Abstract
Slugs and snails specifically secrete mucus to aid their locomotion. This mucus is the contact material between molluscan herbivores and plants. We have recently shown that the locomotion mucus of the slug Deroceras reticulatum contains salicylic acid (SA).1 When applied to wounded leaves of Arabidopsis thaliana this mucus induces the activity of the SA-responsive pathogenesis related 1 (PR1) promotor1. Here we analyzed PR1 promotor activity in response to treatments with locomotion mucus of eight slugs and snails. Although none of the mucus contained SA, their application still elicited PR1 promotor activity. These data provide further insights into the complex interactions between molluscan herbivores and plants.
Keywords: plant, mollusk, slug, snail, PR1, salicylic acid
Molluscan herbivores, such as slugs and snails, are devastating pests in agriculture and small gardens. Slug and snail damage has been reported for a wide range of agriculturally important plants, including many crop plants, such as lettuce, Brussels sprouts, cauliflower, broccoli and spring cabbage. Slug damage to the cabbage crop can represent the largest proportion (40%) of the total reported damage.2 In Europe, various mollusk species are collectively considered as the most important pests for sunflower and the second most important pests for maize in the crop-establishment phase.3 Despite the ecological, cultural and economic relevance of plant interactions with slugs and snails, our current knowledge on chemical cues that mediate plant-mollusk interactions is very limited. Some of the knowledge originated from studies in the 19th century, when the German botanist Ernst Stahl did simple extractions of plant metabolites and tested them as feeding deterrents against gastropods.4 Stahl’s studies represent the first studies on chemical communication between plants and herbivores5 and may represent the first experimental ecological studies. Since Stahl’s seminal studies, our knowledge of the molecular details of how plants perceive and resist insect herbivores has increased tremendously, but our knowledge of how plants interact with slugs and snails remains rudimentary.
Recent studies have shown that plants specifically respond to the mucus of slugs and snails.6,7 Brassica nigra plants grown from seeds that were treated with the mucus and feces of Helix aspersa (also known as Cornu aspersum) were subsequently less damaged by snails, when compared with plants emerged from non-treated seeds, indicating that the plants responded to molluscan cues by increasing their resistance to subsequent attack.6 Treating wounded Arabidopsis thaliana leaves with the mucus residue from Arion lusitanicus induces higher levels of the wound hormone jasmonic acid (JA) and it’s active conjugate JA-isoleucine than wounding alone, suggesting the presence of so called herbivore-associated molecular patterns (HAMPS) in the mucus.7 HAMPS are chemical cues that are associated with herbivores and which can be specifically detected by plants. HAMP perception triggers complex signaling pathways that often lead to the induction of anti-herbivore defense compounds.8,9 However, some herbivore-derived cues can also suppress defense responses, for example by inducing the salicylic acid pathway, which counteracts JA-induced signaling.10,11 We recently analyzed the mucus of 13 slug and snail species, we found that the mucus of one species (Deroceras reticulatum) contained salicylic acid (SA).1 Wounded A. thaliana leaves treated with locomotion mucus of D. reticulatum also increased the promotor activity of the SA-responsive pathogenesis related 1 (PR1) gene. However, given the low concentration of SA (2.8–15 nmol*g mucus fresh mass−1), it was unclear whether the activity of the PR1 promotor was elevated due to the presence of SA or other HAMPS in the mucus. Here we evaluated if the mucus of seven slugs and snails that do not contain SA also activate the PR1 promotor.
We found that treating wounded leaves with mucus from most slugs and snails induced PR1::GUS activity (Fig. 1, indicated by blue staining). For comparison, we also included the results of D. reticulatum that were already published in the accompanying manuscript.1 In addition tothe residual mucus trail of D. reticulatum, four other molluscs (Achatina fulica, Arion fuscus, Lehmannia valentiana and Malacolimax tenellus) consistently induced PR1 promotor activity around wounding sites of A. thaliana leaves. While GUS activity induction was quite variable after leaves were treated with mucus of H. pomatia and S. putris, mucus of the common garden pest D. leave did not induce PR1 promotor activity. We also performed GUS staining with additional promotor lines that were used to study SA-dependent gene expression, including a chitinase gene and senescence-associated gene 13 from A. thaliana.11 However, both lines showed GUS activity in the absence of any treatment, making it difficult to interpret results obtained with these lines (data not shown).
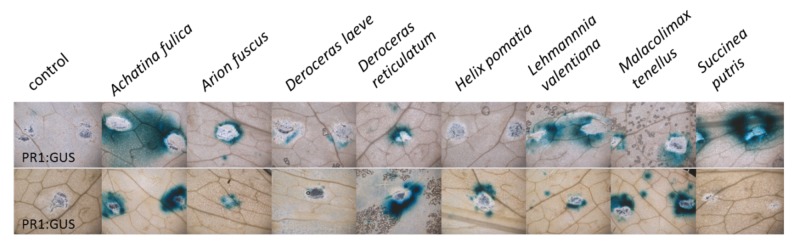
Figure 1.Slug and snail locomotion mucus activates salicylic acid (SA)-dependent marker gene (PR1) in Arabidopsis thaliana. Leaves of A. thaliana PR1-Promotor::GUS plants were wounded with pattern wheel and water (control) or slimes of different slugs and snails were applied to wounds. GUS activity was determined 48 h after start of the experiment.
What is the source of induction of SA-dependent gene expression, if none of the tested mucus contained SA? One possibility could be the presence of slug and snail-derived elicitors that induce the SA pathway, as has been shown for insect eggs.11 These elicitors might be present in the locomotion mucus of the mollusks that consistently induced PR1-promotor activity. Variability in the PR1 promotor induction in leaves treated with locomotion mucus of H. pomatia and S. putris could be explained by different levels of snail-derived elicitors. Alternatively, since SA-induced gene expression is well known to be activated upon pathogen infection in A. thaliana leaves and mollusks are known to present vectors for plant pathogens,13 our results might indicate the presence of microbes in the mucus that may enter the plant via wounding sites. Future experiments are needed to determine the source of the activation of the SA pathway by slugs and snails. As discussed recently,1 mollusk-mediated induction of the SA pathway may change the activation of JA-dependent defenses. Using A. thaliana plants deficient in SA and JA signaling and analyzing the chemical composition of mucus as well as its associated microbial communities will help to shed light on the interaction between plants and molluscan herbivores.
Material and Methods
Mollusk cultivation
The snails and slugs used for experiments included species that occur sympatrically with A. thaliana (Arion fuscus, D. laeve, D. reticulatum, H. pomatia, L. marginata, M. tenellus, S. putris), as well as one exotic species (Achatina fulica). All mollusks were cultivated in a climate chamber (Snijders scientific, Tilburg, The Netherlands), under constant humidity of 80%, a temperature of 16–20 °C, and short day conditions (9.5 h light/ 13.5h dark). All slugs and snails were collected around Jena (GPS: 50.92050°N, 11.61162°E) and Martinfeld (51.28634°N, 10.17949°E, Thuringia, Germany). No specific permissions or approvals were required for collecting slugs and snails at these locations and no endangered or protected species were collected. A. fulica is an important pest in many tropical countries and was provided by Dr. Gustavo Bonaventura. Different numbers of snails were separated in large plastic boxes (OKT easyfresh, Sternwede, Germany; 26.5 x 13 x 15 cm) dependent on their size. Slugs were maintained in smaller boxes (10.5 x 4 x 8 cm) and the number of individuals in one box depended on their size and social compatibility. Potatoes, lettuce and cucumber were provided, with the addition of cuttlebone for calcium (ArtNr.5050, TRIXIE Heimtierbedarf GmbH and Co. KG, Tarp, Germany). The food was changed twice a week and the boxes were cleaned and provided with fresh tissue paper and moistened with tap water. All molluscan species were cultured several weeks under these conditions before they were used for the experiments described in this manuscript.
Plant cultivation
Arabidopsis thaliana plants for experiments were grown in a standard growth substrate (Fruhstorfer Nullerde:vermiculite:sand, 8:1:1) in a climate chamber (21 °C, 55% relative humidity and 130 µmol m-2s-1 photosynthetically active radiation) with a photoperiod of 10h light/14h dark.
GUS-staining experiments
PR1::GUS plants were provided by Philippe Reymond (Department of Plant Molecular Biology, University of Lausanne, CH-1015 Lausanne, Switzerland). Leaves of four weeks old A. thaliana PR1::GUS plants were wounded with a fabric pattern wheel and locomotion mucus was applied by allowing the slugs and snails to crawl over the wounded leaves. For each experiment three leaves from independent plants and three slugs/snails were used. Wounding alone and application of water served as control. Water application was done by gently striking the leaf with one finger (with glove) to mimic slug movement. The sticky nature of the mucus did not allow us to apply it in a similar way as our water treatments. GUS staining was performed 48 h after treatments as described previously.12 Briefly, leaves were incubated overnight at 37 °C in X-Gluc solution (Sigma) and de-stained twice in 99% Ethanol, followed by incubation in chloral hydrate solution (80 g chloral hydrate, 10 ml glycerol, 30 ml water) until leaves were completely transparent. Pictures were taken with a Canon Powershot SD1000 camera (www.canon.de). Results of two representative experiments are shown.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Glossary
Abbreviations:
- SA
salicylic acid
- PR1
pathogenesis-related 1
References
- 1.Kästner J, von Knorre D, Himanshu H, Erb M, Baldwin IT, Meldau S. Salicylic Acid, a plant defense hormone, is specifically secreted by a molluscan herbivore. PLoS One. 2014;9:e86500. doi: 10.1371/journal.pone.0086500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker G. Molluscs as crop pests. Book. 2002;ISBN-10:0851993206. [Google Scholar]
- 3.Chabert A. Importance relative des ravageurs sou terrains en gran des cultures. Resultats de l'enquete d'un groupe de travail. Phytoma. 1994;461:20–4. [Google Scholar]
- 4.Stahl E. Pflanzen und Schnecken. Eine biologische Studie uber die Schutzmittel der Pflanzen gegen Schneckenfrass. Jenaische Zeitschr Mediz Naturwiss Mediz. 1888;22:557–684. [Google Scholar]
- 5.Mitchell-Olds T, Gershenzon J. I B, Boland W. research focus - Chemical ecology in the molecular era. Trends Plant Sci. 1998;3:362–5. doi: 10.1016/S1360-1385(98)01296-5. [DOI] [Google Scholar]
- 6.Orrock JL. Exposure of unwounded plants to chemical cues associated with herbivores leads to exposure-dependent changes in subsequent herbivore attack. PLoS One. 2013;8:e79900. doi: 10.1371/journal.pone.0079900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Falk KL, Kastner J, Bodenhausen N, Schramm K, Paetz C, Vassao DG, Reichelt M, von Knorre D, Bergelson J, Erb M, et al. The role of glucosinolates and the jasmonic acid pathway in resistance of Arabidopsis thaliana against molluscan herbivores. Mol Ecol. 2013 doi: 10.1111/mec.12610. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mithöfer A, Boland W. Plant defense against herbivores: chemical aspects. Annu Rev Plant Biol. 2012;63:431–50. doi: 10.1146/annurev-arplant-042110-103854. [DOI] [PubMed] [Google Scholar]
- 9.Erb M, Meldau S, Howe GA. Role of phytohormones in insect-specific plant reactions. Trends Plant Sci. 2012;17:250–9. doi: 10.1016/j.tplants.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Consales F, Schweizer F, Erb M, Gouhier-Darimont C, Bodenhausen N, Bruessow F, Sobhy I, Reymond P. Insect oral secretions suppress wound-induced responses in Arabidopsis. J Exp Bot. 2012;63:727–37. doi: 10.1093/jxb/err308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruessow F, Gouhier-Darimont C, Buchala A, Metraux JP, Reymond P. Insect eggs suppress plant defence against chewing herbivores. Plant J. 2010;62:876–85. doi: 10.1111/j.1365-313X.2010.04200.x. [DOI] [PubMed] [Google Scholar]
- 12.Little D, Gouhier-Darimont C, Bruessow F, Reymond P. Oviposition by pierid butterflies triggers defense responses in Arabidopsis. Plant Physiol. 2007;143:784–800. doi: 10.1104/pp.106.090837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.ElHamalawi ZA, Menge JA. The role of snails and ants in transmitting the avocado stem canker pathogen, Phytophthora citricola. J Am Soc Hortic Sci. 1996;121:973–7. [Google Scholar]


