Abstract
Changes in ovarian hormones predict changes in emotional eating across the menstrual cycle. However, prior studies have not examined whether the nature of associations varies across dysregulated eating severity. The current study determined whether the strength and/or nature of hormone/dysregulated eating associations differ based on the presence of clinically diagnosed binge episodes (BEs). Participants included 28 women with BEs and 417 women without BEs who provided salivary hormone samples, ratings of emotional eating, and BE frequency for 45 days. Results revealed stronger associations between dysregulated eating and ovarian hormones in women with BEs as compared to women without BEs. The nature of associations also differed, as progesterone moderated the effects of lower estradiol levels on dysregulated eating in women with BEs only. Although hormone/dysregulated eating associations are present across the spectrum of pathology, the nature of associations may vary in ways that have implications for etiological models and treatment.
Keywords: binge eating, ovarian hormones, estrogen, progesterone, emotional eating
Ovarian Hormone Influences on Dysregulated Eating: A Comparison of Associations in Women with versus without Binge Episodes
Past research has suggested that changes in ovarian hormones significantly predict within-person changes in binge-eating phenotypes across the menstrual cycle in women (Edler, Lipson, & Keel, 2007; Klump, Culbert, Edler, & Keel, 2008). These studies initially examined associations indirectly by investigating changes in food intake and binge eating across menstrual cycle phases. Results across samples (including samples from the community and those with bulimia nervosa (BN)) were remarkably consistent in suggesting post-ovulatory peaks in food intake, binge eating, and phenotypes strongly related to binge eating (e.g., emotional eating, or the tendency to overeat in response to negative emotions) (Edler et al., 2007; Gladis & Walsh, 1987; Gong, Garrel, & Calloway, 1989; Klump et al., 2008; Lester, Keel, & Lipson, 2003; Price, Torem, & Dimarzio, 1987).
The consistency of cycle phase findings prompted researchers to examine whether natural changes in ovarian hormones across the menstrual cycle predict these within-person changes in food intake and binge-eating phenotypes. Although sample sizes were small (Maximum N = 9 subjects), initial pilot studies indicated that changes in ovarian hormones accounted for the cycle phase differences observed previously (Edler et al., 2007; Klump et al., 2008). Lower levels of estradiol, and higher levels of progesterone, were associated with increased binge frequency and emotional eating scores in women with BN as well as women drawn from the community, respectively (Edler et al., 2007; Klump et al., 2008). Importantly, these hormonal influences were found to be independent of within-person changes in negative affect and body mass index (BMI) (Edler et al., 2007; Klump et al., 2008; Klump et al., 2013a), suggesting that the effects of hormones on binge eating risk were direct rather than indirectly related to these potentially important third variables.
Until recently, larger scale studies were not available to replicate these direct effects. However, our group recently published the first large-scale study of within-person changes in hormones and emotional eating across the menstrual cycle in a community-based sample (Klump et al., 2013a). The aim of that study was to replicate and extend prior work by examining both the main and interactive effects of hormones on binge eating risk. The focus on interactive effects emanated from results from experimental studies in animals, in which high levels of estrogen caused decreases in food intake when progesterone levels were low; whereas when estrogen and progesterone levels both were high, food intake increased, due to the antagonizing effects of progesterone on estrogen (Blaustein & Wade, 1976; Gray & Wade, 1981; Kemnitz, Gibber, Lindsey, & Eisele, 1989; Varma et al., 1999). Using over 190 women drawn from the community, this study replicated the findings from animal studies by showing that interactions between estrogen and progesterone were the strongest hormonal predictors of within-person changes in emotional eating (Klump et al., 2013a). Emotional eating scores were highest when both estradiol and progesterone levels were high (Klump et al., 2013a). These interactive effects appeared to account for post-ovulatory and mid-luteal phase increases in emotional eating scores, as the mid-luteal phase is characterized by the highest levels of progesterone and the second highest levels of estradiol across the entire menstrual cycle. Overall, these findings were significant in suggesting that estrogen and progesterone act in concert to increase emotional eating levels across the menstrual cycle, most likely through the antagonizing effects of progesterone on estrogen (Klump et al., 2013a).
Nonetheless, our conclusions were limited by the exclusive focus on unselected, community samples of women. Effect sizes were somewhat small in our community sample, as many women did not engage in significant amounts of emotional eating (Klump et al., 2013a). Subgroup analyses of more severely affected subjects (e.g., top 33% of emotional eating scorers) revealed similar patterns of hormone/emotional eating associations, but sample sizes were still modest and conclusions were limited by the focus on emotional eating rather than more traditional indices of binge eating (e.g., binge eating frequency). Thus, while this study contributed important insights into the nature of hormone/dysregulated eating associations in a community sample of women, the generalizability of findings to individuals suffering from more severe types of binge eating that are common in clinical eating disorders such as BN and binge eating disorder (BED) was unclear.
To date, only one small study (N = 9 subjects) has examined binge frequency in a clinically diagnosed sample (i.e., women with BN), and this study did not examine interactions between estrogen and progesterone (Edler et al., 2007). Thus, it remains unknown if the main effects of estrogen, the main effects of progesterone, or the interactive effects of the hormones are most predictive of changes in binge eating in women with clinical levels of pathology. Information regarding similarities and differences in the hormonal milieus that are “risky” for dysregulated eating across the spectrum of severity is needed to inform dimensional models of etiology (e.g., RDoC; Cuthbert, 2005) and the development of novel treatments that consider biological triggers for binge eating (Nillni, Toufexis, & Rohan, 2011; van Elburg & Treasure, 2013).
Given the above, the aim of the current study was to examine hormone-dysregulated eating associations across the menstrual cycle in a clinically diagnosed sample of women. Following previous recommendations (Cuthbert, 2005), we took a dimensional approach by studying women from our community-based sample who endorsed binge episodes (BEs) during study interviews. While some of these women met criteria for a DSM-5 eating disorder (i.e., BN or BED), others did not meet full diagnostic criteria but nonetheless endorsed engaging in BEs. We compared the magnitude and nature of hormone-emotional eating associations in our BE sample (i.e., those with and without eating disorders) to associations observed in our community sample who did not endorse BEs, but who still varied in levels of emotional eating, a prospective risk factor for the development of BEs (Stice, Presnell, & Spangler, 2002). We also examined binge frequencies in the women with BEs only to investigate whether emotional eating and BE frequency exhibit similar patterns of hormone/behavior associations across the menstrual cycle among women with more severe pathology.
Methods
Participants
Participants were 445 same-sex female twins (ages 16–25 years) who participated in the Twin Study of Hormones and Behavior across the Menstrual Cycle project (Klump et al., 2013a) within the Michigan State University Twin Registry (MSUTR; see Burt & Klump, 2013; Klump & Burt, 2006 for a description of recruitment methods). Notably, a sub-set of participants in our full sample (N=196/445, 44%) were included in our previous report (Klump et al., 2013a) that found significant interactive effects of estrogen and progesterone on emotional eating scores (reviewed in the Introduction).
The current report focused on 28 women with a lifetime history of BEs (28/445; 6.3%) and 417 women without BEs (417/445; 93.7%). The diagnostic criteria used to determine the presence of BEs is outlined below (see Binge Episodes). Fifteen (15/445; 3.4%) of the women in the BE sample endorsed a lifetime history of BN (N = 8; 8/445; 1.8%) or BED (N = 7; 7/445; 1.6%) during our diagnostic interviews. Given the small number of BN and BED cases, our analyses focused on the full BE sample (regardless of BN or BED status) to maximize power to detect significant effects. This data aggregation is supported by analyses showing no subgroup differences (i.e., differences between women with BEs only versus women with BEs within the context of BN or BED) in ovarian hormone levels or negative affect scores (all p’s > .10, average d = .26). Although women with BN and BED did report higher emotional eating scores and a higher number of binge episodes than women with BEs only (p’s < .05, d = .47–.77), these group differences would be expected given the more severe pathology in the BN and BED groups.
All participants were required to meet the following inclusion criteria: 1) menstruation every 22–32 days for the past 6 months; 2) no hormonal contraceptive use within the past 3 months; 3) no psychotropic or steroid medications within the past 4 weeks; 4) no pregnancy or lactation within the past 6 months; and 5) no history of genetic or medical conditions known to influence hormone functioning or appetite/weight. Despite these inclusion criteria, participants did not differ meaningfully on continuous measures of binge eating or other disordered eating symptoms (e.g., weight preoccupation, body dissatisfaction) from participants from previous MSUTR studies not requiring these inclusion criteria (average d = .12). Women without BEs were demographically representative of the recruitment region (see Table 1) (Klump et al., 2013a; Racine et al., 2013b). Although women with BEs were primarily Caucasian, there was a similar percentage of individuals of Hispanic ethnicity in the BE sample as compared to the no BE sample. The predominance of Caucasian descent and a more equitable distribution of Hispanic ethnicity in the women with BEs would be expected given previous research suggesting increased rates of binge eating in Caucasians and individuals of Hispanic ethnicity (e.g., Alegria et al., 2007).
Table 1.
Descriptive Statistics.
| Variable | No BEs (N = 417) |
BEs Sample (N = 28) |
|---|---|---|
| Demographic Information | ||
| Age (M (SD)) | 17.81 (1.71) | 18.38 (2.50) |
| Race/Ethnicity | ||
| Caucasian | 350 (83.0%) | 27 (96.4%) |
| African American | 46 (11.0%) | 0 |
| Asian/Pacific Islander | 1 (0.2%) | 1 (3.6%) |
| Native American | 2 (0.5%) | 0 |
| Multiracial | 22 (5.3%) | 0 |
| Non-Hispanic | 379 (90.9%) | 25 (89.3%) |
| Hispanic | 38 (9.1%) | 3 (10.7%) |
| Study Variablesa | ||
| Emotional Eating | ||
| Mean (SD)a | 0.28 (0.38) | 0.62 (0.43) |
| Daily Range | 0–3 | 0–3 |
| Binge Frequency | ||
| Mean (SD)a | -- | 0.22 (0.42) |
| Daily Range | -- | 0–4 |
| Estradiol levels (pg/mL) | ||
| Mean (SD)a | 2.89 (1.41) | 2.82 (2.03) |
| Daily Range | .10–30.93 | .10–19.17 |
| Progesterone (pg/mL) | ||
| Mean (SD)a | 120.11 (75.45) | 124.06 (79.59) |
| Daily Range | 5.01–857.36 | 5.61–700.25 |
| Body Mass Index (BMI) | ||
| Mean (SD)a | 23.69 (5.34) | 25.86 (7.26) |
| Daily Range | 15.55–49.72 | 18.17–47.70 |
| Negative Affect | ||
| Mean (SD)a | 14.97 (3.96) | 17.45 (3.83) |
| Daily Range | 10–47 | 9–44 |
These values are unstandardized (i.e., non-z-scored) means and standard deviations (SD) across the 45 day collection period that index the average level of study variables on any given day.
Procedures
All study measures and procedures were approved by the Michigan State University Institutional Review Board (IRB). Participants provided daily behavioral and hormone data for 45 consecutive days. Salivary samples were used to assay ovarian hormones and were collected within the first 30 minutes of waking using previously established methods (Edler et al., 2007; Klump et al., 2008). Questionnaires were completed each evening (after 5:00 PM) using an online data system or pre-printed scantrons. The timing of data collection ensured that a given day’s hormone collection preceded that day’s behavioral ratings (Edler et al., 2007; Klump et al., 2008).
In addition to daily data collection, all participants completed three in-person visits occurring at the start of the study, halfway through the study (∼day 23), and at the end of data collection (∼day 45). During these in-person assessments, eligibility was re-assessed, height and weight were measured, and completed materials were collected from participants. The diagnostic interview for BEs was administered during the last study visit to ensure that we captured all symptoms that were present during the 45-day study period. Between visits, staff contacted participants 1x/week to answer questions and confirm continued protocol adherence. These procedures were effective at minimizing participant drop-out (< 3%) and missing data (< 6%) as well as identifying individuals who were no longer eligible to participate due to missed periods, medication use, and/or pregnancy (< 3%).
Measures
Binge Episodes (BEs)
A modified version of the eating disorder module from the Structured Clinical Interview for DSM-IV (First, Spitzer, Gibbon, & Williams, 1996) was used to assess the lifetime presence of BEs. Modifications were made to assess both DSM-IV and DSM-5 criteria and to provide additional symptom probes for use with a community sample (e.g., multiple questions were added to assess loss of control over BEs).
All BE cases were required to endorse both the consumption of a large amount of food during a short period of time and the experience of loss of control over eating. Threshold BE cases (N = 15 (54%) met full criteria for both symptoms, i.e., they consumed >1000 calories during a binge episode and reported both behavioral and psychological indicators of loss of control. Subthreshold BE cases (N = 13 (46%)) were defined as those who reported both BEs and a loss of control, but the level of severity was diminished either in terms of the amount of food eaten (i.e., between 600–999 calories) and/or the degree of loss of control reported (i.e., some (but not all) behavioral indicators of loss of control accompanied by endorsement of significant sadness, guilt, or disgust about the potential effects of the BE on body weight or shape). Because of the younger age of participants, and the community-based nature of the sample, we did not enforce DSM-5 frequency requirements for the presence/absence of BEs. However, the range of BE frequencies (i.e., 6x/year to 11x/week) indicated that the full spectrum of severity was present in our participants. Individuals endorsing both current BEs (i.e., BEs in the past month; N = 22 (79%)) and past BEs (i.e., no BEs in the past month; N = 6 (21%)) were included in the study to maximize sample size. The inclusion of past BE cases is supported by prior research suggesting continued difficulties with dysregulated eating (e.g., emotional eating) in individuals with past histories of BE, BN and BED (Fairburn, Cooper, Doll, Norman, & O'Connor, 2000; Keel, Mitchell, Miller, Davis, & Crow, 1999). However, in order to ensure that the inclusion of individuals with past histories of BE did not unduly influence our results, we conducted analyses with and without these past cases. The pattern of results was identical across analyses (data not shown), and thus only findings with the full BE sample (i.e., current and past cases) are reported herein.
The SCID-I was administered by trained interviewers with a master’s level education in clinical psychology or a related mental health field. Training involved viewing SCID instruction videos, studying/reviewing the symptom coding manual, listening to audiotapes of previous interviews, conducting practice interviews, and receiving feedback on interviewing skills via review of audiotapes and live observation. At the end of the training period (i.e., 2–3 months), all interviewers were required to pass a “check-out” interview with a senior interviewer on the project.
All interviews were audiotaped, and each co-twin was assessed by a different interviewer to ensure independence of symptom coding within twin pairs. Codes for all symptoms were reviewed during a weekly diagnostic case conference attended by the interview team and the principal investigator (KLK). Audiotapes of each interview were reviewed (as necessary), and participants were re-contacted when there was insufficient information to code symptoms and/or make diagnoses. As with the interviews themselves, symptom reviews were conducted blind to co-twin status, as co-twin interviews were never reviewed during the same case conference session. Inter-rater reliability was assessed by comparing diagnoses made by an independent interviewer (i.e., an interviewer who was blind to study diagnosis, but who listened to the audiotaped interview) to the final diagnosis. The kappa coefficient (κ = .82) for the presence of BEs (as defined above) indicated substantial inter-rater agreement.
Emotional eating
Emotional eating was assessed daily using the Emotional Eating scale of the Dutch Eating Behavior Questionnaire (DEBQ) (Van Strien, Frijters, Bergers, & Defares, 1986). The Emotional Eating scale assesses eating in response to negative emotions (e.g., “Did you have desire to eat when you were depressed?”) on a 5-point scale ranging from not at all to very often. Internal consistencies for the DEBQ Emotional Eating scale are excellent in previous research (α = .93) (Klump et al., 2008; Racine et al., 2012; Van Strien et al., 1986) and in the current study (45-day average α = .90). Importantly, eating in response to negative emotions is thought to be a core feature of binge eating, and the DEBQ Emotional Eating Scale has demonstrated validity in differentiating between individuals with BN and/or binge eating, overweight individuals, and college students. Furthermore, the DEBQ Emotional Eating scale is correlated with established measures of binge eating (r’s = .55–.69) (Racine, Culbert, Larson, & Klump, 2009; Van Strien, 2000) as well as with palatable food intake (i.e., ice cream) in a laboratory setting (Van Strien, 2000). Similar to previous research (Klump et al., 2008), the instructions for the scale were modified with permission to ask about emotional eating over the current day.
Binge frequency
Participants reported the daily frequency of BEs (0 to 9 or more episodes). In order to help ensure that participants reported accurately on binge frequency, a detailed definition of BEs was provided during the first study session. Specifically, participants were told that binge eating was eating an unusually large amount of food (i.e., something that most people would think is larger than a normal meal) with a sense of loss of control over eating that often is experienced as feeling driven or compelled to eat, not being able to stop eating once started, and/or not being able to keep from eating large amounts of certain kinds of food in the first place. Participants were also quizzed on their understanding of BEs, in that they were provided with four case examples and asked to report on whether BEs and/or loss of control were present for each case according to the definitions given. Past research has suggested that providing BE definitions significantly increases the accuracy of self-reported BEs (Celio, Wilfley, Crow, Mitchell, & Walsh, 2004).
Ovarian hormones
Estradiol and progesterone were assayed from daily saliva samples. Saliva samples are preferred over other methods (e.g., blood spots) because they represent a less invasive collection method, particularly when repeated samples are needed. Previous research has found that saliva samples are associated with higher compliance and more robust hormone-behavior associations than blood spot sampling (Edler et al., 2007).
Saliva samples were processed by Salimetrics, LLC (State College, PA, USA) using enzyme immunoassay kits designed specifically for analyzing saliva. These assays show excellent intra- and inter-assay coefficients of variation (estradiol = 7.1% and 7.5%; progesterone = 6.2% and 7.6%), as well as assay sensitivity (measured by interpolating the mean optical density minus 2 SDs of 10–20 replicates at the 0 pg/mL level; estradiol = 0.10 pg/mL; progesterone = 5 pg/mL) and method accuracy (determined by spike recovery and linearity, estradiol = 104.2% and 99.4%; progesterone = 99.6% and 91.8%). In order to conserve resources, samples were only assayed every other day during menstrual bleeding and early follicular phase when hormones are expected to be low and stable. This process ensured that we captured periods of maximum hormonal change across the menstrual cycle (e.g., mid-late follicular though premenstrual phase) while in turn maximizing the number of participant samples assayed.
Negative affect
The Negative Affect scale (Watson, Clark, & Tellegen, 1988) from the Positive and Negative Affect Schedule (PANAS) was used to assess daily negative affect. This scale consists of 10 items that assess the full range of daily negative emotions (e.g., distress, nervousness, irritability, fear). The degree to which each emotion was experienced was rated on a 5-point scale ranging from very slightly/not at all to extremely. The PANAS Negative Affect scale has exhibited excellent internal consistency as well as good convergent and discriminant validity (Watson et al., 1988). Internal consistency in the current study was excellent (45-day average α = .85).
Body mass index (BMI)
Participants’ height and weight were measured during the three in-person study visits using a wall-mounted ruler and digital scale, respectively. BMI was calculated using the following formula: (BMI = weight (in kilograms)/height (in meters)2).
Statistical Analyses
Data preparation
Data preparation followed methods used in previous studies examining the relationship between ovarian hormones and binge eating/emotional eating across the menstrual cycle (Edler et al., 2007; Klump et al., 2008; Klump et al., 2013a). For our repeated measures (i.e., emotional eating, binge frequency, hormones, negative affect, BMI), five-day rolling averages were calculated and standardized within person. Previous research has used rolling averages, and they are preferred because of their ability to minimize random variation that is present in behavioral data due to environmental circumstances (Gladis & Walsh, 1987). In order to accommodate the fact that BMI was assessed at only three time points across the study, rolling averages were calculated using visit 1 BMI for days in-between the first and second in-person assessments, visit 2 BMI for days in-between the second and third in-person assessment, and visit 3 BMI for the last day of the study. Rolling average variables were then converted to within-person standardized scores based on each individual participant’s overall standard deviation across the study. This standardization meant that statistical modeling results (see below) represent standardized estimates of effects that index the degree to which changes in a woman’s ovarian hormones, relative to her equilibrium, predict changes away from the woman’s equilibrium in emotional eating and binge eating.
Statistical models
Using identical methods as those outlined in Klump et al. (2013), we employed mixed linear models (MLMs) to examine changes in emotional eating and binge eating across menstrual cycle phase (i.e., pre- versus post-ovulation) as well as changes in ovarian hormones as predictors of changes in binge eating. MLMs are ideal for these analyses as we could examine predictive associations while controlling for covariates (i.e., negative affect and BMI) and the non-independence of the repeated measures and twin data. We controlled for the non-independence of the twin data by estimating random intercepts and allowing them to correlate. Because most co-twins were assessed across the same 45-day period, we also estimated a time-specific dyadic correlation that allowed the twins’ residual scores for emotional eating to correlate from day-to-day. For each of these random effects, we specified a compound symmetry covariance structure which estimates a single intercept variance or a single residual variance across twins and time. The models also allowed for random slopes for each of the predictors (i.e., hormones, negative affect, BMI). However, because there was no evidence that these slopes correlated across twins, we fixed the cross-twin correlation to zero for all predictors.
Our first set of MLMs examined changes in emotional eating scores and, for women with BEs, binge frequency across pre- versus post-ovulation, as previous research has found higher binge eating rates in post-ovulatory phases of the menstrual cycle (Edler et al., 2007; Gladis & Walsh, 1987; Klump et al., 2008; Lester et al., 2003; Price et al., 1987). We investigated these changes by including a pre-ovulation (i.e., from the follicular phase through the ovulatory phase) versus post-ovulation (i.e., from post-ovulation through the pre-menstrual phase) variable as the predictor in the MLMs. Pre- versus post-ovulation was coded based on dates of menstrual bleeding and increases/decreases in hormones (see Edler et al., 2007 for coding methods). In order to examine if there were BE/no BE group differences in the magnitude and/or nature of the ovulation/dysregulated eating association, we included a BE “sample” variable (i.e., women with versus without BEs) as a moderator in the models. Significant interactions between the “sample” moderator and ovulation phase indicated the presence of significant group differences in the nature and/or magnitude of changes in emotional eating across ovulation in women who did versus did not binge eat. Importantly, we only conducted these moderator analyses for emotional eating scores, not binge frequency, as women without BEs, by definition, would not be expected to report daily BEs. Consequently, for binge frequency, the models only included the women with BEs and did not include a sample moderator variable.
After examining ovulation phase effects, we then tested whether changes in ovarian hormones (rather than menstrual cycle phase) predicted within-person changes in dysregulated eating by examining estrogen, progesterone, and the estrogen x progesterone interaction as predictors of within-person changes in emotional eating scores and binge frequencies. We initially fit a model that tested only the main effects of estradiol, progesterone, and the covariates (i.e., negative affect and BMI). We then fit a second model that included the estradiol x progesterone interaction in addition to all of the main effects and covariates. Significant hormone interaction effects were graphed using the MLM beta weights (see Dawson & Richter, 2006) in order to determine the specific hormonal conditions (e.g., high versus low levels of estradiol and progesterone) that were associated with the highest emotional eating scores and binge frequencies. As before, we tested for BE/no BE group differences in the nature and/or magnitude of hormone effects by including a BE “sample” moderator (i.e., women with versus without BEs) in the models examining emotional eating scores. Significant interactions between the “sample” variable and the hormones indicated the presence of significant group differences in hormonal influences on emotional eating between women who did and did not report BEs.
Results
Descriptive Statistics
Table 1 includes descriptive statistics for daily, average levels of emotional eating, binge frequencies, ovarian hormones, BMI, and negative affect in the no BE and BE groups. Age differences across the two samples were minimal (t (423) = 1.61, p = .11, d = .27), but as expected, the women with BEs had significantly higher levels of emotional eating (t (443) = 4.49, p = .001, d= .84), higher BMIs (t (443) = 2.00, p = .047, d = .34), and higher levels of negative affect (t (443) = 3.21, p = .001, d = .67) than women without BEs. Importantly, estradiol (t (443) = 0.27, p = .79, d = .04) and progesterone (t (443) = 0.31, p = .76, d = .05) levels were not significantly different between groups.
Changes across Pre- versus Post-Ovulation
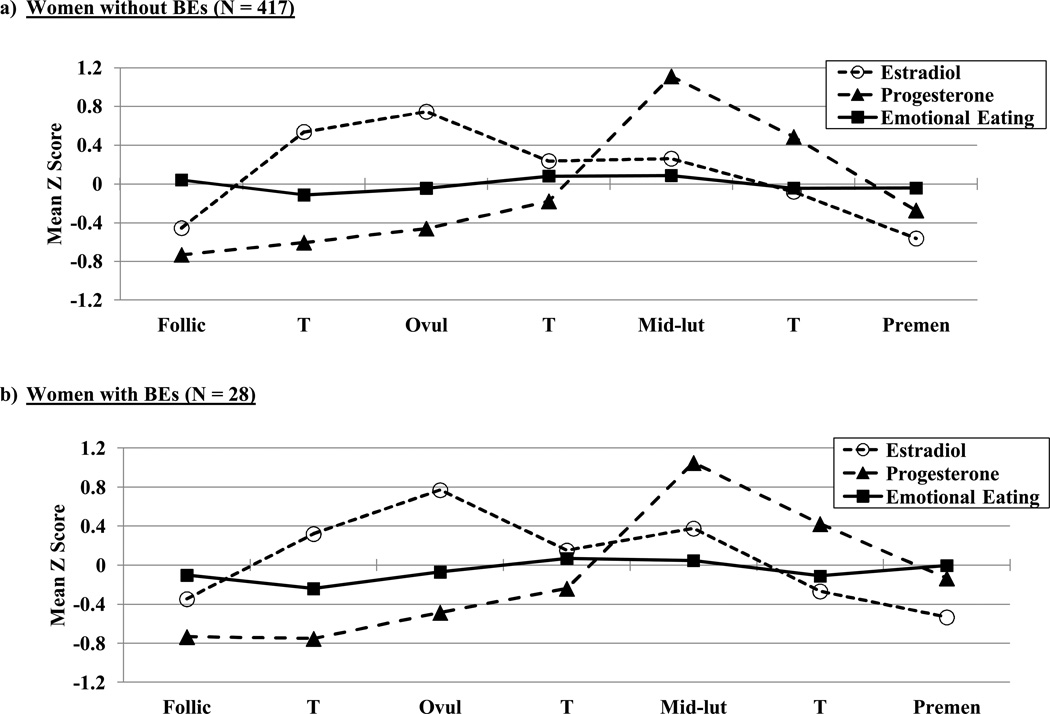
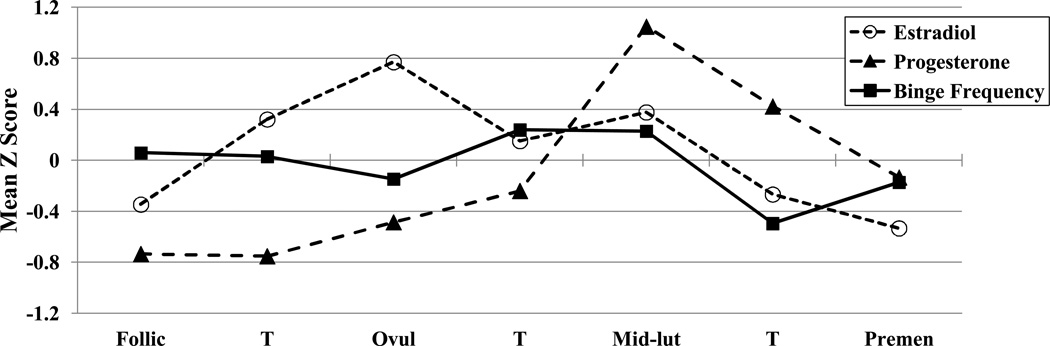
Within-person changes in emotional eating scores, binge frequencies, and ovarian hormones across menstrual cycle phase are shown in Figures 1 and 2. Changes in estradiol and progesterone levels across the menstrual cycle were remarkably similar across groups and followed expectations for hormonal changes in all women (i.e., estradiol peaks before ovulation; progesterone peaks and a secondary estradiol peak in post-ovulation). Within-person changes in emotional eating and binge frequencies were similar across groups as well. There was no significant interaction between the BE “sample” variable (i.e., no BEs versus BEs present) and the ovulation phase variable (i.e., pre- versus post-ovulation) for emotional eating scores (beta (SE) = .08 (.08), p = .30). In the combined subject group (i.e., women with and without BEs), the pre- versus post-ovulation variable was significant (beta (SE) = −.03 (.02), p = .04) and showed significantly lower levels of emotional eating in pre-ovulation (M (SD) across the phase = −.06 (.95)) as compared to post-ovulation (M (SD) across the phase = .01 (1.06)). The same general pattern was observed for binge frequencies in the BE group only (i.e., lower frequencies in pre-ovulation (M (SD) across the phase = −.02 (.92) as compared to post-ovulation (M (SD) across the phase = .11 (1.03)), although smaller sample sizes in the BE group resulted in a non-significant beta estimate despite a higher beta value (beta (SE) = −.07 (.10), p = .49).
Figure 1. Changes in Ovarian Hormone Levels and Emotional Eating across the Menstrual Cycle in women with and without BEs.
Mean Z Score = the mean of the 5-day rolling averages calculated within subjects, then averaged across participants; Follic = follicular phase; Ovul = ovulatory phase; Mid-lut = mid-luteal phase; Premen = premenstrual phase; T = transition days that are in-between phases. The number of days included in each phase varied by participant based on their cycle length, but the days roughly corresponded to the following (first day of menstrual bleeding = +1; previous day = −1): Follicular = +3 to +12; Ovulatory = −15 to −12; Mid-luteal = −9 to −5; Premenstrual = −3 to +1.
Figure 2. Changes in Ovarian Hormone Levels and Binge Frequencies across the Menstrual Cycle in women with BEs.
Mean Z Score = the mean of the 5-day rolling averages calculated within subjects, then averaged across participants; Follic = follicular phase; Ovul = ovulatory phase; Mid-lut = mid-luteal phase; Premen = premenstrual phase; T = transition days that are in-between phases. The number of days included in each phase varied by participant based on their cycle length, but the days roughly corresponded to the following (first day of menstrual bleeding = +1; previous day = −1): Follicular = +3 to +12; Ovulatory = −15 to −12; Mid-luteal = −9 to −5; Premenstrual = −3 to +1.
Associations between Changes in Ovarian Hormones and Changes in Dysregulated Eating
Unlike analyses of cycle phase, there was a significant interaction between the “sample” variable and ovarian hormone levels in the prediction of emotional eating scores. Specifically, the three-way interaction (“sample” x estradiol x progesterone) was statistically significant (beta (SE) = .14 (.04), p = .004), suggesting that the influence of ovarian hormones on emotional eating scores differed in women with versus without BEs. Given these significant interactions, the remaining MLMs examined the no BE and BE groups separately.
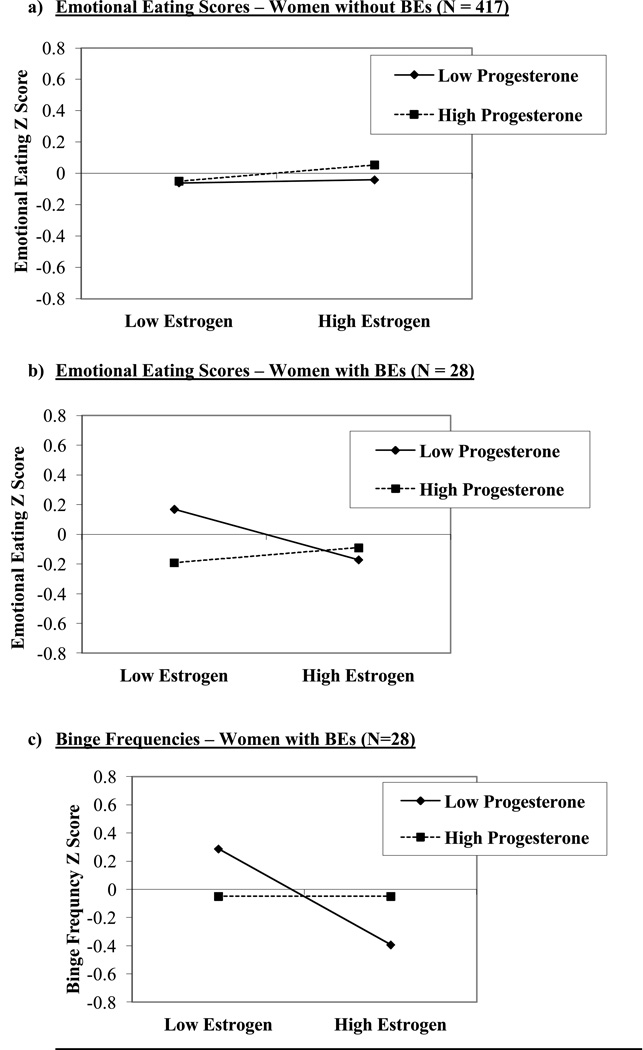
Results for the women without BEs generally corroborated our initial findings from this dataset (Klump et al., 2013a) showing an interaction (but no main effects) between estrogen and progesterone in the prediction of emotional eating scores (see Table 2). The estrogen x progesterone interaction just missed statistical significance (p = .10), but took the form of interactions observed in our previous analysis of the full sample (Klump et al., 2013a). Specifically, emotional eating scores were elevated when both progesterone and estradiol levels were high (see Figure 3a). Although the magnitude of the interaction was diminished compared to that reported previously (see Klump et al., 2013a), this would be expected since the current sample excluded all subjects with clinical levels of binge eating. Thus, the variability and severity of emotional eating (and consequently, associations with ovarian hormones) would be expected to be reduced in the no BE sample.
Table 2.
Predictive Associations between Hormones and Dysregulated Eating.
| Model | b (SE) | t | df | p |
|---|---|---|---|---|
| Emotional Eating Scores | ||||
| Women without BEs (N=417) | ||||
| Main Effects: | ||||
| Intercept | −.02 (.01) | −2.07 | 193 | .04 |
| Estradiol | .03 (.02) | 1.45 | 327 | .15 |
| Progesterone | .03 (.02) | 1.52 | 327 | .13 |
| Negative Affect | .15 (.02) | 7.40 | 329 | <.001 |
| Body Mass Index (BMI) | .05 (.03) | 1.84 | 310 | .07 |
| Interactions: | ||||
| Intercept | −.03 (.01) | −2.10 | 135 | .04 |
| Estradiol | .03 (.02) | 1.60 | 327 | .11 |
| Progesterone | .03 (.02) | 1.34 | 327 | .18 |
| Estrogen x Progesterone | .02 (.01) | 1.61 | 304 | .10 |
| Negative Affect | .15 (.02) | 7.39 | 329 | <.001 |
| BMI | .05 (.03) | 1.64 | 310 | .10 |
| Women with BEs (N=28) | ||||
| Main Effects: | ||||
| Intercept | −.02 (.03) | −0.68 | 644 | .50 |
| Estradiol | −.08 (.04) | −2.23 | 619 | .03 |
| Progesterone | −.04 (.04) | −1.16 | 621 | .24 |
| Negative Affect | .31 (.04) | 8.79 | 645 | <.001 |
| Body Mass Index (BMI) | −.17 (.04) | −4.56 | 635 | <.001 |
| Interactions: | ||||
| Intercept | −.07 (.04) | −1.87 | 616 | .06 |
| Estradiol | −.06 (.04) | −1.62 | 606 | .10 |
| Progesterone | −.07 (.04) | −1.97 | 580 | .05 |
| Estrogen x Progesterone | .11 (.03) | 3.26 | 558 | .001 |
| Negative Affect | .31 (.03) | 8.85 | 632 | <.001 |
| BMI | −.16 (.04) | −4.40 | 620 | <.001 |
| Binge Frequencies | ||||
| Women with BEs (N=28) | ||||
| Main Effects: | ||||
| Intercept | .02 (.05) | 0.36 | 399 | .71 |
| Estradiol | −.20 (.05) | −3.95 | 391 | <.001 |
| Progesterone | .05 (.05) | 1.07 | 391 | .28 |
| Negative Affect | .20 (.05) | 4.09 | 398 | <.001 |
| Body Mass Index (BMI) | −.09 (.05) | −1.80 | 397 | .07 |
| Interactions: | ||||
| Intercept | −.05 (.05) | −0.92 | 381 | .36 |
| Estradiol | −.17 (.05) | −3.45 | 381 | .001 |
| Progesterone | .002 (.05) | 0.04 | 373 | .97 |
| Estrogen x Progesterone | .17 (.05) | 3.47 | 270 | .001 |
| Negative Affect | .20 (.05) | 4.15 | 391 | <.001 |
| BMI | −.08 (.05) | −1.63 | 388 | .10 |
Note. Differences in degrees of freedom (df) across subgroup analyses and dependent variables reflect differences in the samples examined. Lower df in the Women without BEs group resulted from the inclusion of correlated random intercepts and time-specific dyadic correlations to control for the non-independence of the twin data (see Methods). The number of twin pairs was low in the Women with BEs sample (N = 2 pairs) and thus, random intercepts and time-specific dyadic correlations were not included in the MLMs for these women. The lower df for binge frequencies in Women with BEs was due to the presence of some data collection days in which none of the women reported a binge episode.
Figure 3. Interactions between Estradiol and Progesterone in the Prediction of Emotional Eating Scores and Binge Frequencies.
Emotional Eating and Binge Frequency Z Score = 5-day rolling average calculated within subjects, then averaged across participants.
Findings for the women with BEs (see Table 2) were somewhat different. The magnitude of the hormone effects appeared to be larger than those in the women without BEs (see Figures 1, 2, and 3), and the nature of the effects appeared to be somewhat different as well. Specifically, in the main effects models, estrogen showed a significant main effect in women with BEs, with lower levels of estradiol predicting higher emotional eating scores and binge frequencies, even after controlling for changes in negative affect and BMI. These estrogen effects were not observed in the women without BEs or in analyses of unselected twins from this sample (see above and Klump et al., 2013a), but they are consistent with a previous study of women with BN where inverse associations between estradiol levels and binge frequency were observed (Edler et al., 2007).
Nonetheless, follow-up models indicated that the interactive effects of estradiol and progesterone were also predictive of within-person changes in both types of dysregulated eating in women with BEs. As shown in Figures 3b and c, emotional eating scores and binge frequencies were elevated when both progesterone and estradiol levels were low. This pattern was observed for emotional eating scores and binge frequencies, suggesting that the findings are robust and present across a range of dysregulated eating symptoms.
At first glance, these differential hormone effects across the no BE/BE group might seem to contradict findings from analyses of ovulation, since post-ovulation peaks were observed in both women with and without BEs (with no significant group differences in effects). However, closer inspection of Figures 1b and 2 reveals that although emotional eating scores and binge frequencies were highest in post-ovulation in both groups, the post-ovulation peaks and valleys differed somewhat between women with versus without BEs. In women without BEs, the highest post-ovulatory peak was in the mid-luteal phase, whereas the highest peaks for the women with BEs were in the mid-luteal phase as well as the premenstrual phase and the transition phase from ovulation. These latter two phases tend to include lower levels of both hormones (particularly the premenstrual phase), suggesting that the hormonal milieus that are risky for women with BEs include both high levels (during the mid-luteal phase) and low levels (during the premenstrual phase) of hormones. This interpretation is supported by data in Figure 3 showing trends for higher levels of hormones to be associated with the second highest elevations in dysregulated eating in women with BEs. Interestingly, there was a tendency for high estradiol and low progesterone levels to be associated with the lowest levels of dysregulated eating (see Figure 3b and c). This latter finding replicates results from the women without BEs (see Figure 3a) and previous analyses (see Klump et al., 2013a) where the lowest levels of emotional eating were observed at high estradiol and low progesterone levels (Klump et al., 2013a).
Discussion
Our results reveal important similarities as well as differences in hormone/dysregulated eating associations across the spectrum of binge eating severity. Emotional eating scores and binge eating frequencies peaked in the post-ovulatory phases of the menstrual cycle for women with and without BEs, and high levels of hormones appeared to partially account for these peaks. In addition, in the BE sample only, lower levels of hormones (in particular, estradiol) were found to predict within-person increases in emotional eating scores and binge frequencies. Critically, in the BE group, patterns observed for fluctuations in emotional eating were identical to those for fluctuations in binge frequency, suggesting that the continuous measure of emotional eating taps clinically relevant dimensions of binge eating (Racine et al., 2013a; Wardle, 1987) that likely share risk factors with full-threshold binge episodes (Stice et al., 2002).
Nonetheless, variations in hormone/binge eating associations between the no BE and BE samples deserve note. Overall, findings suggested a stronger effect of lower levels of hormones in women with BEs than women without BEs. Estrogen exerted a significant main effect in nearly all models, and interaction graphs indicated that low levels of estrogen and progesterone were most predictive of within-person changes in dysregulated eating in women with BEs. Physiologically, these findings are consistent with previous suggestions that progesterone has few direct effects on binge eating or food intake, but instead acts indirectly by antagonizing estrogen. These antagonizing effects would be expected to be diminished when progesterone levels are low, particularly when estrogen levels also are low (Asarian & Geary, 2006). In these types of hormonal milieus, low levels of progesterone likely act as permissive factors in allowing for the behavioral effects of low estrogen levels (i.e., increased binge eating and food intake – (see Asarian & Geary, 2006) to be manifested.
However, it remains unclear why progesterone would moderate both high and low levels of estradiol in women with BEs, but not in women without BEs. Only one previous study has examined women with BEs (Edler et al., 2007). This study assessed 9 women with BN and found that low levels of estrogen, and high levels of progesterone, were associated with increased binge frequencies across the menstrual cycle. However, this study did not examine estrogen x progesterone interactions and thus, could not investigate how the two hormones act in concert to influence changes in binge eating. Interestingly, in our sample of women with BEs, we saw the same pattern of main effects for binge frequency (e.g., low estradiol and high progesterone levels were associated with increased binge frequency- see Table 2), even though the progesterone main effects were not significant. Thus, although our main effects are largely consistent with the prior study of women with BN, they do not help explain the somewhat different pattern of hormone interactions in women with versus without BEs.
One possible contributing factor is the aberrant eating patterns (e.g., dieting) that often characterize women with BEs. Dieting could affect changes in hormone levels and/or modify associations between hormone changes and changes in dysregulated eating in women with versus without BEs (even if daily hormone levels are equivalent – see Table 1). In order to directly examine this possibility, we conducted post-hoc tests to investigate whether the effects of hormones became less significant when accounting for dietary restraint in the women with BEs. We included rolling averages for dietary restraint scores (measured at three time points using the Eating Disorders Examination Questionnaire (Fairburn & Beglin, 1994)) as covariates and found that the estradiol x progesterone interactions continued to be significant in all models (p’s < .05).
Thus, the bulk of the data thus far indicate that the dual “risky” hormonal milieus in women with BEs (higher and lower hormone levels) are robust and present despite changes in other variables across the cycle (e.g., dietary restraint). Both hormonal milieus would be expected to increase dysregulated eating via the antagonistic effects (at high hormone levels) or permissive effects (at low hormone levels) of progesterone on estrogen influences on behavior. However, future research is needed to determine why the different hormonal milieus are risky for women with BEs versus those without BEs, and to identify factors (e.g., psychological, genetic, neurobiological) that might contribute to this differential pattern of risk.
One potentially interesting possibility in this regard is differential hormone regulation of genetic expression for binge eating in women with versus without BEs. As we have proposed previously (Klump, 2013; Klump et al., 2013a), it is likely that hormones influence dysregulated eating in all women via their regulatory effects on gene expression within the central nervous system. Ovarian hormones in general, and estrogen in particular, act as gene transcription factors to activate and de-activate genes within several neurotransmitter systems (e.g., serotonin, dopamine, opioids) that are believed to be important for binge eating and eating disorders characterized by binge eating (e.g., BN) (Becker, 1999; Craft, 2008; Hildebrandt, Alfano, Tricamo, & Pfaff, 2010; Ostlund, Keller, & Hurd, 2003). Ovarian hormone effects on dysregulated eating in all women therefore might result from the hormones’ effects on the production of these important neurotransmitters, their receptors, or their signal transduction mechanisms. In these scenarios, the key individual difference variable is the presence or absence of susceptibility alleles that are regulated by ovarian hormones. These individual differences would be most evident in hormonal milieus (e.g., during the mid-luteal phase when estradiol and progesterone levels are high) that trigger gene activation or de-activation and result in increased rates of dysregulated eating.
With our current data in hand, we can move beyond theories regarding general effects that might be present in all women and theorize about specific gene x hormone effects that might be present in women who go on to develop full-threshold binge episodes. As noted above, estrogen regulation of neurobiological systems that are important for binge eating appears to be more robust than progesterone regulation of these systems. The differential strength of estrogen versus progesterone maps on nicely to the pattern of phenotypic results presented herein. In all models of women with BEs, estrogen showed stronger phenotypic associations with dysregulated eating than progesterone, and interaction graphs clearly depicted more direct effects of estrogen than progesterone. These strong and direct effects of estrogen in women with BEs was in contrast to the lack of direct estrogen effects observed in women without BEs, where clearly, the interaction between estrogen and progesterone was most important for dysregulated eating.
In aggregate, these findings suggest that perhaps women with BEs have a stronger and/or a different genetic loading for dysregulated eating that translates into even stronger effects of estrogen on binge eating risk. Women with BEs may have a larger number of estrogen-responsive risk genes and/or different genetic variants than women without BEs. Because genetic influences are present across the spectrum of binge eating (Bulik, Sullivan, & Kendler, 1998; Klump, McGue, & Iacono, 2000; Racine, Burt, Iacono, McGue, & Klump, 2011; Racine et al., 2013a), and we see hormone effects across the full range of dysregulated eating (see above and Klump et al., 2013a), we are not proposing that genetic effects are present in women with BEs but not in women without BEs. Clearly, genetic influences are present in both groups. But what might be different is the relative number or type of estrogen-responsive risk genes in women with BEs that translates into greater risk for BEs and a wider range of estrogen milieus that are risky.
What is interesting about this potential mechanistic picture is that in women with BEs, it is low estradiol levels that are risky, particularly in the presence of low progesterone levels. Presumably then, it is the lack of regulation of gene expression in women with BEs that translates into greater risk for binge episodes. This direction of association may seem counterintuitive (lower gene regulation = greater risk); however, estrogen can either activate or de-activate gene transcription. Thus, at lower estradiol levels, there may be lower or higher transcription depending upon the particular role for estrogen within that neurobiological system.
Clearly, future studies are needed to test all of these mechanistic hypotheses, but such studies are possible using both animal and human research. Animal studies could examine the differential effects of estradiol dose on binge eating and gene expression in ovariectomized female animals known to be prone versus resistant to binge eating (see Boggiano et al., 2007; Klump, Racine, Hildebrandt, & Sisk, 2013b; Klump et al., 2011a; Klump, Suisman, Culbert, Kashy, & Sisk, 2011b). A stronger or differential effect of estradiol dose on binge eating and the number/type of genes expressed in binge prone animals would suggest that estrogen regulation may contribute to differences in binge eating proneness. In humans, studies could use our longitudinal, menstrual cycle design to examine changes in heritability and gene expression across the menstrual cycle in large samples of female twins with and without binge eating. Differential changes in heritability and gene expression by estradiol levels would suggest that differences in gene regulation may contribute to differences in the effects of ovarian hormones on dysregulated eating in women with versus without BEs. Importantly, both the animal and human studies could include strong “control” hormonal conditions (high hormone levels for increased risk; high estradiol and low progesterone levels for decreased risk) that do not differ in women with and without BEs in order to determine the specific effect of low estradiol levels on genetic risk in women with BEs.
The trick to completing all of these studies is to identify the most important risk genes for analysis. Unfortunately, given the state of molecular genetic studies of eating disorders (see Trace, Baker, Peñas-Lledó, & Bulik, 2013), there are no replicated risk genes for binge eating or eating disorders characterized by binge eating (e.g., BN). Consequently, the most fruitful approach might be to select genes that already known to be regulated by estrogen that are within key neurobiological systems that likely contribute to the development and phenomenology of binge eating. In our view, genes involved in the reward circuitry contained in the Positive Valence Domain of the Research Domain Criteria (Insel et al., 2010) are likely to be helpful in this regard. Past studies suggest that differential activation of reward areas in the brain (e.g., the nucleus accumbens) likely contribute to binge eating in animals (Berridge, 2009; Gradl, Klump, & Sisk, in preparation) as well as humans (Gearhardt et al., 2011; Stice, Spoor, Ng, & Zald, 2009). Key neurotransmitters in these systems (e.g., dopamine and opioids) show strong estrogen regulation (Becker, 1999; Craft, 2008), highlighting the potential for genes in these systems to exhibit differential responsivity to estrogen in women with and without BEs. Given uncertainty as to which particular dopaminergic and opioid genes may be important, a biological pathway analysis in which all genes within a particular circuit are examined might be a good first step in this line of work (Bralten et al., 2013). Alternatively, a sequential design might be useful in which a series of animal studies are used to identify genetic candidates that are then explored further in studies of women with and without BEs. We particularly like the last combination of animal and human studies, as this approach would identify replicable gene x hormone effects across species that could advance the science of translational research and highlight new areas of interface between basic animal science and neurobiological research in humans.
Before ending, two key study limitations should be noted. First, although our sample of women with BEs was larger than those examined previously, the sample was relatively small for a longitudinal study. Our use of a 45-day collection period somewhat ameliorated these concerns, as the use of repeated measures tends to decrease measurement error (Kazdin, 2002). Nonetheless, replication of our findings in larger samples is needed
Second, because our sample was community-based, we were limited to examining subthreshold in addition to threshold BE cases that might have lacked the severity of binge eating present in treatment-seeking samples. Data in Table 1 suggest that the daily frequency of BEs was lower than that observed in clinical samples (Edler et al., 2007; Lester et al., 2003). Our inclusion of past BE cases (N = 6 (21%)) also likely limited variability in our outcome measures. Although our pattern of results was unchanged when removing past BE cases (see Binge Episodes in Methods), additional research is needed to replicate our results and extend them to more severely affected BE cases.
Acknowledgements
This work was supported by grants from the National Institute of Mental Health (R01 MH082054, T32 MH018269, T32 MH070343). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIMH.
Footnotes
Authorship
K.L.K. and P.K.K. developed the study concept. All authors contributed to the study design. Testing and data collection were performed by K.L.K., S.E.R., and B.H. K.L.K., S.E.R., and B.H. performed the data analysis and interpretation. K.L.K., S.E.R., and B.H. drafted the paper, and P.K.K. and C.L.S. provided critical revisions. All authors approved the final version of the paper for submission.
References
- Alegria M, Woo M, Cao Z, Torres M, Meng Xl, Striegel Moore R. Prevalence and correlates of eating disorders in Latinos in the United States. International Journal of Eating Disorders. 2007;40:S15–S21. doi: 10.1002/eat.20406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asarian L, Geary N. Modulation of appetite by gonadal steroid hormones. Philosophical Transactions of the Royal Society of London Series B: Biological Sciences. 2006;361:1251–1263. doi: 10.1098/rstb.2006.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB. Gender differences in dopaminergic function in striatum and nucleus accumbens. Pharmacology, Biochemistry and Behavior. 1999;64:803–812. doi: 10.1016/s0091-3057(99)00168-9. [DOI] [PubMed] [Google Scholar]
- Berridge KC. 'Liking' and 'wanting' food rewards: Brain substrates and roles in eating disorders. Physiology and Behavior. 2009;97:537–550. doi: 10.1016/j.physbeh.2009.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein JD, Wade GN. Ovarian influences on the meal patterns of female rats. Physiology and Behavior. 1976;17:201–208. doi: 10.1016/0031-9384(76)90064-0. [DOI] [PubMed] [Google Scholar]
- Boggiano MM, Artiga AI, Pritchett CE, Chandler-Laney PC, Smith ML, Eldridge AJ. High intake of palatable food predicts binge-eating independent of susceptibility to obesity: an animal model of lean vs. obese binge-eating and obesity with and without binge-eating. International Journal of Obesity. 2007;31:1357–1367. doi: 10.1038/sj.ijo.0803614. [DOI] [PubMed] [Google Scholar]
- Bralten J, Franke B, Waldman I, Rommelse N, Hartman C, Asherson P, Miranda A. Candidate genetic pathways for attention-deficit/hyperactivity disorder (ADHD) show association to hyperactive/impulsive symptoms in children with ADHD. Journal of the American Academy of Child & Adolescent Psychiatry. 2013;52:1204–1212. doi: 10.1016/j.jaac.2013.08.020. [DOI] [PubMed] [Google Scholar]
- Bulik CM, Sullivan PF, Kendler KS. Heritability of binge-eating and broadly defined bulimia nervosa. Biological Psychiatry. 1998;44:1210–1218. doi: 10.1016/s0006-3223(98)00280-7. [DOI] [PubMed] [Google Scholar]
- Burt SA, Klump KL. The Michigan State University Twin Registry (MSUTR): An Update. Twin Research and Human Genetics. 2013;16:344–350. doi: 10.1017/thg.2012.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celio AA, Wilfley DE, Crow SJ, Mitchell J, Walsh BT. A comparison of the binge eating scale, questionnaire for eating and weight patterns revised, and eating disorder examination questionnaire with instructions with the eating disorder examination in the assessment of binge eating disorder and its symptoms. International Journal of Eating Disorders. 2004;36:434–444. doi: 10.1002/eat.20057. [DOI] [PubMed] [Google Scholar]
- Craft RM. Sex differences in analgesic, reinforcing, discriminative, and motoric effects of opioids. Experimental and Clinical Psychopharmacology. 2008;16:376–385. doi: 10.1037/a0012931. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN. Dimensional models of psychopathology: Research agenda and clinical utility. Journal of Abnormal Psychology. 2005;114:565–569. doi: 10.1037/0021-843X.114.4.565. [DOI] [PubMed] [Google Scholar]
- Dawson JF, Richter AW. Probing three-way interactions in moderated multiple regression: Development and application of a slope difference test. Journal of Applied Psychology. 2006;91:917–926. doi: 10.1037/0021-9010.91.4.917. [DOI] [PubMed] [Google Scholar]
- Edler C, Lipson SF, Keel PK. Ovarian hormones and binge eating in bulimia nervosa. Psychological Medicine. 2007;37:131–141. doi: 10.1017/S0033291706008956. [DOI] [PubMed] [Google Scholar]
- Fairburn CG, Beglin SJ. Assessment of eating disorders: Interview or self-report questionnaire? International Journal of Eating Disorders. 1994;16:363–370. [PubMed] [Google Scholar]
- Fairburn CG, Cooper Z, Doll HA, Norman P, O'Connor M. The natural course of bulimia nervosa and binge eating disorder in young women. Archives of General Psychiatry. 2000;57:659–665. doi: 10.1001/archpsyc.57.7.659. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version (SCID-CV) Washington, DC: American Psychiatric Press, Inc; 1996. [Google Scholar]
- Gearhardt AN, Yokum S, Orr PT, Stice E, Corbin WR, Brownell KD. Neural correlates of food addiction. Archives of General Psychiatry. 2011;68:808–816. doi: 10.1001/archgenpsychiatry.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladis MM, Walsh BT. Premenstrual exacerbation of binge eating in bulimia. American Journal of Psychiatry. 1987;144:1592–1595. doi: 10.1176/ajp.144.12.1592. [DOI] [PubMed] [Google Scholar]
- Gong EJ, Garrel D, Calloway DH. Menstrual cycle and voluntary food intake. American Journal of Clinical Nutrition. 1989;49:252–258. doi: 10.1093/ajcn/49.2.252. [DOI] [PubMed] [Google Scholar]
- Gradl D, Klump KL, Sisk CL. Different neural responses to palatable food in binge eating prone and resistant rats. in preparation doi: 10.1016/j.physbeh.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JM, Wade GN. Food intake, body weight, and adiposity in female rats: actions and interactions of progestins and antiestrogens. American Journal of Physiology-Endocrinology And Metabolism. 1981;240:474–481. doi: 10.1152/ajpendo.1981.240.5.E474. [DOI] [PubMed] [Google Scholar]
- Hildebrandt T, Alfano L, Tricamo M, Pfaff DW. Conceptualizing the role of estrogens and serotonin in the development and maintenance of bulimia nervosa. Clinical Psychology Review. 2010;30:655–668. doi: 10.1016/j.cpr.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Cuthbert BN, Garvey MA, Heinssen RK, Pine DS, Quinn KJ, Wang PS. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. American Journal of Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Kazdin AE. Methodological Issues and Strategies in Clinical Research. American Psychological Association; 2002. [Google Scholar]
- Keel PK, Mitchell JE, Miller KB, Davis TL, Crow SJ. Long-term outcome of bulimia nervosa. Archives of General Psychiatry. 1999;56:63–69. doi: 10.1001/archpsyc.56.1.63. [DOI] [PubMed] [Google Scholar]
- Kemnitz JW, Gibber JR, Lindsey KA, Eisele SG. Effects of ovarian hormones on eating behavior, body weight, and glucoregulation in rhesus monkeys. Hormones and Behavior. 1989;23:235–250. doi: 10.1016/0018-506x(89)90064-0. [DOI] [PubMed] [Google Scholar]
- Klump KL. Puberty as a critical risk period for eating disorders: A review of human and animal studies. Hormones and Behavior. 2013;64:399–410. doi: 10.1016/j.yhbeh.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, Burt SA. The Michigan State University Twin Registry (MSUTR): Genetic, environmental and neurobiological influences on behavior across development. Twin Research and Human Genetics. 2006;9:971–977. doi: 10.1375/183242706779462868. [DOI] [PubMed] [Google Scholar]
- Klump KL, Culbert KM, Edler C, Keel PK. Ovarian hormones and binge eating: Exploring associations in community samples. Psychological Medicine. 2008;38:1749–1757. doi: 10.1017/S0033291708002997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, Keel PK, Racine SE, Burt SA, Neale M, Sisk CL, Hu JY. The interactive effects of estrogen and progesterone on changes in emotional eating across the menstrual cycle. Journal of Abnormal Psychology. 2013a;122:131–137. doi: 10.1037/a0029524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, McGue M, Iacono WG. Age differences in genetic and environmental influences on eating attitudes and behaviors in female adolescent twins. Journal of Abnormal Psychology. 2000;109:239–251. [PubMed] [Google Scholar]
- Klump KL, Racine SE, Hildebrandt B, Sisk CL. Sex differences in binge eating patterns in male and female adult rats. International Journal of Eating Disorders. 2013b;46:729–736. doi: 10.1002/eat.22139. [DOI] [PubMed] [Google Scholar]
- Klump KL, Suisman JL, Culbert KM, Kashy DA, Keel PK, Sisk CL. The effects of ovariectomy on binge eating proneness in adult female rats. Hormones and Behavior. 2011a;59:585–593. doi: 10.1016/j.yhbeh.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL, Suisman JL, Culbert KM, Kashy DA, Sisk CL. Binge eating proneness emerges during puberty in female rats: A longitudinal study. Journal of Abnormal Psychology. 2011b;120:948–955. doi: 10.1037/a0023600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester NA, Keel PK, Lipson SF. Symptom fluctuation in bulimia nervosa: Relation to menstrual-cycle phase and cortisol levels. Psychological Medicine. 2003;33:51–60. doi: 10.1017/s0033291702006815. [DOI] [PubMed] [Google Scholar]
- Nillni YI, Toufexis DJ, Rohan KJ. Anxiety sensitivity, the menstrual cycle, and panic disorder: a putative neuroendocrine and psychological interaction. Clinical Psychology Review. 2011;31:1183–1191. doi: 10.1016/j.cpr.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund H, Keller E, Hurd YL. Estrogen receptor gene expression in relation to neuropsychiatric disorders. Annals of the New York Academy of Sciences. 2003;1007:54–63. doi: 10.1196/annals.1286.006. [DOI] [PubMed] [Google Scholar]
- Price WA, Torem MS, Dimarzio LR. Premenstrual exacerbation of bulimia: 60% increase in the mean number of binge episodes. Psychosomatics. 1987;28:378–379. doi: 10.1016/s0033-3182(87)72511-0. [DOI] [PubMed] [Google Scholar]
- Racine SE, Burt SA, Iacono WG, McGue M, Klump KL. Dietary restraint moderates genetic risk for binge eating. Journal of Abnormal Psychology. 2011;120:119–128. doi: 10.1037/a0020895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine SE, Culbert KM, Keel PK, Sisk CL, Burt SA, Klump KL. Differential associations between ovarian hormones and disordered eating symptoms across the menstrual cycle in women. International Journal of Eating Disorders. 2012;45:333–344. doi: 10.1002/eat.20941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine SE, Culbert KM, Larson CL, Klump KL. The possible influences of impulsivity and dietary restraint on associations between serotonin genes and binge eating. Journal of Psychiatric Research. 2009;43:1278–1286. doi: 10.1016/j.jpsychires.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine SE, Keel PK, Burt SA, Sisk CL, Neale M, Boker S, Klump KL. Exploring the relationship between negative urgency and dysregulated eating: Etiologic associations and the role of negative affect. Journal of Abnormal Psychology. 2013a;122 doi: 10.1037/a0031250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine SE, Keel PK, Burt SA, Sisk CL, Neale M, Boker S, Klump KL. Individual differences in the relationship between ovarian hormones and emotional eating across the menstrual cycle: A role for personality? Eating behaviors. 2013b doi: 10.1016/j.eatbeh.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Presnell K, Spangler D. Risk factors for binge eating onset in adolescent girls: A 2-year prospective investigation. Health Psychology. 2002;21:131–138. [PubMed] [Google Scholar]
- Stice E, Spoor S, Ng J, Zald DH. Relation of obesity to consummatory and anticipatory food reward. Physiology and Behavior. 2009;97:551–560. doi: 10.1016/j.physbeh.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trace SE, Baker JH, Peñas-Lledó E, Bulik CM. The genetics of eating disorders. Annual Review of Clinical Psychology. 2013;9:589–620. doi: 10.1146/annurev-clinpsy-050212-185546. [DOI] [PubMed] [Google Scholar]
- van Elburg A, Treasure J. Advances in the neurobiology of eating disorders. Current Opinion in Psychiatry. 2013;26:556–561. doi: 10.1097/YCO.0b013e328365a2e7. [DOI] [PubMed] [Google Scholar]
- Van Strien T. Ice-cream consumption, tendency toward overeating, and personality. Appetite. 2000;52:380–387. doi: 10.1002/1098-108x(200012)28:4<460::aid-eat16>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Van Strien T, Frijters JE, Bergers GP, Defares PB. The Dutch Eating Behavior Questionnaire (DEBQ) for assessment of restrained, emotional, and external eating behavior. International Journal of Eating Disorders. 1986;5:295–315. [Google Scholar]
- Varma M, Chai JK, Meguid MM, Laviano A, Gleason JR, Yang ZJ, Blaha V. Effect of estradiol and progesterone on daily rhythm in food intake and feeding patterns in Fischer rats. Physiology and Behavior. 1999;68:99–107. doi: 10.1016/s0031-9384(99)00152-3. [DOI] [PubMed] [Google Scholar]
- Wardle J. Eating style: a validation study of the Dutch Eating Behaviour Questionnaire in normal subjects and women with eating disorders. Journal of Psychosomatic Research. 1987;31:161–169. doi: 10.1016/0022-3999(87)90072-9. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]