Abstract
Recent years have witnessed a renaissance in the study of fish immune systems. Such studies have greatly expanded the knowledge of the evolution and diversification of vertebrate immune systems. Several findings in those studies have overturned old paradigms about the immune system and led to the discovery of novel aspects of mammalian immunity. Here I focus on how findings pertaining to immunity in teleost (bony) fish have led to major new insights about mammalian B cell function in innate and adaptive immunity. Additionally, I illustrate how the discovery of the most ancient mucosal immunoglobulin described thus far will help resolve unsettled paradigms of mammalian mucosal immunity.
Nonclassical animal models (invertebrate and vertebrate) have been useful for delineating the evolutionary history of immune reactions and can provide a basis for the discovery of previously unknown molecules and biochemical pathways involved in mammalian immunity. Excellent examples of this are the seminal discoveries by Jules Hoffmann of the Toll and Imd defense molecules and pathways in Drosophila, for which he was awarded the 2011 Nobel Prize in physiology or medicine1. Such studies provided essential insights useful for the later discovery of Toll-like receptors in mammalian systems. Elie Metchnikoff provided a key contribution to the birth of immunology much earlier with his fundamental discoveries about phagocytosis in the amoebocytes of starfish larvae, for which he was also awarded the Nobel Prize in physiology or medicine2. Thus, research invested in studying the immune systems of ‘nontraditional’ animal species would seem to be totally justified, given the Nobel prizes that such research has generated. More critically, such research efforts have generated crucial insights for the understanding of the mammalian immune system and immunity in general.
This Perspective will concentrate mainly on two discoveries pertaining to teleost fish that not only have contributed substantially to the understanding of the evolution of immune responses but also have been key in illuminating new paradigms of mammalian immunology. A new concept that has surfaced from studies of the teleost immune system is the previously unforeseen ability of B cells from vertebrates to act as professional phagocytes. That finding has led to the discovery of phagocytic B cell subsets in mammals. Here I will review the features of mammalian phagocytic B cells and put the implications of such findings into perspective in the context of mammalian innate and adaptive immune responses. I will also discuss the presence of mucosal immunoglobulins and B cells in teleost fish, the most ancient mucosal immunoglobulin-based system thus far described. I will make the argument that additional discoveries about fish mucosal immunity are likely to spark new knowledge applicable to the understanding of unresolved paradigms of mammalian mucosal immunity.
Fundamental features of the teleost fish immune system
The teleost fish immune system includes most if not all of the elements of the innate immune system present in mammals3,4. Evolutionarily speaking, cartilaginous fish (such as sharks) are the earliest living organisms with an adaptive immune system, as they have immunoglobulins, T cell antigen receptors, major histocompatibility complex class I and II molecules, spleen and thymus5. The teleost adaptive immune system also has other features, in addition to the aforementioned elements, which are similar to and in some cases differ from those of the mammalian immune system (Table 1). In terms of lymphoid tissues, despite having a spleen and thymus, teleosts lack lymph nodes and bone marrow6. However, the anterior part of the fish kidney (the head kidney) is considered a functional ortholog of mammalian bone marrow, as it represents the main teleost hematopoietic lymphoid tissue and is thought to be an immunologically responsive organ6. In addition, teleosts do not have germinal centers, and although they can express the cytidine deaminase AID, they lack antibody class-switch recombination. The gut, skin and gills of teleosts contain mucosa-associated lymphoid tissue that serves a pivotal role in the maintenance of mucosal homeostasis7. Although the teleost repertoire of innate immune molecules—for example, lectins, complement and natural killer cell receptors—is more diverse than that of mammals8-10, the opposite is true for immunoglobulins. Thus far, only three immunoglobulins classes have been identified in teleosts: immunoglobulin M (IgM), IgD and IgT (called ‘IgZ’ in some teleosts)11,12. I have summarized additional key features of teleost immunoglobulins (Box 1), as well as some of the latest key findings about teleost immunology relevant to the understanding of mammalian immunity (Box 2). Further details of teleost immunity are available in a 2011 journal issue devoted entirely to reviewing all aspects of teleost immunology13.
Table 1.
Fundamental features of adaptive immune systems of teleost fish and mammals
| Teleosts | Mammals | |
|---|---|---|
| Immunoglobulin | IgM, IgD and IgT (or IgZ) | IgM, IgG, IgA, IgD and IgE |
| AID | Yes | Yes |
| Class-switch recombination | No | Yes |
| Somatic hypermutation | +++ | +++ |
| Affinity maturation | + | +++ |
| Memory responses | + | +++ |
| TCR, CD4, CD8 | Yes | Yes |
| MHC class I and II | Yes | Yes |
| CD28, CD40, CD80, CD86, ICOS | Yes | Yes |
| TH1, TH2 and TH17 cytokines | Yes | Yes |
| Spleen, thymus and bone marrow | Spleen and thymus but no true bone marrow | Yes |
| Mucosa-associated lymphoid tissue | Yes | Yes |
| Germinal centers and lymph nodes | No | Yes |
Comparison of key elements of immunoglobulin-based adaptive immune systems of teleost fish and mammals. MHC, major histocompatibility complex; ICOS, inducible costimulator; TH1, TH2 and TH17, subsets of helper T cells.
Box 1. Teleost immunoglobulins.
IgM: Teleost IgM is a tetrameric molecule and is by far the most prevalent immunoglobulin in plasma59,60. Gut and skin mucus are reported to have very low concentrations of IgM61-63. IgM tetramers in teleosts are formed in the apparent absence of intermolecular interactions mediated by immunoglobulin joining chains. Moreover, these tetramers are in various oxidation states that seem to influence their effector functions64. All teleost B cells that express surface IgM also express surface IgD. Teleost IgM is secreted mainly by plasmablasts and plasma-like cells that are located mostly in the head kidney and are key to eliciting memory IgM responses65. After booster immunization, teleost fish undergo a substantial increase in IgM titers59,60, a process that is temperature dependent. However, teleosts show a moderate increase in IgM affinity60,66 relative to the maturation responses of very high affinity noted for IgG.
IgD: The organization of the gene encoding IgD in teleosts is very variable, a fact reflected in the variety of secreted IgD isoforms with different molecular masses that seem to be present as monomers in serum67. Although the role of teleost IgD remains obscure, it has been postulated that catfish IgD functions as a PRR, as secretory IgD lacks a variable region67. However, secretory IgD in trout does contain a variable region and thus could potentially be involved in the adaptive recognition of antigens68.
IgT: Teleost IgT is specialized in gut mucosal immunity49. IgT is present in serum as monomers, whereas in the gut mucus it forms mainly multimers similar in mass to those of IgM. However, IgT multimers are associated in a noncovalent manner. An additional lineage of teleost B cells that uniquely express surface IgT has been identified in rainbow trout and has been found to represent the main B cell subset in the gut49. Mucosal IgT and IgM in trout associate with a polymeric immunoglobulin receptor for their transport into the gut lumen49. Consistent with the prevalent roles of IgT in gut immunity, most bacteria in the gut lumen of rainbow trout are coated with IgT, and IgT responses to gut parasites are measurable only in the gut, whereas IgM responses are detected only in serum49. Although it is suspected that IgT has a key role in other mucosal areas (such as the skin and gills), this remains to be investigated.
Box 2. Additional findings of teleost immunology that have contributed to the understanding of mammalian immunity.
New discoveries of IgD function: Studies of catfish have shown the presence of a unique IgM−IgD+ B cell subset69 that is phenotypically similar to a human IgM−IgD+ subset found in tonsillar and nasal tissues70. Moreover, IgD-armed granulocytes have been detected in catfish. Those observations led to the critical identification of IgM−IgD+ B cell subsets and IgD-armed basophils in humans70. Those subsequent studies showed that crosslinkage of human IgD on basophils in the upper respiratory mucosa induces the release of antimicrobial, opsonizing, inflammatory and B cell–stimulating factors70
Unusual affinity maturation in trout: Studies have shown that affinity maturation occurs in trout apparently via modification of the redox state of IgM71. That study found a strong association between greater IgM affinity and more disulfide polymerization in the IgM molecule. This work suggests that given the affinity of the interaction of antigen with IgM on the surface of B cells, intracellular signals probably modify the activity of the enzymes involved in the formation of disulfide bonds. Although this work is still at its inception, further research may provide insights to novel mechanisms by which the affinity of the BCR can modify the general cell biology of B lymphocytes.
Possibility of primitive germinal centers in teleosts: Although teleosts are thought to be devoid of germinal centers, a study has shown the presence of discrete cell clusters in the spleen and kidney of the channel catfish, which were suggested to represent primordial germinal centers72. Those cell clusters contain cells that express AID and melano-macrophages that express the receptor for colony-stimulating factor 1, as well as B cells and CD4+ T cells. Study of these putatively ancient germinal centers may provide clues for understanding primordially conserved mechanisms of germinal center formation as well as insight into how germinal centers form in species devoid of lymph nodes.
NITRs of teleosts: NITRs (novel immune-type receptors) are encoded by clusters of multigene families that encode innate immune receptors with a close structural resemblance to antigen receptors9. These have been identified only in teleosts. There is evidence that some NITRs participate in allogenic recognition73 and thus it has been postulated that NITRs could be the teleost equivalent of some natural killer cell receptors. Elucidation of the structure and function of NITRs in innate immunity will probably demonstrate mechanisms and strategies by which these receptors regulate cell-mediated cytoxicity.
Phagocytic B cells in vertebrates: a paradigm shift
In mammals and other vertebrates, including fish, professional phagocytosis is achieved mainly by polymorphonuclear cells, monocytes and macrophages14,15. Other nonprofessional phagocytes (such as mammalian epithelial cells, fibroblasts, natural killer T cells and plasmacytoid dendritic cells) have also been shown to ingest microbes, albeit with a greatly restricted capacity15-17. It was initially believed that primary B cells from vertebrates were incapable of phagocytosis. However, that paradigm was broken by the finding that the primary IgM+ B cells of teleosts have potent phagocytic ability and are able to engulf and kill microbes18. All teleost species analyzed thus far have phagocytic B cells, which suggests that this is a general feature of teleost B cells18,19. Those findings have also been extended to the amphibian Xenopus laevis, in which a large proportion of blood IgM+ B cells are able to engulf large particles18. Moreover, small numbers of blood phagocytic IgM+ B cells have been found in a reptile: the red-eared slider turtle20.
The discovery of phagocytic B cells in fish and their evolutionary preservation in amphibians and reptiles led to the investigation of whether such phagocytic ability is conserved in subsets of mammalian B cells. That does indeed seem to be the case because the peritoneal cavity of mice has a large proportion of phagocytic B cells (~10–17%), although they can also be found in other lymphoid sites but at a lower frequency (~1.6%)21. Most of the phagocytic B cells in the peritoneal cavity are B-1 cells, with relatively very few phagocytic B-2 cells. Phagocytosis occurs in a manner independent of the B cell antigen receptor (BCR), which leaves the nature of the phagocytic receptors unknown21. Both phagocytic B-1 cell subsets (B-1a and B-1b) are able to mature their phagosomes into phagolysosomes and are able to kill internalized bacteria. In addition to taking up large particles, B-1a and B-1b cells in the peritoneal cavity are able to efficiently present antigen from internalized particles to CD4+ T cells. Similar to what has been found for dendritic cells and macrophages, the ability of both B-1 subsets in the peritoneal cavity to present particulate antigen is vastly superior to their ability to present the same amount of soluble antigen21. Although macrophages in the peritoneal cavity are the leukocytes with the greatest phagocytic ability in mice, they are considerably less potent antigen-presenting cells than are B-1 cells in the peritoneal cavity, whereas dendritic cells in the bone marrow are the most efficient. Whether phagocytic B cells can also cross-present to CD8+ T cells remains to be investigated.
Most of the aforementioned findings about phagocytic B-1 cells in the peritoneal cavity21 have been confirmed by an additional study22, although that second study did not compare the phagocytic, bactericidal and antigen-presenting abilities of B-1 cells in the peritoneal cavity with those of macrophages and bone marrow dendritic cells. A further study has reported the existence of phagocytic B cells in the liver and spleen of mice23, although this work did not address the presence of such cells in the peritoneal cavity. In agreement with the findings about the mouse peritoneal cavity21, most phagocytic B cells in liver and spleen belong to the B-1 subset. However, the observed percentage of phagocytic B-2 cells in liver and spleen23 is greater than that reported for B-2 cells in the peritoneal cavity and spleen21,22. The reasons for the differences noted for phagocytic B-2 cells in those three studies21-23 remain unknown. Whether phagocytic B cells in mouse liver and spleen can act as antigen-presenting cells remains to be investigated, although phagocytic B cells in the liver, but not those in the spleen, have been shown to secrete interleukin 12 (IL-12)23.
From an evolutionary perspective, the innate traits of B-1 cells that have been identified (such as their phagocytic and microbicidal ability) might suggest that the most ancient B cell lineage had many B-1 cell–like features shared by fish B cells and B-1 cells. Accordingly, B-1 cells would have subsequently evolved from that primordial fish B-1 cell–like lineage. That hypothesis would fit well with the concept of an evolutionarily layered immune system24 in which B-1 cells would constitute the evolutionarily oldest layer and would thus have more innate features than B-2 cells have. Because of the more specialized role of B-2 cells in adaptive immunity, B-2 cells would have emerged later and would thus represent the most evolved layer of all B cell subsets. Alternatively, it is possible that other B cell lineages exist in fish, some of which are perhaps unable to perform phagocytosis or perhaps lack other innate B-1 cell–like roles. If such a B cell lineage were to be found in fish, then the hypothesis that B-1-like B cells are the oldest B cells in evolution might not hold true. Therefore, the evolutionary origins of B-1 and B-2 cell lineages will be delineated only after the composition, roles and relationships of B cell lineages in fish and other ectothermic vertebrates are fully understood.
New paradigms emerge from studies of phagocytic B cells
The discovery of the phagocytic ability of B-1 cells, the main producers of ‘natural’ antibodies with low affinity but broad specificity for both foreign and self antigens25,26, raises the intriguing possibility that non-BCR uptake of particles by these cells enhances the secretion of polyreactive IgM or IgA. Antibody secretion is probably triggered through the engagement of pattern-recognition receptors (PRRs) by microbe-derived ligands (pathogen-associated molecular patterns). However, the possibility that antibody production occurs in response to signals derived from the phagocytic process or even from signals provided by T cells during the process of antigen presentation cannot be ruled out. Nonetheless, this apparently evolutionarily preserved strategy would generate ‘innate’ polyreactive antibodies that act as pattern-recognition molecules by a mechanism analogous to the polyclonal activation induced by lipopolysaccharide in B-1 cells27,28. Such a strategy would prove advantageous in circumstances of acute infection, in which such natural antibodies serve a key role as the first line of defense. However, although such a polyclonal response may be broadly protective, it may simultaneously promote autoimmune reactivity. Indeed, B-1 cells have been linked to autoimmune diseases29. Nonetheless, the production of such polyreactive immunoglobulins by phagocytic B-1 cells might have different outcomes depending on the nature of the phagocytosed particle and the lymphoid tissue in which the B cell is located (Fig. 1).
Figure 1.

Hypothetical roles of phagocytic B-1 cells in antibody secretion and cytokine production after phagocytosis of microbes in a BCR-independent manner. (a) Commensal bacteria that have leaked from the gut lumen as a result of injury or inflammation are released into the peritoneal cavity, where phagocytic B-1 cells engulf them. (b) In a different scenario, the B-1 cells phagocytose pathogenic bacteria that have intruded into the peritoneal cavity or the liver. As a result, those B-1 cells may differentiate directly into antibody-secreting cells or they may activate, proliferate and turn into antibody-secreting cells (a,b). Those antibody-secreting cells will probably produce low-affinity polyreactive IgM that can recognize leaked commensals found in the peritoneal cavity (a) or pathogenic microbes (b). In addition, part of the secreted IgM will probably exit to the periphery, where it will add to the pool of natural antibodies (a,b). It may be possible that some of the secreted IgM recognizes self antigens and thus serves a role in autoimmunity (a,b). Alternatively, the phagocytic B-1 cells may migrate into the gut lamina propria (a) or to the spleen and peripheral lymph nodes (b). In those sites, the phagocytic B cells may directly differentiate into antibody-secreting cells and produce IgM (a,b), or IgA (a). IgA and IgM produced in the lamina propria may serve a role in immune exclusion (a). Phagocytic B-1 cells may also express pro- or anti-inflammatory cytokines depending on the tissue in which they are located (a,b). Ab, antibody; TNF, tumor-necrosis factor; IFN-γ, interferon-γ; TGF-β, transforming growth factor-β; M cell, microfold cell; CSR, class-switch recombination; sIgM and sIgA, secretory IgM and IgA.
In one possible scenario (Fig. 1a), peritoneal cavity B-1 cell–mediated phagocytosis of commensal bacteria (that have leaked from the gut as a result of injury or inflammatory damage) promotes PRR-dependent activation by the B cell. Such activation might then promote the proliferation of B cells and their secretion of polyreactive IgM to neutralize the leaked microbes. Moreover, phagocytic B-1 cells in the peritoneal cavity are likely to migrate into the lamina propria of the gut, where they may continue to produce polyreactive IgM or will probably switch classes and begin to secrete polyreactive low-affinity IgA. Both IgM and IgA would then be transported into the gut lumen via the polymeric immunoglobulin receptor, where they would coat microbes and contribute to immune exclusion (the process by which immunoglobulin-coated bacteria are prevented from traversing the gut epithelium). Relevant to this scenario (Fig. 1a), the presence of IgA+ plasma cells with monocytic features in the lamina propria of mice has been reported30. Among their other monocytic attributes, these cells produce tumor-necrosis factor and inducible nitric oxide synthase. Thus, it is possible that they are phagocytic B cells that have migrated from the peritoneal cavity and have matured into IgA+ plasma cells.
Alternatively, not only will phagocytosis of a pathogenic microbe by B-1 cells in the peritoneal cavity (Fig. 1b) promote the production of polyreactive IgM in the peritoneum, but the phagocytic B-1 cell in the peritoneal cavity will also migrate to the spleen or a peripheral lymph node. Indeed, such migration in response to microbial infection has already been documented28,31. Whether the proliferation of phagocytic B cells will occur in the peritoneal cavity is questionable; instead, it is more likely that activated B cells migrate, clonally expand and produce IgM in the periphery. The polyreactive IgM locally produced and transported to other sites would serve to destroy microbes through IgM-mediated effector mechanisms. It is also likely that B-1 cells engulfing pathogenic microbes can migrate to the gut lamina propria and serve roles similar to those described above (Fig. 1a).
In addition to phagocytosis of bacteria, B-1 cells may also be able to ingest apoptotic bodies (Fig. 2). Indeed, apoptotic bodies can bind to B-1 cells through the receptor TIM-4 (ref. 32), although their phagocytosis has not been shown directly. If B-1 cells can internalize apoptotic bodies, this may promote the secretion of polyreactive IgM able to bind to apoptotic bodies and thus facilitate their removal from circulation. Such action would greatly contribute to tissue homeostasis, and indeed IgM-deficient mice have a greater incidence of autoimmunity33. In the same vein, it has been demonstrated that subsets of natural IgM can recognize apoptotic bodies34 and that complement C1q can then bind to IgM, which leads to phagocytosis of the apoptotic bodies by C1q receptor–expressing macrophages or immature dendritic cells35. That same study has shown that the natural IgM that recognizes apoptotic bodies also reacts with a broad range of determinants associated with the pathogenesis of autoimmune disease. Thus, it is possible that phagocytosis of apoptotic bodies by B-1 cells contributes to the generation of autoreactive IgM. The generation of natural IgM that recognizes apoptotic bodies would lead to the formation of IgM–apoptotic body complexes able to activate the classical pathway of complement, which in turn would lead to the deposition of iC3b (the proteolytically inactive product of the complement cleavage fragment C3b) onto the complexes to generate IgM–apoptotic body–iC3b complexes. Conceivably such complexes could be taken up by the complement receptor CR3 on B-1 cells, macrophages or dendritic cells (with or without the cooperation of TIM-4 or other receptors), which might lead to the presentation of autoantigens in an inflammatory milieu, a process that could result in the generation of autoreactive antibodies. However, it should be emphasized that the internalization of apoptotic body–containing immunocomplexes by dendritic cells induces self-tolerance in CD4+ T cells and CD8+ T cells36. Therefore, the uptake of apoptotic bodies by B-1 cells might also lead to a process of self-tolerance instead of autoreactivity.
Figure 2.
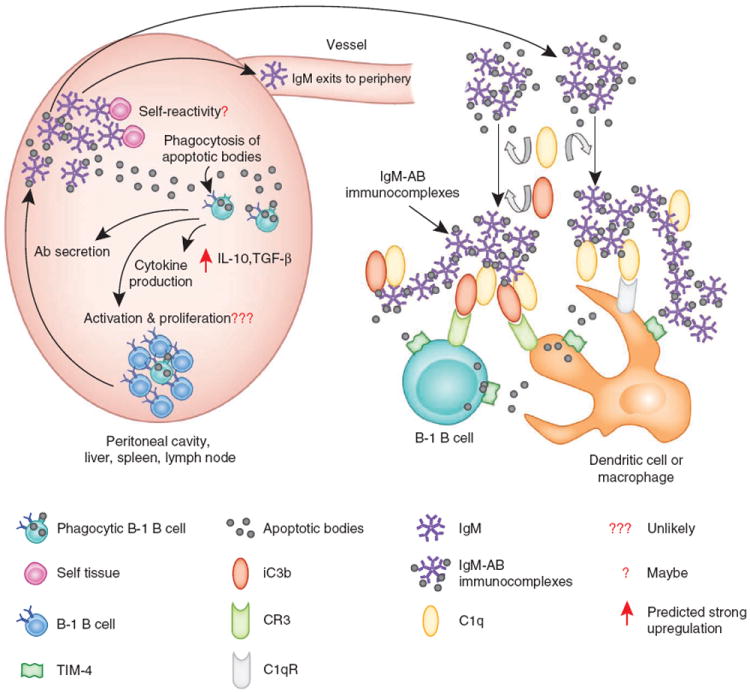
Hypothetical roles of phagocytic B-1 cells in antibody secretion and cytokine production after phagocytosis of apoptotic bodies. Apoptotic bodies (AB) are engulfed by phagocytic B-1 cells from various lymphoid sites. As a result, those cells may differentiate directly into antibody-secreting cells or they may activate, proliferate and turn into antibody-secreting cells. Antibody-secreting cells will produce low-affinity polyreactive IgM that may recognize apoptotic bodies. It is also possible that some of the secreted IgM will recognize self antigens and thus have a role in autoimmunity. Moreover, part of the secreted IgM will probably exit to the periphery, where it will add to the pool of natural antibodies. After IgM binds to the apoptotic bodies, the IgM–apoptotic body complexes may bind C1q through the Fc portion of IgM to form C1q–IgM–apoptotic body complexes. Those complexes can activate the classical pathway of complement, which leads to the deposition of C3 activation fragments (such as iC3b) on the complexes. The resulting complexes can then be taken up by B-1 cells or other leukocytes (such as dendritic cells or macrophages). As presented here, they may be recognized by the C1q receptor (C1qR), the complement receptor CR3 and/or TIM-4. However, other receptors not shown here are also able to recognize apoptotic bodies, either directly or through other IgM, complement or lectin receptors.
Finally, enrichment of the B-1 cell repertoire for self and non-self polyreactive specificities could be partially explained by the potential ability of these cells to phagocytose apoptotic bodies. B-1 cells that have phagocytosed apoptotic bodies might undergo a positive selection event, as has already been shown for B-1 cells that produce autoantibodies37. Along the same lines, it will be of great interest to determine whether the phagocytosis of apoptotic bodies influences the shape of the BCR repertoire and to what degree this contributes to the pool of autoreactive B-1 cells.
Although the scenarios above focused on non–BCR-mediated phagocytosis and the polyclonal production of polyreactive antibodies by B-1 cells, additional mechanisms may also contribute to the functional importance of phagocytic B-1 cells. In addition to their roles in killing microbes and in antigen presentation, phagocytic B cells might produce cytokines. It is well established that the phagocytosis of microbes or self material (such as apoptotic bodies) promotes the production of proinflammatory cytokines or anti-inflammatory cytokines, respectively38. In this context, B cells produce many of these cytokines (such as tumor-necrosis factor, IL-1, IL-6, IL-10, interferon-γ and transforming growth factor-β) in addition to others (such as IL-2, IL-4, IL-12 and IL-13) expressed in response to different stimuli39,40. Thus, it is plausible that phagocytic B cells could upregulate their production of proinflammatory cytokines after uptake of commensal or pathogenic microbes (Fig. 1a,b). It is also possible that depending on the microenvironment (that is, gut lamina propria; Fig. 1a) and additional signals received, phagocytic B cells could produce anti-inflammatory cytokines (such as IL-10 and transforming growth factor-β). However, phagocytosis of apoptotic bodies would probably lead to the production of anti-inflammatory cytokines, similar to what has been shown for ‘classic’ phagocytes (Fig. 2). In that sense, it will be of great interest to determine whether uptake of apoptotic bodies activates IL-10-producing regulatory B cells41,42 or other B cell subsets. In addition to exacerbating or ameliorating inflammation, the production of some of these cytokines could have potent immunomodulatory effects on the immune response. Along similar lines, cytokines produced by effector or regulatory B cells in response to cytokines secreted by T helper type 1 and T helper type 2 cells can either enhance or suppress T cell responses39. It is worth noting that the discussion above did not include cytokines that might be released by phagocytic B cells during the process of presenting antigen to T cells. Thus, it is possible that when presenting particulate antigen to naive T cells, the phagocytic B cell produces cytokines (such as IL-12 or IL-4) that modulate T cell responses.
In contrast to non–BCR-mediated phagocytosis that would lead to the polyclonal activation of B cells, it is possible that uptake of particles by B-1 cells may also occur in a BCR-dependent manner22. In this case, BCR-mediated signal transduction would probably not result in proliferative responses, as B-1 cells are unable to proliferate in response to BCR crosslinking43. Nonetheless, there are examples in which microbes (such as Bacillus hermsii) or microbial products (such as pneumococcal polysaccharide type 3) in the peritoneal cavity induce BCR-specific clonal expansion of B-1b cells44,45. Moreover, it is possible that coengagement of the BCR and PRRs binding to particulate pathogen-associated molecular patterns could induce the clonal expansion of phagocytic B-1 cells. However, prolonged activation and proliferative responses induced by coengagement of the BCR and PRRs on the B-1 cell may affect homeostasis and favor the generation of autoimmune disorders. The BCR-dependent clonal expansion of B-1 cells and enhanced secretion of antigen-specific IgM would act in synergy during the clearance of microbes. Moreover, as microbes would be recognized by both the BCR and other PRRs in this scenario, the threshold of activation of these cells may well be lower than when recognition and internalization of the pathogen occurs only through PRRs. As for other potential outcomes (such as migration to other sites or cytokine production), it is likely that all of the possibilities proposed above for non–BCR-mediated phagocytosis would also apply to these cells, although at present it is unclear whether BCR-mediated signals have comparable effects on B-1 cell trafficking46. Finally, to what degree B-2 cells are phagocytic and whether uptake in these cells is mediated solely by the BCR are issues that warrant thorough evaluation. Most probably, phagocytic B-2 cells internalize particles in a mostly BCR-mediated manner, with functional consequences similar to those noted above for B-1 cells that phagocytose through their BCR. In that case, the origin or location of the B-2 cell may have an influence on such outcomes, as B-2 cells in the peritoneal cavity are functionally similar to B-1 cells in the peritoneal cavity and thus may function differently from peripheral B-2 cells after phagocytosis47. Although most of the discussion in this section is based on findings and knowledge derived from mouse models, it will be of paramount interest to investigate the presence of phagocytic B cells in humans. B-1 cells have been described in humans48 and thus these cells are obvious candidates with which to begin such studies. It is apparent that the functional consequences of phagocytosis by B cells in innate and adaptive immunity can be intricate. Thus, although elucidation of the roles of mammalian phagocytic B cells is expected to be provided mainly by studies of mice, the use of phylogenetically older species such as teleost fish should also provide valuable insights, given the demonstrated conservation of key roles of these cells in fish and mammals18,21.
Teleosts answer unresolved questions of mucosal immunity
Immunoglobulin isotypes have evolved to serve many roles in either the mucosal or systemic compartment. In mammals and birds, IgA is the main participant in mucosal tissues, whereas in amphibians, that role is served by IgX5. In teleosts, IgM was thought to be the only immunoglobulin class that responds to antigenic challenge in both the systemic and mucosal compartments. However, it has now been demonstrated that teleost IgT serves a specialized role in gut mucosal immunity49. That finding clearly overturned the paradigm that specialized mucosal isotypes arose during tetrapod evolution. The gut-associated lymphoid tissue (GALT) of fish and mammals share some properties but also differ in some ways (Table 2).
Table 2.
Fundamental features of mammalian and teleost fish GALT
| Teleosts | Mammals | |
|---|---|---|
| Main GALT immunoglobulin | Polymeric IgT | Polymeric IgA |
| Main GALT B cell subset | IgT+ B cells | IgA-producing B-1 and B-2 cells |
| GALT ultrastructure | LP, IELs, No PP and MLNs | LP, IELs, PP, MLNs |
| Main immunoglobulin that coats gut commensals | IgT | IgA |
| pIgR used to transport sIg to gut lumen | Yes | Yes |
| Generation of specific IgT or IgA responses to gut parasites | Yes | Yes |
LP, lamina propria; IELs, intraepithelial lymphocytes; PP, Peyer’s patches; MLNs, mesenteric lymph nodes; pIgR, polymeric immunoglobulin receptor; sIg, secretory immunoglobulin.
Teleost IgT represents the phylogenetically oldest known immunoglobulin that is specialized for gut mucosal responses. Studies of IgT should therefore uncover fundamental mechanisms of action common to the mucosal immune systems of both fish and humans. The mucosal immune systems of both fish and mammals emerged in response to common physiological demands related to the need to protect these vital exchange surfaces from the multitude of potentially pathogenic microbes to which they are exposed. Thus, it is apparent that the selective forces (such as host-commensal, pathogenic or mutualistic interactions) and physical constraints that have shaped fish and mammalian mucosal surfaces are very similar. Therefore, the novel immunological solutions that have evolved to protect these surfaces (such as GALT and dedicated mucosal immunoglobulins) probably share fundamental structural and mechanistic aspects. In fact, the mammalian intestinal IgA is considered a “primitive form of adaptive immunity that regulates microbial communities in the gut”50,51. Notably, this ‘primitive’ quality is not an exclusive feature of GALT, as other elements of the mammalian immune system, such as B-1 lymphocytes, γδ T cells and natural killer T cells, among others, have retained ‘primitive’ characteristics that are essential for host protection. Although the general ultrastructure of the teleost GALT is similar to that of mammals, it lacks Peyer’s patches and mesenteric lymph nodes7. Because of the simpler organization of the GALT in fish, a lower spatial and functional complexity of the local mucosal responses would be expected. Because of the reasons stated above, in-depth comparative and functional analysis of fish and mammalian GALT will probably reveal the most ‘generic’ or primordial features and perhaps some unidentified critical features of mucosal immunity in mammals. For example, it is now apparent that gut follicular structures (such as Peyer’s patches and mesenteric lymph nodes) initially thought to be important for mammalian mucosal IgA responses may not in fact be essential52. Accordingly, the lamina propria of genetically engineered mice lacking Peyer’s patches, mesenteric lymph nodes and isolated lymphoid follicles53 is alone sufficient to support IgA production even in the absence of those secondary lymph tissues. Those and other findings have indicated that the GALT initiates T cell–dependent and T cell–independent IgA responses through both follicular and extrafollicular pathways52,54. However, the T cell–dependent and T cell–independent extrafollicular pathways of IgA production are not yet well defined. In addition, it is unclear at present how B-1 cells home to those extrafollicular sites in the gut. Because teleosts lack Peyer’s patches and mesenteric lymph nodes, they represent a valuable model with which to assess extrafollicular production of IgT and, by extension, IgA.
Another area of mucosal immunity to which studies of teleost fish have already made important contributions and should continue to do so is the understanding of host-commensal interactions at mucosal interfaces. For example, substantial inroads have been made with the use of gnotobiotic zebrafish, in which responses to commensal colonization are analogous to those of gnotobiotic mice55. Because of the physical characteristics of the zebrafish (their transparency), this model allows real-time imaging of the dynamic interactions of commensals, pathogens and the immune system at mucosal interfaces, including the gut56. In addition, zebrafish also permit access to early developmental stages and provide the possibility of mutagenesis screens for distinct aberrant phenotypes. Finally, the use of transcriptomics and metagenomics in combination with germ-free fish should provide answers to the question of how the mucosal microbiota is controlled by IgT or other immunological mechanisms, as well as how microbial communities (such as probiotics) specifically affect both mucosal and systemic immune responses.
Teleosts should therefore add a new phylogenetic dimension to the field, providing essential insight into unresolved paradigms of mucosal immunity in mammals.
Concluding remarks
This Perspective has highlighted findings obtained with teleost fish that have led to the identification of previously unrecognized functions and potential new paradigms for mammalian B cells and mucosal immune reactions, respectively. It would seem that regardless of their phylogenetic differences, the general schemes used by jawed vertebrate immune systems operate under the guidance of primordially conserved principles. This applies even to comparisons of jawless and jawed vertebrates for which ‘general design principles’ of adaptive immunity can be drawn after comparison of their adaptive immune systems, as has been elegantly portrayed before57. Therefore, although there may be differences among the immune systems of jawed vertebrates, they share immunologically relevant body areas (such as gut mucosal surfaces) that throughout evolutionary time have been subjected to very similar selective pressures. Thus, the study of such sites in phylogenetically primitive species will probably identify canonical structural and functional aspects of immunity that are common to all jawed vertebrates. Therefore, I wish to conclude this Perspective with a quote that eloquently reflects one of the key messages I hope to have conveyed here: “The use of comparative approaches will provide information about the ways that innate and adaptive immunity are interwoven and affect the constantly evolving relationships among humans, commensal organisms and microbial pathogens”58.
Acknowledgments
I thank L. King and D. Parra for critical reading and editing of the manuscript. Supported by the National Science Foundation (NSF-MCB-0719599), the US National Institutes of Health (R01GM085207-01), the US Department of Agriculture (USDA-NRI 2006-01619 and USDA-NRI 2007-01719).
Footnotes
COMPETING FINANCIAL INTERESTS
The author declares no competing financial interests.
References
- 1.Hoffmann J. Antifungal defense in Drosophila. Nat Immunol. 2007;8:543–545. doi: 10.1038/ni0607-543. [DOI] [PubMed] [Google Scholar]
- 2.Kaufmann SH. Immunology’s foundation: the 100-year anniversary of the Nobel Prize to Paul Ehrlich and Elie Metchnikoff. Nat Immunol. 2008;9:705–712. doi: 10.1038/ni0708-705. [DOI] [PubMed] [Google Scholar]
- 3.Magnadóttir B. Innate immunity of fish (overview) Fish Shellfish Immunol. 2006;20:137–151. doi: 10.1016/j.fsi.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Magor BG, Magor KE. Evolution of effectors and receptors of innate immunity. Dev Comp Immunol. 2001;25:651–682. doi: 10.1016/s0145-305x(01)00029-5. [DOI] [PubMed] [Google Scholar]
- 5.Flajnik MF, Kasahara M. Origin and evolution of the adaptive immune system: genetic events and selective pressures. Nat Rev Genet. 2010;11:47–59. doi: 10.1038/nrg2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zapata A, Amemiya CT. Phylogeny of lower vertebrates and their immunological structures. Curr Top Microbiol Immunol. 2000;248:67–107. doi: 10.1007/978-3-642-59674-2_5. [DOI] [PubMed] [Google Scholar]
- 7.Salinas I, Zhang YA, Sunyer JO. Mucosal immunoglobulins and B cells of teleost fish. Dev Comp Immunol. 2011;35:1346–1365. doi: 10.1016/j.dci.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vasta GR, et al. Structural and functional diversity of the lectin repertoire in teleost fish: relevance to innate and adaptive immunity. Dev Comp Immunol. 2011;35:1388–1399. doi: 10.1016/j.dci.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoder JA, Litman GW. The phylogenetic origins of natural killer receptors and recognition: relationships, possibilities, and realities. Immunogenetics. 2011;63:123–141. doi: 10.1007/s00251-010-0506-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sunyer JO, Zarkadis IK, Lambris JD. Complement diversity: a mechanism for generating immune diversity? Immunol Today. 1998;19:519–523. doi: 10.1016/s0167-5699(98)01341-3. [DOI] [PubMed] [Google Scholar]
- 11.Danilova N, Bussmann J, Jekosch K, Steiner LA. The immunoglobulin heavy-chain locus in zebrafish: identification and expression of a previously unknown isotype, immunoglobulin Z. Nat Immunol. 2005;6:295–302. doi: 10.1038/ni1166. [DOI] [PubMed] [Google Scholar]
- 12.Hansen JD, Landis ED, Phillips RB. Discovery of a unique Ig heavy-chain isotype (IgT) in rainbow trout: Implications for a distinctive B cell developmental pathway in teleost fish. Proc Natl Acad Sci USA. 2005;102:6919–6924. doi: 10.1073/pnas.0500027102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sunyer JO, editor. Special issue on teleost fish immunology. Dev Comp Immunol. 2011;35:1193–1400. doi: 10.1016/j.dci.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 14.Neumann NF, Stafford JL, Barreda D, Ainsworth AJ, Belosevic M. Antimicrobial mechanisms of fish phagocytes and their role in host defense. Dev Comp Immunol. 2001;25:807–825. doi: 10.1016/s0145-305x(01)00037-4. [DOI] [PubMed] [Google Scholar]
- 15.Rabinovitch M. Professional and non-professional phagocytes: an introduction. Trends Cell Biol. 1995;5:85–87. doi: 10.1016/s0962-8924(00)88955-2. [DOI] [PubMed] [Google Scholar]
- 16.Wu Y, et al. Human gamma delta T cells: a lymphoid lineage cell capable of professional phagocytosis. J Immunol. 2009;183:5622–5629. doi: 10.4049/jimmunol.0901772. [DOI] [PubMed] [Google Scholar]
- 17.Ochando JC, et al. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat Immunol. 2006;7:652–662. doi: 10.1038/ni1333. [DOI] [PubMed] [Google Scholar]
- 18.Li J, et al. B lymphocytes from early vertebrates have potent phagocytic and microbicidal abilities. Nat Immunol. 2006;7:1116–1124. doi: 10.1038/ni1389. [DOI] [PubMed] [Google Scholar]
- 19.Øverland HS, Pettersen EF, Ronneseth A, Wergeland HI. Phagocytosis by B-cells and neutrophils in Atlantic salmon (Salmo salar L.) and Atlantic cod (Gadus morhua L.) Fish Shellfish Immunol. 2009;28:193–204. doi: 10.1016/j.fsi.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 20.Zimmerman LM, Vogel LA, Edwards KA, Bowden RM. Phagocytic B cells in a reptile. Biol Lett. 2010;6:270–273. doi: 10.1098/rsbl.2009.0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parra D, et al. Pivotal Advance: Peritoneal cavity B-1 B cells have phagocytic and microbicidal capacities and present phagocytosed antigen to CD4+ T cells. J Leukoc Biol. 2012;91:525–536. doi: 10.1189/jlb.0711372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao XM, et al. A novel function of murine B1 cells: Active phagocytic and microbicidal abilities. Eur J Immunol. 2012;42:982–992. doi: 10.1002/eji.201141519. [DOI] [PubMed] [Google Scholar]
- 23.Nakashima M, et al. Pivotal Advance: characterization of mouse liver phagocytic B cells in innate immunity. J Leukoc Biol. 2012;91:537–546. doi: 10.1189/jlb.0411214. [DOI] [PubMed] [Google Scholar]
- 24.Herzenberg LA, Kantor AB, Herzenberg LA. Layered evolution in the immune system. A model for the ontogeny and development of multiple lymphocyte lineages. Ann NY Acad Sci. 1992;651:1–9. doi: 10.1111/j.1749-6632.1992.tb24588.x. [DOI] [PubMed] [Google Scholar]
- 25.Casali P, Schettino EW. Structure and function of natural antibodies. Curr Top Microbiol Immunol. 1996;210:167–179. doi: 10.1007/978-3-642-85226-8_17. [DOI] [PubMed] [Google Scholar]
- 26.Zhou ZH, et al. The broad antibacterial activity of the natural antibody repertoire is due to polyreactive antibodies. Cell Host Microbe. 2007;1:51–61. doi: 10.1016/j.chom.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murakami M, et al. Oral administration of lipopolysaccharides activates B-1 cells in the peritoneal cavity and lamina propria of the gut and induces autoimmune symptoms in an autoantibody transgenic mouse. J Exp Med. 1994;180:111–121. doi: 10.1084/jem.180.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ha SA, et al. Regulation of B1 cell migration by signals through Toll-like receptors. J Exp Med. 2006;203:2541–2550. doi: 10.1084/jem.20061041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Viau M, Zouali M. B-lymphocytes, innate immunity, and autoimmunity. Clin Immunol. 2005;114:17–26. doi: 10.1016/j.clim.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 30.Fritz JH, et al. Acquisition of a multifunctional IgA+ plasma cell phenotype in the gut. Nature. 2012;481:199–203. doi: 10.1038/nature10698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Itakura A, et al. An hour after immunization peritoneal B-1 cells are activated to migrate to lymphoid organs where within 1 day they produce IgM antibodies that initiate elicitation of contact sensitivity. J Immunol. 2005;175:7170–7178. doi: 10.4049/jimmunol.175.11.7170. [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez-Manzanet R, et al. T and B cell hyperactivity and autoimmunity associated with niche-specific defects in apoptotic body clearance in TIM-4-deficient mice. Proc Natl Acad Sci USA. 2010;107:8706–8711. doi: 10.1073/pnas.0910359107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boes M, et al. Accelerated development of IgG autoantibodies and autoimmune disease in the absence of secreted IgM. Proc Natl Acad Sci USA. 2000;97:1184–1189. doi: 10.1073/pnas.97.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ehrenstein MR, Notley CA. The importance of natural IgM: scavenger, protector and regulator. Nat Rev Immunol. 2010;10:778–786. doi: 10.1038/nri2849. [DOI] [PubMed] [Google Scholar]
- 35.Chen Y, Park YB, Patel E, Silverman GJ. IgM antibodies to apoptosis-associated determinants recruit C1q and enhance dendritic cell phagocytosis of apoptotic cells. J Immunol. 2009;182:6031–6043. doi: 10.4049/jimmunol.0804191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steinman RM, Turley S, Mellman I, Inaba K. The induction of tolerance by dendritic cells that have captured apoptotic cells. J Exp Med. 2000;191:411–416. doi: 10.1084/jem.191.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hayakawa K, et al. Positive selection of natural autoreactive B cells. Science. 1999;285:113–116. doi: 10.1126/science.285.5424.113. [DOI] [PubMed] [Google Scholar]
- 38.Henson PM. Dampening inflammation. Nat Immunol. 2005;6:1179–1181. doi: 10.1038/ni1205-1179. [DOI] [PubMed] [Google Scholar]
- 39.Lund FE, Randall TD. Effector and regulatory B cells: modulators of CD4+ T cell immunity. Nat Rev Immunol. 2010;10:236–247. doi: 10.1038/nri2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barr TA, Brown S, Ryan G, Zhao J, Gray D. TLR-mediated stimulation of APC: distinct cytokine responses of B cells and dendritic cells. Eur J Immunol. 2007;37:3040–3053. doi: 10.1002/eji.200636483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mizoguchi A, Bhan AK. A case for regulatory B cells. J Immunol. 2006;176:705–710. doi: 10.4049/jimmunol.176.2.705. [DOI] [PubMed] [Google Scholar]
- 42.Hoehlig K, et al. Immune regulation by B cells and antibodies a view towards the clinic. Adv Immunol. 2008;98:1–38. doi: 10.1016/S0065-2776(08)00401-X. [DOI] [PubMed] [Google Scholar]
- 43.Morris DL, Rothstein TL. Abnormal transcription factor induction through the surface immunoglobulin M receptor of B-1 lymphocytes. J Exp Med. 1993;177:857–861. doi: 10.1084/jem.177.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haas KM, Poe JC, Steeber DA, Tedder TF. B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity. 2005;23:7–18. doi: 10.1016/j.immuni.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 45.Gil-Cruz C, et al. The porin OmpD from nontyphoidal Salmonella is a key target for a protective B1b cell antibody response. Proc Natl Acad Sci USA. 2009;106:9803–9808. doi: 10.1073/pnas.0812431106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baumgarth N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat Rev Immunol. 2011;11:34–46. doi: 10.1038/nri2901. [DOI] [PubMed] [Google Scholar]
- 47.Hastings WD, Tumang JR, Behrens TW, Rothstein TL. Peritoneal B-2 cells comprise a distinct B-2 cell population with B-1b-like characteristics. Eur J Immunol. 2006;36:1114–1123. doi: 10.1002/eji.200535142. [DOI] [PubMed] [Google Scholar]
- 48.Griffin DO, Holodick NE, Rothstein TL. Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+ CD27+ CD43+ CD70. J Exp Med. 2011;208:67–80. doi: 10.1084/jem.20101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang YA, et al. IgT, a primitive immunoglobulin class specialized in mucosal immunity. Nat Immunol. 2010;11:827–835. doi: 10.1038/ni.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Macpherson AJ, et al. A primitive T cell-independent mechanism of intestinal mucosal IgA responses to commensal bacteria. Science. 2000;288:2222–2226. doi: 10.1126/science.288.5474.2222. [DOI] [PubMed] [Google Scholar]
- 51.Suzuki K, Ha SA, Tsuji M, Fagarasan S. Intestinal IgA synthesis: a primitive form of adaptive immunity that regulates microbial communities in the gut. Semin Immunol. 2007;19:127–135. doi: 10.1016/j.smim.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 52.Cerutti A, Chen K, Chorny A. Immunoglobulin responses at the mucosal interface. Annu Rev Immunol. 2011;29:273–293. doi: 10.1146/annurev-immunol-031210-101317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kang HS, et al. Signaling via LTbetaR on the lamina propria stromal cells of the gut is required for IgA production. Nat Immunol. 2002;3:576–582. doi: 10.1038/ni795. [DOI] [PubMed] [Google Scholar]
- 54.Macpherson AJ, McCoy KD, Johansen FE, Brandtzaeg P. The immune geography of IgA induction and function. Mucosal Immunol. 2008;1:11–22. doi: 10.1038/mi.2007.6. [DOI] [PubMed] [Google Scholar]
- 55.Rawls JF, Samuel BS, Gordon JI. Gnotobiotic zebrafish reveal evolutionarily conserved responses to the gut microbiota. Proc Natl Acad Sci USA. 2004;101:4596–4601. doi: 10.1073/pnas.0400706101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kanther M, Rawls JF. Host-microbe interactions in the developing zebrafish. Curr Opin Immunol. 2010;22:10–19. doi: 10.1016/j.coi.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boehm T. Design principles of adaptive immune systems. Nat Rev Immunol. 2011;11:307–317. doi: 10.1038/nri2944. [DOI] [PubMed] [Google Scholar]
- 58.Litman GW, Cooper MD. Why study the evolution of immunity? Nat Immunol. 2007;8:547–548. doi: 10.1038/ni0607-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Warr GW. The adaptive immune system of fish. Dev Biol Stand. 1997;90:15–21. [PubMed] [Google Scholar]
- 60.Solem ST, Stenvik J. Antibody repertoire development in teleosts-a review with emphasis on salmonids and Gadus morhua L. Dev Comp Immunol. 2006;30:57–76. doi: 10.1016/j.dci.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 61.Cain KD, Jones DR, Raison RL. Antibody-antigen kinetics following immunization of rainbow trout (Oncorhynchus mykiss) with a T-cell dependent antigen. Dev Comp Immunol. 2002;26:181–190. doi: 10.1016/s0145-305x(01)00063-5. [DOI] [PubMed] [Google Scholar]
- 62.Cain KD, Jones DR, Raison RL. Characterisation of mucosal and systemic immune responses in rainbow trout (Oncorhynchus mykiss) using surface plasmon resonance. Fish Shellfish Immunol. 2000;10:651–666. doi: 10.1006/fsim.2000.0280. [DOI] [PubMed] [Google Scholar]
- 63.Maki JL, Dickerson HW. Systemic and cutaneous mucus antibody responses of channel catfish immunized against the protozoan parasite Ichthyophthirius multifiliis. Clin Diagn Lab Immunol. 2003;10:876–881. doi: 10.1128/CDLI.10.5.876-881.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kaattari S, Evans D, Klemer J. Varied redox forms of teleost IgM: an alternative to isotypic diversity? Immunol Rev. 1998;166:133–142. doi: 10.1111/j.1600-065x.1998.tb01258.x. [DOI] [PubMed] [Google Scholar]
- 65.Ye J, Kaattari I, Kaattari S. Plasmablasts and plasma cells: reconsidering teleost immune system organization. Dev Comp Immunol. 2011;35:1273–1281. doi: 10.1016/j.dci.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 66.Kaattari SL, Zhang HL, Khor IW, Kaattari IM, Shapiro DA. Affinity maturation in trout: clonal dominance of high affinity antibodies late in the immune response. Dev Comp Immunol. 2002;26:191–200. doi: 10.1016/s0145-305x(01)00064-7. [DOI] [PubMed] [Google Scholar]
- 67.Edholm ES, Bengten E, Wilson M. Insights into the function of IgD. Dev Comp Immunol. 2011;35:1309–1316. doi: 10.1016/j.dci.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 68.Ramirez-Gomez F, et al. Discovery and characterization of secretory IgD in rainbow trout: secretory IgD is produced through a novel splicing mechanism. J Immunol. 2012;188:1341–1349. doi: 10.4049/jimmunol.1101938. [DOI] [PubMed] [Google Scholar]
- 69.Edholm ES, et al. Identification of two IgD+ B cell populations in channel catfish, Ictalurus punctatus. J Immunol. 2010;185:4082–4094. doi: 10.4049/jimmunol.1000631. [DOI] [PubMed] [Google Scholar]
- 70.Chen K, et al. Immunoglobulin D enhances immune surveillance by activating antimicrobial, proinflammatory and B cell-stimulating programs in basophils. Nat Immunol. 2009;10:889–898. doi: 10.1038/ni.1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ye J, Bromage ES, Kaattari SL. The strength of B cell interaction with antigen determines the degree of IgM polymerization. J Immunol. 2010;184:844–850. doi: 10.4049/jimmunol.0902364. [DOI] [PubMed] [Google Scholar]
- 72.Saunders HL, Oko AL, Scott AN, Fan CW, Magor BG. The cellular context of AID expressing cells in fish lymphoid tissues. Dev Comp Immunol. 2010;34:669–676. doi: 10.1016/j.dci.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 73.Cannon JP, et al. A bony fish immunological receptor of the NITR multigene family mediates allogeneic recognition. Immunity. 2008;29:228–237. doi: 10.1016/j.immuni.2008.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]


