Abstract
Two types of adaptive immune strategies are known to have evolved in vertebrates: the VLR-based system, which is present in jawless organisms and is mediated by VLRA and VLRB lymphocytes, and the BCR/TCR-based system, which is present in jawed species and is provided by B and T cell receptors expressed on B and T cells, respectively. Here we summarize features of B cells and their predecessors in the different animal phyla, focusing the review on B cells from jawed vertebrates. We point out the critical role of nonclassical species and comparative immunology studies in the understanding of B cell immunity. Because nonclassical models include species relevant to veterinary medicine, basic science research performed in these animals contributes to the knowledge required for the development of more efficacious vaccines against emerging pathogens.
Keywords: adaptive immunity, innate immunity, immunoglobulin, variable lymphocyte receptor (VLR), phagocytosis, antibody diversification
INTRODUCTION
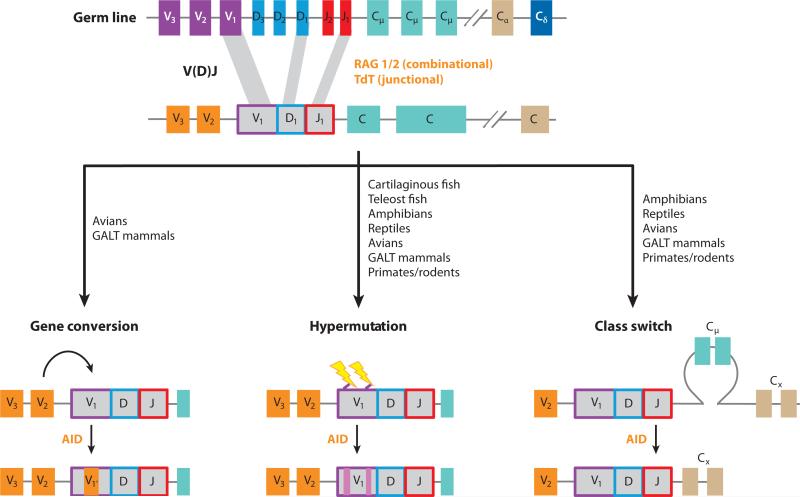
Evolution of the immune system is driven by exposure of organisms to new pathogenic insults that arise from physiological or environmental changes. Two forms of immunity, innate and adaptive, exist. Innate immunity is present in all plants and metazoans, although some forms of innate immunity are present even in eukaryotic unicellular organisms such as amoebas (1). Moreover, even prokaryotic organisms, such as bacteria, are considered to bear immune mechanisms against pathogens (2). Hallmarks of innate responses include: (a) microorganism recognition by germ line–encoded and nonrearranging receptors, such as pattern-recognition receptors (PRRs), which have both broad and uniquely exquisite specificities for several pathogen-associated molecular patterns (PAMPs); and (b) rapid effector mechanisms that involve phagocytosis, proteolytic activation cascades, and synthesis of potent antimicrobial molecules (3). However, after these innate processes are in place, adaptive immunity is activated and endowed with immunological memory to cope with previously encountered antigens. Adaptive immunity is based on antigen receptors assembled through somatic recombination of gene segments present in the germ line. These antigen receptors are expressed as one type of receptor on a lymphocyte in a monoallelic fashion and are highly specific for a great diversity of antigens. Currently, two types of adaptive immune strategies are known to have evolved in vertebrates (4): (a) the VLR-based system, which is present in jawless organisms and is mediated by VLRA and VLRB lymphocytes, and (b) the B cell receptor (BCR)/T cell receptor (TCR)-based system, which is present in jawed organisms and is provided by BCRs and TCRs expressed by B and T cells, respectively. One of the hallmarks of adaptive immunity is the enormous variability that immunoglobulins (Igs), TCRs, and variable lymphocyte receptors (VLRs) present, which allows them to recognize almost any antigen. In Igs, different mechanisms such as junctional diversity, somatic hypermutation (SHM), gene conversion, and class switch recombination (CSR) generate this variability (Figure 1). They all introduce several sequence variations in the Ig loci, generating diversity in Ig structure and amplifying vastly the antibody repertoire.
Figure 1.
Antibody diversification mechanisms. In addition to the combinatorial diversity of variable (diversity) joining [V(D)J] segments and heavy/light chains, antibodies are diversified by different mechanisms, including gene conversion, somatic hypermutation, and class switch recombination. The main enzymes implicated in each process are shown in orange. Animal names represent species in which a mechanism has been experimentally demonstrated. Abbreviations: AID, activation-induced cytidine deaminase; GALT, gut-associated lymphoid tissue.
Evolution of B cell immunity from jawed fish to mammals has led to increased diversity, specificity, and affinity of Igs, which has thus fine-tuned adaptive immune responses against almost any pathogen. This evolutionary trend may have been fueled, at least partially, by the emergence of warm-blooded vertebrates on earth (5). The capacity of microbes to grow and mutate faster in warmer hosts probably acted as a selective force to improve, diversify, and fine-tune the adaptive immune system tool kit present in cold-blooded vertebrates (6). In higher vertebrates this led to the evolutionary emergence of B cell subsets highly specialized in adaptive immunity (i.e., B-2 B cells). More recently described innate features (i.e., phagocytic and microbicidal capacities) of some B cell subsets indicate that B cells have conserved attributes from primordial phagocytes with which they likely share a common ancestor (7, 8). In the same vein, and probably reflecting both their ancient and more modern origins in their subsets, in the past few years it has become apparent that B cells do not only produce antibodies; in addition to their newly described phagocytic and microbicidal capacities, subsets of cytokine-secreting effector and regulatory B cells have been shown to modulate T cell responses to microbes and self-antigens (9). These novel B cell functions have opened up fresh research avenues in the basic understanding of B cell function as well as new opportunities for the improvement and development of new vaccines and prophylactics.
It is important to point out the critical role of nonclassical species and comparative immunology studies in the understanding of B cell immunity both in the past and in recent years. For example, B cells were first characterized in birds (10), and phagocytic B cells were first described in teleost fish (8). In this review, we summarize features of B cells and their predecessors in the different animal phyla, from invertebrates and agnathans, which lack bona fide B cells, to mammals, in which adaptive immune responses mediated by B cells are very sophisticated. We discuss features that B cells have acquired during evolution as well as highlight the conserved features that they have retained from their ancestors (Table 1).
Table 1.
Overview of B cell immunity in vertebrates
| Agnathans | Cartilaginous fish | Teleost fish | Amphibians | Reptiles | Birds | GALT mammals | Classical mammals | ||
|---|---|---|---|---|---|---|---|---|---|
| Primary lymphoid tissues | Thymus (or equivalent) | Gill | + | + | + | + | + | + | + |
| Bone marrow (or equivalent) | Typhlosole/Kidney | Epigonal/Leydig | Head kidney | Liver | + | Bursa of Fabricius | +/IPP/Appendix | + | |
| Secondary lymphoid tissues | Spleen | – | + | + | + | + | + | + | + |
| Peyer's patches | – | – | – | – | – | + | + | + | |
| Lymph nodes | – | – | – | – | – | + (Except chicken) | + | + | |
| Immuno globulin (IG) (orthologs in lower vertebrates) | IgM | – | + | + | + | + | + | + | + |
| IgD | – | + | + | + | + | – | + (Except rabbit) | + | |
| IgA | – | – | IgT | IgX | + | + | + | + | |
| IgG | – | – | – | IgY | IgY | IgY | + | + | |
| IgE | – | – | – | + | + | ||||
| Species-specific Ig | – | IgNAR | IgF (frog) IgP (newt) |
IgO (Monotreme) hcIgG (Camelid) |
|||||
| IgL | σ | – | σ, σ-cart | + | + | – | – | – | – |
| k | – | + | + | + | + | – | + | + | |
| λ | – | + | + | + | + | + | + | + | |
| Hallmark features of adaptive immune systems | Recombination-activated gene (RAG) | – | + | + | + | + | + | + | + |
| Terminal deoxynucleotidyl transferase (TdT) | – | + | + | + | + | + | + | + | |
| Activation-induced cytidine deaminase (AID) | – (but have CDA) | + | + | + | + | + | + | + | |
| Class switch recombination | – | – | – | + | + | + | + | + | |
| Somatic hypermutation | – | + | + | + | + | + | + | + | |
| Allelic exclusion | + | + | + | + | + | + | + | + | |
| Germinal centers | – | – | – | – | – | + | + | + | |
| Affinity maturation | +b | + | + | + | ND | ++ | +++ | +++ | |
| Rearrangement throughout life | ND | +a | +a | +a (Except axolotl) | +a | – | – | + | |
| Phagocytic B cell subsets | – | ND | + | + | + | ND | ND | + |
This feature is presumed (but not demonstrated) because of the persistence of RAG expression throughout life in primary lymphoid tissue.
Demonstrated in vitro.
Abbreviation: ND, not determined.
PREDECESSORS OF JAWED VERTEBRATE LYMPHOCYTES
Upon microbial challenge, invertebrates respond with both cellular and humoral components. Invertebrates appear to lack bona fide adaptive immune responses and lymphocytes, although this cannot be established conclusively until more species are thoroughly analyzed. Thus, some processes reminiscent of acquired vertebrate responses have been described in invertebrates, which suggests that invertebrate species could have some memory and specificity in their responses (11, 12).
Recombination-activated gene (RAG)-1 and RAG-2 proteins regulate recombination of variable, diversity, and joining (VDJ) segments in B and T cells to generate the acquired immune repertoire (13). However, the lack of lymphocytes in invertebrates raises the question of how the RAG system evolved. V(D)J recombination presumably arose owing to transposon insertion of an ancestral RAG gene (14). Whether the insertion of this primordial RAG gene took place in a TCR- or BCR-like gene is unknown. This gene may be more similar to TCR than BCR, because although lampreys lack V(D)J recombination, they contain a single TCR-like gene with characteristics of V and J regions (15); hence, this TCR-like gene might have been the target of the transposon insertion (14). The BCR might have evolved by gene duplication from this ancient RAG-containing, TCR-like gene cluster, and both genes would have gradually diverged into contemporary BCRs and TCRs. However, this transposon insertion event possibly occurred in species from which jawed vertebrates emerged. These species contained distinct populations of cytotoxic killer cells and phagocytes, and both cell populations would have had a BCR/TCR prototype. Subsequent to the transposon insertion event, the cytotoxic killer and the phagocytes would have evolved into T and B cells, respectively (7). In that regard, the presence of B cells with phagocytic abilities in fish may reinforce the aforementioned scenario (8).
Interestingly, sequence analysis of the complete sea urchin genome has revealed a gene encoding RAG-1-like protein adjacent to a gene encoding a functional RAG-2-like protein. Because sea urchins do not apparently possess an adaptive immune system, this data may suggest that RAG proteins have had alternative functions in invertebrates and that in vertebrates, RAG function evolved to play a role in V(D)J recombination (16). While more work is needed to address the roles of sea urchin RAG1/2, the aforementioned possible evolutionary pathway of invertebrate RAG may question the validity of the transposon model.
Jawless fish (agnathans) represent the most primitive living vertebrates. BCR, TCR, and RAG orthologs have not been identified in these species. Instead, agnathans express VLRs, named for their expression on lymphocyte-like cells and their considerable sequence diversity (17).
To date, two lymphocyte populations have been identified in lamprey based on their cell-surface expression of VLRA or VLRB molecules. VLRA+ lymphocytes resemble T cells in some functional regards, because they can proliferate after PHA stimulation (a widely used T cell mitogen) and they don't secrete VLRA. By contrast, VLRB+ lymphocytes, similar to mammalian B cells, can secrete VLRB molecules into plasma. After immunization with bacteria or human erythrocytes, VLRB+ lymphocytes show proliferation, lymphoblastoid transformation, and differentiation into plasma-like cells that secrete antigen-specific VLRBs (34). VLRB+ cells also bind cognate bacteria through endogenous antigen receptor, analogous to binding by the BCR in B cells (18). VLRB+ lymphocytes also express transcripts of orthologous genes related to B cell activation and differentiation. In particular, transcripts for TLRs, IL-8, and IL-17R are expressed in VLRB+ lymphocytes, which implies the involvement of VLRB+ lymphocytes in innate immunity (18). Intriguingly, VLRA+ lymphocytes can express IL-17 and IL-8R transcripts, which suggests the functional interactions between VLRA+ and VLRB+ lymphocytes.
Jawless fish have no lymph nodes (LNs) or true thymus, but the blood, typhlosole (an invagination of the intestinal epithelium), kidneys, and gills all contain lymphoid cells. Whereas gill filament tips and the neighboring secondary lamellae of lamprey larvae were identified as candidates for hematopoietic tissue for VLRA+ lymphocytes, termed thymoids (17), the typhlosole and/or kidney may be the tissues for VLRB+ lymphocyte development through CDA2 enzyme expression. Accordingly, VLRB+ lymphocytes outnumber VLRA+ lymphocytes in kidney and typhlosole, whereas these populations are comparable in number in the gill. This implies that, similar to T and B lymphocytes, VLRA and VLRB lymphocytes individually develop in spatially distinct tissues.
CARTILAGINOUS FISH B CELLS
Cartilaginous fish (Chondrichthyes), the Holocephali (chimeras and ratfish) and the Elasmobranchii (sharks, skates, and rays), are the first jawed vertebrate group within living gnathostomes and diverged from the common ancestor of other jawed vertebrates approximately 500 Mya. Cartilaginous fish are the oldest living vertebrate species in which essential molecules for BCR/TCR-based adaptive immunity [including major histocompatibility complex (MHC), Ig, TCR, and RAG] have been identified.
Immunoglobulins
Three Ig isotypes, designated IgM, IgNAR, and IgD, have been identified in cartilaginous fish to date, together with four light chain (IgL) isotypes, κ, λ, σ, and σ-cart. CSR is not found in cartilaginous fish. IgM is the major antibody in serum and is secreted as two forms, a monomeric (7S) and a pentameric (19S) form, that are equally present and can constitute as much as half of the total serum protein in an adult (19). On B cells, surface IgM is expressed exclusively as a monomeric form. In nurse sharks, a subclass of IgM, termed IgM1gj, is encoded by a germ line–joined, nondiverse VDJ gene. It is found predominantly in neonatal serum and is secreted by neonatal splenocytes and cells from the epigonal organ. As neonates mature, IgM1gj expression decreases in the serum and spleen, but it is still detectable in the adult epigonal organ (20). IgNAR is a unique, heavy-chain isotype in elasmobranchs that forms disulfide-bonded dimers of two identical heavy chains without IgL. The dimers are reminiscent of camelid heavy-chain V domains, which also have no IgLs (19). Serum IgNAR levels are much lower than those of IgM. IgD was referred to previously as IgW, IgNARC, IgX, and IgR, depending on the species in which it was found. It is now known to be orthologous to other, vertebrate IgD, based on phylogenetic analysis (21). The function of IgD in elasmobranchs remains to be investigated. Interestingly, monomeric IgM and IgNAR are present in the yolk of nurse sharks and may be transferred from the mother to the embryo via the egg yolk (19).
B Cell Development
Cartilaginous fish are known to have bona fide thymus and spleen as lymphoid organs, although they lack bone marrow and LNs. Moreover, elasmobranchs contain unique lymphoid tissues, such as the epigonal organ (a tissue connected to the gonads) and the Leydig organ (associated with the esophagus). Continuous transcript expression of RAG, terminal deoxynucleotidyl transferase (TdT), and T/B cell–specific transcription factors are found in thymus and the aforementioned elasmobranch-specific tissues (22, 23). Thus, Leydig and epigonal organs of elasmobranch are regarded as a primary lymphoid organ for B cells.
In dogfish shark embryos, although conventional Ig expression is first identified in the liver, during early development, the kidney is thought to be the most important site for the differentiation of large populations of B cells (19). As the hatch time approaches, B cells are observed in the kidney, spleen, and Leydig/epigonal organs. The number of kidney leukocytes declines sharply at the posthatch stage, at which time the main hematopoietic tissue is apparently shifted to the spleen. In nurse shark, the epigonal organ is the main site for neonatal B lymphopoiesis. Notably, adult elasmobranch spleen shows clearly compartmentalized red and white pulp areas. The white pulp areas in nurse shark compose the central T cell zone and surrounding B cell zone and are thought to be a major site of antibody synthesis following antigen stimulation (22).
B Cell Immunity
Sera from unimmunized nurse shark include high levels of IgM capable of innately recognizing and binding to diverse antigens (24). The pentameric IgM, which is induced earlier than other isotypes during an immune response, shows a low-affinity interaction with various antigens and localizes mainly in plasma. In contrast, monomeric IgM, which is capable of entering tissues, appears to be the main Ig involved in antigen-specific responses. IgNAR also shows increased titer and high specificity to antigen after immunization. IgNAR and IgM reportedly are expressed by different B cell subsets, although there are no B cells that coexpress both B cell receptors. However, nothing is known at this point regarding the IgD+ B cell (25). It is unclear whether a B cell subset producing multimeric IgM can switch to monomeric IgM production or whether distinct lineages of IgM+ B cells are specialized to make either multimeric or monomeric IgM. In summary, although the kinetics of the humoral immune response of sharks to antigen is much slower than that of mammals, Ig isotype diversity and the capacity to generate specific and memory responses exists in this primitive jawed vertebrate (24).
TELEOST FISH B CELLS
Telostei arose during the Triassic period, approximately 420 Mya, and are considered the oldest living vertebrates with bony skeletons as well as the largest class of vertebrates in existence today. Similar to cartilaginous fish, they have a BCR/TCR-based adaptive immune system.
Immunoglobulins
In 2005, an in-depth analysis of the IgH loci in two teleost species (rainbow trout and zebrafish) revealed a novel IgH genomic architecture: Another set of VHDJC elements was found upstream of the known (VH)-DJCμCδ elements. This new set encodes for τ heavy chain in trout (26) and for ζ heavy chain in zebrafish (27). Thus, bony fish present three IgH, designated as μ, τ/ζ, and δ, which are part of the IgM, IgT/Z, and IgD isotypes, respectively. In addition, teleosts are known to contain at least three IgL: κ, λ, and σ. As opposed to the pentameric mammalian IgM, teleost IgM is a tetramer formed apparently in the absence of the immunoglobulin joining (J) chains (28). Moreover, the IgM tetramers are found in different oxidation states that depend on the degree of interdisulfide-bond formation among the Ig monomers believed to be important for function (29). In some teleosts, IgM can also be present as a low–molecular weight Ig in serum, although the nature of this Ig is not well understood (29). Teleost IgM is by far the most abundant Ig in serum and is the Ig isotype that plays the most prevalent role in systemic immunity. In addition, IgM also plays a role in intestinal and cutaneous immune responses (29). IgT is a monomer in serum and a multimer (associated by noncovalent bonds) in gut (30) and skin mucus (Z. Xu, D. Parra, D. Gomez, I. Salinas, Y.A. Zhang, G.L. Jørgensen, R.D. Heinecke, K. Buchmann, S.L. LaPatra, J.O. Sunyer, unpublished data). Its concentration in serum is much lower, approximately 1,000 times less, than that of IgM, but in gut mucus the IgT/IgM ratio is almost 100 times higher than in serum. Importantly, IgT represents the most ancient vertebrate mucosal Ig identified to date (30), breaking the paradigm that compartmentalization of immune responses (systemic versus mucosal) arose during tetrapod evolution. Interestingly, secreted IgD (sIgD) has been characterized in two teleost species, catfish and trout, and its structure is very different. Thus, catfish sIgD does not possess Cμ1 or variable domains (31), whereas these domains are present in trout IgD (32). In addition, IgD is secreted as a monomer in at least two different isoforms in trout and is produced through a novel splicing mechanism (32). In all species analyzed except catfish, IgD is coexpressed with IgM on B cells. However, in catfish, the existence of B cells uniquely expressing IgD has been reported (33). The function of IgD in bony fish is still unclear, although the IgD Fc-region of catfish may play a role as a pattern-recognition molecule. In addition, catfish have also been shown to contain IgD-armed granulocytes (31). In mucosal surfaces, including the gut and skin, IgT and IgM associate with a polymeric immunoglobulin receptor (pIgR) that probably mediates their transport into the gut lumen and skin epithelial-mucosal surface (30, 34).
B Cell Development
The structure of the trout and zebrafish IgH locus predicts that the expression of IgT/IgZ cannot occur in IgM/IgD-expressing cells. Our lab confirmed this in a recent study in which we characterized a novel lineage of B cells in trout uniquely expressing IgT (30). Similarly, genetic evidence for two distinct lineages expressing IgM or IgZ has also been reported in zebrafish (35). Two populations of IgD+ B cells have been described in teleost: IgD+/IgM– B cells, found only in catfish (33), and IgD+/IgM+ B cells, which have been shown in all teleosts analyzed thus far (29).
The development of B cells at early stages in teleost fish varies between different species. Based on the expression of specific markers important to B cell development, several studies have reported different results. Early studies suggested that B cells originate in the kidney (36), although no RAG-1 expression could be detected in early stages of development in the kidney (37), which suggests a lack of progenitors in this organ. However, making use of GFP expression technology, in zebrafish the kidney may be the first organ (after thymus) that expresses RAG-2 [8 days post-fecundation (dpf)] (38). Danilova & Steiner (39) observed RAG-1 expression and the first rearrangement of genes encoding Igμ at 4 dpf and 10 dpf, respectively, in zebrafish pancreas, although the same authors later suggest that the rearrangement likely corresponds to Igζ instead of Igμ (27). Thus, B cell differentiation may start in the pancreas and move to the kidney early in the development, but eventually this could vary depending on the species, and nevertheless, the results on fish pancreas are limited to only one study. Similarly, in different fish species lymphocytes differentiate within the lymphoid organs at different times relative to hatching. However, some features can be generalized: (a) Ig-producing cells first appear in the kidney, followed by the spleen, and mucosal-associated lymphoid tissues (MALTs) are the last place where Ig-producing cells migrate; (b) it seems that cytoplasmic Ig is produced later than surface Ig; and (c) Ig-producing cells appear earlier in freshwater species than in marine species (37, 29).
Thymus and kidney (anterior and posterior) are the primary lymphoid tissues in teleost fish after hatching, whereas the spleen, gut-associated lymphoid tissue (GALT), and kidney most likely act as secondary lymphoid tissues (38). Teleost fish have no germinal centers (GCs) or organized lymph structures, such as LNs and Peyer's patches (PPs). However, small aggregates of intraepithelial lymphocytes, mainly composed of T cells and a few B cells, and lymphocyte populations in lamina propria (LP) have been described in GALT (29).
B Cell Immunity
IgM+ B cells are the major subset of B cells in the spleen, kidney, blood, and peritoneal cavity, whereas IgT+ B cells outnumber IgM+ in gut (53) and skin (Z. Xu, D. Parra, D. Gomez, I. Salinas, Y.A. Zhang, G.L. Jørgensen, R.D. Heinecke, K. Buchmann, S.L. LaPatra, J.O. Sunyer, unpublished data). In addition, recent studies appear to suggest an important role of IgT+ B cells in the gills, which are considered to be another fish mucosal surface (40). All teleost B cells expressing surface IgM also express surface IgD, and from this perspective, these cells resemble mammalian B-1 B cells. Similar to mammalian B-1 B cells, both IgT+ and IgM+ head kidney B cells can proliferate rapidly and secrete IgT and IgM, respectively, in response to microbial stimulation (30).
The IgM antibodies generated in teleost fish are of limited heterogeneity, and the response time is generally longer than that in mammals (41). Thus, IgM titers are not normally seen until after the third or fourth week of immunization (42), although that appears to be a temperature-dependent process. Teleost IgM is secreted mainly by plasmablasts and plasma-like cells that are localized mostly within the head kidney, where they play a key role in eliciting memory IgM responses (42). It is well known that after booster immunizations, teleost fish show significant increases in IgM (41). Nevertheless, it is generally accepted that the IgM response shows poor anamnestic properties, and that even after repeated immunizations, teleost fish show little increase in IgM affinity (43). Finally, in addition to their important role in systemic responses, IgM plays a role in mucosal responses, because the presence of antigen-specific IgM, albeit low, has been demonstrated in the gut and skin mucus (29).
The gut of vertebrates is inhabited by large, complex microbial communities. Antibodies play a pivotal role in the maintenance of gut homeostasis in mammals. In mammals, IgA, and to a lesser extent IgM and IgG, coats commensal intestinal bacteria, precluding their translocation into the intestinal epithelium by a process known as immune exclusion. The gut of fish also contains bacteria at extremely high density. Analogous to mammalian IgA, a very interesting characteristic of IgT is its capacity to coat a large percentage of intestinal luminal (44) and skin (Z. Xu, D. Parra, D. Gomez, I. Salinas, Y.A. Zhang, G.L. Jørgensen, R.D. Heinecke, K. Buchmann, S.L. LaPatra, J.O. Sunyer, unpublished data) with commensal bacteria, which thus suggests a role of IgT in performing bacteria-immune exclusion in these mucosal surfaces. Further reinforcing the role of IgT in gut immunity, we have shown that infection of trout with Ceratomyxa shasta, a gut parasite, induces a massive accumulation of IgT+ B cells in the LP and gut epithelium in surviving fish. Moreover, IgT-specific titers against the parasite are confined to gut mucus, whereas IgM is the only isotype involved in serum responses (30). Overall, these studies provided the first evidence for compartmentalization of Ig isotypes into mucosal (IgT) and systemic (IgM) areas in response to pathogenic challenge in a nontetrapod species. Although it is tempting to speculate that IgT will be the main Ig class responding in other mucosal sites (e.g., skin, gills), this remains to be investigated.
Notably, our group first described phagocytic B cells in teleost fish (8). Until recently, vertebrate primary B cells were believed to be incapable of performing phagocytosis. In 2006, we broke this paradigm by showing for the first time that teleost primary IgM+ B cells had a potent phagocytic capacity and were able to kill engulfed microbes (8). Later studies showed that rainbow trout IgT+ B cells also contained subsets with phagocytic and bactericidal capacities (30). In addition to rainbow trout, other teleost fish species, including catfish, cod, and Atlantic salmon, contain phagocytic B cells, which suggests that this is a general feature of teleost B cells (8, 45).
AMPHIBIAN B CELLS
Amphibians, including Anuran, Caudata/Urodela, and Gymnophiona, are the most primitive ectothermic tetrapods and diverged from other tetrapods ~350 Mya. Most amphibians undergo metamorphosis from a water-breathing larval form to an adult air-breathing form, at which time they change their lifestyle as well as their body structure. Therefore, a reconstruction of the immune system is also required to cope with different types of agents and to acquire immune tolerance to new, adult-specific self-antigens. However, such reconstitution may render frogs vulnerable to infection, because postmetamorphic defenses are not yet mature (46).
Immunoglobulins
Most amphibians have five Ig isotypes, IgM, IgY, IgX, IgD, and IgF, and three IgL genes, ρ, σ, and type III (ρ and type III are orthologous isotypes to the other vertebrate κ and λ, respectively). IgM is the most abundant isotype and associates mainly with J chain and forms hexamers (47). IgM and IgX are present in thymectomized animals, which thus indicates that their production is T cell independent (47). IgY is present as a monomer in serum and is regarded as a counterpart to both mammalian IgE and IgG. The production of IgY is thymus dependent (48). IgX is abundant in the gut epithelium and is considered an important Ig in amphibian mucosal immunity (6). Xenopus IgD was identified in 2006 (21, 49) and was shown to be orthologous to IgW from cartilaginous and lungfish. It was concluded that, like IgM, IgD/W was present in the ancestor of all living jawed vertebrates. IgD is expressed on the surface of Xenopus IgM+ B cells (21). IgF was cloned in Xenopus tropicalis and has only two constant domains, similar to the first and fourth constant domains of IgY, which suggests IgF generation occurs by tandem duplication of IgY followed by a loss of internal constant domains (49). The functions of amphibian IgD and IgF are unknown.
Pleurodeles waltl has been shown to have three Ig isotypes, IgM, IgY, and IgP, but no IgX to date (50). Transcript levels for IgY were found higher in intestine than in spleen, an observation that may suggest an important role of IgY in gut immunity. IgP is unique to P. waltl and is mainly expressed during larval life, and its expression decreases after metamorphosis. Based on primary sequence analysis, IgP appears to be similar to IgD (31). Interestingly, in Ambystoma mexicanum, another urodele amphibian, no IgP has been found, whereas IgM, IgX, and IgY are present. The involvement of IgX in mucosal immunity in A. mexicanum was suggested at the transcript level (51).
The Ig gene locus in Xenopus and mammals has a similar organization and usage to that of somatic combinatorial joining of V(D)J segments. CSR and allelic exclusion operate in Xenopus (46). However, like other ectothermic vertebrates, the immune system of Xenopus, including CSR, is affected by temperature; for example, the switch from IgM to IgY is prevented at 19°C (46). Interestingly, similar to those of fish and birds, X. laevis eggs contain maternal antibodies with antigen specificity that help during the first stages of larvae (48).
B Cell Development
B cell lymphopoiesis in Xenopus larvae occurs first in the liver, where IgM heavy chain as well as transcripts of RAG, AID, and rearranged IgH chain are detected five days after fertilization (46), and later it moves to the spleen. At later developmental stages (12 days postfertilization and onward) larvae are immunocompetent, display a diverse Ig repertoire distinct from adults, and are able to undergo SHM (46). Importantly, the immune system of tadpoles is considered more ancestral than that of adult frogs. In that regard, CSR from IgM to IgY in tadpoles is poor. In addition, compared with adults, larvae have a lower V-region diversity and antibody affinity (46). In the tadpole of Rana pipiens, B cell development is observed predominantly in the pronephros and mesonephros (36). In Xenopus tadpoles, IgY is present in serum (IgY was detected three days after IgM detection at the developmental stage). Moreover, IgY induction was observed after tadpole immunization (52).
In Mexican axolotl, B cells are first observed in spleen seven weeks after fertilization. Interestingly, TdT as well as RAG-1 expressions in spleen and liver are detected in juvenile axolotl but diminish in 15-month-old axolotl. Thus, gene rearrangement occurs in early stages of development to diversify the repertoire of B cell antigen receptors, and the Ig repertoire is established during the first year of life in axolotl (53). The spleen of urodele amphibians is not clearly divided into white and red pulp (54), eliciting low IgM antibody heterogeneity and poor humoral responses to antigens when compared with anuran amphibians (51).
Although the immune system of adult amphibians is similar in many instances to that of mammals, no equivalent of mammalian GCs, LNs, or PPs are observed in amphibians. The spleen in Xenopus is the main peripheral lymphoid organ, and B cell accumulation in the white pulp has been detected during immune responses to bacteria and virus, whereas no accumulation of B cells was observed in bone marrow (52). Therefore, Xenopus bone marrow is not considered to be a bona fide hematopoietic organ, and the liver and spleen mostly serve in B cell differentiation (46). In adult R. pipiens, bone marrow reportedly is used as a site for B cell development (36). In fact, IgY+ B cells are found in the liver, spleen, and blood, but not in the intestine, whereas IgM+ B cells are widely located in most tissues (55). In contrast, main distribution of Xenopus IgX+ B cells was detected in intestine (55). Recently, the aforementioned isotypes, IgD, IgF, and IgP, have been identified in amphibians. Thus, in addition to IgM+, IgY+, and IgX+ B cells, other B cell subsets expressing IgD, IgF, and IgP may exist. However, their expression pattern on B cells remains to be determined, although the expression of IgD on IgM+ B cells was indicated at transcript levels (21).
B Cell Immunity
Antibody affinity maturation in Xenopus is poor when compared with that of mammals, perhaps owing to its lack of LNs and GCs. However, in viral infection, especially after secondary infection, a significant increase of splenic B cells and IgY specific to the virus is induced along with AID upregulation (56). Moreover, immunization with heat-killed Batrachochytrium dendrobatidis, a chytrid fungus, can induce production of IgM and class-switch to IgY, detectable for at least one month after the last immunization (57). Notably, larval B cells can upregulate IgY and AID mRNA upon bacterial stimulation or viral infection (52). In addition, effective memory responses are known to occur in amphibians (47, 58).
Skin mucus from X. laevis exposed to mucosal pathogens contains significant amounts of IgM, IgY, and IgX antibodies capable of binding specifically to the pathogen, with IgX antibody being the most effective (57). Recently, it was revealed that in contrast to intracoelomic injection, oral immunization of antigen in frogs induces IgX in plasma (59). In contrast, following intraperitoneal immunization with DNP, specific anti-DNP IgM and IgY, but not IgX, were significantly induced in serum (55). These studies suggest that oral immunization specifically induces mucosal Ig in amphibians and that amphibian IgX is probably a functional homolog of mammalian IgA. Similar to teleost fish, B cells from X. laevis are phagocytic, although the percentage of blood B cells with phagocytic capacity was lower than that found in teleost fish (8).
REPTILIAN B CELLS
Reptiles emerged ~320 Mya, in the carboniferous period. They are ectodermic vertebrates presenting scales and/or scutum covering their skin. Four living orders exist presently: Crocodilia, Squamata, Sphenodontia, and Testudines. In contrast to amphibians that must return to water to lay eggs, reptiles are amniotes, whose eggs possess a shell that allows them to be laid on land. Thus, they were better adapted to the new drier conditions after the Carboniferous rainforest collapse. Lymphoid tissues in reptiles include the thymus, spleen, MALT, and bone marrow (60). Structurally, the lymphoid tissues of reptiles vary with the seasons (61).
Immunoglobulins
Reptiles have four Ig classes, IgM, IgY, IgD, and IgA-like (62, 63), which contain λ and κ IgLs (64). Evidence of CSR and SHM was found in reptiles (Pseudemys scripta) after a study on the organization and complexity of the Ig gene system (65). IgM is formed as a pentamer and has been referred to as the secretory Ig in reptiles (66), although this remains to be confirmed. So far, it is the only surface Ig described in reptilian B cells (67). Several species produce two forms of IgY, a 7.5S molecule and a truncated form lacking an Fc region, IgY(ΔFc), although the function of the latter remains unknown (68). In snakes, 3 subclasses (IgYA, IgYB, and IgYC) have been described recently (63). IgY is transferred from the mother to the embryo via the yolk (69). The presence and function of IgY+ B cells are unknown. The function of IgD is not entirely understood. Its expression on the surface of B cells has not been described thus far, although mRNA transcript expression levels and distribution are similar to those of IgM (70). An IgA-like antibody was recently found in the intestine of the leopard gecko (71). Authors considered this an IgA-like Ig based on the high transcript levels of this molecule in the intestine (whereas no expression was detected in liver or blood) and on its homology in primary sequence with Xenopus IgX and bird IgA. Interestingly, the authors suggested that a process of recombination between IgY and IgM was responsible for the evolutionary emergence of this IgA in the leopard gecko. However, no IgA was identified in Anolis carolinensis, another lizard (68), which suggests a degree of variation between species. Studies at the protein level are necessary to demonstrate the functional role of reptilian IgA-like molecule as an Ig that specializes in mucosal immunity.
B Cell Development
Initial reptilian B cell differentiation is produced in fetal liver (72), whereas the main hematopoietic tissue in the studied reptile species is the bone marrow (62, 73). However, in adults of some species, the spleen reportedly can contribute to the production of lymphocytes (74). In terms of secondary lymphoid organs, and similar to that of mammals, the spleen of reptiles is constituted mostly by red pulp, a small fraction of white pulp, and a marginal zone, although this may vary among species. Thus, in snakes, white pulp prevails over red pulp (75). In reptiles, GALT can be observed along the entire gastrointestinal tract (76).
B Cell Immunity
Reptiles have no LNs or GCs, even after repeated immunization. Information regarding antibody responses in reptiles is primarily derived from experiments using fixed pathogens or soluble proteins as antigen (62, 77). IgM is the predominant systemic Ig in primary responses and has a slow response relative to that observed in mammals. After the initial IgM response, reptiles utilize IgY as the major Ig, similar to the mammalian secondary IgG response. Unlike in amphibians, no functional mucosal Ig has been described thus far, although, as indicated previously, an IgA-like molecule has been cloned in the leopard gecko (71). However, IgM likely plays a role in mucosal responses, owing to its presence in gut mucus and its role as secretory Ig (66, 68). Splenectomy abrogates the humoral response, which demonstrates the functional importance of the spleen in immune responses in the lizard Scincus scincus (78). Notably, the spleen is subject to seasonal variation in the snake Psammophis schokari: The white pulp is involuted during summer, and winter slows the immune response (79). Natural antibodies have been identified in several reptiles, which increase throughout the lifetime of the reptile (61). However, little is known about their function in reptiles.
Similarly to teleost fish and amphibian, Zimmerman and collaborators (80) have recently reported that reptilian B cells can phagocytose latex beads, although like in amphibians, the percentage of phagocytic B cells is much lower than that reported for fish.
AVIAN B CELLS
Birds diverged from mammals ~330 Mya. In 1956, Glick et al. (81) revealed that a majority of bursectomized chickens injected with Salmonella typhimurium O antigen failed to produce antibodies to the antigen, which indicates that the bursa of Fabricius, discovered 350 years earlier by Hieronymus Fabricius, plays a crucial role in the development of humoral immunity (82). Approximately ten years later, Cooper and collaborators (83) demonstrated that thymus- and bursa-dependent systems are critical for cellular and humoral immunity, respectively. Thus, experiments in chickens were the first to reveal (a) lymphocyte specialization for antibody production and (b) the existence of dual arms of adaptive immunity–based T and B cells. These findings led to the identification of the mammalian equivalent for the bursa of Fabricius (83).
Immunoglobulins
Birds possess three different Ig isotypes, IgM, IgA, and IgY, although only one IgL isotype (λ) has been identified in chicken, duck, and zebra finch (84). IgM and IgA are clearly homologous to their mammalian counterparts, although no IgD has been identified in avians. IgM is predominantly pentameric, although tetrameric structures are also observed. IgM is the major isotype expressed on avian B cells and is generated first during a primary antibody response. IgA is present in serum and secretions, such as bile. In serum, ~80% is dimeric and 20% is monomeric (85). In bile, chicken and duck IgA may exist in dimeric (350 kDa) and tetrameric (890 kDa) forms, respectively (86). IgY is monomeric, similar to mammalian IgG and IgE, and is produced during secondary antibody responses, which indicates a functional equivalency to mammalian IgG. Ducks express a truncated IgY, which lacks the two C-terminal domains of its heavy chains (87), and its function remains unknown.
Primary antibody repertoires in the chicken are generated through a process of somatic gene conversion mediated by AID. For heavy and light chains, VDJH and VJL genes are functionally rearranged, and diversity arises through gene conversion from an upstream pool of pseudogenes, which include approximately 80 pseudo-VH genes and 26 pseudo-VL genes in the IgH and IgL loci, respectively (88). This gene conversion for the B cell repertoire is also used in GALT species, such as rabbit, cattle, and sheep, which utilize the GALT as the primary lymphoid organ (see section on GALT Animals, below).
B Cell Development
The bursa of Fabricius, a critical organ involved in avian B cell development, is unique to the avian system, although the appendix and ileal PP (IPP) in some mammals have similar function (25). Contrary to its role in humans or mice, chicken bone marrow does not play a main role in B cell development, although B cell precursors have been described in chick bone marrow (89). Chickens have only one functional V and J segment for both the heavy and light Ig chains, and the rearrangement occurs only during a short period of embryonic development. Ig gene rearrangement begins in the yolk sac and is seen throughout embryogenesis in all hematopoietic tissues. After reaching the bursa, B cells begin to migrate across the basement membrane and cluster between this membrane and the epithelium (86). B cells that productively rearrange Ig genes are selected for subsequent expansion in bursal follicles. After the colonization of bursal follicles by B cells, gene conversion is induced to diversify the IgH and IgL genes. Around hatching, only 5% of bursal B cells survive and migrate from the bursa to the periphery (86). Gene conversion most likely continues until the bursa involutes four to six months after hatching. The IgA-producing B cells are derived from IgM+ B cells generated in the bursa of Fabricius around hatching (90) and colonize the intestine between the third and seventh day after hatching. IgA-producing B cells are the prevalent B cell subset in MALT but are sparse in spleen, blood, and thymus (91). Moreover, the peripheral seeding of IgA-producing B cells appears to require T cell help, especially in expressing the V1 TCR, whereas IgY production level is not impaired by thymectomy (92). IgY+ B cells are first observed in bursa in 21-day embryos and expand sharply on the eighth day after hatching (93). In adult chickens, this subset is widely distributed in lymphoid organs, including MALT (91).
B Cell Immunity
As in other tetrapods, the type of antibody response is dependent on the microorganisms as well as the infection or the vaccine delivery route (94). As a systemic immune response, the IgM is initially induced in the avian primary immune responses, and IgY is the predominant isotype with antigen specificity in the secondary response. In addition, infection or vaccination via a mucosal route induces local IgA (94). Similar to other vertebrates, chicken humoral immune responses are induced in the secondary lymphoid organs, such as spleen, as well as head-associated lymphoid tissues, including the Harderian gland and conjunctiva-associated lymphoid tissues, nasal-associated lymphoid tissues, bronchus-associated lymphoid tissues, GALTs (pharyngeal, esophageal, pyloric, and caecal tonsils; PPs; and Meckel's diverticulum), skin-associated lymphoid tissues, and pineal-associated lymphoid tissues (95).
Spleen and all MALTs present GCs, in which CSR of IgM to IgY or to IgA, in addition to affinity maturation of the Ig genes, occurs during an immune response (88, 96). Notably, the mechanism of affinity maturation in chicken Ig is different from that in mice and humans B cells; in these species, SHM is the main mechanism for affinity maturation, whereas gene conversion and SHM both contribute to the affinity maturation in chicken (97). Moreover, when compared with murine GCs, a higher rate of hypermutation of Ig genes occurs in chicken GCs, which causes the affinity maturation of chicken Ig to tend toward more diversification and less selection (98). These processes are important to maintain the Ig diversification in the adult chicken, in which peripheral B cells are maintained by self-renewal (97), although, in terms of affinity maturation, the efficiency of these processes may be low (98).
The duck is a natural host and reservoir of influenza virus. Humoral immunity to influenza in ducks is generated through hemagglutination inhibition and neutralization by full-length IgY, IgM, and IgA but not by truncated IgY. The inability of truncated IgY to carry out these effector functions may contribute to the susceptibility of ducks to this virus (87). Interestingly, ducks appear to show antibody responses with higher affinity maturation when compared with chickens (99). This might result from the lack of LNs in chickens (99, 100).
Natural antibodies, mainly IgA secreted from the Harderian gland, are believed to be transported to the nasal cavity and to be involved in immune exclusion in the upper airways (101). Natural antibodies in GALT were enhanced following probiotic treatment (102).
Application of IgY to Passive Immunization and Human/Veterinary Medicine
Maternal transfer of antibodies has been well characterized in chickens (103). Prior to ovulation, the developing embryo is protected from potential pathogens by IgY antibodies transferred from the hen's blood to the egg yolk by IgY-specific receptors on the yolk membrane. In a separate process, IgM and IgA antibodies are transferred to the chicken egg white and then later to the embryonic digestive tract.
Notably, egg yolk IgY has attracted considerable attention for prophylaxis and control of disease, because its production is cost-effective and convenient and results in high yields relative to the analogous production of mammalian IgG. Moreover, in contrast to antibiotics, IgY does not promote antibiotic-resistant bacteria or leave toxic residues in animal products (104). Potential applications of IgY to human and veterinary medicine are currently under way, and oral administration of a specific IgY antibody to livestock as a feed additive has been effective against a variety of enteric and nonenteric infections of bacterial and viral origin (104). Several IgY antibodies and hyperimmune egg products are available commercially to treat specific diseases in veterinary medicine (104).
MAMMALIAN B CELLS
The first mammals appeared in the Late Triassic, approximately 200 Mya. There are two mammalian subclasses: Prototheria and Theria. In Theria, there are two infraclasses: Meta-theria and Eutheria. In this review, we focus on Eutheria, mammals with real placenta, which represents more than 95% of the treated animals in veterinary medicine. One of the key features that distinguish mammals from other groups is the appearance of mammary glands that produce milk to feed young offspring. Passive transfer of Igs from mother to newborn through the colostrum (first milk produced in mammary glands after birth) or milk is crucial to provide protection for the newborn.
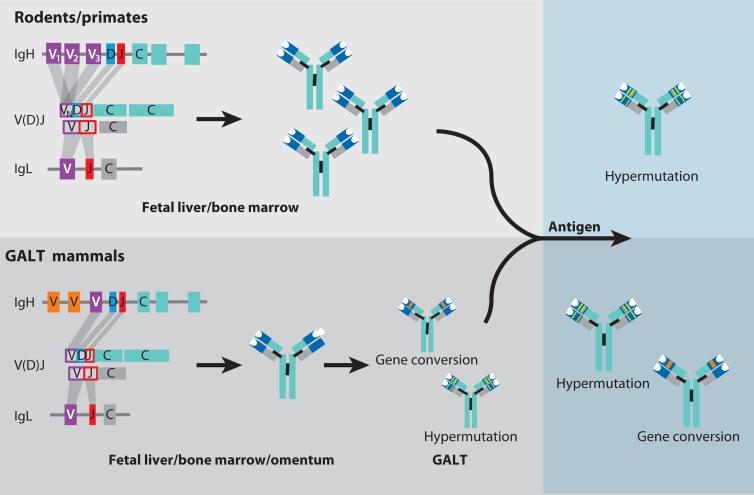
Based on B cell–development pathways, Eutheria mammals can be divided into two groups: (a) mammals that continually replenish the B cell repertoire in the bone marrow throughout life, represented by rodents and primates, and (b) GALT mammals, in which gene diversification is terminated in the perinatal period and which utilize the GALT as the primary lymphoid organ for repertoire diversification before or after birth (Figure 2). Thus, there are significant differences in the development and capacity of antibody diversification between mammalian species. However, once the B cell repertoire is assembled, there is less variation in immune response mechanisms. In this section, we summarize the main features of the most representative animals in both groups. It is important to point out that this classification is based in the presence of ileum IPPs in GALT animals. IPPs are characterized by an accumulation of B cells, by a high rate of apoptosis, and by involution a few days or weeks after birth. However, the evidence that B cells need IPPs for maintenance and to create antibody diversity has only been demonstrated experimentally in sheep (107). In the other animals usually included in this group (swine, cattle, horse, rabbit, and dog) the IPP function has been inferred based only on the similar anatomy to sheep IPPs or chicken bursa and the existence of large numbers of B cells inside the tissue. Therefore, functional studies are required in several animals to determine whether IPPs are really necessary for B cell development and whether they act as a primary lymphoid organ or can be considered as only a secondary lymphoid organ. This is the case for swine, which we do not consider to be GALT animals (Table 2), because recent reports suggest that in these animals IPPs are not necessary for maintenance of B cells but rather function as a secondary lymph organ (108, 109).
Figure 2.
Immunoglobulin (Ig) repertoire diversification in rodents/primates and gut-associated lymphoid tissue (GALT) mammals. In primates and rodents, VH genes from many VH families rearrange to one diversity (D) and one joining (J) segment, which thus leads to the generation of enormous diversity through combinatorial joining and the rearrangement process itself (junctional diversity). In the GALT species, a single or very few variable (V) genes rearrange with D and J segments, generating a poor repertoire that is further modified and amplified in the GALT (e.g., the ileum Peyer's patches in sheep and the appendix in rabbit) in a nonantigen-dependent process. After antigen encounter and as a secondary response, the mammalian repertoire is diversified by somatic hypermutation (SHM) in rodents and primates and SHM and gene conversion in GALT mammals. Abbreviation: C, constant region.
Table 2.
B cell immunity of veterinarily relevant mammalian species
| Mouse | Sheep | Swine | Cattle | Dog | Cat | Horse | ||
|---|---|---|---|---|---|---|---|---|
| IgH | IgGl–4, IgM, IgA, IgE, IgD | IgGl–3, IgM, IgA, IgE, IgD | IgGl–4, IgM, IgA, IgE, IgD | IgGl–3, IgM, IgA, IgE, IgD | IgGl–4, IgM, IgA, IgEl, IgE2, IgD | IgGl–3, IgM, IgAl, IgA2, IgEl, IgE2 | IgGl–7, IgM, IgA, IgE, IgD | |
| IgL usage | k > λ | λ > k | k > λ | λ > k | λ > k | λ > k | λ > k | |
| Primary lymph tissue | Fetal liver/Bone marrow | Fetal liver/IPP | Bone marrow | Bone marrow/IPP | Bone marrow/IPP | Bone marrow | Bone marrow/IPP | |
| Antibody diversification | Comb >Junct > SHM>>GC | SHM > Junct > GC > Comb | Junct > SHM > Comb | SHM > Junct > GC > Comb | SHM > Comb | Comb > SHM | SHM > Junct > Comb | |
| Mother-fetus transfer | IgG | No | No | No | Few IgG | Few IgG | No | |
| Mother-offspring transfer | Colostrum Milk | IgG>>IgM > IgA IgG > IgA > IgM |
IgG>>IgM>IgA IgG > IgA > IgM |
IgG>>IgA > IgM IgA > IgG > IgM |
IgG>>IgM > IgA IgG > IgA > IgM |
IgA > IgG > IgM IgA > IgM > IgG |
IgG>>IgA > IgM IgA > IgG > IgM |
IgG > IgA > IgM IgA > IgG > IgM |
| Main mucosal Ig | IgA | IgA | IgA | IgA | IgA | IgA | IgA | |
| Phagocytic B cells | Yes | ND | ND | ND | ND | ND | ND | |
Abbreviations: Comb, combinatorial diversity; GC, gene conversion; Ig, immunoglobulin; IPP, ileal Peyer's patch; Junct, junctional diversity; ND, not determined; SHM, somatic hypermutation.
MICE AND HUMANS
Immunoglobulins
Human Ig isotypes include IgA (with 2 subclasses, IgA1 and IgA2), IgG (with 4 subclasses, IgG1, IgG2, IgG3, and IgG4), IgM, IgD, and IgE. Mice have the same Ig classes, but two IgG2 (IgG2a and IgG2b) and only one IgA subclass have been described. Humans use κ and λ IgLs equally, while mice preferentially utilize the κ IgL. Importantly, CSR in both mice and humans is mediated by external stimuli, such as antigens or cytokines. Thus, in contrast to GALT mammals, there is little CSR in fetal stages of mice or humans, although CSR can occur in early development outside of GCs in lymphoid tissues (108). IgM is the first antibody produced in primary humoral responses. IgM is present in pentameric form and can induce a very strong activation of the complement system. IgM is the most prevalent isotype that acts as a natural polyreactive antibody secreted by B-1 B cells in pleural and peritoneal cavities. IgG is monomeric, which facilitates its diffusion into extravascular sites, and is the most abundant isotype in serum. IgG is believed to have evolved from bird IgY, although no evidence is available to explain how IgG acquired hinge region, which is not present in IgY. Hinge region introduces flexibility to the Fab region of the Ig, thus facilitating antigen binding. Different IgG subclasses in mice and humans show distinct effector functions. IgE is monomeric and, like IgG, it probably evolved via gene duplication and subsequent evolution from bird IgY. IgE cannot activate complement. However, it is implicated in allergic reactions through its binding to mast cell receptor FcεRI. Its concentration is very low in serum, but after helminth parasite infection, large amounts of IgE are produced. Moreover, several studies indicate an important role for IgE in fighting parasite infection through opsonization of the parasite (109, 110). IgA in humans is monomeric (in blood) or dimeric (in secretions, called secretory IgA or sIgA). In mice, serum IgA concentration is very low, and sIgA represent the majority of IgA. One of the main functions of sIgA is that of a neutralizing antibody, and it is also known to be an anti-inflammatory Ig. sIgA critically prevents infection in mucosal surfaces, such as intestinal, respiratory, or reproductive tracts, by inhibiting the adhesion of pathogens to the epithelial cells lining these surfaces in a process called immune exclusion. Similar to IgT in fish or IgX in amphibians, sIgA is the main mucosal Ig in mammals (111). Human IgA in serum presents different effector functions than mucosal sIgA. Thus, serum IgA can be inflammatory and can bind to FcαR (CD89) in myeloid cells, including neutrophils, dendritic cells, Kupffer cells, eosinophils, monocytes, and several macrophage subsets (e.g., alveolar, tonsilar, and splenic, but not small intestine macrophages). So far, CD89 has not been described in mice. Plasma IgA is involved in the opsonization of microbes, which can then be taken up by phagocytes expressing CD89. Signaling pathways of CD89 binding to IgA-immune complexes modulate several processes, such as gene expression by activation of several transcription factors (including nuclear factor-B, AP-1, and Sp1), phagocytosis, respiratory burst, degranulation, antigen presentation, and release of cytokines and inflammatory lipid mediators (112, 113). Interestingly, binding of IgA to CD89 in absence of antigen downregulates IgG-mediated phagocytosis, bactericidal activity, oxidative burst, and cytokine release (114). Thus, crosslinking of FcαR with IgA-opsonized pathogens (i.e., during an infection) may result in proinflammatory responses, whereas serum IgA that is not complexed with an antigen induces inhibitory signals through FcαR to regulate exacerbated immune responses (114). IgD is secreted as a monomer and, together with IgM, is coexpressed on the surface of mature B cells. Coexpression is achieved through a process of alternative splicing of μ and δ exons, although its regulation is not well understood. After antigen encounter, μ is expressed abundantly and transcription of δ exon is repressed, which explains the low levels of IgD+ cells found in GCs of lymphoid tissues (115). As described previously in catfish, an IgM–IgD+ population has been described in the human respiratory tract (116). Expression of IgD in this subset is produced by CSR. Although the roles of IgD in immunity are not completely understood, it was shown recently that IgD participates in mucosal immunity in the respiratory tract by binding bacteria and their products and that IgD can bind to basophils and mast cells through a still-unknown receptor and activate proinflammatory responses (116).
B Cell Development
Mice and humans have different B cell subsets that differ not only in surface markers but also in their development pathways, function, and location. Thus, naïve B cells generally are divided into three subsets: B-2 (which includes follicular B cells and B-2 B cells from peritoneal and pleural cavities), B-1, and marginal zone (MZ). After activation, memory B cells (long-lived B cells primed against a specific antigen) and plasma cells (which secrete high amounts of specific Igs) are produced from naïve cells. A new B cell subset, the regulatory B cell population (Breg), was described recently, although it is not clear whether it represents a committed subset or whether all B cells can behave as Bregs under the appropriate conditions (117).
B-2 B cell development begins in the fetal liver and continues in the bone marrow throughout the life of the animal. Once a B cell expresses a functional nonautoreactive BCR, IgM, and IgD, it can mature and survive in the periphery. The majority of B-2 B cells in the periphery are found in the spleen or in LNs, mainly as follicular B cells. After antigen recognition, B-2 B cells can either differentiate into plasma or memory cells or initiate processes, such as SHM or CSR, to increase antibody repertoire and affinity.
B-1 and MZ B cells seem to share a common progenitor distinct from that of B-2 B cells (118). While both populations emerge from fetal progenitors in the liver, some B-1 progenitors have been observed in spleen (119) and in bone marrow (120) in adults. However, B-1 lymphopoiesis is not sustained at constant levels throughout life because hematopoietic stem cells and common lymphoid progenitors, typical precursors of B cells in bone marrow, produce very few B-1 B cells (121). Thus, B-1 B cells are still considered mainly self-renewing, while MZ B cells are long-lived. The last are present in spleen, representing approximately 15% of the total splenic B cell population, whereas B-1 B cells predominate in pleural and peritoneal cavities, representing 60–80% of all B cells, and exist in very low percentages in spleen (approximately 2% of total B cells). MZ B cells have been described in humans (122), whereas only one recent publication (123) has reported the existence of B-1 B cells in humans. (For further details on B-1 and MZ subsets, please refer to reviews 124 and 125.) Development of the Breg subset is not fully understood. Bregs share some characteristics of B-1 B cells (IL-10 production) and MZ B cells (surface markers), and they are also considered innate-like cells. For more details see Mauri & Ehrenstein (126).
B Cell Immunity
Adaptive immune responses are initiated in secondary lymphoid tissues. In mice and humans, secondary lymphoid tissues consist of (a) LNs that collect antigens from the tissues; (b) spleen, which collects antigen from the bloodstream; and (c) MALT, which collects antigen from the respiratory, gastrointestinal, and urogenital tracts. B cell responses in MALT tissues differ from those in spleen or LNs. In MALT, adaptive responses are primarily mediated by IgA (111, 127), whereas IgM is the first Ig in primary systemic responses and IgG is utilized in secondary responses (128).
B-2, B-1, and MZ B cells have different functions in immunity. In terms of antibody diversity, B-2 B cells produce more diversified antibodies than B-1 or MZ B cells. Thus, B-1 and MZ B cells present a more restricted V-region repertoire and a lower SHM rate than B-2 cells. Furthermore, B-2 B cells have extensive N regions in contrast to B-1 cells, in which there are few. Owing to their capacity to secrete natural antibodies, which participate in immune exclusion in the gut as well as in protection in several infections, and to respond to T-independent antigens (129, 130), MZ and B-1 B cells are considered innate-like B cells. Recent findings reinforced this role in innate immunity, describing for the first time the capacity of mouse peritoneal cavity (PerC) B-1 B cells to perform phagocytosis and kill internalized bacteria (131, 132). Critically, we showed that phagocytic B-1 B cells were able to efficiently present antigen from internalized particles to CD4+ T cells (131). Interestingly, the capacity to present particulate antigen of phagocytic B cells was significantly greater than that of macrophages, although it was inferior to that of bone marrow–derived dendritic cells. Gao and collaborators (132) recently confirmed most of the above findings on phagocytic PerC B-1 B cells. Nakashima et al. (133) also identified phagocytic B cells in the mouse liver and spleen. Most phagocytic B cells in liver and spleen belonged to the B-1 B cell subset. However, many phagocytic B-2 B cells in these organs were also identified in greater numbers than those seen in PerC and spleen B-2 B cells in other studies (131, 132). Whether murine liver and spleen phagocytic B cells can function as antigen-presenting cells remains to be defined. It is worth mentioning that liver, but not spleen, phagocytic B cells secrete IL-12 (133). Together these data suggest that phagocytic murine B cells resemble phagocytic B cells described in fish, amphibians, and reptiles, which supports the hypothesis proposed by us and others that B cells evolved from a phagocytic predecessor (134).
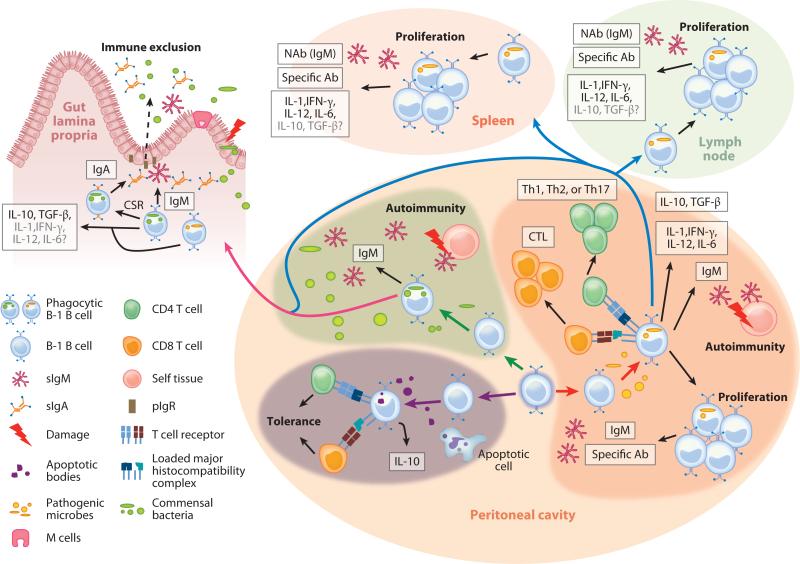
The function of these phagocytic B-1 B cells is not fully understood. Depending on the scenario, the immune responses could be diverse (Figure 3). For example, if commensal bacteria leak from gut as the result of an injury, PerC B-1 B cells may phagocytose them, which initiates an anti-inflammatory response either in situ or following migration into the gut (i.e., LP or PPs) in which they may produce anti-inflammatory cytokines and secrete IgA and IgM antibodies to enhance immune exclusion and thus prevent bacterial entry. However, if PerC B-1 B cells encounter pathogenic microbes, they could produce polyreactive antibodies and proinflammatory cytokines in the peritoneum, although the production of anti-inflammatory cytokines by these cells cannot be ruled out. These cytokines could be released by the phagocytic B-1 B cells or by T cells primed by the B-1 B cells after antigen presentation. Depending on the nature of the pathogen, phagocytic B-1 B cells could migrate to spleen, LNs, or gut (LP, PPs, or MLNs) and elicit roles similar to those described above. Finally, phagocytosis of apoptotic bodies likely leads to production of anti-inflammatory cytokines and induction of self-tolerance in CD4+ and CD8+ T cells. B-1 B cells have been implicated broadly in autoimmune diseases (135). Phagocytic B-1 B cells may play a key role in this process owing to the secretion of polyreactive antibodies induced by the phagocytosis of microbes or apoptotic bodies.
Figure 3.
Hypothetical roles of phagocytic B-1 B cells. In peritoneal cavity (PerC), B-1 B cells phagocytose apoptotic bodies (purple arrows) generated from apoptotic cells, which may lead to the secretion of IL-10 by these cells. Subsequent antigen presentation of epitopes from apoptotic bodies will likely induce T cell tolerance. In a different scenario, and as a result of injury or inflammatory damage, commensal bacteria may leak from the gut lumen into the PerC, where phagocytic B-1 B cells engulf them (green arrows). Alternatively, these cells could phagocytose pathogenic bacteria that have intruded into the PerC (red arrows). As result, phagocytic B-1 B cells may secrete polyreactive immunoglobulin M (IgM), which can recognize either leaked commensals or pathogenic microbes found in the PerC. It is also possible that after clonal expansion, they secrete specific antibodies against pathogenic bacteria. In addition, some of the secreted IgM may recognize self-antigens and thus play a role in autoimmunity. After ingesting commensals or pathogens, phagocytic B-1 B cells may migrate into the gut lamina propria (pink arrow) or the spleen or peripheral lymph nodes (blue arrows). In those sites, the phagocytic B cells may produce polyreactive IgM or IgA [after class switch recombination (CSR)]. The produced IgA and IgM will be transported into the gut lumen (dashed arrow) to coat commensals, thus playing a role in immune exclusion. In addition, phagocytosis by B-1 B cells may lead to their expression of proinflammatory cytokines in PerC, spleen, and lymph nodes, whereas their expression of anti-inflammatory cytokines is less likely (indicated in gray with question mark). Conversely, production of anti-inflammatory cytokines by phagocytic B-1 B cells that have migrated into the gut lamina propria likely is more probable than production of proinflammatory cytokines.
Although many of the immune roles of phagocytic B cells, as well as their in vivo significance, remain undefined, their identification has unveiled a wide range of potential new roles in B cell function that require further investigations. Notably, B-1 B cells have been described recently in humans (123). Thus, it would be interesting to determine whether these human B-1 B cells or other human B cell subsets have retained the phagocytic and microbicidal capacities described above for murine B-1 B cells.
Role of B Cells in Xenotransplantation
Because of the similarities between swine and humans, swine are used occasionally as xenogeneic organdonors (136) and as a source of xenotissues, including skin trans plantation for temporary cover of severe skin burns (137) and replacement of human heart valves (138). However, a major problem for xenotranspla ntation is rejection by the xenoreactive natural antibodies (XNA) that human B cells produce (139). Genetic manipulation of pigs to remove antigens recognized by XNA (136) and induction of tolerance of human B cells are current strategies being used to avoid rejection (140).
GALT ANIMALS
Sheep, cattle, rabbit, horse, and dog are the representative species in the GALT group. However, whereas B cell development has been well studied in farmed animals (i.e., sheep and cattle), significantly less is known about development in dogs and horses, owing in part to ethics-related problems derived from working with early stages of the fetus. Thus, we mainly focus on farmed animals and only mention the main features of B cell responses in other species (Table 2).
Immunoglobulins
GALT mammals express IgM, IgD, IgG, IgE, and IgA, except rabbit, in which no IgD has been described so far. In sheep, nine VH gene segments, including three pseudogenes, have been identified, and these VH genes belong to only one family (141). Similarly, single VH gene dominates the Ig heavy-chain repertoire in cattle (142). In rabbit, the repertoire is slightly more diversified, although one predominant segment is rearranged most frequently (143). This limited use of VH regions led to a very low Ig diversification after V(D)J recombination, and GALT animal repertoire diversification is generated in the GALT, hence the name. Igl is the major IgL isotype used in sheep and cattle, whereas in rabbits the κ and λ IgL loci are duplicated. There are two IgG allotypes in sheep, three in cattle, and one in rabbit. Interestingly, rabbits do not have IgD but do have 13 IgA allotypes.
B Cell Development
In sheep, the yolk sac and fetal liver serve as the earliest sites of hematopoiesis. The first B cells can be identified in the fetal spleen by gestational day 48 (144). In early fetal life, the spleen appears to be the major organ of B cell proliferation (145). In rabbit, B lymphopoiesis begins in the fetal liver and omentum and switches, shortly before birth, to the bone marrow. In all GALT animals, B cells from the bone marrow, as well as from fetal liver or omentum, migrate to the GALT before birth. These GALT tissues, including the appendix and sacculus rotundus in rabbit and IPP in cattle and sheep, are considered the primary lymphoid tissues in which the B cell repertoire undergoes expansion and diversification (by gene conversion and SHM) (Figure 2). Although spleen is the major organ of B cell proliferation, splenectomy in early fetal development does not influence the development of IPP (145). Generation of diversity occurs in the rabbit GALT only after gut colonization (146), probably driven by superantigen-like molecules (147). In sheep and cattle, diversity is generated prior to gut colonization, although gut bacteria can play an important role. B cell progenitors in bone marrow disappear approximately 18 weeks after birth, almost completely ceasing lymphopoiesis in adult animals. However, signs of some de novo formation of B cells have been found in adult rabbit bone marrow and spleen (148, 149).
As we mentioned before, B cell lymphopoiesis in GALT animals declines drastically during adulthood. Consequently, the repertoire of the adult peripheral B cell pool remains constant throughout life. This situation resembles that of B-1 B cells in mice, which are generated pre- and perinatally, and although they are capable of self-renewal, de novo formation of B-1 B cells during adulthood is very restricted (124). This feature, together with the fact that some B cells—approximately 4% in sheep, 5–10% in cattle and more than 90% in rabbit—express CD5 on their surface have led some authors to claim the existence of B-1 B cells in GALT animals. Although CD5 is a good marker for the B-1a B cell subset in mice, it cannot be used as a unique marker for B-1 B cells in other species. Thus, human B-2 B cells can express CD5 after activation (124), and some human B cells present CD5 on their surface, but they do not possess B-1 B features (123). More functional studies are necessary to confirm the existence of B-1 B cells in GALT animals. No MZ B cells have been described in GALT animals so far. However, a recent report suggests the existence of MZ B cell in sheep, based only on morphological analysis (150). Thus, more studies in GALT animals are necessary to determine the presence of the different naïve subsets above described for mice and humans. Recently a new subset of B cells expressing IL-10 has been described in PPs in sheep (151) that may represent B regulatory cells.
Passive Transfer of Immunity
In GALT animals (except for rabbit), the placenta is impermeable to large molecules, and Igs are not transferred to the fetus (Table 2). Antibodies are transferred to newborns by absorption through the gut. In sheep, the major Ig in colostrum is IgG, and IgM and IgA are also found in low concentrations. As colostrum is replaced by milk in sheep, IgG1 becomes the main Ig, and there is a slight increase in IgA and IgM (152). The situation in cattle is very similar, although IgA and IgM are relatively higher in colostrum. However, in rabbit there is active transfer of maternal antibodies to the fetus through the yolk-sac splanchnopleure (153). Rabbit colostrum contains IgG and IgA, which are also transferred to the newborn.
B Cell Immunity
There are no major variations in the immune responses of different mammal species. Thus, as we described in mice and humans, in GALT animals, antigen-specific IgM (primary responses) and IgG (secondary responses) are generated during systemic responses against pathogens (154–158), whereas IgA predominates in mucosal responses (159). Similar to mice and humans, the administration route is important to maintain this compartmentalization between mucosal and systemic responses (160). Generation of IgA responses in rabbit is different than in other GALT animals. Thus, IgA is produced in the gut, and it can diffuse to the bloodstream and enter the liver. Hepatocytes in rabbit express pIgR that binds IgA and releases it into the bile canaliculi (112). Thus, bile is the major route by which IgA reach the intestine in rabbit, whereas in the other GALT mammals it is mainly through the pIgR localized in intestinal enterocytes. As we described for mice and humans, immune responses in some parasitized GALT animals are mediated predominantly by IgE (161, 162). Natural antibodies are present in GALT animals (163, 164); however, they have been poorly studied.
CONCLUDING REMARKS
Innate and adaptive immunity have coevolved in response to changing environments that expose organisms to new antigenic insults. Whereas innate immunity is present in all plants and metazoans, and even in some unicellular organisms, adaptive immunity seems to have evolved along with the emergence of vertebrates. Two distinct forms of adaptive immunity exist, both of which generate immune diversity via rearrangement of gene segments. Thus the VLR- and Ig/TCR-based adaptive immune systems arose in the jawless and jawed vertebrates, respectively. V(D)J recombination, a hallmark of Ig/TCR-based adaptive immunity, emerged through the acquisition of functional RAG recombinase activities. However, the jawless fish appear to have no RAG genes; CDAs, homologs to AID, are used in lamprey for VLR diversification through a gene conversion–like mechanism. Thus, the emergence of the AID/APOBEC family in vertebrates (165) appears to represent the turning point at which adaptive immunity arose in evolutionary time. Interestingly, although CDA1 serves to generate VLRA diversity in lamprey VLRA+ lymphocytes (analogous to T cells of jawed vertebrates), only B cells in jawed vertebrates utilize AID for affinity maturation and memory through gene conversion, SHM, and CSR (25, 166, 167). Interestingly, fish AID can mediate CSR in mouse cells, although CSR does not exist in teleosts (168). Whether an ancestor of VLR and Ig/TCR systems existed in a primordial lymphocyte-like cell with features of both adaptive immune responses is still unclear; however, VLR and Ig/TCR systems likely have evolved through a process of convergent evolution (169). It is worth mentioning that the presence of phagocytic B cells in teleosts, amphibians, reptiles, and mammals supports the hypothesis that B cells evolved from an ancient phagocytic predecessor (7, 8). It would be very interesting, therefore, to determine whether lampreys have phagocytic VLRB+ lymphocytes, an observation that would support further the phagocytic ancestry of cells containing rearranging immune receptors.
Although the ancestral cell lineage from which Ig-producing B cells evolved is still unknown (as discussed above), fish B cells clearly represent the cell predecessors from which amphibian, reptilian, avian, and mammalian B cells evolved. In that regard, there are obvious morphological and functional similarities between mammalian B-1 B cells and fish IgM-bearing B cells: (a) They can innately secrete IgM when activated by several PAMPs; (b) they have phagocytic and microbicidal capacities; and (c) upon antigenic or pathogenic challenge, they generate antigen-specific IgM in a T cell–independent manner. Thus, it is tempting to conjecture that B-1 B cells evolved from IgM-bearing B cells of cold-blooded vertebrates and that the B-2 B cell lineage emerged later as a more advanced B cell subset, which gradually specialized to perform a dominant role in adaptive immunity (170). The aforementioned scenario can be reconciled with the concept of an “evolutionarily layered immune system,” first proposed by Herzenberg & Kantor (171). According to this concept, the B-1 B cells represent the oldest B cell layer evolutionarily; thus, they play a more innate role than B-2 B cells, the most evolved layer of all B cell subsets, which bear a key role in adaptive immunity. This concept, and these suggested paths of B cell evolution, could also account for the much lower phagocytic capacity observed in B-2 versus B-1 B cells (131, 132), because phagocytosis is a key mechanism of innate immunity. However, the predecessor of B-1 and B-2 lineages also might have evolved concomitantly from B cell subsets that have not yet been uncovered in other poikilothermic vertebrates. Thus, the evolutionary origins of B-1 and B-2 B cell lineages may not be mapped out precisely until all existing B cell lineages of lower vertebrates, as well as their specific roles in innate and adaptive immune reactions, are investigated.
In addition to the evolutionary modifications in the B cell itself, changes in immunological structures in the animal have been key to driving the evolution of B cell immunity. Thus, the faster and more robust adaptive responses that birds and mammals present, compared with those of poikilothermic vertebrates, owe greatly to the acquisition of LNs and GCs, in which B cells and dendritic cells can encounter antigen easily and interact more efficiently with T cells. This in turn results in a vastly expanded antibody repertoire with increased affinity and specificity for the antigen.
Many questions are still unanswered regarding the evolution of B cells as well as their roles in immunity. In that regard, old paradigms that were generally accepted have been challenged recently. For example, the old concept of one type of adaptive immunity, based on Ig/TCR molecules that emerged first in jawed vertebrates, has been refuted with the finding of VLR-mediated immunity in jawless vertebrates (17). The old belief that compartmentalization of immune responses in mucosal and systemic sites emerged in tetrapods has been changed with the recent finding that fish contain an Ig specialized in mucosal immunity (30). The old dogma that vertebrate B cells were not phagocytic has been overturned recently with findings that B cells from fish, amphibians, reptiles, and mammals are in fact phagocytic (8, 80, 131). And the old view that B cells are cells mainly dedicated to the production of antibodies has now changed in view of recent reports showing not only their phagocytic capacity but also their ability to modulate adaptive immunity through the secretion of an array of pro- or anti-inflammatory cytokines (i.e., effector and regulatory B cells) (9, 172). These changed dogmas open a vast array of new questions regarding the evolution and thus far unrecognized roles of B cells in innate and adaptive immunity. It is important to recognize that many of the new discoveries that have helped overturn some old B cell paradigms have been made in nonclassical animals, which indicates the importance of comparative immunology studies in the understanding of B cell function and evolution. Moreover, many nonclassical models include species relevant to veterinary medicine. Thus, basic science research performed in these animals will also lead to the knowledge required for the development of more efficacious vaccines and prophylactic strategies to combat infectious diseases in many veterinarily relevant species.
In conclusion, through evolutionary time, all vertebrate species, from agnathans to mammals, have been exposed to many common selective pressures that have led to similar immunological solutions, through either parallel or convergent evolution. Thus, general design principles of adaptive immunity can be discerned after comparing the adaptive immune systems of even jawless and jawed species, as already asserted by Boehm (173). Hence, although significant disparities are evident among jawed vertebrate immune systems, the study of immuno-relevant body areas that have been subjected to very similar selective pressures (i.e., gut mucosal surfaces) will probably unveil conserved structural and functional aspects of B cell biology.
Junctional diversity: a mechanism to generate diversity through the addition and subtraction of nucleotides at the joints between different gene segments
Somatic hypermutation (SHM): a mechanism to generate immunoglobulin diversity by point mutation, which allows the affinity of the antibody response to increase
Gene conversion: a process of diversifying immunoglobulins by replacing homologous sequences in rearranged immunoglobulin genes with pseudogene-derived sequences
Class switch recombination (CSR): a mechanism in which antigen-activated B cells change the isotype from IgM to another isotype; provides antibody with the same antigen specificity but distinct effector function
Recombination-activated gene (RAG): encodes enzymes essential for the rearrangement and recombination of Ig and TCR genes during the process of V(D)J recombination
Terminal deoxynucleotidyl transferase (TdT): enzyme that inserts nontemplated nucleotides or N-nucleotides into the junctions between gene segments in immunoglobulin V-region genes
Primary (central) lymphoid organ: the site for development of lymphocyte from immature progenitor cells and for selection of lymphocyte to prevent autoimmune reactions
Germinal centers (GCs): the lymphoid follicles in peripheral lymphoid tissues for intense B cell proliferation, differentiation, somatic hypermutation, and class switching during antibody responses
Immune exclusion: an ability of soluble IgA, IgM, and IgT to prevent microbial pathogens and antigens from entering the body, especially in intestine
Allelic exclusion: expression of only single immunoglobulin molecules in a single B cell by selecting one of the two alleles
AID: Activation-induced cytidine deaminase; an enzyme essential for the somatic hypermutation, class switch recombination, and gene conversion
Gene rearrangement [V(D)J recombination]: recombination of V(D)J gene segments in the Ig and TCR loci to produce a functional variable region
Secondary (peripheral) lymphoid organ: the site where mature naïve lymphocytes are maintained and adaptive immune responses are initiated; e.g., lymph nodes, spleen, and mucosal lymphoid tissues
Natural antibody: an antibody present without any infection, vaccination, or passive immunization that has a broad specificity for self- and microbial antigens
ACKNOWLEDGMENTS
This work was supported by the National Science Foundation (NSF-MCB-0719599 to J.O.S.), the National Institutes of Health (R01GM085207-01 to J.O.S.), and the United States Department of Agriculture (USDA-NRI 2006-01619 and USDA-NRI 2007-01719 to J.O.S.).
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Chen G, Zhuchenko O, Kuspa A. Immune-like phagocyte activity in the social amoeba. Science. 2007;317:678–81. doi: 10.1126/science.1143991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhaya D, Davison M, Barrangou R. CRISPR-Cas systems in bacteria and archaea: versatile small RNAs for adaptive defense and regulation. Annu. Rev. Genet. 2011;45:273–97. doi: 10.1146/annurev-genet-110410-132430. [DOI] [PubMed] [Google Scholar]
- 3.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–26. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 4.Pancer Z, Cooper MD. The evolution of adaptive immunity. Annu. Rev. Immunol. 2006;24:497–518. doi: 10.1146/annurev.immunol.24.021605.090542. [DOI] [PubMed] [Google Scholar]
- 5.Matsunaga T, Rahman A. What brought the adaptive immune system to vertebrates?—The jaw hypothesis and the seahorse. Immunol. Rev. 1998;166:177–86. doi: 10.1111/j.1600-065x.1998.tb01262.x. [DOI] [PubMed] [Google Scholar]
- 6.Hsu E. Mutation, selection, and memory in B lymphocytes of exothermic vertebrates. Immunol. Rev. 1998;162:25–36. doi: 10.1111/j.1600-065x.1998.tb01426.x. [DOI] [PubMed] [Google Scholar]
- 7.Kawamoto H, Katsura Y. A new paradigm for hematopoietic cell lineages: revision of the classical concept of the myeloid-lymphoid dichotomy. Trends Immunol. 2009;30:193–200. doi: 10.1016/j.it.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Li J, Barreda DR, Zhang YA, Boshra H, Gelman AE, et al. B lymphocytes from early vertebrates have potent phagocytic and microbicidal abilities. Nat. Immunol. 2006;7:1116–24. doi: 10.1038/ni1389. [DOI] [PubMed] [Google Scholar]
- 9.Lund FE, Randall TD. Effector and regulatory B cells: modulators of CD4+ T cell immunity. Nat. Rev. Immunol. 2010;10:236–47. doi: 10.1038/nri2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooper MD, Peterson RD, Good RA. Delineation of the thymic and bursal lymphoid systems in the chicken. Nature. 1965;205:143–46. doi: 10.1038/205143a0. [DOI] [PubMed] [Google Scholar]
- 11.Rowley AF, Powell A. Invertebrate immune systems—Specific, quasi-specific, or nonspecific? J. Immunol. 2007;179:7209–14. doi: 10.4049/jimmunol.179.11.7209. [DOI] [PubMed] [Google Scholar]
- 12.Little TJ, Hultmark D, Read AF. Invertebrate immunity and the limits of mechanistic immunology. Nat. Immunol. 2005;6:651–54. doi: 10.1038/ni1219. [DOI] [PubMed] [Google Scholar]
- 13.Alt FW, Rathbun G, Oltz E, Taccioli G, Shinkai Y. Function and control of recombination-activating gene activity. Ann. N.Y. Acad. Sci. 1992;651:277–94. doi: 10.1111/j.1749-6632.1992.tb24626.x. [DOI] [PubMed] [Google Scholar]
- 14.Boehm T, Bleul CC. The evolutionary history of lymphoid organs. Nat. Immunol. 2007;8:131–35. doi: 10.1038/ni1435. [DOI] [PubMed] [Google Scholar]
- 15.Pancer Z, Mayer WE, Klein J, Cooper MD. Prototypic T cell receptor and CD4-like coreceptor are expressed by lymphocytes in the agnathan sea lamprey. Proc. Natl. Acad. Sci. USA. 2004;101:13273–78. doi: 10.1073/pnas.0405529101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fugmann SD, Messier C, Novack LA, Cameron RA, Rast JP. An ancient evolutionary origin of the Rag1/2 gene locus. Proc. Natl. Acad. Sci. USA. 2006;103:3728–33. doi: 10.1073/pnas.0509720103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boehm T, McCurley N, Sutoh Y, Schorpp M, Kasahara M, Cooper MD. VLR-based adaptive immunity. Annu. Rev. Immunol. 2012;30:203–20. doi: 10.1146/annurev-immunol-020711-075038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirano M, Das S, Guo P, Cooper MD. The evolution of adaptive immunity in vertebrates. Adv. Immunol. 2011;109:125–57. doi: 10.1016/B978-0-12-387664-5.00004-2. [DOI] [PubMed] [Google Scholar]
- 19.Dooley H, Flajnik MF. Antibody repertoire development in cartilaginous fish. Dev. Comp. Immunol. 2006;30:43–56. doi: 10.1016/j.dci.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 20.Rumfelt LL, Avila D, Diaz M, Bartl S, McKinney EC, Flajnik MF. A shark antibody heavy chain encoded by a nonsomatically rearranged VDJ is preferentially expressed in early development and is convergent with mammalian IgG. Proc. Natl. Acad. Sci. USA. 2001;98:1775–80. doi: 10.1073/pnas.98.4.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohta Y, Flajnik M. IgD, like IgM, is a primordial immunoglobulin class perpetuated in most jawed vertebrates. Proc. Natl. Acad. Sci. USA. 2006;103:10723–28. doi: 10.1073/pnas.0601407103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rumfelt LL, McKinney EC, Taylor E, Flajnik MF. The development of primary and secondary lymphoid tissues in the nurse shark Ginglymostoma cirratum: B-cell zones precede dendritic cell immigration and T-cell zone formation during ontogeny of the spleen. Scand. J. Immunol. 2002;56:130–48. doi: 10.1046/j.1365-3083.2002.01116.x. [DOI] [PubMed] [Google Scholar]
- 23.Miracle AL, Anderson MK, Litman RT, Walsh CJ, Luer CA, et al. Complex expression patterns of lymphocyte-specific genes during the development of cartilaginous fish implicate unique lymphoid tissues in generating an immune repertoire. Int. Immunol. 2001;13:567–80. doi: 10.1093/intimm/13.4.567. [DOI] [PubMed] [Google Scholar]
- 24.Dooley H, Flajnik MF. Shark immunity bites back: affinity maturation and memory response in the nurse shark, Ginglymostoma cirratum. Eur. J. Immunol. 2005;35:936–45. doi: 10.1002/eji.200425760. [DOI] [PubMed] [Google Scholar]
- 25.Flajnik MF. Comparative analyses of immunoglobulin genes: surprises and portents. Nat. Rev. Immunol. 2002;2:688–98. doi: 10.1038/nri889. [DOI] [PubMed] [Google Scholar]
- 26.Hansen JD, Landis ED, Phillips RB. Discovery of a unique Ig heavy-chain isotype (IgT) in rainbow trout: implications for a distinctive B cell developmental pathway in teleost fish. Proc. Natl. Acad. Sci. USA. 2005;102:6919–24. doi: 10.1073/pnas.0500027102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Danilova N, Bussmann J, Jekosch K, Steiner LA. The immunoglobulin heavy-chain locus in zebrafish: identification and expression of a previously unknown isotype, immunoglobulin Z. Nat. Immunol. 2005;6:295–302. doi: 10.1038/ni1166. [DOI] [PubMed] [Google Scholar]
- 28.Flajnik MF, Kasahara M. Origin and evolution of the adaptive immune system: genetic events and selective pressures. Nat. Rev. Genet. 2010;11:47–59. doi: 10.1038/nrg2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salinas I, Zhang YA, Sunyer JO. Mucosal immunoglobulins and B cells of teleost fish. Dev. Comp. Immunol. 2011;35:1346–65. doi: 10.1016/j.dci.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang YA, Salinas I, Li J, Parra D, Bjork S, et al. IgT, a primitive immunoglobulin class specialized in mucosal immunity. Nat. Immunol. 2010;11:827–35. doi: 10.1038/ni.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edholm ES, Bengten E, Wilson M. Insights into the function of IgD. Dev. Comp. Immunol. 2011;35:1309–16. doi: 10.1016/j.dci.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 32.Ramirez-Gomez F, Greene W, Rego K, Hansen JD, Costa G, et al. Discovery and characterization of secretory IgD in rainbow trout: secretory IgD is produced through a novel splicing mechanism. J. Immunol. 2012;188:1341–49. doi: 10.4049/jimmunol.1101938. [DOI] [PubMed] [Google Scholar]
- 33.Edholm ES, Bengten E, Stafford JL, Sahoo M, Taylor EB, et al. Identification of two IgD+ B cell populations in channel catfish, Ictalurus punctatus. J. Immunol. 2010;185:4082–94. doi: 10.4049/jimmunol.1000631. [DOI] [PubMed] [Google Scholar]
- 34.Hamuro K, Suetake H, Saha NR, Kikuchi K, Suzuki Y. A teleost polymeric Ig receptor exhibiting two Ig-like domains transports tetrameric IgM into the skin. J. Immunol. 2007;178:5682–89. doi: 10.4049/jimmunol.178.9.5682. [DOI] [PubMed] [Google Scholar]
- 35.Schorpp M, Bialecki M, Diekhoff D, Walderich B, Odenthal J, et al. Conserved functions of Ikaros in vertebrate lymphocyte development: genetic evidence for distinct larval and adult phases of T cell development and two lineages of B cells in zebrafish. J. Immunol. 2006;177:2463–76. doi: 10.4049/jimmunol.177.4.2463. [DOI] [PubMed] [Google Scholar]
- 36.Hansen JD, Zapata AG. Lymphocyte development in fish and amphibians. Immunol. Rev. 1998;166:199–220. doi: 10.1111/j.1600-065x.1998.tb01264.x. [DOI] [PubMed] [Google Scholar]
- 37.Zapata A, Diez B, Cejalvo T, Gutiérrez-de Frías C, Cortés A. Ontogeny of the immune system of fish. Fish Shellfish Immunol. 2006;20:126–36. doi: 10.1016/j.fsi.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 38.Trede NS, Langenau DM, Traver D, Look AT, Zon LI. The use of zebrafish to understand immunity. Immunity. 2004;20:367–79. doi: 10.1016/s1074-7613(04)00084-6. [DOI] [PubMed] [Google Scholar]
- 39.Danilova N, Steiner LA. B cells develop in the zebrafish pancreas. Proc. Natl. Acad. Sci. USA. 2002;99:13711–16. doi: 10.1073/pnas.212515999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olsen MM, Kania PW, Heinecke RD, Skjoedt K, Rasmussen KJ, Buchmann K. Cellular and humoral factors involved in the response of rainbow trout gills to Ichthyophthirius multifiliis infections: molecular and immunohistochemical studies. Fish Shellfish Immunol. 2011;30:859–69. doi: 10.1016/j.fsi.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 41.Warr GW. The adaptive immune system of fish. Dev. Biol. Stand. 1997;90:15–21. [PubMed] [Google Scholar]
- 42.Ye J, Kaattari I, Kaattari S. Plasmablasts and plasma cells: reconsidering teleost immune system organization. Dev. Comp. Immunol. 2011;35:1273–81. doi: 10.1016/j.dci.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 43.Kaattari SL, Zhang HL, Khor IW, Kaattari IM, Shapiro DA. Affinity maturation in trout: clonal dominance of high affinity antibodies late in the immune response. Dev. Comp. Immunol. 2002;26:191–200. doi: 10.1016/s0145-305x(01)00064-7. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Y-A, Salinas I, Sunyer JO. Recent findings on the structure and function of teleost IgT. Fish Shellfish Immunol. 2011;31:627–34. doi: 10.1016/j.fsi.2011.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Øverland HS, Pettersen EF, Rønneseth A, Wergeland HI. Phagocytosis by B-cells and neutrophils in Atlantic salmon (Salmo salar L.) and Atlantic cod (Gadus morhua L.). Fish Shellfish Immunol. 2010;28:193–204. doi: 10.1016/j.fsi.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 46.Du Pasquier L, Robert J, Courtet M, Mussmann R. B-cell development in the amphibian Xenopus. Immunol. Rev. 2000;175:201–13. doi: 10.1111/j.1600-065x.2000.imr017501.x. [DOI] [PubMed] [Google Scholar]
- 47.Du Pasquier L, Schwager J, Flajnik MF. The immune system of Xenopus. Annu. Rev. Immunol. 1989;7:251–75. doi: 10.1146/annurev.iy.07.040189.001343. [DOI] [PubMed] [Google Scholar]
- 48.Robert J, Ohta Y. Comparative and developmental study of the immune system in Xenopus. Dev. Dyn. 2009;238:1249–70. doi: 10.1002/dvdy.21891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao Y, Pan-Hammarstrom Q, Yu S, Wertz N, Zhang X, et al. Identification of IgF, a hinge-region-containing Ig class, and IgD in Xenopus tropicalis. Proc. Natl. Acad. Sci. USA. 2006;103:12087–92. doi: 10.1073/pnas.0600291103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schaerlinger B, Bascove M, Frippiat JP. A new isotype of immunoglobulin heavy chain in the urodele amphibian Pleurodeles waltl predominantly expressed in larvae. Mol. Immunol. 2008;45:776–86. doi: 10.1016/j.molimm.2007.06.356. [DOI] [PubMed] [Google Scholar]
- 51.Schaerlinger B, Frippiat JP. IgX antibodies in the urodele amphibian Ambystoma mexicanum. Dev. Comp. Immunol. 2008;32:908–15. doi: 10.1016/j.dci.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 52.Marr S, Morales H, Bottaro A, Cooper M, Flajnik M, Robert J. Localization and differential expression of activation-induced cytidine deaminase in the amphibian Xenopus upon antigen stimulation and during early development. J. Immunol. 2007;179:6783–89. doi: 10.4049/jimmunol.179.10.6783. [DOI] [PubMed] [Google Scholar]
- 53.Golub R, André S, Hassanin A, Affaticati P, Larijani M, Fellah JS. Early expression of two TdT isoforms in the hematopoietic system of the Mexican axolotl. Implications for the evolutionary origin of the N-nucleotide addition. Immunogenetics. 2004;56:204–13. doi: 10.1007/s00251-004-0681-2. [DOI] [PubMed] [Google Scholar]
- 54.Fellah JS, Vaulot D, Tournefier A, Charlemagne J. Ontogeny of immunoglobulin expression in the Mexican axolotl. Development. 1989;107:253–63. doi: 10.1242/dev.107.2.253. [DOI] [PubMed] [Google Scholar]
- 55.Mussmann R, Du Pasquier L, Hsu E. Is Xenopus IgX an analog of IgA? Eur. J. Immunol. 1996;26:2823–30. doi: 10.1002/eji.1830261205. [DOI] [PubMed] [Google Scholar]
- 56.Maniero GD, Morales H, Gantress J, Robert J. Generation of a long-lasting, protective, and neutralizing antibody response to the ranavirus FV3 by the frog Xenopus. Dev. Comp. Immunol. 2006;30:649–57. doi: 10.1016/j.dci.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 57.Ramsey JP, Reinert LK, Harper LK, Woodhams DC, Rollins-Smith LA. Immune defenses against Batrachochytrium dendrobatidis, a fungus linked to global amphibian declines, in the South African clawed frog, Xenopus laevis. Infect. Immun. 2010;78:3981–92. doi: 10.1128/IAI.00402-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen G, Robert J. Antiviral immunity in amphibians. Viruses. 2011;3:2065–86. doi: 10.3390/v3112065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Du CC, Mashoof SM, Criscitiello MF. Oral immunization of the African clawed frog (Xenopus laevis) upregulates the mucosal immunoglobulin IgX. Vet. Immunol. Immunopathol. 2012;145:493–98. doi: 10.1016/j.vetimm.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kvell K, Cooper EL, Engelmann P, Bovari J, Nemeth P. Blurring borders: innate immunity with adaptive features. Clin. Dev. Immunol. 2007;2007:83671. doi: 10.1155/2007/83671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zimmerman LM, Paitz RT, Vogel LA, Bowden RM. Variation in the seasonal patterns of innate and adaptive immunity in the red-eared slider (Trachemys scripta). J. Exp. Biol. 2010;213:1477–83. doi: 10.1242/jeb.037770. [DOI] [PubMed] [Google Scholar]
- 62.Zimmerman LM, Vogel LA, Bowden RM. Understanding the vertebrate immune system: insights from the reptilian perspective. J. Exp. Biol. 2010;213:661–71. doi: 10.1242/jeb.038315. [DOI] [PubMed] [Google Scholar]
- 63.Gambón-Deza F, Sánchez-Espinel C, Mirete-Bachiller S, Magadán-Mompó S. Snakes antibodies. Dev. Comp. Immunol. 2012;38:1–9. doi: 10.1016/j.dci.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 64.Wu Q, Wei Z, Yang Z, Wang T, Ren L, et al. Phylogeny, genomic organization and expression of l and k immunoglobulin light chain genes in a reptile, Anolis carolinensis. Dev. Comp. Immunol. 2010;34:579–89. doi: 10.1016/j.dci.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 65.Turchin A, Hsu E. The generation of antibody diversity in the turtle. J. Immunol. 1996;156:3797–805. [PubMed] [Google Scholar]
- 66.Portis JL, Coe JE. IgM the secretory immunoglobulin of reptiles and amphibians. Nature. 1975;258:547–48. doi: 10.1038/258547a0. [DOI] [PubMed] [Google Scholar]
- 67.Kawaguchi S, Hiruki T, Harada T, Morikawa S. Frequencies of cell-surface or cytoplasmic IgM-bearing cells in the spleen, thymus and peripheral blood of the snake, Elaphe quadrivirgata. Dev. Comp. Immunol. 1980;4:559–63. doi: 10.1016/s0145-305x(80)80057-7. [DOI] [PubMed] [Google Scholar]
- 68.Wei Z, Wu Q, Ren L, Hu X, Guo Y, et al. Expression of IgM, IgD, and IgY in a reptile, Anolis carolinensis. J. Immunol. 2009;183:3858–64. doi: 10.4049/jimmunol.0803251. [DOI] [PubMed] [Google Scholar]
- 69.Warr GW, Magor KE, Higgins DA. IgY: clues to the origins of modern antibodies. Immunol. Today. 1995;16:392–98. doi: 10.1016/0167-5699(95)80008-5. [DOI] [PubMed] [Google Scholar]
- 70.Gambón-Deza F, Sánchez Espinel C. IgD in the reptile leopard gecko. Mol. Immunol. 2008;45:3470–76. doi: 10.1016/j.molimm.2008.02.027. [DOI] [PubMed] [Google Scholar]
- 71.Gambón-Deza F, Sánchez Espinel C, Valdueza Beneitez J. A novel IgA-like immunoglobulin in the reptile Eublepharis macularius. Dev. Comp. Immunol. 2007;31:596–605. doi: 10.1016/j.dci.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 72.Natarajan K, Muthukkaruppan VR. Distribution and ontogeny of B cells in the garden lizard, Calotes versicolor. Dev. Comp. Immunol. 1985;9:301–10. doi: 10.1016/0145-305x(85)90121-1. [DOI] [PubMed] [Google Scholar]
- 73.Dbrowski Z, Sano Martins IS, Tabarowski Z, Witkowska-Pelc E, Spadacci Morena DD, et al. Haematopoiesis in snakes (Ophidia) in early postnatal development. Cell Tissue Res. 2007;328:291–99. doi: 10.1007/s00441-006-0303-4. [DOI] [PubMed] [Google Scholar]
- 74.Sano-Martins IS, Dabrowski Z, Tabarowski Z, Witkowska-Pelc E, Spadacci Morena DD, Spodaryk K. Haematopoiesis and a new mechanism for the release of mature blood cells from the bone marrow into the circulation in snakes (Ophidia). Cell Tissue Res. 2002;310:67–75. doi: 10.1007/s00441-002-0557-4. [DOI] [PubMed] [Google Scholar]
- 75.Zapata A, Leceta J, Barrutia MG. Ultrastructure of splenic white pulp of the turtle, Mauremys caspica. Cell Tissue Res. 1981;220:845–55. doi: 10.1007/BF00210466. [DOI] [PubMed] [Google Scholar]
- 76.Cooper EL, Klumpau AE, Zapata AG. In: Reptilian immunity. In Biology of the Reptilia. Gans C, editor. Academic Press; New York: 1985. pp. 599–678. [Google Scholar]
- 77.Xu Z, Wang GL, Nie P. IgM, IgD and IgY and their expression pattern in the Chinese soft-shelled turtle Pelodiscus sinensis. Mol. Immunol. 2009;46:2124–32. doi: 10.1016/j.molimm.2009.03.028. [DOI] [PubMed] [Google Scholar]
- 78.Hussein MF, Badir N, el-Ridi R, el-Deeb S. Effect of splenectomy on the humoral immune response in the lizard, Scincus scincus. Experientia. 1979;35:869–70. doi: 10.1007/BF01955118. [DOI] [PubMed] [Google Scholar]
- 79.El-Ridi R, Badir N, El-Rouby S. Effect of seasonal variations on the immune system of the snake, Psammophis schokari. J. Exp. Zool. 1981;216:357–65. [Google Scholar]
- 80.Zimmerman LM, Vogel LA, Edwards KA, Bowden RM. Phagocytic B cells in a reptile. Biol. Lett. 2010;6:270–73. doi: 10.1098/rsbl.2009.0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Glick B, Chang TS, Jaap RG. The bursa of Fabricius and antibody production. Poult. Sci. 1956;35:224–25. [Google Scholar]
- 82.Ribatti D, Crivellato E, Vacca A. The contribution of Bruce Glick to the definition of the role played by the bursa of Fabricius in the development of the B cell lineage. Clin. Exp. Immunol. 2006;145:1–4. doi: 10.1111/j.1365-2249.2006.03131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cooper MD. A life of adventure in immunobiology. Annu. Rev. Immunol. 2010;28:1–19. doi: 10.1146/annurev-immunol-030409-101248. [DOI] [PubMed] [Google Scholar]
- 84.Das S, Mohamedy U, Hirano M, Nei M, Nikolaidis N. Analysis of the immunoglobulin light chain genes in zebra finch: evolutionary implications. Mol. Biol. Evol. 2010;27:113–20. doi: 10.1093/molbev/msp212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Watanabe H, Kobayashi K, Isayama Y. Peculiar secretory IgA system identified in chickens. II. Identification and distribution of free secretory component and immunoglobulins of IgA, IgM, and IgG in chicken external secretions. J. Immunol. 1975;115:998–1001. [PubMed] [Google Scholar]
- 86.Ratcliffe MJ. Antibodies, immunoglobulin genes and the bursa of Fabricius in chicken B cell development. Dev. Comp. Immunol. 2006;30:101–18. doi: 10.1016/j.dci.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 87.Magor KE. Immunoglobulin genetics and antibody responses to influenza in ducks. Dev. Comp. Immunol. 2011;35:1008–16. doi: 10.1016/j.dci.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 88.Arakawa H, Buerstedde J-M. Activation-induced cytidine deaminase-mediated hypermutation in the DT40 cell line. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009;364:639–44. doi: 10.1098/rstb.2008.0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brand A, Galton J, Gilmour DG. Committed precursors of B and T lymphocytes in chick embryo bursa of Fabricius, thymus, and bone marrow. Eur. J. Immunol. 1983;13:449–55. doi: 10.1002/eji.1830130604. [DOI] [PubMed] [Google Scholar]
- 90.Kincade PW, Cooper MD. Immunoglobulin A: site and sequence of expression in developing chicks. Science. 1973;179:398–400. doi: 10.1126/science.179.4071.398. [DOI] [PubMed] [Google Scholar]
- 91.Lawrence EC, Arnaud-Battandier F, Koski IR, Dooley NJ, Muchmore AV, Blaese RM. Tissue distribution of immunoglobulin-secreting cells in normal and IgA-deficient chickens. J. Immunol. 1979;123:1767–71. [PubMed] [Google Scholar]
- 92.Cihak J, Hoffmann-Fezer G, Ziegler-Heibrock HW, Stein H, Kaspers B, et al. T cells expressing the Vb1 T-cell receptor are required for IgA production in the chicken. Proc. Natl. Acad. Sci. USA. 1991;88:10951–55. doi: 10.1073/pnas.88.23.10951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kincade PW, Cooper MD. Development and distribution of immunoglobulin-containing cells in the chicken. An immunofluorescent analysis using purified antibodies to μ, γ and light chains. J. Immunol. 1971;106:371–82. [PubMed] [Google Scholar]
- 94.Muir WI, Bryden WL, Husband AJ. Immunity, vaccination and the avian intestinal tract. Dev. Comp. Immunol. 2000;24:325–42. doi: 10.1016/s0145-305x(99)00081-6. [DOI] [PubMed] [Google Scholar]
- 95.Casteleyn C, Doom M, Lambrechts E, Van den Broeck W, Simoens P, Cornillie P. Locations of gut-associated lymphoid tissue in the 3-month-old chicken: a review. Avian Pathol. 2010;39:143–50. doi: 10.1080/03079451003786105. [DOI] [PubMed] [Google Scholar]
- 96.Imamura K, Yasuda M, Riwar B, Inui S, Ekino S. Characteristic cellular composition of germinal centers. Comp. Immunol. Microbiol. Infect. Dis. 2009;32:419–28. doi: 10.1016/j.cimid.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 97.Arakawa H, Furusawa S, Ekino S, Yamagishi H. Immunoglobulin gene hyperconversion ongoing in chicken splenic germinal centers. EMBO J. 1996;15:2540–46. [PMC free article] [PubMed] [Google Scholar]
- 98.Mehr R, Edelman H, Sehgal D, Mage R. Analysis of mutational lineage trees from sites of primary and secondary Ig gene diversification in rabbits and chickens. J. Immunol. 2004;172:4790–96. doi: 10.4049/jimmunol.172.8.4790. [DOI] [PubMed] [Google Scholar]
- 99.Hofmann J, Greter M, Du Pasquier L, Becher B. B-cells need a proper house, whereas T-cells are happy in a cave: the dependence of lymphocytes on secondary lymphoid tissues during evolution. Trends Immunol. 2010;31:144–53. doi: 10.1016/j.it.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 100.Lundqvist ML, Middleton DL, Radford C, Warr GW, Magor KE. Immunoglobulins of the nongalliform birds: antibody expression and repertoire in the duck. Dev. Comp. Immunol. 2006;30:93–100. doi: 10.1016/j.dci.2005.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Smialek M, Tykalowski B, Stenzel T, Koncicki A. Local immunity of the respiratory mucosal system in chickens and turkeys. Pol. J. Vet. Sci. 2011;14:291–97. doi: 10.2478/v10181-011-0047-2. [DOI] [PubMed] [Google Scholar]
- 102.Haghighi HR, Gong J, Gyles CL, Hayes MA, Zhou H, et al. Probiotics stimulate production of natural antibodies in chickens. Clin. Vaccine Immunol. 2006;13:975–80. doi: 10.1128/CVI.00161-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ulmer-Franco AM, Cherian G, Quezada N, Fasenko GM, McMullen LM. Hatching egg and newly hatched chick yolk sac total IgY content at 3 broiler breeder flock ages. Poult. Sci. 2012;91:758–64. doi: 10.3382/ps.2011-01757. [DOI] [PubMed] [Google Scholar]
- 104.Kovacs-Nolan J, Mine Y. Egg yolk antibodies for passive immunity. Annu. Rev. Food Sci. Technol. 2012;3:163–82. doi: 10.1146/annurev-food-022811-101137. [DOI] [PubMed] [Google Scholar]
- 105.Gerber HA, Morris B, Trevella W. The role of gut-associated lymphoid tissues in the generation of immunoglobulin-bearing lymphocytes in sheep. Aust. J. Exp. Biol. Med. Sci. 1986;64(Pt. 3):201–13. doi: 10.1038/icb.1986.22. [DOI] [PubMed] [Google Scholar]
- 106.Butler JE, Santiago-Mateo K, Sun XZ, Wertz N, Sinkora M, Francis DH. Antibody repertoire development in fetal and neonatal piglets. XX. B cell lymphogenesis is absent in the ileal Peyer's patches, their repertoire development is antigen dependent, and they are not required for B cell maintenance. J. Immunol. 2011;187:5141–49. doi: 10.4049/jimmunol.1101871. [DOI] [PubMed] [Google Scholar]
- 107.Sinkora M, Stepanova K, Butler JE, Francis D, Santiago-Mateo K, et al. Ileal Peyer's patches are not necessary for systemic B cell development and maintenance and do not contribute significantly to the overall B cell pool in swine. J. Immunol. 2011;187:5150–61. doi: 10.4049/jimmunol.1101879. [DOI] [PubMed] [Google Scholar]
- 108.Han JH, Akira S, Calame K, Beutler B, Selsing E, Imanishi-Kari T. Class switch recombination and somatic hypermutation in early mouse B cells are mediated by B cell and Toll-like receptors. Immunity. 2007;27:64–75. doi: 10.1016/j.immuni.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gurish MF, Bryce PJ, Tao H, Kisselgof AB, Thornton EM, et al. IgE enhances parasite clearance and regulates mast cell responses in mice infected with Trichinella spiralis. J. Immunol. 2004;172:1139–45. doi: 10.4049/jimmunol.172.2.1139. [DOI] [PubMed] [Google Scholar]
- 110.Watanabe N, Bruschi F, Korenaga M. IgE: a question of protective immunity in Trichinella spiralis infection. Trends Parasitol. 2005;21:175–78. doi: 10.1016/j.pt.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 111.Cerutti A, Chen K, Chorny A. Immunoglobulin responses at the mucosal interface. Annu. Rev. Immunol. 2011;29:273–93. doi: 10.1146/annurev-immunol-031210-101317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Snoeck V, Peters IR, Cox E. The IgA system: a comparison of structure and function in different species. Vet. Res. 2006;37:455–67. doi: 10.1051/vetres:2006010. [DOI] [PubMed] [Google Scholar]
- 113.Bakema JE, van Egmond M. The human immunoglobulin A Fc receptor FcaRI: a multifaceted regulator of mucosal immunity. Mucosal Immunol. 2011;4:612–24. doi: 10.1038/mi.2011.36. [DOI] [PubMed] [Google Scholar]
- 114.Pasquier B, Launay P, Kanamaru Y, Moura IC, Pfirsch S, et al. Identification of FcaRI as an inhibitory receptor that controls inflammation: dual role of FcRg ITAM. Immunity. 2005;22:31–42. doi: 10.1016/j.immuni.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 115.Chen K, Cerutti A. New insights into the enigma of immunoglobulin D. Immunol. Rev. 2010;237:160–79. doi: 10.1111/j.1600-065X.2010.00929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chen K, Cerutti A. The function and regulation of immunoglobulin D. Curr. Opin. Immunol. 2011;23:345–52. doi: 10.1016/j.coi.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gray D, Gray M. What are regulatory B cells? Eur. J. Immunol. 2010;40:2677–79. doi: 10.1002/eji.201040961. [DOI] [PubMed] [Google Scholar]
- 118.Yoshimoto M, Montecino-Rodriguez E, Ferkowicz MJ, Porayette P, Shelley WC, et al. Embryonic day 9 yolk sac and intra-embryonic hemogenic endothelium independently generate a B-1 and marginal zone progenitor lacking B-2 potential. Proc. Natl. Acad. Sci. USA. 2011;108:1468–73. doi: 10.1073/pnas.1015841108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ghosn EEB, Sadate-Ngatchou P, Yang Y, Herzenberg LA. Distinct progenitors for B-1 and B-2 cells are present in adult mouse spleen. Proc. Natl. Acad. Sci. USA. 2011;108:2879–84. doi: 10.1073/pnas.1019764108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Esplin BL, Welner RS, Zhang Q, Borghesi LA, Kincade PW. A differentiation pathway for B1 cells in adult bone marrow. Proc. Natl. Acad. Sci. USA. 2009;106:5773–78. doi: 10.1073/pnas.0811632106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Barber CL, Montecino-Rodriguez E, Dorshkind K. Reduced production of B-1-specified common lymphoid progenitors results in diminished potential of adult marrow to generate B-1 cells. Proc. Natl. Acad. Sci. USA. 2011;108:13700–4. doi: 10.1073/pnas.1107172108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Weill JC, Weller S, Reynaud CA. Human marginal zone B cells. Annu. Rev. Immunol. 2009;27:267–85. doi: 10.1146/annurev.immunol.021908.132607. [DOI] [PubMed] [Google Scholar]
- 123.Griffin DO, Holodick NE, Rothstein TL. Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+CD27+CD43+CD70−. J. Exp. Med. 2011;208:67–80. doi: 10.1084/jem.20101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Baumgarth N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat. Rev. Immunol. 2011;11:34–46. doi: 10.1038/nri2901. [DOI] [PubMed] [Google Scholar]
- 125.Pillai S, Cariappa A, Moran ST. Marginal zone B cells. Annu. Rev. Immunol. 2005;23:161–96. doi: 10.1146/annurev.immunol.23.021704.115728. [DOI] [PubMed] [Google Scholar]
- 126.Mauri C, Ehrenstein MR. The ‘short’ history of regulatory B cells. Trends Immunol. 2008;29:34–40. doi: 10.1016/j.it.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 127.Bemark M, Boysen P, Lycke NY. Induction of gut IgA production through T cell-dependent and T cell-independent pathways. Ann. N.Y. Acad. Sci. 2012;1247:97–116. doi: 10.1111/j.1749-6632.2011.06378.x. [DOI] [PubMed] [Google Scholar]
- 128.Murphy KP, Travers P, Walport M, Janeway C. Janeway's Immunobiology. Garland Science; New York: 2012. [Google Scholar]
- 129.Lang VR, Mielenz D, Neubert K, Böhm C, Schett G, et al. The early marginal zone B cell-initiated T-independent type 2 response resists the proteasome inhibitor bortezomib. J. Immunol. 2010;185:5637–47. doi: 10.4049/jimmunol.1001040. [DOI] [PubMed] [Google Scholar]
- 130.Martin F, Oliver AM, Kearney JF. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity. 2001;14:617–29. doi: 10.1016/s1074-7613(01)00129-7. [DOI] [PubMed] [Google Scholar]
- 131.Parra D, Rieger AM, Li J, Zhang YA, Randall LM, et al. Pivotal advance: Peritoneal cavity B-1 B cells have phagocytic and microbicidal capacities and present phagocytosed antigen to CD4+ T cells. J. Leukoc. Biol. 2012;91:525–36. doi: 10.1189/jlb.0711372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gao J, Ma X, Gu W, Fu M, An J, et al. Novel functions of murine B1 cells: active phagocytic and microbicidal abilities. Eur. J. Immunol. 2012;42:982–92. doi: 10.1002/eji.201141519. [DOI] [PubMed] [Google Scholar]
- 133.Nakashima M, Kinoshita M, Nakashima H, Habu Y, Miyazaki H, et al. Pivotal advance: characterization of mouse liver phagocytic B cells in innate immunity. J. Leukoc. Biol. 2012;91:537–46. doi: 10.1189/jlb.0411214. [DOI] [PubMed] [Google Scholar]
- 134.Kawamoto H. A close developmental relationship between the lymphoid and myeloid lineages. Trends Immunol. 2006;27:169–75. doi: 10.1016/j.it.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 135.Duan B, Morel L. Role of B-1a cells in autoimmunity. Autoimmun. Rev. 2006;5:403–8. doi: 10.1016/j.autrev.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 136.Ekser B, Ezzelarab M, Hara H, van der Windt DJ, Wijkstrom M, et al. Clinical xenotransplantation: The next medical revolution? Lancet. 2012;379:672–83. doi: 10.1016/S0140-6736(11)61091-X. [DOI] [PubMed] [Google Scholar]
- 137.Dvorankova B, Broz L, Pafcuga I, Kapounkova Z, Konigova R. The role of skin bank in the treatment of severely burnt patients. Acta Chir. Plast. 2004;46:51–55. [PubMed] [Google Scholar]
- 138.Chen RH, Adams DH. Transgenic porcine valves show no signs of delayed cardiac xenograft rejection. Ann. Thorac. Surg. 2001;71:S389–92. doi: 10.1016/s0003-4975(01)02505-x. [DOI] [PubMed] [Google Scholar]
- 139.Bracy JL, Cretin N, Cooper DK, Iacomini J. Xenoreactive natural antibodies. Cell. Mol. Life Sci. 1999;56:1001–7. doi: 10.1007/s000180050489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ohdan H, Sykes M. B cell tolerance to xenoantigens. Xenotransplantation. 2003;10:98–106. doi: 10.1034/j.1399-3089.2003.02108.x. [DOI] [PubMed] [Google Scholar]
- 141.Dufour V, Malinge S, Nau F. The sheep Ig variable region repertoire consists of a single VH family. J. Immunol. 1996;156:2163–70. [PubMed] [Google Scholar]
- 142.Sinclair MC, Gilchrist J, Aitken R. Bovine IgG repertoire is dominated by a single diversified VH gene family. J. Immunol. 1997;159:3883–89. [PubMed] [Google Scholar]
- 143.Becker RS, Knight KL. Somatic diversification of immunoglobulin heavy chain VDJ genes: evidence for somatic gene conversion in rabbits. Cell. 1990;63:987–97. doi: 10.1016/0092-8674(90)90502-6. [DOI] [PubMed] [Google Scholar]
- 144.Jenne CN, Kennedy LJ, Reynolds JD. Antibody repertoire development in the sheep. Dev. Comp. Immunol. 2006;30:165–74. doi: 10.1016/j.dci.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 145.Press CM, McCullagh P, Landsverk T. Effect of early fetal splenectomy on prenatal B-cell development in sheep. Immunology. 2001;102:131–36. doi: 10.1046/j.1365-2567.2001.01153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Knight KL, Winstead CR. Generation of antibody diversity in rabbits. Curr. Opin. Immunol. 1997;9:228–32. doi: 10.1016/s0952-7915(97)80140-9. [DOI] [PubMed] [Google Scholar]
- 147.Severson KM, Mallozzi M, Driks A, Knight KL. B cell development in GALT: role of bacterial superantigen-like molecules. J. Immunol. 2010;184:6782–89. doi: 10.4049/jimmunol.1000155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Jasper PJ, Zhai SK, Kalis SL, Kingzette M, Knight KL. B lymphocyte development in rabbit: progenitor B cells and waning of B lymphopoiesis. J. Immunol. 2003;171:6372–80. doi: 10.4049/jimmunol.171.12.6372. [DOI] [PubMed] [Google Scholar]
- 149.Sehgal D, Schiaffella E, Anderson AO, Mage RG. Analyses of single B cells by polymerase chain reaction reveal rearranged VH with germline sequences in spleens of immunized adult rabbits: implications for B cell repertoire maintenance and renewal. J. Immunol. 1998;161:5347–56. [PubMed] [Google Scholar]
- 150.Lee F, Chen J-L, Lin C-M, Wang F-I. Presence of bluetongue virus in the marginal zone of the spleen in acute infected sheep. Vet. Microbiol. 2011;152:96–100. doi: 10.1016/j.vetmic.2011.04.025. [DOI] [PubMed] [Google Scholar]
- 151.Booth JS, Griebel PJ, Babiuk LA, Mutwiri GK. A novel regulatory B-cell population in sheep Peyer's patches spontaneously secretes IL-10 and downregulates TLR9-induced IFNa responses. Mucosal Immunol. 2009;2:265–75. doi: 10.1038/mi.2009.6. [DOI] [PubMed] [Google Scholar]
- 152.Hurley WL, Theil PK. Perspectives on immunoglobulins in colostrum and milk. Nutrients. 2011;3:442–74. doi: 10.3390/nu3040442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Shek PN, Dubiski S. Maternal-foetal transfer of normal IgM in the rabbit. Immunology. 1975;29:365–69. [PMC free article] [PubMed] [Google Scholar]
- 154.Angulo-Valadez CE, Ascencio F, Jacquiet P, Dorchies P, Cepeda-Palacios R. Sheep and goat immune responses to nose bot infestation: a review. Med. Vet. Entomol. 2011;25:117–25. doi: 10.1111/j.1365-2915.2010.00911.x. [DOI] [PubMed] [Google Scholar]
- 155.Villa-Mancera A, Méndez-Mendoza M. Protection and antibody isotype responses against Fasciola hepatica with specific antibody to pIII-displayed peptide mimotopes of cathepsin L1 in sheep. Vet. J. 2012;194:108–12. doi: 10.1016/j.tvjl.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 156.Nguyen TK, Wieland W, Santema W, Hoeboer J, van Eden W, et al. Immune response of cattle immunized with a conjugate of the glycolipid glucose monomycolate and protein. Vet. Immunol. Immunopathol. 2011;142:265–70. doi: 10.1016/j.vetimm.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 157.Ogunrinade AF. IgG response in natural and experimental infections of cattle with Fasciola gigantica. Vet. Parasitol. 1983;13:325–32. doi: 10.1016/0304-4017(83)90048-1. [DOI] [PubMed] [Google Scholar]
- 158.Mosier DA, Simons KR, Confer AW, Panciera RJ, Clinkenbeard KD. Serum IgG and IgM antibody response in cattle to antigens of Pasteurella haemolytica. Vet. Immunol. Immunopathol. 1989;22:53–65. doi: 10.1016/0165-2427(89)90163-3. [DOI] [PubMed] [Google Scholar]
- 159.Henderson NG, Stear MJ. Eosinophil and IgA responses in sheep infected with Teladorsagia circumcincta. Vet. Immunol. Immunopathol. 2006;112:62–66. doi: 10.1016/j.vetimm.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 160.Corbeil LB, Anderson ML, Corbeil RR, Eddow JM, BonDurant RH. Female reproductive tract immunity in bovine trichomoniasis. Am. J. Reprod. Immunol. 1998;39:189–98. doi: 10.1111/j.1600-0897.1998.tb00353.x. [DOI] [PubMed] [Google Scholar]
- 161.Shaw RJ, Gatehouse TK, McNeill MM. Serum IgE responses during primary and challenge infections of sheep with Trichostrongylus colubriformis. Int. J. Parasitol. 1998;28:293–302. doi: 10.1016/s0020-7519(97)00164-1. [DOI] [PubMed] [Google Scholar]
- 162.Gershwin LJ. Bovine immunoglobulin E. Vet. Immunol. Immunopathol. 2009;132:2–6. doi: 10.1016/j.vetimm.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 163.Ploegaert TC, Tijhaar E, Lam TJ, Taverne-Thiele A, van der Poel JJ, et al. Natural antibodies in bovine milk and blood plasma: variability among cows, repeatability within cows, and relation between milk and plasma titers. Vet. Immunol. Immunopathol. 2011;144:88–94. doi: 10.1016/j.vetimm.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 164.Schwabacher H. The occurrence of natural antibodies in rabbit sera in relation to the paracolon group of organisms. J. Hyg. 1949;47:115–18. doi: 10.1017/s0022172400014364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Conticello SG. The AID/APOBEC family of nucleic acid mutators. Genome Biol. 2008;9:229. doi: 10.1186/gb-2008-9-6-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Malecek K, Brandman J, Brodsky JE, Ohta Y, Flajnik MF, Hsu E. Somatic hypermutation and junctional diversification at Ig heavy chain loci in the nurse shark. J. Immunol. 2005;175:8105–15. doi: 10.4049/jimmunol.175.12.8105. [DOI] [PubMed] [Google Scholar]
- 167.Zhu C, Feng W, Weedon J, Hua P, Stefanov D, et al. The multiple shark Ig H chain genes rearrange and hypermutate autonomously. J. Immunol. 2011;187:2492–501. doi: 10.4049/jimmunol.1101671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Barreto VM, Magor BG. Activation-induced cytidine deaminase structure and functions: a species comparative view. Dev. Comp. Immunol. 2011;35:991–1007. doi: 10.1016/j.dci.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 169.Cooper MD, Alder MN. The evolution of adaptive immune systems. Cell. 2006;124:815–22. doi: 10.1016/j.cell.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 170.Sunyer JO. Evolutionary and functional relationships of B cells from fish and mammals: insights into their novel roles in phagocytosis and presentation of particulate antigen. Infect. Disord. Drug Targets. 2012;12:200–12. doi: 10.2174/187152612800564419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Herzenberg LA, Kantor AB. Layered evolution in the immune system. A model for the ontogeny and development of multiple lymphocyte lineages. Ann. N.Y. Acad. Sci. 1992;651:1–9. doi: 10.1111/j.1749-6632.1992.tb24588.x. [DOI] [PubMed] [Google Scholar]
- 172.Barr TA, Brown S, Ryan G, Zhao J, Gray D. TLR-mediated stimulation of APC: distinct cytokine responses of B cells and dendritic cells. Eur. J. Immunol. 2007;37:3040–53. doi: 10.1002/eji.200636483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Boehm T. Design principles of adaptive immune systems. Nat. Rev. Immunol. 2011;11:307–17. doi: 10.1038/nri2944. [DOI] [PubMed] [Google Scholar]