrations of DSF alone or in combination with BCS. E, 22RV1 cells were treated for 72 hr. with increasing concentrations of MDV3100 alone or in combination with R1881. The AR negative PC3 (F) or the normal immortalized prostate RWPE-1 (G) cell lines were treated for 72 hr. with increasing concentrations of DSF alone or in the presence of the copper chelator BCS. The results from representative experiments are shown and are expressed as relative fluorescence ± SE (n=3).
The growth inhibitory activity of DSF is absolutely dependent on copper
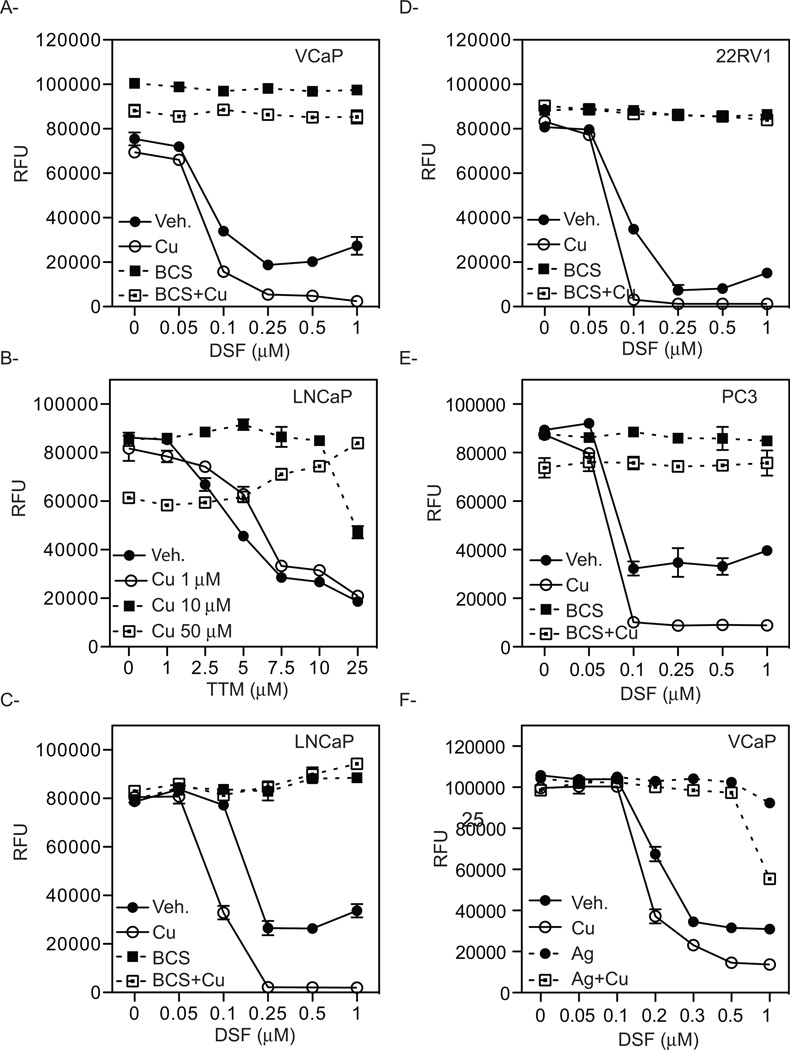
Considering the copper binding specificity of the two chelators (TTM and BCS) utilized in our studies, it was likely that the growth inhibitory activity of DSF required its interaction with copper. We therefore asked whether copper supplementation could enhance the growth inhibitory activity of DSF. To this end, VCaP cells were treated with increasing concentrations of DSF alone or with copper supplementation. In this manner, we showed that copper supplementation enhances the cytotoxic effect of DSF (Fig. 3A). Thus, as opposed to the ability of passive copper chelation by TTM to inhibit PCa cell proliferation (Fig. 1), the inhibitory activity of DSF was attenuated by the co-administration of copper, highlighting the distinct mechanisms of action of the two drugs (Fig. 3B). The ability of copper to enhance DSF cytotoxicity was confirmed in the AR-positive PCa cell lines LNCaP and 22RV1, as well as in the AR-negative PC3 cells (Fig. 3C–E). As expected, the treatment with the copper chelator BCS abrogated the cytotoxic effect of DSF in the presence of additional copper in all cells. Together, these results suggest that although the available copper in the media used in these studies is sufficient for DSF action, the efficacy of the drug can be enhanced significantly by additional copper supplementation.
Figure 3. Copper supplementation enhances the cytotoxic effect of DSF.
A, VCaP cells were treated for 72 hr. with increasing concentrations of DSF alone or in the presence of 0.1 µM CuSO4. Copper supplementation enhances the cytocidal effect of DSF and treatment with the copper chelator BCS reversed the anti-proliferative effect of DSF alone or when in the presence of copper. B, LNCaP cells were treated for 72 hr. with increasing concentrations of the copper chelator TTM. Copper supplementation reverses the anti-proliferative effect of TTM. LNCaP cells (C), 22RV1 (D), and PC3 (E) prostate cancer cells were treated for 72 hr. with increasing concentration of DSF. Co-treatment with 0.1 µM of CuSO4 enhances the cytocidal effect of DSF. F, VCaP cells were treated with increasing concentration of DSF alone or in the presence of copper. Co-treatment with excess of Ag+NO3+ reverses the cytotoxic effects of DSF.
Reduction of copper from Cu(II) to Cu(I) by the metalloreductases is an obligate step for efficient transport across the plasma membrane by the high-affinity transporter CTR1 [30, 31]. Indeed it has been shown previously that divalent metal ions do not inhibit copper uptake by CTR1, whereas an excess of silver ions that are isoelectronic and of similar size to Cu(I) block the CTR1-mediated copper uptake [32]. We therefore assessed the ability of Ag(I) to block DSF-mediated inhibition of cell growth. To this end, VCaP cells were treated with increasing concentrations of DSF alone or in the presence of additional copper to enhance the anti-proliferative effect of DSF. Interestingly, co-administration of excess silver ions abolishes the cytotoxicity induced by both DSF alone or DSF in the presence of copper (Fig. 3F). This result is consistent with a role for CTR1-mediated uptake of copper in regulating the cytotoxic actions of DSF. In previous studies using cellular models of melanoma it has been shown that DSF:Au and DSF:Ag complexes are growth inhibitory [33]. However, the ability to reverse DSF:Cu activity with Ag suggests that the DSF:Cu complex is functioning in a different manner in PCa cells. These data also suggest that tumors with high intrinsic copper levels, or those which can be manipulated to uptake copper, are likely to exhibit the greatest therapeutic response to DSF.
DSF induces prostate cancer cell apoptosis
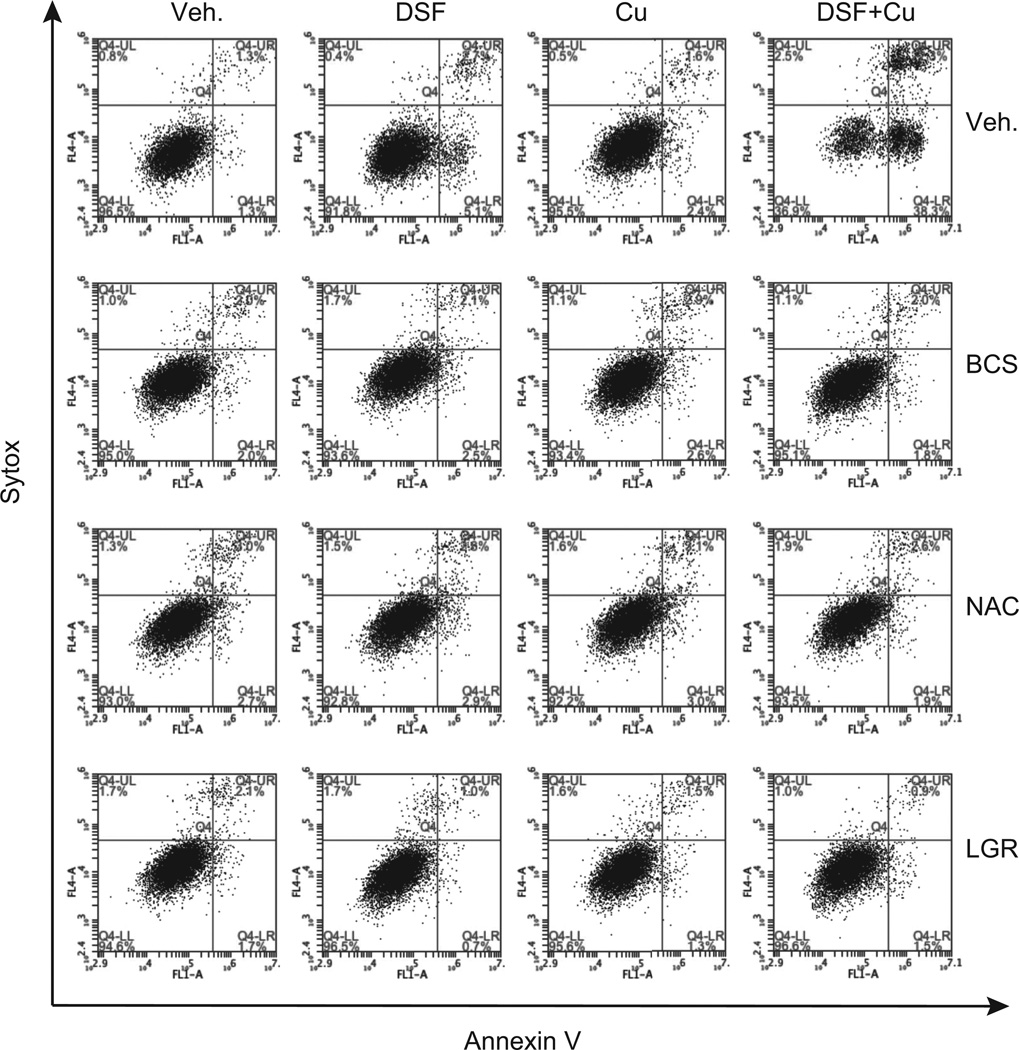
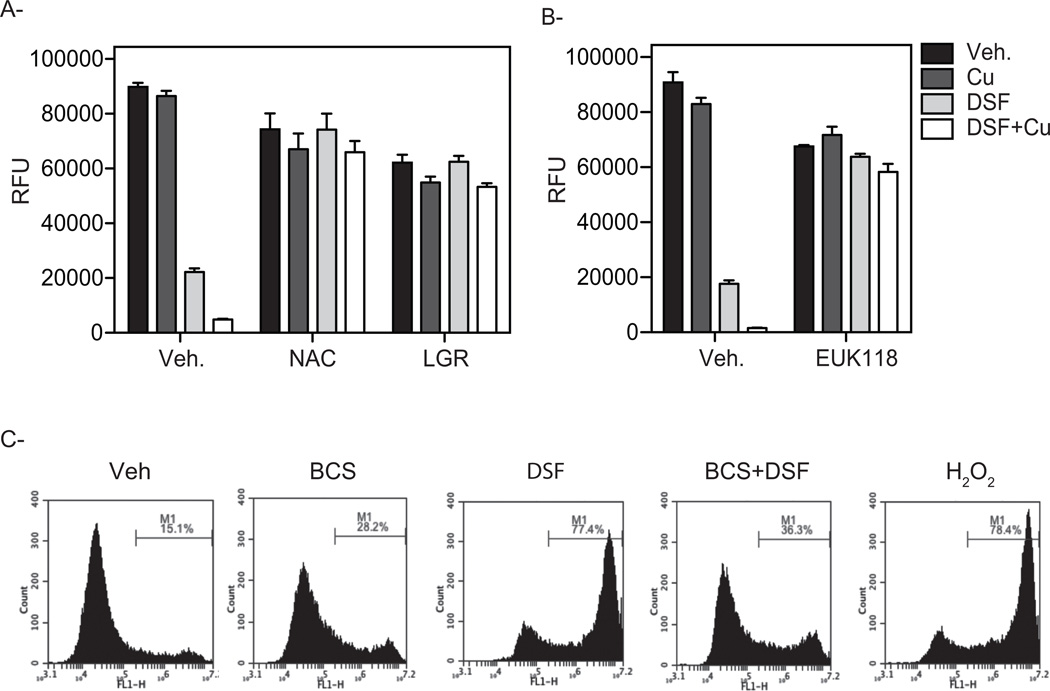
The impact of DSF and DSF:Cu on PCa cell apoptosis was evaluated by measuring Annexin V staining using flow cytometry. As shown in Figure 4, (and supplemental Fig. S1) treatment of VCaP cells for 24 hr. with DSF alone increased the Annexin V-positive cell population (5.1% of Annexin V+/Sytox−, and 2.7% of Annexin V+/Sytox+) as compared to control treated cells (1.3% of Annexin V+/Sytox−, and 1.3% of Annexin V+/Sytox+). Even more pronounced effects of DSF on VCaP cells were observed at later time points (supplemental Fig. S2 (72hr. time point)). As expected the pro-apoptotic activity of DSF was dramatically enhanced in the presence of copper (DSF alone: 5.1% of Annexin V+/Sytox−, and 2.7% of Annexin V+/Sytox+, DSF + Cu: 38.8% of Annexin V+/Sytox−, and 22.3% of Annexin V+/Sytox+, (Fig. 4)). Addition of copper alone was without effect. In all cases DSF-dependent apoptosis was abrogated by the copper chelator BCS (Fig. 4). Together, these results indicate that DSF-mediated inhibition of prostate cancer cell growth can be attributed in large part to its ability to induce apoptosis in a copper-dependent manner.
Figure 4. Disulfiram (DSF) induces prostate cancer cell apoptosis.
VCaP cells were incubated with vehicle, DSF, copper alone or DSF in combination with copper. The effects of the copper chelation (BCS) as well as the antioxidants (NAC and LGR) were evaluated. Following 24 hr. drug treatments cells were harvested, stained with Annexin V-Alexa Fluor® 488 and Sytox red dead cells staining, and analyzed by flow cytometry. The populations of Annexin V−/Sytox−, Annexin V+/Sytox−, and Annexin V+/Sytox+ correspond to live cells, early apoptotic cells, and late apoptotic cells respectively. Representative plots of one set of triplicate experiments are presented.
ROS as a mediator of the copper-dependent cytotoxic effects of DSF
Copper, a transition metal with multiple oxidation states, can participate in single electron transfer reactions. Therefore, given the chemistry of the interaction between copper and DSF, we hypothesized that the inherent redox shuttling of copper (Cu(I)/Cu(II)/Cu(III) within Cu-dithiocarbamate complexes could generate free radicals that would facilitate ROS formation [34–36]. It was of significance, therefore, that treatment of cells with the free-radical scavenger N-acetyl-L-cysteine (NAC) blocked cell death induced by DSF:Cu, Similar results were obtained when PCa cells were treated with the free-radical scavenger, L-Glutathione reduced (LGR) in the presence of DSF alone or in combination with supplementary copper (Fig. 4). These antioxidants also blocked the cytotoxic actions of DSF:Cu in other PCa cells (Fig. S2 and data not shown).
Both of the antioxidants NAC and LGR reversed the anti-proliferative effect of DSF in the presence or absence of added copper (Fig. 5A). Further, co-treatment of the VCaP cells with EUK118, a synthetic catalytic scavenger of ROS with superoxide dismutase and catalase mimetic activity [37] blocked DSF-mediated inhibition of cell growth (Fig. 5B). Together, these results implicate ROS in DSF-mediated cell death. Thus, we directly measured intracellular free radical production in DSF-treated cells using the cell permeable oxidation sensitive dye CM-H2DCFDA. For this assay, VCaP cells treated for 2 days with H2O2 were used as positive controls. Using this approach, we were able to establish that addition of DSF resulted in robust CM-H2DCFDA oxidation when compared with control-treated cells (Fig. 5C). To analyze whether elevation of intracellular copper is a prerequisite for the induction of oxidative stress, VCaP cells were co-incubated with the copper chelator BCS. As seen in Figure 5C, co-treatment with the copper chelator BCS blocked both DSF-dependent generation of ROS and apoptosis. These data are consistent with the idea that DSF:Cu induces PCa cell apoptosis secondary to its ability to induce intracellular ROS production.
Figure 5. Antioxidants reverse the inhibitory effect of DSF on prostate cancer cell growth.
VCaP cells were treated for 72 hr. with increasing concentrations of DSF alone or in combinations with 0.1 µM of CuSO4. A, Co-treatment with the antioxidants NAC or LGR reverse the inhibitory growth activity exerted by DSF on VCaP cells. B, EUK118, a synthetic catalytic scavenger of ROS with superoxide dismutase and catalase mimetic activity blocked DSF mediated inhibition of cell growth. C, Disulfiram induces ROS production. VCaP cells were incubated for 48 hr. with vehicle or DSF either alone or in combination with copper. The effect of the copper chelator BCS was also evaluated. As a positive control VCaP cells were treated with 500 µM H2O2. Cells were then incubated with 10 µM of CM-H2DCFDA for 60 min at 37°C, washed twice with PBS and the intensity of fluorescence was measured using flow cytometry. A representative result from one of three experiments is shown.
Copper enhances the growth inhibitory activity of DSF in xenograft models of prostate cancer
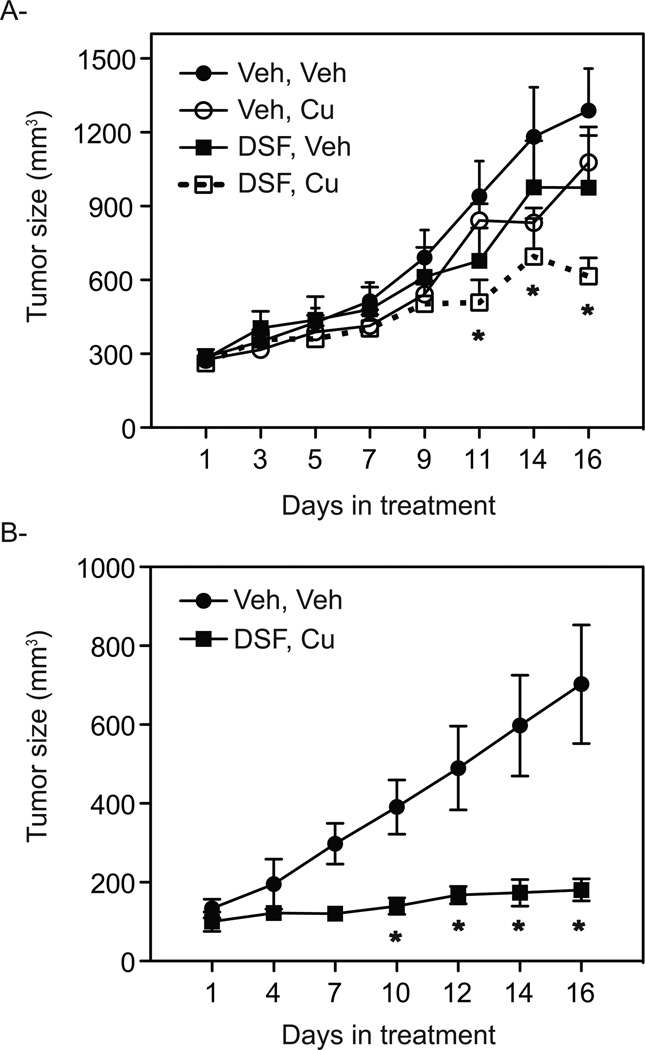
In agreement with our in vitro data, it has been demonstrated, using positron emission tomography (PET) imaging, that human PCa xenografts propagated as tumors in mice have a high capacity to uptake and accumulate copper [23, 24]. We therefore asked whether the therapeutic activity of DSF could be enhanced using copper supplementation to increase intratumoral copper within VCaP cells propagated as xenografts in immunodeficient mice. To this end, the effect of DSF alone or in combination with copper treatment was evaluated. For comparative purposes, a vehicle control group and a copper alone group were also included in this study. In this manner, it was shown that while DSF alone had only marginal effects on tumor growth, treatment with a combination of DSF and copper significantly decreased tumor growth (Fig. 6A). Subsequently, the effect of DSF and copper in a well-validated model of CRPC was evaluated. To this end, 22RV1 cells, which express a constitutively active AR-splice variant, were propagated as xenografts in castrated, immunodeficient mice. Similar to what we observed in VCaP cells, DSF:Cu efficiently inhibited the growth of 22RV1 tumors when compared to vehicle treated mice (Fig. 6B). Taken together, the in vivo data are consistent with the in vitro data and reinforce the concept that the combined treatment of DSF and copper has superior activity in targeting PCa cells than either agent alone with no observable increase in animal toxicity or weight loss.
Figure 6. Copper enhances the inhibitory effect of Disulfiram on tumor growth.
A. Tumor growth rate of a subcutaneous VCaP xenograft in male NOD SCID gamma mice is represented. Tumor size was allowed to proceed until they reached 0.2 cm3, at which time mice were randomized into 4 groups (n=12) and treated with either vehicle, copper, DSF alone or DSF in combination with copper. B. Mice bearing 22RV1 xenograft tumors were grown until ~ 0.15 cm3 tumor volume, at which time mice were randomized into two group (n=5) to receive daily treatment with either vehicle or DSF in combination with copper. Data points are mean of tumor volume in each experimental group; error bars are SE. Statistical significance from Veh, Veh is denoted by stars (*), (p<0.05).
AR upregulates the expression of key proteins required for cellular copper homeostasis
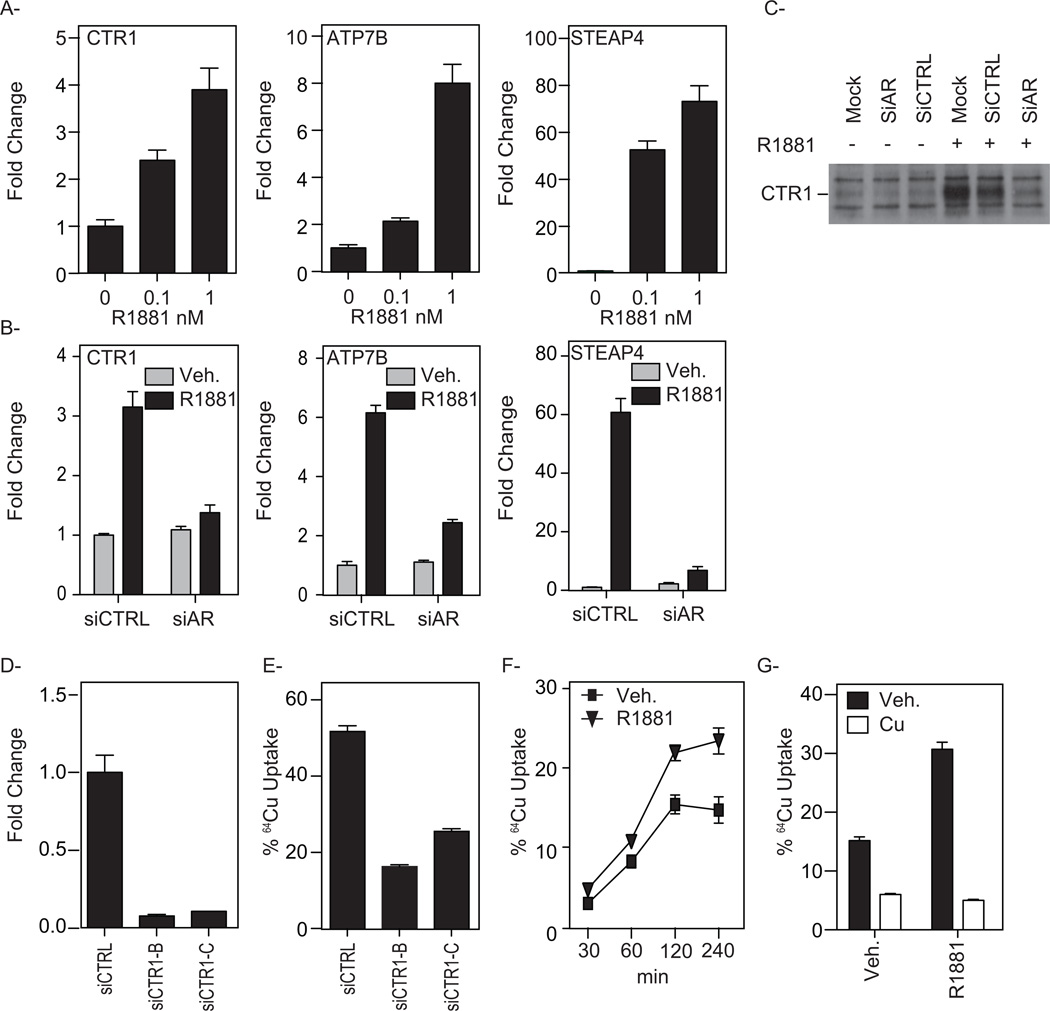
Whereas the antiproliferative activities of DSF observed were not restricted to AR-positive PCa cells, we were intrigued by the observation that the expression of several proteins involved in the uptake and trafficking of copper were upregulated by androgens in VCaP cells. Specifically, using qPCR we determined that the synthetic androgen R1881 increased the transcript levels of CTR1 (copper uptake) ATP7B (copper trafficking) and STEAP4 (metallo/copper reductase) (Fig. 7A). Furthermore, small interfering RNAs (siRNAs) targeting AR dramatically reduced the androgen-mediated up-regulation of these transcripts (Fig. 7B), Importantly, androgens also increased CTR1 protein levels, an effect that was also dependent on AR (Fig. 7C). Additionally, at a concentration that inhibits the expression of secondary androgen responsive target genes (ex. INSIG1), cycloheximide treatment did not block the R1881-mediated increase in CTR1, ATP7B or STEAP4 mRNA levels (supplemental Fig. S4). In LNCaP cells, only STEAP4 was shown to be upregulated by androgens (supplemental Fig. S5). This may relate to the differences in AR expression in the two models with VCaP cells exhibiting AR overexpression, and thus a good model of late stage CRPC, and LNCaP expressing less AR, modeling an earlier stage in the disease. This finding is significant as AR expression has been found to be consistently upregulated during tumor progression, and that a 2- to 3-fold increase in AR protein levels is sufficient to facilitate the transition of prostate cancer cells from a hormone-sensitive to a hormone-refractory state in experimental animal models [8]. Thus, we engineered LNCaP cells to express higher levels of AR and found that this resulted in significantly higher levels of CTR1 and ATP7B expression in response to androgen treatment (supplemental Fig. S5). Interestingly, similar results were obtained when the immortalized RWPE-1 prostate epithelial cell line was engineered to express high levels of AR (supplemental Fig. S5). Collectively, these findings validate CTR1, ATP7B and STEAP4 as bona fide AR target genes in prostate cancer cells. However, the insensitivity of RWPE-1-AR cells to DSF indicates that while androgens can increase the expression of proteins involved in copper homeostasis, this activity alone is not sufficient to confer sensitivity to these agents. Although it does suggest that in cells that have an inherent sensitivity to DSF, that upregulation of AR-target gene expression as occurs in late stage disease may sensitize cells to DSF:Cu.
Figure 7. Androgen up-regulates the expression of genes required for copper uptake and the maintenance of intracellular copper homeostasis.
A. VCaP cells were treated for 12 hr. with either vehicle or increasing concentrations of R1881, and the mRNA expression of CTR1, ATP7B or STEAP4 were evaluated using qPCR. Results are expressed as fold induction over vehicle-treated cells ±SE (n=3). The data shown are representative of three independent experiments. B. VCaP cells were transiently transfected with a Stealth siRNA-control targeting a negative control (siCTRL) or AR (siAR). Two days later, cells were treated overnight with either vehicle (white bars) or 10 nM R1881 (black bars). The mRNA expression of CTR1, ATP7B or STEAP4 was assessed using qPCR. C. VCaP cells were transfected as described in B with mock, siCTRL or siAR and treated for 24 hr. Whole-cell extracts were subjected to Western immunoblot analysis using antibodies direct against CTR1 or GAPDH (loading control). D. VCaP cells were transiently transfected with a nonspecific siRNA-control (siCTRL) or a siRNA targeting CTR1 (siCTR1-B; siCTR1-C). qPCR analysis confirming siRNA CTR1 knockdown at 72 hr. post-transfection is represented. E. VCaP cells, transiently transfected with a nonspecific siRNA-control (siCTRL) or a siRNA targeting CTR1 (siCTR1-B; siCTR1-C), were incubated with 5µCi/ml 64CuCl2 for 2 hr. 64Cu accumulation was measured using a γ-counter and normalized to the protein concentration of the cell lysates. F. VCaP Cells were treated for 24 hr. with either vehicle or 10 nM R1881 and incubated with 5Ci/ml 64CuCl2 for 30–240 min at 37°C. 64Cu uptake was quantified and normalized to protein concentrations of cells lysates. G. 100-fold molar excess of nonradioactive copper (CuSO4) was added to the media with 5µCi/ml 64CuCl2 and incubated for 2 hr. 64Cu uptake was measured and compared with control cells incubated in the absence of cold copper and presented as mean ±SE, n=3.
Androgens increase cellular copper uptake
CTR1 is the primary copper transporter in mammalian cells and we have shown that it is significantly expressed in PCa cells and that androgens can further increase its expression. Given this observation, we sought to determine the importance of CTR1 on cellular copper uptake in PCa cells using radioactive copper (64Cu). As expected, siRNA-mediated CTR1 knockdown resulted in a significant decrease in 64Cu uptake (Fig. 7E) indicating that copper uptake into PCa cells is largely dependent on CTR1 expression. Next, we investigated the effect of androgen treatment on cellular copper uptake. As expected, there is a significant time-dependent increase in 64Cu uptake into PCa cells in the basal state and this uptake is accentuated when cells are pretreated with an androgen agonist (Fig. 7G). The uptake of 64Cu was quantitatively inhibited by co-incubation with a 100-fold molar excess of nonradioactive copper, a result that supports the involvement of a specific, saturable transporter in regulating copper uptake (Fig. 7G). We have also found that addition of DSF to the incubation media further increases the copper accumulation in cells (supplemental Fig. S6). However, it is unclear whether this is a consequence of DSF trapping copper within cells or if it is related to the reported metal ionophore activity of this compound [38, 39]. Regardless, these results suggest that the increased accumulation of copper in PCa cells can be attributed to a significant expression of CTR1, and that the expression and functional activity of this transporter can be upregulated by androgens. The latter finding is of particular significance, as it suggests CRPC cells take up a significantly higher level of copper and would by inference exhibit increased sensitivity to DSF.
DISCUSSION
A common objective in the development of chemotherapeutics is to identify compounds whose cytostatic/cytotoxic actions require, or are reinforced by, proteins or processes that are selectively expressed in malignant cells. It was of considerable importance therefore, that several investigators noted that PCa has a particular proclivity for copper uptake that was not apparent in normal prostate [17–19]. These findings, together with the observation that copper was required for angiogenesis in PCa, led to the initiation of several clinical trials that evaluated the impact of copper chelation in patients with CRPC. Unfortunately, no significant impact on clinical outcomes in PCa was observed using this therapeutic strategy [40, 41]. Copper chelation using TTM is currently being evaluated in several clinical trials, alone or in combination with chemotherapy/radiation, in patients with advanced solid tumors, including breast, lung, esophageal, colorectal, and hepatocellular carcinoma (www.clinicaltrials.gov). It will be of interest to see whether this strategy works in other cancers and if information can be drawn from these studies that would inform better approaches to use chelation therapy in PCa. However, considering the narrow therapeutic window we observed in the sensitivity of normal vs. malignant prostate cancer cells to copper chelators in vitro, it is unlikely that copper chelation, as currently contemplated, will play a significant role in the management of PCa.
Recently, we made the unexpected finding that both AR-positive and AR-negative PCa cells overexpress many of the proteins required for copper uptake and trafficking and that in AR-positive PCa cells, the expression of these proteins can be accentuated by androgens. Further, a robust uptake of copper into PCa cells was observed; an activity that was increased further by androgens. With a view to exploiting this propensity for copper uptake we developed a “conditional lethal” screen to identify compounds whose cytotoxic activities required copper. Using this approach two drugs, both members of the dithiocarbamate family of metal binding compounds, were identified. The actions of both of these drugs were inhibited by classical copper chelators; a finding that highlights their unique mechanism of action. One of the compounds identified, DSF, an FDA approved drug, has extensive clinical and pharmacologic experience and is used for the treatment of alcohol abuse [27, 28, 42, 43]. Interestingly, this drug was identified previously in a screen for PCa therapeutics and was subsequently evaluated in a multicenter phase II clinical study in men with non-metastatic recurrent prostate cancer after local therapy. The results of this trial were disappointing and no significant benefit of the drug was observed [44]. However, in studies performed both in vitro and in vivo we have found that the activity of DSF absolutely requires copper. Using Positron PET imaging and 64Cu as an imaging agent it was observed by others that PCa tumors propagated as xenografts have a particularly high capacity to accumulate copper [23, 24]. However, notwithstanding this ability to accumulate copper, we demonstrated that DSF has a minimal impact on tumor growth unless animals were supplemented with copper. Thus, although PCa cells express the transporters that enable them to uptake copper, it appears as if the available copper in the blood of unsupplemented animals, and by inference humans, is not sufficient to confer sensitivity to DSF. This has important implications with respect to the interpretation of the recently completed clinical trials of DSF as it brings into question whether or not the treated tumors had copper levels sufficient to confer sensitivity to the drug. To address this issue we have designed, and will soon enroll into, a clinical trial (FDA approval IND 116012) to examine the antitumor activity of DSF in the setting of parenteral copper supplementation. The primary goal of this trial is to evaluate the feasibility of manipulating intratumoral copper levels in patients with PCa as a means to sensitize the tumors to DSF.
In preclinical studies, several mechanisms of action underlying the anti-proliferative effect of DSF has been reported including generation of ROS, inhibition of DNA methyltransferase and ubiquitin-proteasome pathway, activities that are potentiated by copper supplementation [45–50]. However, these actions were only observed using drug concentrations far exceeding that which we demonstrated to effectively inhibit PCa cell growth. In our studies, it was demonstrated that intracellular ROS production is dramatically elevated in cells upon co-treatment with DSF and copper. This activity is likely to be important for therapeutic efficacy as PCa cell apoptosis induced by DSF:Cu can be inhibited by blocking ROS production using free radial scavengers. Although the mechanisms underlying ROS production were not addressed in our study, it is likely due to the free radical formation that results from the inherent redox shuttling of copper (Cu(I)/Cu(II)) within Cu-dithiocarbamate complexes [34, 35, 38]. These highly reactive species target a variety of biological molecules including DNA, RNA, cholesterol, lipids, carbohydrates, and proteins. In normal cells, free radical generation is balanced by processes that facilitate antioxidant defense. However, this equilibrium is upset in prostate cancer cells, favoring oxidation and increased ROS production. A question that remains unanswered is why the DSF:Cu complex is selectively toxic to malignant vs. non-malignant PCa cells. The ability of PCa to accumulate copper is certainly important; however, it is also likely that the highly oxidative environment within cancer cells predisposes them to ROS generation [51–55]. Understanding the mechanism(s) underlying this specificity will likely inform the optimal use of this drug or a related drug:metal conjugate.
The mechanism(s) by which copper is taken up into cells has been elucidated in detail. In blood, copper (Cu(II)) is highly protein bound being associated with ceruloplasmin or albumin. At the cell surface metalloreductases (STEAP family members) convert Cu(II) to Cu(I) allowing it to be transported into cells by the CTR1 [16]. Once inside the cell, carrier cytoplasmic metallochaperones deliver copper to specific cellular targets and compartments. One of the surprising findings of our study is that the expression of all of the key regulators of copper homeostasis (CTR1, STEAP4, ATP7B and ATP7A) are either expressed at a high basal level and/or can be induced upon AR activation. Not surprisingly, we have shown that copper uptake into PCa cells can be increased by androgens although it is important to note that the basal expression of these proteins enables the uptake of enough copper to sensitize cells to DSF. Interestingly, we have shown that DSF treatment increases overall copper accumulation into PCa cells. However, it is unclear whether this reflects an ability of DSF to trap copper within cells or if DSF can facilitate copper uptake by functioning as an ionophore [38, 39].
In summary, we have demonstrated that PCa cells accumulate copper and that this can be exploited to sensitize cells to the cytotoxic actions of dithiocarbamates. The absolute requirement of copper supplementation for the positive therapeutic action of DSF in animals has informed a clinical trial which will explore the impact of copper supplementation on DSF efficacy in PCa patients with advanced disease.
Supplementary Material
Acknowledgments
Grant Support: this work was supported by the NIH grants CA139818 (D.P. McDonnell), CA42324 (M.R. Zalutsky) and RO1GM084176 (K.J. Franz). We thank Dr. Dennis J. Thiele for the gift of CTR1 antibody and for helpful scientific input. We also thank the members of the McDonnell lab for their useful discussions and critical reading of the manuscript.
Footnotes
Disclosure of Potential Conflicts of Interest:
The authors have no potential conflicts of interest to disclose.
REFRENCES
- 1.George D, Moul JW. Emerging treatment options for patients with castration-resistant prostate cancer. Prostate. 2012;72(3):338–349. doi: 10.1002/pros.21435. [DOI] [PubMed] [Google Scholar]
- 2.Loriot Y, et al. Antitumour activity of abiraterone acetate against metastatic castration-resistant prostate cancer progressing after docetaxel and enzalutamide (MDV3100) Ann Oncol. 2013;24(7):1807–1812. doi: 10.1093/annonc/mdt136. [DOI] [PubMed] [Google Scholar]
- 3.Pezaro CJ, Mukherji D, De Bono JS. Abiraterone acetate: redefining hormone treatment for advanced prostate cancer. Drug Discov Today. 2012;17(5–6):221–226. doi: 10.1016/j.drudis.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 4.Scher HI, et al. Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1–2 study. Lancet. 2010;375(9724):1437–1446. doi: 10.1016/S0140-6736(10)60172-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stein MN, Goodin S, Dipaola RS. Abiraterone in prostate cancer: a new angle to an old problem. Clin Cancer Res. 2012;18(7):1848–1854. doi: 10.1158/1078-0432.CCR-11-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cai C, et al. Intratumoral de novo steroid synthesis activates androgen receptor in castration-resistant prostate cancer and is upregulated by treatment with CYP17A1 inhibitors. Cancer Res. 2011;71(20):6503–6513. doi: 10.1158/0008-5472.CAN-11-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Locke JA, et al. Androgen levels increase by intratumoral de novo steroidogenesis during progression of castration-resistant prostate cancer. Cancer Res. 2008;68(15):6407–6415. doi: 10.1158/0008-5472.CAN-07-5997. [DOI] [PubMed] [Google Scholar]
- 8.Chen CD, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10(1):33–39. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 9.Golias C, et al. Amplification and co-regulators of androgen receptor gene in prostate cancer. Exp Oncol. 2009;31(1):3–8. [PubMed] [Google Scholar]
- 10.Lee GT, et al. Macrophages induce neuroendocrine differentiation of prostate cancer cells via BMP6-IL6 Loop. Prostate. 2011 doi: 10.1002/pros.21369. [DOI] [PubMed] [Google Scholar]
- 11.Nordin A, et al. Midkine is associated with neuroendocrine differentiation in castration-resistant prostate cancer. Prostate. 2013;73(6):657–667. doi: 10.1002/pros.22607. [DOI] [PubMed] [Google Scholar]
- 12.Dehm SM, Tindall DJ. Alternatively spliced androgen receptor variants. Endocr Relat Cancer. 2011;18(5):R183–R196. doi: 10.1530/ERC-11-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo Z, et al. A novel androgen receptor splice variant is up-regulated during prostate cancer progression and promotes androgen depletion-resistant growth. Cancer Res. 2009;69(6):2305–2313. doi: 10.1158/0008-5472.CAN-08-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun S, et al. Castration resistance in human prostate cancer is conferred by a frequently occurring androgen receptor splice variant. J Clin Invest. 2010;120(8):2715–2730. doi: 10.1172/JCI41824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beltran H, et al. Targeted next-generation sequencing of advanced prostate cancer identifies potential therapeutic targets and disease heterogeneity. Eur Urol. 2013;63(5):920–926. doi: 10.1016/j.eururo.2012.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Logothetis CJ, et al. Molecular classification of prostate cancer progression: foundation for marker-driven treatment of prostate cancer. Cancer Discov. 2013;3(8):849–861. doi: 10.1158/2159-8290.CD-12-0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Habib FK, Dembinski TC, Stitch SR. The zinc and copper content of blood leucocytes and plasma from patients with benign and malignant prostates. Clinica chimica acta; international journal of clinical chemistry. 1980;104(3):329–335. doi: 10.1016/0009-8981(80)90390-3. [DOI] [PubMed] [Google Scholar]
- 18.Nayak SB, et al. Copper and ceruloplasmin status in serum of prostate and colon cancer patients. Indian journal of physiology and pharmacology. 2003;47(1):108–110. [PubMed] [Google Scholar]
- 19.Gupte A, Mumper RJ. Elevated copper and oxidative stress in cancer cells as a target for cancer treatment. Cancer treatment reviews. 2009;35(1):32–46. doi: 10.1016/j.ctrv.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 20.Leone N, et al. Zinc, copper, and magnesium and risks for all-cause, cancer, and cardiovascular mortality. Epidemiology. 2006;17(3):308–314. doi: 10.1097/01.ede.0000209454.41466.b7. [DOI] [PubMed] [Google Scholar]
- 21.Turski ML, et al. A novel role for copper in Ras/mitogen-activated protein kinase signaling. Mol Cell Biol. 2012;32(7):1284–1295. doi: 10.1128/MCB.05722-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nose Y, et al. Ctr1 is an apical copper transporter in mammalian intestinal epithelial cells in vivo that is controlled at the level of protein stability. J Biol Chem. 2010;285(42):32385–32392. doi: 10.1074/jbc.M110.143826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peng F, et al. PET of human prostate cancer xenografts in mice with increased uptake of 64CuCl2. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2006;47(10):1649–1652. [PubMed] [Google Scholar]
- 24.Jorgensen JT, et al. High tumor uptake of (64)Cu: implications for molecular imaging of tumor characteristics with copper-based PET tracers. Nucl Med Biol. 2013;40(3):345–350. doi: 10.1016/j.nucmedbio.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 25.Kim BE, Nevitt T, Thiele DJ. Mechanisms for copper acquisition, distribution and regulation. Nature chemical biology. 2008;4(3):176–185. doi: 10.1038/nchembio.72. [DOI] [PubMed] [Google Scholar]
- 26.Iljin K, et al. High-throughput cell-based screening of 4910 known drugs and drug-like small molecules identifies disulfiram as an inhibitor of prostate cancer cell growth. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15(19):6070–6078. doi: 10.1158/1078-0432.CCR-09-1035. [DOI] [PubMed] [Google Scholar]
- 27.Johansson B. A review of the pharmacokinetics and pharmacodynamics of disulfiram and its metabolites. Acta psychiatrica Scandinavica. Supplementum. 1992;369:15–26. doi: 10.1111/j.1600-0447.1992.tb03310.x. [DOI] [PubMed] [Google Scholar]
- 28.Chick J. Safety issues concerning the use of disulfiram in treating alcohol dependence. Drug safety : an international journal of medical toxicology and drug experience. 1999;20(5):427–435. doi: 10.2165/00002018-199920050-00003. [DOI] [PubMed] [Google Scholar]
- 29.Chen D, et al. Inhibition of prostate cancer cellular proteasome activity by a pyrrolidine dithiocarbamate-copper complex is associated with suppression of proliferation and induction of apoptosis. Front Biosci. 2005;10:2932–2939. doi: 10.2741/1749. [DOI] [PubMed] [Google Scholar]
- 30.Ohgami RS, et al. The Steap proteins are metalloreductases. Blood. 2006;108(4):1388–1394. doi: 10.1182/blood-2006-02-003681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gomes IM, Maia CJ, Santos CR. STEAP proteins: from structure to applications in cancer therapy. Mol Cancer Res. 2012;10(5):573–587. doi: 10.1158/1541-7786.MCR-11-0281. [DOI] [PubMed] [Google Scholar]
- 32.Lee J, et al. Biochemical characterization of the human copper transporter Ctr1. J Biol Chem. 2002;277(6):4380–4387. doi: 10.1074/jbc.M104728200. [DOI] [PubMed] [Google Scholar]
- 33.Brar SS, et al. Disulfiram inhibits activating transcription factor/cyclic AMP-responsive element binding protein and human melanoma growth in a metal-dependent manner in vitro, in mice and in a patient with metastatic disease. Molecular Cancer Therapeutics. 2004;3(9):1049–1060. [PubMed] [Google Scholar]
- 34.Halliwell B, Gutteridge JM. Role of free radicals and catalytic metal ions in human disease: an overview. Methods in enzymology. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]
- 35.Aust SD, Morehouse LA, Thomas CE. Role of metals in oxygen radical reactions. Journal of free radicals in biology & medicine. 1985;1(1):3–25. doi: 10.1016/0748-5514(85)90025-x. [DOI] [PubMed] [Google Scholar]
- 36.Hendrickson AR, Martin RL, Rohde NM. Dithiocarbamates of Cu(I), Cu(Ii), and Cu(Iii) - Electrochemical Study. Inorganic Chemistry. 1976;15(9):2115–2119. [Google Scholar]
- 37.Doctrow SR, et al. Salen-manganese complexes as catalytic scavengers of hydrogen peroxide and cytoprotective agents: structure-activity relationship studies. Journal of Medicinal Chemistry. 2002;45(20):4549–4558. doi: 10.1021/jm020207y. [DOI] [PubMed] [Google Scholar]
- 38.Cen D, et al. Disulfiram facilitates intracellular Cu uptake and induces apoptosis in human melanoma cells. J Med Chem. 2004;47(27):6914–6920. doi: 10.1021/jm049568z. [DOI] [PubMed] [Google Scholar]
- 39.Ding WQ, Lind SE. Metal ionophores - an emerging class of anticancer drugs. IUBMB Life. 2009;61(11):1013–1018. doi: 10.1002/iub.253. [DOI] [PubMed] [Google Scholar]
- 40.Henry NL, et al. Phase II trial of copper depletion with tetrathiomolybdate as an antiangiogenesis strategy in patients with hormone-refractory prostate cancer. Oncology. 2006;71(3–4):168–175. doi: 10.1159/000106066. [DOI] [PubMed] [Google Scholar]
- 41.Lin J, et al. A non-comparative randomized phase II study of 2 doses of ATN-224, a copper/zinc superoxide dismutase inhibitor, in patients with biochemically recurrent hormone-naive prostate cancer. Urologic oncology. 2011 doi: 10.1016/j.urolonc.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burnett GB, Reading HW. The pharmacology of disulfiram in the treatment of alcoholism. Br J Addict Alcohol Other Drugs. 1970;65(4):281–288. doi: 10.1111/j.1360-0443.1970.tb03946.x. [DOI] [PubMed] [Google Scholar]
- 43.Petersen EN. The pharmacology and toxicology of disulfiram and its metabolites. Acta Psychiatr Scand Suppl. 1992;369:7–13. doi: 10.1111/j.1600-0447.1992.tb03309.x. [DOI] [PubMed] [Google Scholar]
- 44.Schweizer MT, et al. Pharmacodynamic study of disulfiram in men with non-metastatic recurrent prostate cancer. Prostate Cancer Prostatic Dis. 2013 doi: 10.1038/pcan.2013.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen D, et al. Disulfiram, a clinically used anti-alcoholism drug and copper-binding agent, induces apoptotic cell death in breast cancer cultures and xenografts via inhibition of the proteasome activity. Cancer Res. 2006;66(21):10425–10433. doi: 10.1158/0008-5472.CAN-06-2126. [DOI] [PubMed] [Google Scholar]
- 46.Lin J, et al. Disulfiram is a DNA demethylating agent and inhibits prostate cancer cell growth. Prostate. 2011;71(4):333–343. doi: 10.1002/pros.21247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morrison BW, et al. Disulfiram induces copper-dependent stimulation of reactive oxygen species and activation of the extrinsic apoptotic pathway in melanoma. Melanoma Res. 2010;20(1):11–20. doi: 10.1097/CMR.0b013e328334131d. [DOI] [PubMed] [Google Scholar]
- 48.Yip NC, et al. Disulfiram modulated ROS-MAPK and NFkappaB pathways and targeted breast cancer cells with cancer stem cell-like properties. Br J Cancer. 2011;104(10):1564–1574. doi: 10.1038/bjc.2011.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cen D, et al. Disulfiram induces apoptosis in human melanoma cells: a redox-related process. Mol Cancer Ther. 2002;1(3):197–204. [PubMed] [Google Scholar]
- 50.Chen SH, et al. Oxidative stress and c-Jun-amino-terminal kinase activation involved in apoptosis of primary astrocytes induced by disulfiram-Cu(2+) complex. Eur J Pharmacol. 2001;414(2–3):177–188. doi: 10.1016/s0014-2999(01)00792-0. [DOI] [PubMed] [Google Scholar]
- 51.Schumacker PT. Reactive oxygen species in cancer cells: live by the sword, die by the sword. Cancer Cell. 2006;10(3):175–176. doi: 10.1016/j.ccr.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 52.Szatrowski TP, Nathan CF. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991;51(3):794–798. [PubMed] [Google Scholar]
- 53.Kumar B, et al. Oxidative stress is inherent in prostate cancer cells and is required for aggressive phenotype. Cancer research. 2008;68(6):1777–1785. doi: 10.1158/0008-5472.CAN-07-5259. [DOI] [PubMed] [Google Scholar]
- 54.Khandrika L, et al. Oxidative stress in prostate cancer. Cancer Lett. 2009;282(2):125–136. doi: 10.1016/j.canlet.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gupta-Elera G, et al. The role of oxidative stress in prostate cancer. Eur J Cancer Prev. 2012;21(2):155–162. doi: 10.1097/CEJ.0b013e32834a8002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.