Abstract
Sensing and responding toward gravity vector is a complicated and multistep process. Gravity is a constant factor feeding plants with reliable information for the spatial orientation of their organs. Auxin, cytokinin, ethylene and BRs have been the most explored hormones in relation to gravitropism. We have previously shown that glucose (Glc) could promote brassinosteroid (BR) signaling thereby inducing changes in root directional growth. Auxin signaling and polar transport components are also involved in Glc induced changes in root directional growth. Here, we provide evidence for involvement of cytokinin and ethylene signaling components in regulation of root directional growth downstream to Glc and BR. Altogether, Glc mediated change in root direction is an adaptive feature which is a result of a collaborative effort integrating phytohormonal signaling cues.
Keywords: Arabidopsis, glucose, brassinosteroid, cytokinin, ethylene
Considering the complexity of interactions during various physiological changes, the differential growth responses of plants also involve a coordinated action of phytohormones to regulate the growth process.1 Plant hormones such as auxin, cytokinin, ethylene, and BR have been shown to play important roles in affecting root directionality.2-6 Cytokinin plays an inhibitory effect on root gravitropism.3 Ethylene also inhibits gravity response by altering flavonoid synthesis.5 It has been shown that along with root growth and development in general, Glc can also influence root directional responses via both HXK -dependent and -independent signal transduction pathways. Glc employs auxin response machinery to modulate root directional growth.2 Recently, we have shown that BR and Glc could also modulate root directional growth.7 There are few reports for BR-cytokinin interaction in Arabidopsis.8-11 The Arabidopsis brx-2 mutant was insensitive for cytokinin induced lateral root initiation arrest which can be rescued by embryonic application of brassinolide (BL).8 Ectopic expression of CYTOKININ OXIDASE/ DEHYDROGENASE3 (CKX3) and BRASSINOSTEROID INSENSITIVE1 (BRI1) can synergistically enhance both leaf and root growth.9 BR can also regulate genes of the cytokinin signaling pathway by increasing cytokinin levels.11 Similarly, BR and ethylene have also been shown to regulate many physiological responses such as, primary root growth, etiolated hypocotyl growth, apical hook formation and shoot gravitropism.10,12-15 Based on previous reports and our present findings, here we provide a hormonal cascade in controlling Glc mediated root directional growth.
Involvement of Known Hormone Signaling Components in Regulating Glc Induced Root Deviation Response
In our previous studies, we have shown the involvement of BR signaling, auxin signaling and auxin transport components during Glc induced root directional growth.2,7 Here, a hormonal hierarchy has been established downstream to Glc and BR involved in regulation of root growth direction. We tested the possibility that in addition to Glc, BR and auxin, whether other potential phytohormones which are already known to be involved in controlling root gravitropism such as; cytokinin and ethylene3,5 could also contribute to root deviation response. To investigate how cytokinin and ethylene affect Glc-induced root directional growth, the WT, cytokinin mutants and ethylene mutant seeds were surface sterilized and imbibed at 4 °C for 48h. Imbibed seeds were germinated and grown vertically on petri dishes containing ½ MS supplemented with 1% sucrose and solidified with 0.8% agar for 5d under climate-controlled growth rooms in a long day condition, with 22 °C ± 2 °C temperature and 60 µmoles/m2/s light intensity. 5d old light grown seedlings were then transferred to the treatment medium (Glc/hormones/inhibitors) and kept vertically in culture room conditions for 72h to monitor root directional changes.
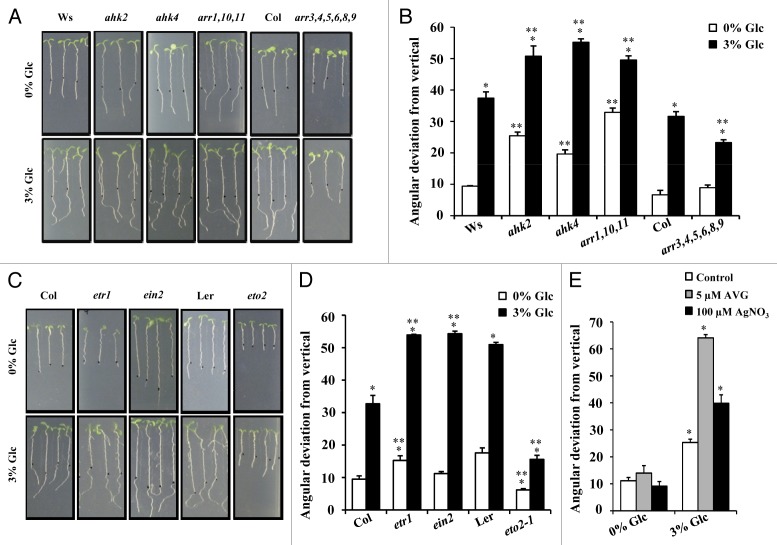
The cytokinin receptor mutants; Arabidopsis histidine kinase2 (ahk2), ahk4 and Type B response regulator (ARRs) triple mutant; arr1,10,11, showed an enhanced Glc induced root direction reset response. While the Type A ARRs sextuple mutant arr3,4,5,6,8,9, showed a reduced response as compared with the WT (Fig. 1A and 1B). Similarly, the ethylene receptor mutant ethylene resistant1–1 (etr1–1) and signaling mutant ethylene insensitive2 (ein2–1) also exhibited an exaggerated Glc induced root directional response, whereas the ethylene overproducer2 (eto2) mutant showed very less response as compared with their respective WTs (Fig. 1C and 1D). Also, inhibiting ethylene biosynthesis using amino-ethoxy-vinyl-glycine (AVG) and signaling via AgNO3 application along with 3% Glc treatment could enhance the response significantly (Fig. 1E). These results suggest that both cytokinin and ethylene signal transduction pathways might act antagonistically to Glc for regulating root directional growth.
Figure 1. Involvement of cytokinin and ethylene signaling in controlling Glc-induced root deviation response. (A) Pictures and (B) quantification of Glc-induced root deviation response in cytokinin mutants. Cytokinin receptors (ahk2, ahk4) and Type B ARR mutant arr1,10,11 show enhanced root deviation while response of Type A ARR mutant arr3,4,5,6,8,9 was very less as compared with WT. (C) Pictures and (D) quantification of Glc-induced root deviation response in ethylene mutants. In presence of 3% Glc, ethylene receptor mutant etr1–1 and signaling mutant ein2 show enhanced root deviation; while response of eto2 mutant was very less as compared with WT. (E) Inhibitors of ethylene perception (AgNO3) and biosynthesis (AVG) could enhance Glc induced root deviation of WT (Col-0). Data shown is the average of two biological replicate having atleast 30 seedlings; error bars represent SE; (Student’s t test; P < 0.05), * control vs. treatment; ** WT vs. mutant.
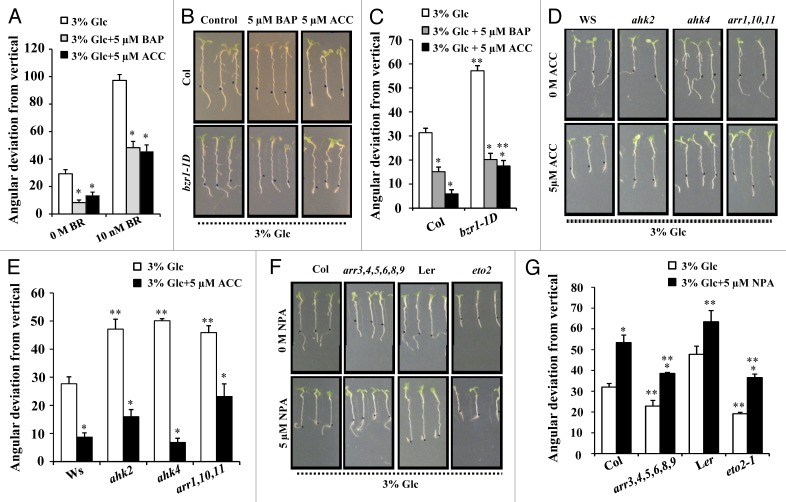
Glc and BR were found to act synergistically to regulate Glc induced root directional growth.7 Since ethylene and cytokinin seem to exert an antagonistic effect on Glc induced deviation response, we analyzed the effect of cytokinin (6-benzylaminopurine, BAP) and ethylene (1-aminocyclopropane-carboxylic acid hydrochloride, ACC) on Glc and BR induced root directional growth. Both BAP as well as ACC were able to abolish the effect of Glc and BR in terms of root directional growth (Fig. 2A) suggesting that both BAP and ACC might act antagonistically with BR also to regulate this response. We also tested the effect of BAP and ACC supplementation to a mutant with endogenously high BR signaling; brassinazole resistant1–1D (bzr1–1D) which also shows hyper responsiveness toward Glc induced root directional response. The exaggerated root deviation response of bzr1–1D mutant in presence of Glc was highly reduced on both BAP as well as ACC inclusion (Fig. 2B and 2C). This confirms that both cytokinin and ethylene works antagonistically to- and downstream to- BR for controlling this response. Further, ACC could inhibit the exaggerated Glc-induced root directional response of cytokinin mutants ahk2, ahk4 and arr1,10,11 suggesting that cytokinin antagonize the Glc induced root directional response via ethylene mediated machinery (Fig. 2D and 2E).
Figure 2. Phytohormone hierarchy in controlling Glc-induced root deviation response. (A) Cytokinin and ethylene can reduce BR induced root deviation in presence of Glc. (B) and (C) CK and ethylene signaling work downstream to BR as Glc-induced root deviation of bzr1–1D was dramatically reduced in BAP and ACC treatment. (D) and (E) ACC treatment reduces Glc-induced root deviation in CK mutants significantly. (F) and (G) NPA treatment along with 3% Glu could cause root deviation in arr3,4,5,6,8,9 and eto2–1 mutants which are resistant to Glc-induced root deviation response. Data shown is the average of two biological replicate having atleast 30 seedlings; error bars represent SE; (Student’s t test; P < 0.05), * control vs. treatment; ** WT vs. mutant.
Interestingly, application of auxin polar transport inhibitor; 1-N-naphthylphthalamic acid (NPA) along with 3% Glc could induce root deviation response in arr3,4,5,6,8,9 and eto2 mutants, indicating that auxin transport lies further downstream to both cytokinin and ethylene (Fig. 2F and 2G). So far our result suggests that root deviation due to Glc involves a hormonal cascade in function with auxin transport modulation. The auxin transport machinery lies downstream to cytokinin and ethylene to control root directional growth.
A Testable Model Depicting Relationship between Glucose and Phytohormone Signals Controlling Root Directional Growth
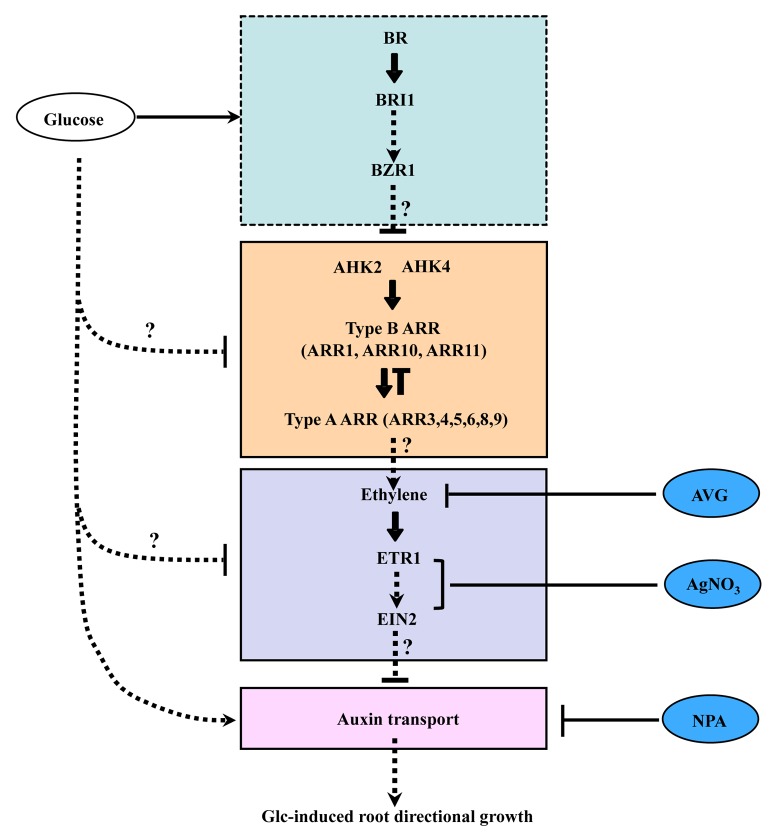
Glc enhances BR signaling to induce root deviation response by increasing BR receptor BRI1 internalization.7 Both cytokinin and ethylene signaling can antagonize this response. The antagonistic effect of cytokinin involves the two-component system which employs the AHK receptors and type A and type B ARRs (Fig. 1A and 1B). Cytokinin signaling works downstream to BR and act via ethylene biosynthesis/signaling as evident by its ability to abolish the enhanced Glc-induced root deviation response in BR treated WT seedlings as well as the enhanced Glc-induced root deviation response of bzr1–1D mutant seedlings (Fig. 2B and 2C). Ethylene biosynthesis as well as signaling components such as ETR1 and EIN2 work downstream to cytokinin for transducing the signal further (Fig. 1C, 1D and Figure 2D, 2E). Differential distribution of auxin lies downstream of all the above mentioned factors, since auxin transport inhibitor NPA can evoke the Glc-induced root deviation response even in those BR, cytokinin and ethylene mutants which otherwise show resistance/less response for Glc application in terms of root directional growth (Fig. 2F and 2G). Compiling our experimental evidences and previous findings, we propose a model for Glc-phytohormonal signal network involved in regulating root directional growth (Fig. 3). Our findings have allowed us to verify the possible BR-cytokinin and BR-ethylene interaction during root directional growth. The antagonistic nature of interaction between BR, cytokinin and ethylene might help to fine tune the optimal root directional growth for resource utilization and stability. This model will provide a foundation for testing and for discovery of additional routes available for the regulation of root directional growth.
Figure 3. A testable model based on previous and present findings. Glc regulates BR biosynthesis and BRI1 endocytosis to regulate BR signaling and modulate root directional response.7 Cytokinin signaling and ethylene signaling antagonizes BR to reduce this response and lies downstream to BR signaling cascade. Auxin transport lies downstream to Glc as Glc can regulate auxin distribution.2,7 Differential distribution of auxin lies downstream to ethylene and CK signaling since NPA-treatment to seedlings shows enhanced response even in eto2 and arr3,4,5,6,8,9 mutants which are otherwise resistant to Glc induced root deviation. Dotted arrows and question marks represent the possibility of additional routes and routes that are also consistent with the data.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was financially supported by the grant from Department of Biotechnology, Ministry of Science and Technology, Government of India (BT/PR3302/AGR/02/814/2011), NIPGR core grant and Council of Scientific and Industrial Research, India (research fellowships to A.G. and M.S.)
References
- 1.Philosoph-Hadas S, Friedman H, Meir S. Gravitropic bending and plant hormones. Vitam Horm. 2005;72:31–78. doi: 10.1016/S0083-6729(05)72002-1. [DOI] [PubMed] [Google Scholar]
- 2.Mishra BS, Singh M, Aggrawal P, Laxmi A. Glucose and auxin signaling interaction in controlling Arabidopsis thaliana seedlings root growth and development. PLoS One. 2009;4:e4502. doi: 10.1371/journal.pone.0004502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aloni R, Langhans M, Aloni E, Ullrich CI. Role of cytokinin in the regulation of root gravitropism. Planta. 2004;220:177–82. doi: 10.1007/s00425-004-1381-8. [DOI] [PubMed] [Google Scholar]
- 4.Li L, Xu J, Xu ZH, Xue HW. Brassinosteroids stimulate plant tropisms through modulation of polar auxin transport in Brassica and Arabidopsis. Plant Cell. 2005;17:2738–53. doi: 10.1105/tpc.105.034397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buer CS, Sukumar P, Muday GK. Ethylene modulates flavonoid accumulation and gravitropic responses in roots of Arabidopsis. Plant Physiol. 2006;140:1384–96. doi: 10.1104/pp.105.075671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strohm AK, Baldwin KL, Masson PH. Multiple roles for membrane-associated protein trafficking and signaling in gravitropism. Front Plant Sci. 2012;3:274. doi: 10.3389/fpls.2012.00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh M, Gupta A, Laxmi A. Glucose control of root growth direction in Arabidopsis thaliana. J Exp Bot. 2014 doi: 10.1093/jxb/eru146. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li J, Mo X, Wang J, Chen N, Fan H, Dai C, Wu P. BREVIS RADIX is involved in cytokinin-mediated inhibition of lateral root initiation in Arabidopsis. Planta. 2009;229:593–603. doi: 10.1007/s00425-008-0854-6. [DOI] [PubMed] [Google Scholar]
- 9.Vercruyssen L, Gonzalez N, Werner T, Schmülling T, Inzé D. Combining enhanced root and shoot growth reveals cross talk between pathways that control plant organ size in Arabidopsis. Plant Physiol. 2011;155:1339–52. doi: 10.1104/pp.110.167049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta A, Singh M, Jones AM, Laxmi A. Hypocotyl directional growth in Arabidopsis: a complex trait. Plant Physiol. 2012;159:1463–76. doi: 10.1104/pp.112.195776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kudryakova NV, Efimova MV, Danilova MN, Zubkova NK, Khripach VA, Kusnetsov VV, Kulaeva ON. Exogenous brassinosteroids activate cytokinin signaling pathway gene expression in transgenic Arabidopsis thaliana. Plant Growth Regul. 2013;70:61–9. doi: 10.1007/s10725-012-9778-z. [DOI] [Google Scholar]
- 12.Müssig C, Shin GH, Altmann T. Brassinosteroids promote root growth in Arabidopsis. Plant Physiol. 2003;133:1261–71. doi: 10.1104/pp.103.028662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Grauwe L, Vandenbussche F, Tietz O, Palme K, Van Der Straeten D. Auxin, ethylene and brassinosteroids: tripartite control of growth in the Arabidopsis hypocotyl. Plant Cell Physiol. 2005;46:827–36. doi: 10.1093/pcp/pci111. [DOI] [PubMed] [Google Scholar]
- 14.Gendron JM, Haque A, Gendron N, Chang T, Asami T, Wang ZY. Chemical genetic dissection of brassinosteroid-ethylene interaction. Mol Plant. 2008;1:368–79. doi: 10.1093/mp/ssn005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deslauriers SD, Larsen PB. FERONIA is a key modulator of brassinosteroid and ethylene responsiveness in Arabidopsis hypocotyls. Mol Plant. 2010;3:626–40. doi: 10.1093/mp/ssq015. [DOI] [PubMed] [Google Scholar]