Abstract
The root causes of regional variation in medical spending are poorly understood and vary by clinical condition. To identify drivers of regional spending variation for Medicare patients with advanced cancer, we used linked Surveillance, Epidemiology, and End Results (SEER) program–Medicare data from 2004–10. We broke down Medicare spending into thirteen cancer-relevant service categories. We then calculated the contribution of each category to spending and regional spending variation. Acute hospital care was the largest component of spending and the chief driver of regional spending variation, accounting for 48 percent of spending and 67 percent of variation. In contrast, chemotherapy accounted for 16 percent of spending and 10 percent of variation. Hospice care comprised 5 percent of spending; however variation in hospice spending was fully offset by opposing variation in other categories. Our analysis suggests that the strategy with the greatest potential to improve the value of care for patients with advanced cancer is to reduce reliance on acute hospital care for this patient population.
Improving the value of health care services is a critical imperative for the US health care system. Unexplained regional variation in per capita health care spending has been identified as a marker of inefficient, low-value care.,[1–3] However, the appropriate policy prescriptions to address regional spending variation remain uncertain. The observation that within-area spending variation is at least as substantial as between-area variation also erodes the rationale for instituting geographically targeted payment incentives.[4]
Instead of looking to regional spending variation as an indicator of the value of care delivered in particular geographic regions, an alternative approach is to use regional variation to better understand the processes of care that are associated with high- and low-value service utilization.[5] When applied to a clinically well-defined population of patients, such as patients with advanced cancer, this approach offers the potential to identify specific clinical and policy approaches with the potential to improve the quality and efficiency of care.
Spending for cancer care in the United States exceeds $125 billion annually.[6] Spending is highest for patients with advanced cancer,[7] a stage at which disease generally is not amenable to curative treatments. Clinicians face considerable uncertainty in choosing appropriate treatment approaches for patients with advanced cancer, and previous work has shown that there is substantial regional variation in per capita Medicare spending for this population. Furthermore, regional variation in advanced cancer spending was not associated with survival differences in elderly patients with advanced cancer,[8] supporting the conclusion that the marginal value of high-intensity practice patterns in the advanced cancer setting is low. Here, we examine regional variation in Medicare spending for elderly patients (ages sixty-five and older) with advanced cancer, with spending claims broken down into thirteen distinct cancer-relevant service categories. Using this framework, we were able to identify service categories that were drivers of regional spending variation. A better understanding of these drivers should facilitate the design of clinical and policy interventions to improve the value of advanced-cancer care.
Study Data And Methods
Data Source And Study Subjects
We used linked data from the Surveillance, Epidemiology, and End Results (SEER) program and Medicare to study the costs of care in the first six months after advanced cancer diagnosis. The SEER program is a network of seventeen population-based cancer registries, covering 28 percent of the US population.[9] Linkage to Medicare claims permits analysis of health care utilization and spending among subjects diagnosed with cancer and enrolled in traditional fee-for-service Medicare. Our study used SEER records of new cancer diagnoses from 2004 to 2009 and associated Medicare claims records from 2004 to 2010. The research plan was reviewed and approved by the institutional review board of the Dana-Farber/Harvard Cancer Center.
We included subjects ages sixty-five and older with newly diagnosed advanced-stage non-small-cell lung, colorectal, breast, prostate, or pancreas cancer. These five cancer sites account for 53 percent of all newly diagnosed cancers and 54 percent of all cancer deaths in the United States.[10] Advanced cancer was defined to include the stages at which cancer treatment is generally of palliative intent. Operationally, this was defined as stage IV for all cancers, as well as stage IIIB for lung cancer and stage III for pancreas cancer. Stages were determined using the AJCC Cancer Staging Manual (6th edition).[11] To ensure complete Medicare claims data, all subjects were continuously enrolled in traditional Medicare Parts A and B from the time of diagnosis until whichever came first, death or six months after diagnosis.
We assigned each subject to a hospital referral region based on the subject’s zip code of residence. Hospital referral regions (HRRs) are geographic regions that reflect patterns of tertiary care,[12] and the SEER registries substantially overlap with eighty of 306 US HRRs. We limited our study to patients residing within these eighty regions.
Categorization And Estimation Of Spending
We used Medicare claims data to estimate total Medicare spending in the six months following diagnosis of advanced cancer. We studied patients with advanced cancer because this population has high treatment costs and high mortality, and because prior analysis has demonstrated that regional variation in advanced-cancer spending is not associated with differences in survival.[8] A six-month interval was selected to serve as a prospective and clinically relevant observation period during which decisions about critical components of advanced cancer care (such as initial therapy with surgery, radiation, and chemotherapy) are made. Medicare claims files included the Medicare Provider, Analysis, and Review (MedPAR) (acute and postacute hospital care), outpatient, physician, durable medical equipment, home health, and hospice files.
We assigned all claims to one of thirteen mutually exclusive and cancer-relevant service categories, as listed in Exhibit 1. Claims were assigned to service categories using the Berenson-Eggers Type of Service classification system,[13] with modifications to improve relevance to oncology care (see online Appendix for details.)[14]
Exhibit 1.
Advanced Cancer Spending And Regional Spending Variation, By Cancer-Relevant Service Category
| Service category |
Description | Mean spending, 2011 USD |
Spending variation (ratio, quintile 5 to quintile 1) |
|---|---|---|---|
| Acute hospital care |
Acute hospital and inpatient physician services |
$16,953 | 1.60 |
| Chemotherapy | Intravenous and oral-equivalent chemotherapies |
5,705 | 1.61 |
| Outpatient procedures |
Outpatient surgery and other outpatient procedures |
2,281 | 1.38 |
| Imaging | X-ray, ultrasound, CT, MRI, and nuclear medicine |
1,837 | 1.40 |
| Radiation therapy |
Radiation therapy | 1,832 | 1.53 |
| Hospice | All hospice services | 1,743 | 2.73 |
| Home health | Home care services, excluding hospice |
870 | 2.41 |
| Outpatient physician services |
Outpatient physician evaluation and management |
865 | 1.42 |
| Diagnostics | Laboratory and pathology testing and evaluation |
864 | 1.46 |
| Part B medications |
Part B medications, excluding chemotherapy |
804 | 3.58 |
| Postacute facility |
Skilled nursing facilities and rehabilitation hospitals |
611 | 16.70 |
| Other Part B | Ambulance, vision, and hearing services, etc. |
406 | 2.07 |
| Durable medical equipment |
Durable medical equipment for home use |
351 | 2.10 |
| Total | All service Categories |
35,257 | 1.31 |
SOURCE 2004–10 Medicare claims linked to Surveillance, Epidemiology, and End Results data for 61,838 subjects with advanced cancer.
Medicare spending for each claim was estimated from actual reimbursements. Patient copayments were not included in spending amounts. Disproportionate share and indirect medical education payments were subtracted from reimbursements for inpatient claims, as these payments are hospital cross-subsidies (for uncompensated care and graduate medical education, respectively) and not payments for direct patient care. Spending amounts from individual claims were adjusted for inflation, geographic location of care delivery, and patient demographic characteristics, as detailed below.
Because Medicare payments vary based on the geographic location of service provision, we performed geographic adjustment of all claims amounts to approximate a geographically normalized payment. We used the capital geographic adjustment factor to adjust Part A expenditures and the geographic practice cost indices to adjust Part B expenditures.[15] These indices are calculated annually by the Department of Health and Human Services.[16] For inflation adjustment, we used the Hospital Input Price Index for Part A expenditures and the Medicare Economic Index for Part B expenditures.[15] All spending is expressed in 2011 US dollars.
After adjusting for inflation and geographic location of care delivery, we further adjusted spending estimates from individual claims to account for patient demographic characteristics. We adapted the demographic adjustment approach of the Dartmouth Atlas of Health Care,[1] creating demographic cells to capture strata of patients with similar characteristics by sex, age (65–69, 70–74, 75–79, and eighty or older), and race (black vs. nonblack). The mean spending in each cell was then divided by the overall mean, and this ratio was used to calculate the demographically standardized spending amount. Demographic spending adjustments were completed separately for each primary cancer site.
Analysis Of Regional Spending Variation
We aggregated the claims-level spending estimates to calculate the mean six-month per capita spending for each HRR, as well as the mean spending for each of the thirteen service categories. Due to differences in cancer case mix across regions, mean spending was calculated as a weighted average matching the overall distribution of the cancers across SEER areas. Regional variation in category spending was calculated as the ratio of spending for regions in the highest compared with the lowest quintile of spending (the Q5:Q1 ratio). In addition to the primary analysis (with spending calculated as a weighted average across all included cancer sites), we also conducted subgroup analyses of mean regional spending and regional spending variation within the five included cancer sites.
The primary study outcome was the percentage of regional variation in advanced-cancer spending that could be attributed to each service category. Specifically, we sought to identify the health care service categories that were drivers of regional spending variation in patients with advanced cancer. We defined driver service categories as those contributing proportionally more to regional spending variation than to total spending, such that the observed contribution of a category to spending variation was greater than expected. Total spending variation attributable to individual spending categories was determined by calculating the percentage “reduction in variance” associated with eliminating regional spending variation for a given service category.[17,18]
We performed sensitivity analyses to test key assumptions of our analytic approach. In the first sensitivity analysis, we removed race from our demographic adjustment approach (adjusting only for sex and age group). In the second additional analysis, we assessed the influence of outlier costs (category spending greater than the ninety-ninth percentile) on our estimates of spending and spending variation. Results of the sensitivity analyses were not substantially different from the primary analysis and do not affect the main study findings; details are available in the online Appendix.[14]
Limitations
We studied patients with advanced-stage cancer, and our findings should not be extended to the clinically distinct setting of early-stage cancer. Our study population was restricted to patients ages sixty-five and older with fee-for-service Medicare living within areas included in the SEER program of population-based cancer registries. As such, the generalizability of our findings outside this population is uncertain. Indeed, Mireille Jacobson and colleagues have argued that SEER regions are not representative of the range of variation in US health care practice.[19] This observation would suggest that our report underestimates the range of regional spending variation in Medicare patients with advanced cancer.
Furthermore, our analysis does not include spending for prescription medications from Medicare Part D (which was available for only a portion of our study period) or other payment sources. As a result, our data underreport spending for oral chemotherapy agents that do not have an intravenous equivalent and are, therefore, ineligible for coverage under Medicare Part B. Additionally, spending for chemotherapy delivered in the inpatient setting could not be distinguished from other acute hospital spending and, therefore, is not captured as chemotherapy spending.
Prior studies have found that regional differences in baseline health status are an important determinant of regional spending variation in general medical populations.[20,21] However, attempts to identify regional differences in baseline health status through Medicare claims may be confounded by regional variation in diagnosis coding.[22] Because we studied a homogeneous population of patients with advanced cancer, we assumed that advanced cancer dominated other comorbidities as a determinant of medical spending, and we did not adjust for regional differences in baseline health.[23]
Study Results
The study cohort included 61,838 subjects diagnosed with advanced-stage lung (59 percent), colorectal (16 percent), pancreas (12 percent), breast (6 percent), or prostate (8 percent) cancer between 2004 and 2009. Demographic details of the cohort composition are included in Appendix Exhibit A5.[14] Mean adjusted total spending in the six months after diagnosis of advanced cancer was $35,123.
Mean six-month spending for each advanced-cancer service category, as well as the regional spending variation for each category, is shown in Exhibit 1. Acute hospital care was the largest component of advanced-cancer spending, at $16,953. Spending for Part B chemotherapy drugs made up the second largest amount, at $5,705. Postacute facility care exhibited the largest regional spending variation. Regions in the highest quintile of spending for postacute facility care had spending that was sixteen times greater than regions in the lowest quintile. The second most variable service category was nonchemotherapy Part B medications, and hospice care showed the third-highest variation. Seven of the thirteen categories had comparatively modest spending variation: Chemotherapy, acute hospital care, radiation therapy, diagnostics, outpatient physician services, imaging, and outpatient procedure categories all showed less than twofold variation between regions in the highest and lowest quintiles of category spending.
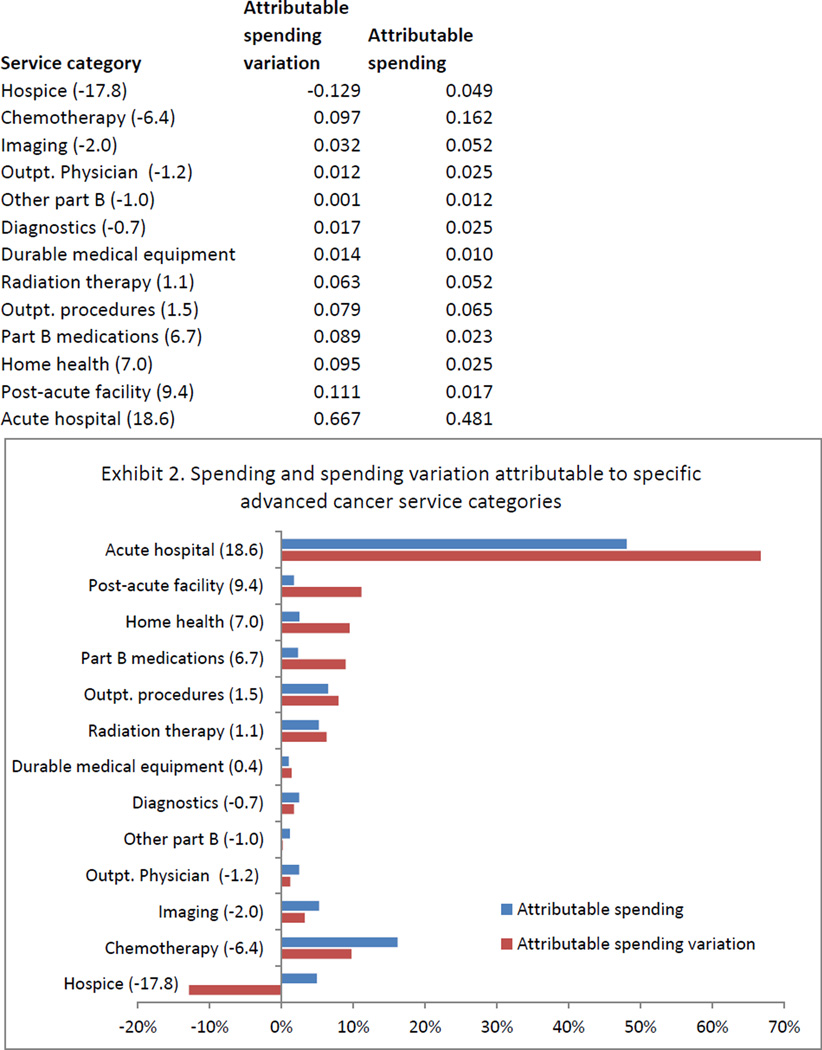
The percentage of spending and spending variation attributable to each service category are shown in Exhibit 2. Seven of the thirteen service categories contributed proportionally more to regional spending variation than to total spending, meeting our a priori definition as drivers of regional spending variation. Driver service categories included acute hospital care, postacute facility, home health, nonchemotherapy Part B medications, outpatient procedures, radiation therapy, and durable medical equipment. Acute hospital care was the largest driver of regional spending variation, accounting for 67 percent of spending variation and 48 percent of total spending. Postacute facility care was the second largest driver category, accounting for 11 percent of spending variation despite comprising just 2 percent of spending. After accounting for covariance between categories, the seven driver service categories accounted for 67 percent of spending and 82 percent of spending variation.
EXHIBIT 2. Spending And Spending Variation Attributable To Specific Advanced Cancer Service Categories.
Source/Notes: SOURCE 2004–10 Medicare claims linked to Surveillance, Epidemiology, and End Results data for 61,838 subjects with advanced cancer. NOTES Numbers in parentheses beside the category label indicate the difference between the percentage of attributable spending variation and the percentage of attributable spending for the associated category. When this number is positive, the category is identified as a net driver of regional spending variation. Attributable variation sums to greater than 100 percent due to covariance terms.
Nondriver service categories (where spending variation was proportionally less than the category contribution to total spending) included chemotherapy, imaging, diagnostics, outpatient physician services, other Part B services, and hospice. Notably, hospice was the only service category that accounted for a net negative percentage of spending variation (−13 percent), meaning that increased spending for hospice services was more than completely offset by larger spending decreases in other service categories.
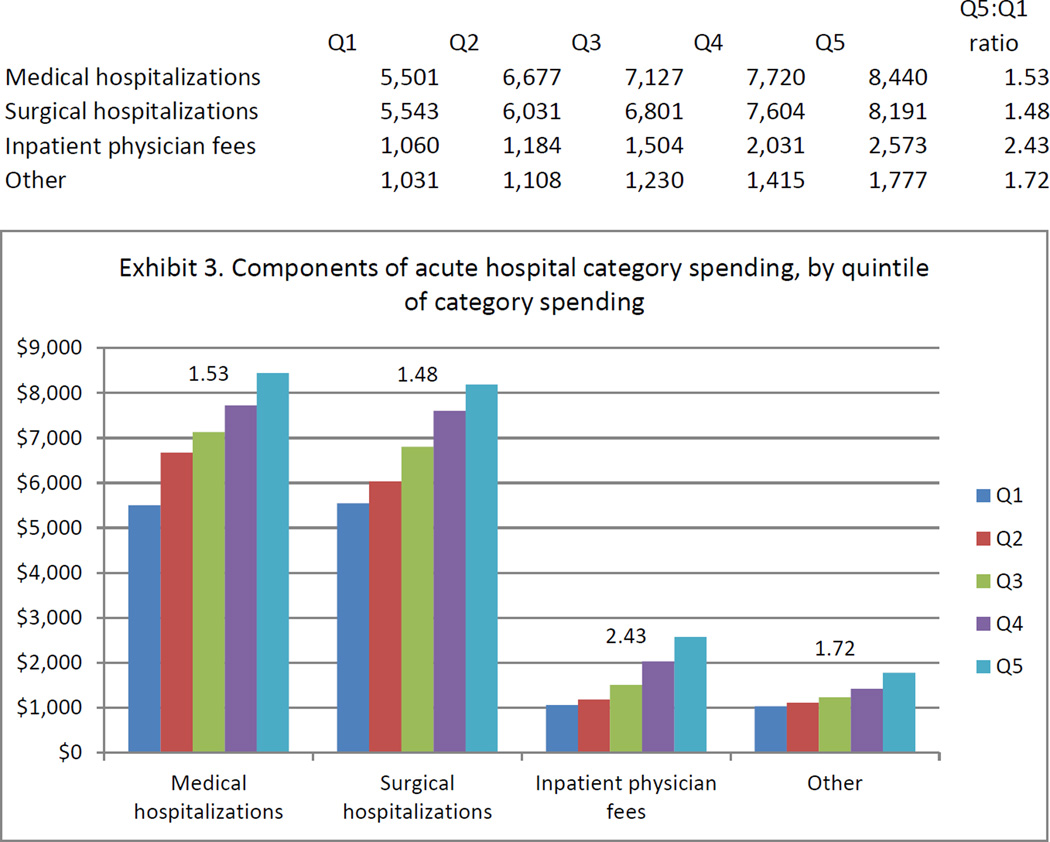
Because acute hospital spending was identified as the chief driver of regional spending variation, we further broke down spending in this category into subcategories including surgical hospitalization, medical hospitalization, inpatient physician fees, and other acute hospital spending. Spending in each of these subcategories increased successively across quintiles of acute hospital category spending (Exhibit 3). The cross-quintile gradient was strongest for inpatient physician fees, where spending was 2.4 times greater in the highest compared to the lowest quintile of acute hospital spending. Increasing acute hospital spending was associated with both more frequent hospitalization and higher spending per hospitalization (Appendix Exhibit A6).[14]
EXHIBIT 3. Components Of Acute Hospital Category Spending, By Quintile Of Category Spending.
Source/Notes: SOURCE 2004–10 Medicare claims linked to Surveillance, Epidemiology, and End Results data for 61,838 subjects with advanced cancer. NOTES The vertical axis shows per capita spending over the first six months after advanced-cancer diagnosis. The number above each series indicates the ratio of spending between quintile 5 (Q5) and quintile 1 (Q1) for the component of category spending represented by that series.
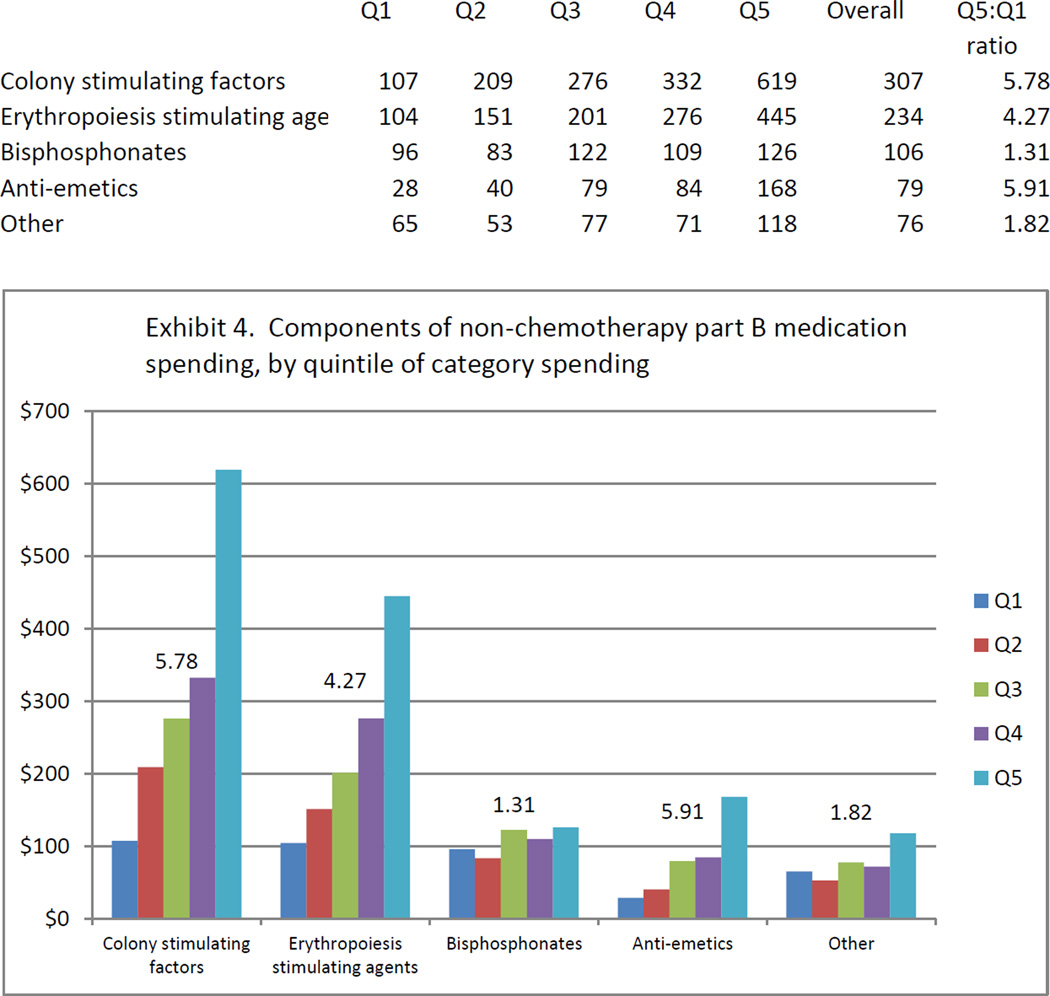
Drivers of regional spending variation in the nonchemotherapy Part B medication category are shown in Exhibit 4. Medications in four discrete classes accounted for 91 percent of nonchemotherapy Part B medication spending (white blood cell colony stimulating factors, erythropoiesis-stimulating agents, bisphosphonates, and anti-emetic medications). Colony stimulating factors, erythropoiesis-stimulating agents, and anti-emetic medications were strong drivers of spending variation within the Part B medication category, while bisphosphonates exhibited only modest spending variation.
EXHIBIT 4. Components Of Nonchemotherapy Part B Medication Spending, By Quintile Of Category Spending.
Source/Notes: SOURCE 2004–10 Medicare claims linked to Surveillance, Epidemiology, and End Results data for 61,838 subjects with advanced cancer. NOTES The vertical axis shows per capita spending over the first six months after advanced-cancer diagnosis. The number above each series indicates the ratio of spending between quintile 5 (Q5) and quintile 1 (Q1) for the component of category spending represented by that series.
Subgroup analysis of attributable spending variation for the individual cancer site subgroups was generally consistent with the findings of the overall analysis. Acute hospital care was the largest driver of spending variation for all subgroups except colorectal cancer. Full results of the subgroup analysis are shown in the online Appendix Exhibit A7.[14]
Discussion
We sought to understand the specific components of Medicare spending that account for regional spending variation in patients with advanced cancer. Acute hospital care was responsible for the largest percentage of spending (48 percent) and was also the chief driver of spending variation, accounting for a disproportionate 67 percent of variation. Although it represents a smaller component of total spending (just 2 percent), postacute facility care was the second-largest driver of spending variation, accounting for 11 percent of variation.
Although chemotherapy spending has been the target of many efforts to improve the value of advanced-cancer care,[24–26] we did not find chemotherapy to be a driver of regional spending variation. In fact, variation in chemotherapy spending was much less than variation in the nonchemotherapy Part B medication category, even though both classes of medications are primarily prescribed by oncologists in office settings. These observations suggest that blunt efforts to reduce variation in chemotherapy spending may have limited spillover effects on aggregate advanced-cancer spending.
Our study demonstrates a provocative relationship between hospice spending and regional spending variation. In contrast to prior studies,[5,17] we analyzed spending variation for hospice services separately from other postacute care services. While postacute facility care, home health, and durable medical equipment were confirmed as drivers of regional spending variation, variation in the use of hospice care demonstrated the opposite association, actually offsetting total spending variation. This observation suggests that some regions substitute hospice care for other more costly management strategies. The inverse relationship between hospice utilization and total medical spending has been previously observed in multiple research contexts.[27–29]
Regional variation in aggregate Medicare spending has been well documented.[1–3,20] However, the degree of variation in spending for specific types of health care services is not as well understood. Using health care service categories similar to those in our study, James D. Reschovsky and colleagues found that home health services and durable medical equipment were the leading drivers of regional variation in the general Medicare population.[5] Acute hospital care accounted for 40 percent of total spending but was not found to be a driver of regional variation. The Institute of Medicine (IOM) recently assessed the contribution of seven distinct service categories to regional spending variation among Medicare recipients.[17] The IOM’s report found that postacute care (inclusive of postacute facility care, hospice care, home health, and durable medical equipment) was the major driver of regional spending variation; acute hospital care was identified as a secondary driver of regional variation.[17] We confirmed the findings from these prior studies of large between-region variations in postacute care services, including postacute facility care, home health, hospice, and durable medical equipment. However, the role of acute hospital care as the dominant driver of regional spending variation in advanced-cancer patients appears to be distinct from the pattern seen in the general Medicare population.
In an era of emphasis on accountable care, our findings have important implications for providers and health systems that care for patients with advanced cancer. The role of acute hospital care as the chief driver of regional spending variation emphasizes the need for health systems to develop strategies for judicious use of the acute hospital setting in patients with advanced cancer. The hospital environment is designed for patients who present with acute and potentially reversible complaints. While symptoms of advanced cancer frequently respond to palliation, the underlying disease process leading to hospitalization is rarely reversible. As such, hospitalization exposes patients with advanced cancer to myriad risks (including infections, procedural complications, and prolonged time away from home and family) without the likelihood of substantial long-term benefit.
Although little emphasis has yet been placed on reducing acute hospital care in cancer patients, this concept appears to be feasible. In a single-center study, 19 percent of hospital admissions for patients with advanced gastrointestinal cancers were determined to be potentially avoidable based on medical record review by practicing oncologists.[30] An analysis from Medicare’s Physician Group Practice Demonstration showed that participating practices were successful in reducing costs among patients with cancer and that savings appeared to be entirely attributable to reductions in inpatient hospital admissions.[31]
A recent report from the IOM identified a number of policy approaches for incentivizing high-quality, high-value cancer care, including bundled payments, value-based reimbursement, and other organizational innovations.[32] These approaches have in common a reorientation away from high-volume practice and toward more deliberative, value-oriented care. During this transition, it will be essential to develop and monitor metrics that define high-quality, advanced-cancer care--both to promote desirable changes (such as earlier referral to palliative and hospice care) and to prevent underutilization of costly but highly effective treatments.
Conclusion
Acute hospital care is the chief driver of regional spending variation among Medicare patients with advanced cancer, while chemotherapy spending was not a driver of regional spending variation. Furthermore, variation in spending for hospice care actually served to offset regional variation in total spending, consistent with a substitution effect between hospice care and other components of advanced-cancer care.
These findings illustrate the complexity of delivering advanced-cancer care. Different regions use distinct mixes of services to care for patients with advanced cancer, resulting in substantially different levels of spending for similar patient populations. Our analysis suggests that the strategy with the greatest potential to improve the value of care for patients with advanced cancer is to reduce reliance on acute hospital care for this patient population. Policies that incentivize high-quality, outpatient-based advanced-cancer care (for example, through bundled payments or value-based purchasing) have the potential to motivate health system changes to improve the value of advanced-cancer care.
Supplementary Material
Acknowledgment
Gabriel Brooks was supported by a Young Investigator Award from the Conquer Cancer Foundation of the American Society of Clinical Oncology and by a program grant from the National Cancer Institute of the National Institutes of Health (Grant no. R25CA92203). Contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. This study used the linked Surveillance, Epidemiology, and End Results (SEER)–Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Program, National Cancer Institute; the Office of Research, Development, and Information, Centers for Medicare and Medicaid Services; Information Management Services, Inc.; and the Surveillance, Epidemiology, and End Results program tumor registries in the creation of the SEER-Medicare database.
Biographies
Gabriel A. Brooks (gabriel_brooks@dfci.harvard.edu) is an instructor in medicine at Harvard Medical School and a research scientist at the Dana-Farber Cancer Institute, in Boston, Massachusetts.
Ling Li is a data analyst and statistician at the Dana-Farber Cancer Institute.
Hajime Uno is an assistant professor of biostatistics at the Harvard School of Public Health and a research scientist at the Dana-Farber Cancer Institute.
Michael J. Hassett is an assistant professor of medicine at Harvard Medical School and a research scientist at the Dana-Farber Cancer Institute.
Bruce E. Landon is an associate professor in the Department of Health Care Policy at Harvard Medical School, in Boston.
Deborah Schrag is a professor of medicine at Harvard Medical School and a research scientist at the Dana-Farber Cancer Institute.
Footnotes
This study was presented as a research abstract at the AcademyHealth Annual Meeting in Baltimore, Maryland, June 24, 2013.
NOTES
- 1.Fisher ES, Wennberg DE, Stukel TA, Gottlieb DJ, Lucas FL, Pinder EL. The implications of regional variations in Medicare spending. Part 1: the content, quality, and accessibility of care. Ann Intern Med. 2003;138(4):273–287. doi: 10.7326/0003-4819-138-4-200302180-00006. [DOI] [PubMed] [Google Scholar]
- 2.Fisher ES, Wennberg DE, Stukel TA, Gottlieb DJ, Lucas FL, Pinder EL. The implications of regional variations in Medicare spending. Part 2: health outcomes and satisfaction with care. Ann Intern Med. 2003;138(4):288–298. doi: 10.7326/0003-4819-138-4-200302180-00007. [DOI] [PubMed] [Google Scholar]
- 3.Baicker K, Chandra A. Medicare spending, the physician workforce, and beneficiaries’ quality of care. Health Aff (Millwood) 2004;23(Suppl):w4-184–w4-197. doi: 10.1377/hlthaff.w4.184. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y, Baik SH, Fendrick AM, Baicker K. Comparing local and regional variation in health care spending. N Engl J Med. 2012;367(18):1724–1731. doi: 10.1056/NEJMsa1203980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reschovsky JD, Ghosh A, Stewart KA, Chollet DJ. Durable medical equipment and home health among the largest contributors to area variations in use of Medicare services. Health Aff (Millwood) 2012;31(5):956–964. doi: 10.1377/hlthaff.2011.0243. [DOI] [PubMed] [Google Scholar]
- 6.Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 2011;103(2):117–128. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yabroff KR, Lamont EB, Mariotto A, Warren JL, Topor M, Meekins A, et al. Cost of care for elderly cancer patients in the United States. J Natl Cancer Inst. 2008;100(9):630–641. doi: 10.1093/jnci/djn103. [DOI] [PubMed] [Google Scholar]
- 8.Brooks GA, Li L, Sharma DB, Weeks JC, Hassett MJ, Yabroff KR, et al. Regional variation in spending and survival for older adults with advanced cancer. J Natl Cancer Inst. 2013;105(9):634–642. doi: 10.1093/jnci/djt025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Cancer Institute. Surveillance, epidemiology, and end results program [Internet] Bethesda (MD): National Cancer Institute; [[cited 2014 Jul 9]]. Available from: http://seer.cancer.gov/about/overview.html. [Google Scholar]
- 10.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 11.Green FL, editor. AJCC Cancer Staging Manual. 6 ed. Chicago (IL): Springer; 2002. [Google Scholar]
- 12.Wennberg JE, editor. The Dartmouth atlas of health care. Chicago (IL): American Hospital Publishing, Inc.; 1996. [PubMed] [Google Scholar]
- 13.Centers for Medicare and Medicaid Services. Berenson-Eggers type of service (BETOS) [Internet] Baltimore (MD): CMS; [[cited 2014 Jul 9]]. Available from: http://www.cms.gov/Medicare/Coding/HCPCSReleaseCodeSets/BETOS.html. [Google Scholar]
- 14.To access the Appendix, click on the Appendix link in the box to the right of the article online.
- 15.Brown ML, Riley GF, Schussler N, Etzioni R. Estimating health care costs related to cancer treatment from SEER-Medicare data. Med Care. 2002;40(8 Suppl):IV-104–IV-117. doi: 10.1097/00005650-200208001-00014. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Medicare and Medicaid Services. Wage index files [Internet] Baltimore (MD): CMS; [[cited 2014 Jul 9]]. Available from: http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Wage-Index-Files.html. [Google Scholar]
- 17.Newhouse JP, Garber AM, Graham RP, McCoy MA, Mancher M, Kibria A, editors. Variation in health care spending: target decision making, not geography (2013) Washington (DC): National Academies Press; 2013. [PubMed] [Google Scholar]
- 18.Attributable variation is calculated as ([V1-V2]/V1), where V1 is the between-region variance in total spending, and V2 is the variance in total spending calculated when variation in the specified service category is held constant at the mean value across all regions.
- 19.Jacobson M, Earle CC, Newhouse JP. Geographic variation in physicians’ responses to a reimbursement change. N Engl J Med. 2011;365(22):2049–2052. doi: 10.1056/NEJMp1110117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zuckerman S, Waidmann T, Berenson R, Hadley J. Clarifying sources of geographic differences in Medicare spending. N Engl J Med. 2010;363(1):54–62. doi: 10.1056/NEJMsa0909253. [DOI] [PubMed] [Google Scholar]
- 21.Reschovsky JD, Hadley J, O’Malley AJ, Landon BE. Geographic variations in the cost of treating condition specific episodes of care among Medicare patients. Health Serv Res. 2014;49(1):32–51. doi: 10.1111/1475-6773.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song Y, Skinner J, Bynum J, Sutherland J, Wennberg JE, Fisher ES. Regional variations in diagnostic practices. N Engl J Med. 2010;363(1):45–53. doi: 10.1056/NEJMsa0910881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Neill CB, Atoria CL, O’Reilly EM, LaFemina J, Henman MC, Elkin EB. Costs and trends in pancreatic cancer treatment. Cancer. 2012;118(20):5132–5139. doi: 10.1002/cncr.27490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramsey S, Shankaran V. Managing the financial impact of cancer treatment: the role of clinical practice guidelines. J Natl Compr Canc Netw. 2012;10(8):1037–1042. doi: 10.6004/jnccn.2012.0106. [DOI] [PubMed] [Google Scholar]
- 25.Bach PB, Mirkin JN, Luke JJ. Episode-based payment for cancer care: a proposed pilot for Medicare. Health Aff (Millwood) 2011;30(3):500–509. doi: 10.1377/hlthaff.2010.0752. [DOI] [PubMed] [Google Scholar]
- 26.Newcomer LN. Changing physician incentives for cancer care to reward better patient outcomes instead of use of more costly drugs. Health Aff (Millwood) 2012;31(4):780–785. doi: 10.1377/hlthaff.2012.0002. [DOI] [PubMed] [Google Scholar]
- 27.Kelley AS, Deb P, Du Q, Aldridge Carlson MD, Morrison RS. Hospice enrollment saves money for Medicare and improves care quality across a number of different lengths-of-stay. Health Aff (Millwood) 2013;32(3):552–561. doi: 10.1377/hlthaff.2012.0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brumley R, Enguidanos S, Jamison P, Seitz R, Morgenstern N, Saito S, et al. Increased satisfaction with care and lower costs: results of a randomized trial of in-home palliative care. J Am Geriatr Soc. 2007;55(7):993–1000. doi: 10.1111/j.1532-5415.2007.01234.x. [DOI] [PubMed] [Google Scholar]
- 29.Bergman J, Saigal CS, Lorenz KA, Hanley J, Miller DC, Gore JL, et al. Hospice use and high-intensity care in men dying of prostate cancer. Arch Intern Med. 2011;171(3):204–210. doi: 10.1001/archinternmed.2010.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brooks GA, Abrams TA, Meyerhardt JA, Enzinger PC, Sommer K, Dalby CK, et al. Identification of potentially avoidable hospitalizations in patients with GI cancer. J Clin Oncol. 2014;32(6):496–503. doi: 10.1200/JCO.2013.52.4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colla CH, Lewis VA, Gottlieb DJ, Fisher ES, editors. Cancer spending and accountable care organizations: evidence from the Physician Group Practice Demonstration. Healthcare. 2013;1(3–4):100–107. doi: 10.1016/j.hjdsi.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levit L, Balogh E, Nass S, Ganz PA, editors. Delivering high-quality cancer care: charting a new course for a system in crisis. Washington (DC): Institute of Medicine; 2013. Sep, [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.