Abstract
The contribution of Glutathione (GSH) in drought stress tolerance is an established fact. However, the proteins which are directly or indirectly related to the increased level of GSH in response to drought stress are yet to be known. To explore this, here, transgenic tobacco plants (NtGp11) overexpressing gamma-glutamylcysteine synthetase (γ-ECS) was tested for tolerance against drought stress. NtGp11 conferred tolerance to drought stress by increased germination rate, water retention, water recovery, chlorophyll, and proline content compared with wild-type plants. Semi-quantitative RT-PCR analysis revealed that the transcript levels of stress-responsive genes were higher in NtGp11 compared with wild-type in response to drought stress. Two-dimensional gel electrophoresis (2-DE) coupled with MALDI TOF-TOF MS/MS analysis has been used to identify 43 differentially expressed proteins in response to drought in wild-type and NtGp11 plants. The results demonstrated the up-accumulation of 58.1% of proteins among which 36%, 24%, and 20% of them were related to stress and defense, carbon metabolism and energy metabolism categories, respectively. Taken together, our results demonstrated that GSH plays an important role in combating drought stress in plants by inducing stress related genes and proteins like HSP70, chalcone synthase, glutathione peroxidase, thioredoxin peroxidase, ACC oxidase, and heme oxygenase I.
Keywords: Drought stress, GSH, mass spectrometry, transgenic Nicotiana
Introduction
Environmental stress is the major limiting factor in plant productivity. Considering agricultural perspectives, drought stress is one of the most significant factors responsible for substantial and unpredictable losses in crop production. Drought, salinity, and osmotic stresses cause adverse effects on the growth and photosynthesis, oxidative damage, hormonal changes, and the accumulation of numerous stress-related proteins. These changes are usually the result of tissue dehydration. Tissue dehydration occurs when there is an imbalance between root water uptake and leaf transpiration.1 Dehydrated plants generally start closing their stomata; however, under some environmental situations or in specific plant genotypes, modification of root water uptake capacity plays a more important role compared with stomatal closure in avoiding stress-induced growth reduction.2,3 Again, guard cell signaling is of critical importance because it is a key denominator within the plant water budget in drought stress responses.4
Drought stress induces the overproduction of reactive oxygen species (ROS) which are highly reactive and toxic, which must be minimized to protect the cell from oxidative damage. The cell organelles, particularly chloroplast and mitochondria are the major sites of ROS production in plants where excessive rate of electron flow takes place. Plant cells are well equipped to efficiently scavenge ROS and its reaction products by the coordinated and concerted action of antioxidant machinery constituted by vital enzymatic and non-enzymatic antioxidant components.5,6 Prolonged drought stress results in ROS production in plant cell which overwhelm the scavenging action of the antioxidant system resulting in extensive cellular damage and death.7 Recently, it is also reported that drought is responsible for the development of oxidative stress in plant cell which induces various associated genes.8
Cellular redox homeostasis is essential for plant growth, development as well as for the resistance to biotic and abiotic stresses.9 Glutathione is a key water-soluble antioxidant and plays a central part in ROS scavenging through the GSH-ascorbate cycle and as an electron donor to glutathione peroxidase (GPx). It is the storage form and the long-distance transport form of reduced sulfur, is involved in the detoxification of heavy metals and xenobiotics and in the regulation of the cell cycle. Previous studies have shown that GSH acts as a signaling molecule and mitigates biotic stress through non-expressor of PR genes 1 (NPR1)-dependent/independent salicylic acid (SA)-mediated pathway.10-13 In our recent investigation it was also noted that GSH may act through multistep signaling pathways to mitigate environmental stresses.14 The protective role of GSH against low temperature stress was also demonstrated by chemical or genetic manipulation of its level.15 Besides an increase in the size of the glutathione pool, a high GSH/GSSG ratio is also necessary for the efficient removal of the reactive oxygen species generated under stress conditions. The higher redox status (ascorbate, glutathione under reduced and oxidized forms) and increase in the activities of antioxidative enzymes (superoxide dismutase, catalase, ascorbate peroxidase, dehydroascorbate reductase, glutathione reductase) plays an important role in mitigating osmotic and drought stress.16,17 Keyster et al.18 also shown that exogenously applied nitric oxide improves maize tolerance to long-term salt stress by inducing elevated antioxidant enzyme activity to maintain redox homeostasis.
In plants synthesis of GSH is mainly mediated by 2 enzymes, γ-ECS [EC:6.3.2.2] and glutathione synthetase (GSH-S) [EC:6.3.2.3] in the presence of amino acids and ATP. γ-ECS and GSH-S are upregulated in peas, tobacco, maize, and tomatoes exposed to abiotic stress.15,19,20
In our previous study the transgenic tobacco lines of chloroplast targeted overexpressing γ -ECS (NtGp1 and NtGp11) were developed by applying the genetic engineering approach and enhanced GSH content was estimated from both lines. Among the transgenic tobacco lines, HPLC analysis confirmed the higher GSH content in NtGp11 than NtGp1 and better biotic stress tolerance potential of the NtGp11 line was also established.14 Therefore, in the present investigation we aim to obtain an insight into the role of GSH in mitigating abiotic stress by considering NtGp11 plants. Until now, limited data are available about the stress-elicited changes in plants with enhanced GSH content, at the proteome level. Here, proteome analysis of the wild-type and NtGp11 plant along with the identification of changes at the protein expression level in response to drought stress has been performed. The proteomic data will supply the important information on drought stress condition and provide new clues about the role of GSH in mitigating drought stress in planta.
Results
Drought and salinity stress analysis
Seeds from NtGp11 and wild-type were germinated on 1/2MS plates supplemented with different concentrations of NaCl (data not shown) and mannitol (data not shown). Results showed that in comparison to the wild-type, NtGp11 showed a significantly higher germination rate on the medium containing 200 mM NaCl and 200 mM mannitol (Fig. 1A). Hence, 200 mM concentration of NaCl and mannitol were selected for further investigation.
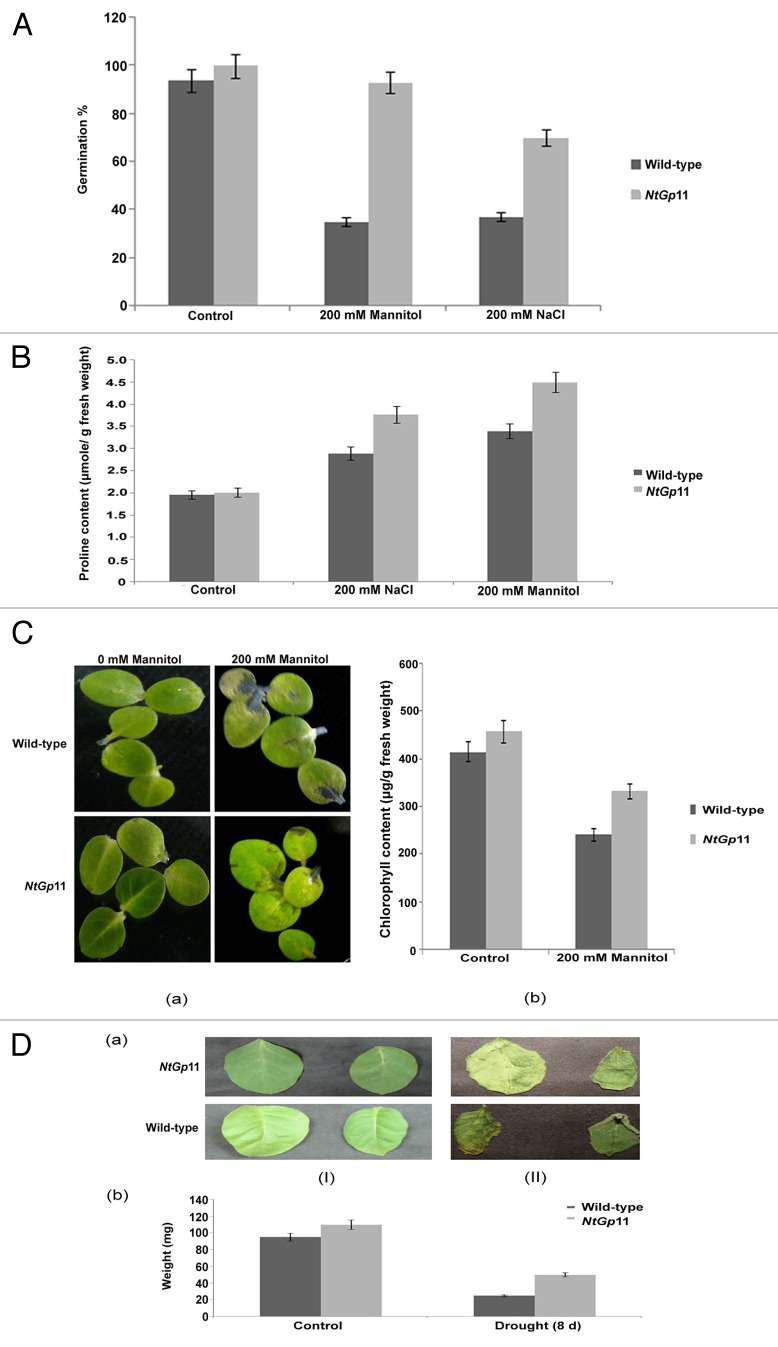
Figure 1. Drought and salinity stress analysis. (A) Germination percentage of 72 h 200 mM mannitol and NaCl treated wild-type and NtGp11 seeds. (B) Proline content of 72 h 200 mM mannitol and NaCl treated wild-type and NtGp11 plants. (C) Effect of 200 mM mannitol on (a) morphology (b) chlorophyll content of the detached leaves of wild-type and NtGp11 plants after 72 h. (D) Transpirational water loss assay of the detached leaves from both wild-type and NtGp11 plants after (I) 0 d and (II) 8 d at room temperature.
Under drought and salinity stress conditions, proline content in NtGp11 plant leaves was significantly higher than those of wild-type (Fig. 1B). The chlorophyll content was higher in 200 mM mannitol treated NtGp11 plants leaves than the wild-type plants (Fig. 1C). In addition, the rate of the loss of fresh weight from detached leaves of NtGp11 plants was lower than that of wild-type plants after 8 d in room temperature. So, it can be assumed that the rate of water loss was also lesser in NtGp11 than the wild-type plant in the above condition (Fig. 1D). To confirm further the drought tolerance of transgenic plants at the vegetative growth stage, water was withheld from the 2-mo-old wild-type and NtGp11 plants for 10 d. Results showed that after 10 d of drought treatment, wild-type plants were completely wilted, whereas NtGp11 plants were less affected. Moreover, under drought stress conditions, NtGp11 plants showed a mild increase in growth over the wild-type plants (Fig. 2B). When they were watered again after the drought treatment, the NtGp11 plants recovered faster than the wild-type plants, although many leaves did not recover completely (Fig. 2C).

Figure 2. The phenotypes of wild-type and NtGp11 plants after (A) 0 d of drought (B) 10 d of drought (C) Recovery after 3 d of water treatment.
Semiquantitative RT-PCR analysis
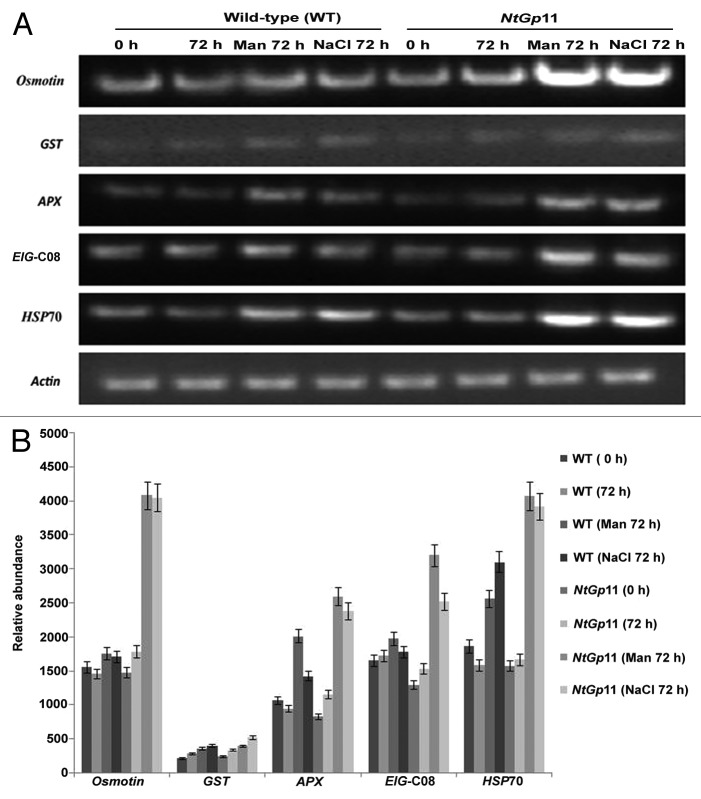
HPLC analysis further confirmed the enhanced GSH content in NtGp11 as reported earlier.14 In response to drought and salinity stress γ-ECS was found upregulated at the transcript level in NtGp11 (Fig. 3A). The expression level of all the 6 abiotic stress induced genes like osmotin, HSP70, ascorbate peroxidase (APX), glutathione-S-transferase (GST), and glutathione peroxidase (EIG-C08) were higher in NtGp11 plant than the wild-type plant in response to 72 h 200 mM mannitol and NaCl treatment (Fig. 4). This clearly illustrated the effect of enhanced GSH level on the elevated expression of abiotic stress related genes in response to drought stress in NtGp11.
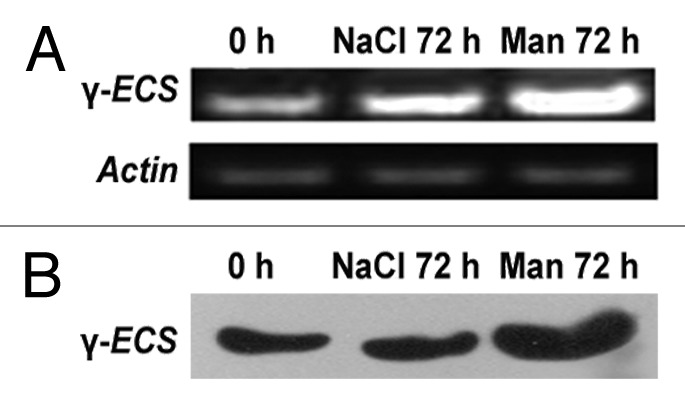
Figure 3. Expression level of γ-ECS in NtGp11 plant after 72 h of 200 mM mannitol and NaCl treatment by (A) Semi-quantitative RT-PCR (B) western blotting.
Figure 4. (A) Expression level of stress related genes in response to 200 mM mannitol and NaCl treatment in wild-type and NtGp11 plants after 72 h. (B) Densitometric study of gene expression in response to stress condition by Quantity One Software.
Western Blot analysis of γ-ECS
Here, enhanced accumulation of γ-ECS was noted in drought stress treated NtGp11 than the salinity-stress treated plants (Fig. 3B, Fig. S1) and this also corroborates with the γ-ECS expression data.
Comparative Proteomics Analysis
NtGp11 was noted with better drought tolerance potential than salinity, higher expression of γ-ECS as well as abiotic stress induced genes, so further comparative proteomic profiling was performed with NtGp11 in response to drought stress. The protein samples for 2-DE analyses were isolated from drought stress treated wild-type and NtGp11 leaf samples. The differentially accumulated spots as identified by comparative protein profiling (Fig. 5) were further identified. The number of resolved spots from drought stress treated wild-type and NtGp11 were approximately 246 and 272 respectively. 122 spots matched successfully and 144 spots were found to be altered in intensity between the drought stress treated wild-type and NtGp11. The overall mean coefficient of variation for intensity of the spots matched was determined to be 38.7. Spots showing statistically significant increase or decrease in response to stress treatment were excised from gels of the drought stress treated wild-type and NtGp11 samples were digested with trypsin and identified using MALDI TOF-TOF MS/MS. Among the 43 identified differentially accumulated protein spots, 58.13% and 23.25% spots were found up-accumulated, i.e., spot intensity in NtGp11 is higher than the wild-type (control) by 2-fold or more, and down-accumulated, i.e., spot intensity in NtGp11 is lower than wild-type (control) by 2-fold or more, respectively, in response to drought stress. 9.3% uniquely induced protein spots in response to drought stress from stress treated NtGp11 plants and 9.3% protein spots that were restricted only to wild-type plants were also identified (Fig. 6). Several proteins remained unidentified because the number and/or intensity of the fragment ions obtained by MS/MS were insufficient for a significant hit. For further confirmation on the role of GSH in mitigating drought stress, the identified differentially accumulated proteins in response to stress condition were distributed according to their functional categories and proteomics data are presented in Table 1. These proteins placed mostly into stress and defense, energy metabolism, carbon metabolism, gene and protein regulation, hypothetical and unnamed proteins categories. Among the up-accumulated proteins in response to drought stress treatment in NtGp11 about 36%, 24%, and 20% of the proteins were placed in stress and defense, carbon metabolism and energy metabolism category respectively (Fig. 7). About 50% of down-accumulated proteins against stress condition in NtGp11 were related to carbon metabolism. Among uniquely induced proteins in response to stress in NtGp11 50% and 25% of them were grouped under energy metabolism and hormonal response categories respectively (Table 1). Several spots appeared more than once which might be due to different post-translational modifications of proteins.
Figure 5. Representative 2-DE gel images of 200 mM mannitol treated wild-type (A) and NtGp11 (B) plant leaves.

Figure 6. Pie chart of differentially accumulated protein spots in response to 200 mM mannitol treatment in wild-type and NtGp11 plant after 72 h.
Table 1. MALDI TOF-TOF MSMS based identification of protein differentially expressed in wild-type (control) and NtGp11 in response to the treatment of 200 mM mannitol.
| SSP no.a (Spot no.) |
Th. Mr/pI Exp. Mr/pI b | Avg Fold change c | Protein (Taxonomy) | Accession number d | Mascot Score e |
Sequence Coverage (%) f |
|---|---|---|---|---|---|---|
| Stress and defense | ||||||
| 1801 (4) |
76.14/5.19 70.13/5.01 |
3.80 | Heat shock protein 70 (Spenacia oleracia) | gi|2654208 | 329 | 17 |
| 4604 (10) |
28.90/7.79 35.24/6.67 |
2.77 | Chalcone synthase (Camellia fascicularis) | gi|28565125 | 37 | 31 |
| 2601 (12) |
18.25/6.72 37.09/5.69 |
2.16 | Glutathione peroxidase (Hordeum vulgare) | gi|6179604 | 46 | 38 |
| 1402 (13) |
21.90/5.07 30.90/5.16 |
2.19 | Putative heat shock protein (Oryza sativa var Japonica) | gi|31432124 | 45 | 34 |
| 403 (14) |
36.80/6.11 28.40/4.76 |
2.55 | 1-Aminocyclopropane-1-carboxylic acid oxidase (Stellaria logipes) | gi|2293550 | 35 | 33 |
| 8002 (22) |
25.22/5.41 45.13/6.97 |
7.32 | Chalcone synthase (Leibnitzia anandria) | gi|1403057 | 44 | 25 |
| 201 (16) |
29.80/8.20 22.40/4.69 |
2.70 | Thioredoxin peroxidase (Nicotiana. tabacum) | gi|21912927 | 74 | 42 |
| 1104 (18) |
20.34/5.07 17.30/5.18 |
2.77 | Elicitor induciible protein EIG-J7 (Capsicum annum) | gi|40287496 | 74 | 15 |
| 1702 (39) |
11.20/6.35 38.43/5.43 |
2.41 | Heme oxygenase I (N. tabacum) | gi|18874688 | 37 | 43 |
| Energy metabolism | ||||||
|---|---|---|---|---|---|---|
| 4802 (5) |
51.47/5.12 54.41/6.05 |
4.32 | ATPase β subunit (Aristolochia gigantea) | gi|52082881 | 80 | 27 |
| 2101 (19) |
26.78/8.96 18.94/5.34 |
2.75 | Chloroplast ATP synthase delta subunit (N. tabacum) | gi|19787 | 95 | 41 |
| 3001 (21) |
14.59/5.18 15.21/5.83 |
2.75 | ATP synthase CF1epsilon chain (Atropa belladonna) | gi|28261723 | 80 | 54 |
| 8202 (23) |
14.54/6.40 23.36/ 6.92 |
3.00 | Putative beta4 proteasome subunit (N. tabacum) | gi|14594929 | 64 | 32 |
| 3804 (34) |
55.45/5.26 65.42/5.67 |
177.96 | ATP synthase CF1 α chain (A. belladonna) | gi|28261702 | 80 | 14 |
| 2503 (36) |
35.19/5.6 32.48/5.27 |
150.88 | Oxygen evolving complex 33kDa photosystem II (N. tabatum) | gi|30013657 | 41 | 28 |
| 9403 (41) |
33.09/9.23 35.54/8.30 |
0 | Allinase (Allium cepa) | gi|4512105 | 48 | 29 |
| Carbon metabolism | ||||||
|---|---|---|---|---|---|---|
| 8901 (1) |
51.95/6.41 99.00/6.88 |
2.92 | RUBISCO large subunit (A. belladonna) | gi|475728 | 371 | 53 |
| 5801 (8) |
48.35/5.06 45.08/6.18 |
3.49 | RUBISCO Activase II (Gossypium hirsutum) | gi|12620883 | 62 | 32 |
| 5701 (9) |
42.74/5.50 43.58/6.18 |
3.64 | RUBISCO Activase (A. thaliana) | gi|445628 | 248 | 43 |
| 7901 (2) |
51.95/6.41 100.86/6.67 |
2.54 | RUBISCO large subunit (A. belladonna) | gi|475728 | 467 | 53 |
| 3801 (3) |
20.28/7.57 75.60/5.75 |
2.31 | RUBISCO small subunit (N. tabacum) | gi|30013663 | 417 | 62 |
| 7801 (6) |
51.94/6.41 53.70/6.67 |
3.34 | RUBISCO large subunit (Solandra gradiflora) | gi|6093933 | 206 | 40 |
| 7802 (7) |
51.95/6.41 52.85/6.77 |
2.68 | RUBISCO large subunit (A. belladonna) | gi|475728 | 553 | 55 |
| 0004 (17) |
20.28/7.57 15.75/4.7 |
15.49 | RUBISCO small subunit (N. tabacum) | gi|30013663 | 89 | 28 |
| 2705 (11) |
44.46/5.71 40.63/5.62 |
2.24 | Phosphoribulokinase precursor (A. thaliana) | gi|23197622 | 59 | 9 |
| 4801 (37) |
14.55/5.59 76.00/6.00 |
1.56 | Chain S, Crystal structure of unactivated Tobacco RUBISCO with bound phosphate ion (N. tabacum) | gi|7546556 | 351 | 91 |
| 4102 (24) |
18.70/5.59 21.05/6.11 |
0.50 | Chlorophyll a/b- binding protein type-1 (Asarina barclaiana) | gi|7271945 | 75 | 15 |
| 5001 (25) |
19.55/5.39 15.21/6.22 |
0.05 | Photosystem I assembly protein Ycf3 (N. tabacum) | gi|11465956 | 46 | 28 |
| 5503 (27) |
42.62/8.82 32.05/6.19 |
0.38 | At1g49970/F2J10_5 (A. thaliana) | gi|23308343 | 50 | 33 |
| 6603 (30) |
37.49/8.87 34.33/6.43 |
0.44 | Putative PrMC3 (O. sativa var Japonica) | gi|51535276 | 38 | 10 |
| 1304 (33) |
28.40/5.68 26.31/5.21 |
0.11 | Light harvesting chlorophyll a/b-binding protein (N. sylvestris) | gi|3036951 | 41 | 21 |
| Gene regulation | ||||||
|---|---|---|---|---|---|---|
| 6204 (38) |
42.38/5.98 23.14/6.49 |
310.46 | Homeobox transcription factor KN3 (Populus balsamifera) | gi|55276122 | 43 | 21 |
| Hypothetical / unnamed and other protein | ||||||
|---|---|---|---|---|---|---|
| 1303 (20) |
28.29/5.48 26.31/5.11 |
2.34 | Unnamed protein product (N. tabacum) | gi|19829 | 153 | 62 |
| 5301 (26) |
12.26/10.02 24.51/6.2 |
0.49 | Hypothetical protein (Zea mays) | gi|40795123 | 39 | 56 |
| 5501 (28) |
11.48/10.03 34.04/6.17 |
0.03 | Hypothetical protein (O. sativa var Japonica) | gi|50253252 | 54 | 47 |
| 6501 (29) |
23.20/5.07 32.00/6.5 |
0.31 | Hypothetical protein (A. thaliana) | gi|2829909 | 46 | 36 |
| 7503 (31) |
34.14/8.53 33.07/6.8 |
0.36 | Hypothetical protein(O. sativa varjaponica) | gi|56202236 | 38 | 26 |
| 8602 (32) |
11.02/4.68 34.60/6.94 |
0.05 | Hypothetical protein (Pennisetum glaucum) | gi|33321026 | 43 | 41 |
| 6505 (42) |
28.34/8.75 32.08/6.54 |
0.50 | P0004D12.20 (O. sativa var Japonica) | gi|34906958 | 45 | 23 |
| 5805 (35) |
50.32/5.72 54.90/6.18 |
1595.7 | ARF2 (A. thaliana) | gi|12484201 | 37 | 14 |
| 302 (15) |
29.60/4.75 26.40/3.65 |
2.56 | 29kD Ribonucleoprotein (N. sylvestris) | gi|19754 | 153 | 58 |
| 8601 (43) |
80.20/10.59 36.01/6.91 |
0 | Hypothetical protein (O. sativa varjaponica) | gi|56784271 | 41 | 56 |
| 9703 (44) |
13.67/10.57 42.55/9.21 |
0 | Hypothetical protein (O. sativa varjaponica) | gi|50944661 | 44 | 38 |
Identified differentially accumulated proteins in wild-type and NtGp11 plants in response to 200 mM mannitol were categorized in stress and defense, energy metabolism, carbon metabolism, and hypothetical/ unnamed and other protein groups. aAssigned sample protein and spot number as indicated in Figure 5. bTh Mr/pI, theoretical mass of protein in kDa and pI; Exp Mr/pI, experimental mass of protein in kDa and pI. cAverage fold change represents the ratio of change of spot intensity in comparison to the wild-type. dNCBI accession number of identified protein spots. eStatistical probability of true positive identification of predicted proteins calculated by MASCOT (http://www.matrixscience.com). fSequence coverage%: percentage of predicted protein sequence covered by matched peptides.
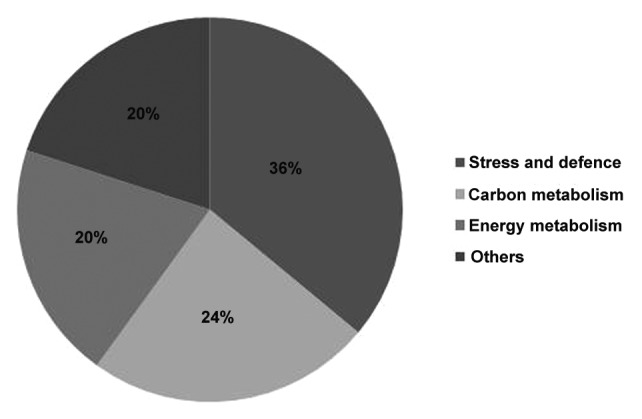
Figure 7. Functional categorization of identified up-accumulated proteins in response to 200 mM mannitol treatment in NtGp11 after 72 h.
Gene Expression analysis by semi-quantitative RT-PCR
The proteomic profiling data obtained from comparative proteomic analysis was confirmed through semi-quantitative RT-PCR analysis for 6 stress and defense related genes viz. HSP70, glutathione peroxidase, thioredoxin peroxidase, chalcone synthase, ACC oxidase, and heme oxygenase I. Results indicated that the enhanced expression of these genes were in accordance with proteomic result (Fig. 8), thus supporting the comparative proteomic profile data.
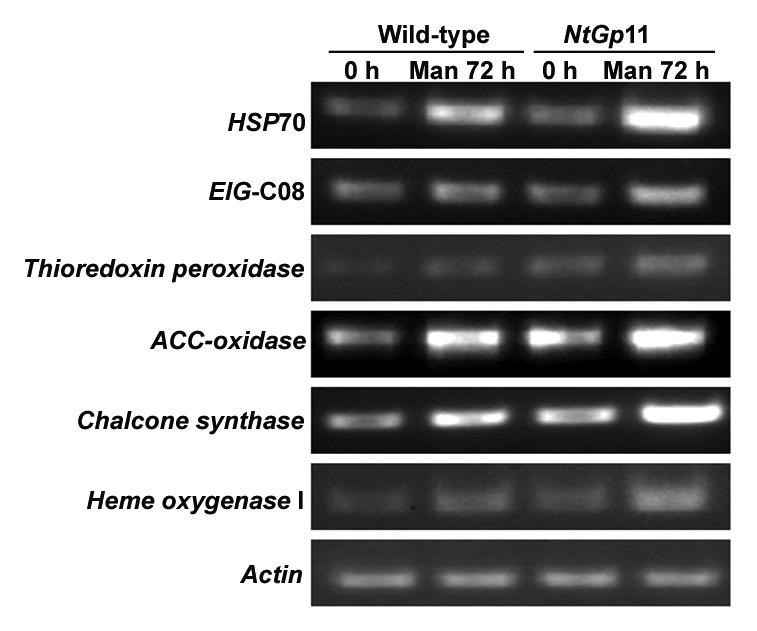
Figure 8. Accumulation of genes viz. HSP70, EIG-C08, thioredoxin peroxidase, ACC oxidase, chalcone synthase and heme oxygenase I after 72 h mannitol treatment in wild-type and NtGp11 plant.
Discussion
In plant cells reduced GSH is the most abundant source of non-protein thiols, which plays major role in various plant functions like cell differentiation, cell death, pathogen resistance, formation of phytochelatins, detoxification of xenobiotics etc. Out of stable chloroplast-targeted γ-ECS overexpressed transgenic tobacco lines, NtGp11 demonstrated better potential in GSH content; stress-responsive transcripts/proteins accumulation as well as disease-resistance capacity14 and hence was further evaluated here to assess the abiotic stress tolerance prospective.
In this study, seed germination, drought assay and higher expression level of stress related genes in response to drought stress than the salinity stress depicted that NtGp11 plants was more resistant to drought stress in comparison to salinity stress. To study the effect of drought and salinity stress induction at preliminary transcript level, the expression of few well known stress related genes have been analyzed after 72 h of stress treatment and compared with that of 0 min wild-type, i.e., basal expression level. Expression level of osmotin, APX, HSP70, GST, and EIG-C08 were higher in NtGp11 than wild-type plant after 72 h of stress treatment. The expression pattern of these stress related genes were similar to the drought tolerant plants as reported earlier.21-24
Among the up-accumulated proteins in response to drought stress in NtGp11 spot no. 4 and 13 had identified to HSP70 like proteins and spot no. 12 identified to glutathione peroxidase which also showed correlation with its gene expression in response to drought stress. HSP70 is stress-inducible genes respond to environmental abiotic stresses such as high-/low-temperature, drought and high salinity.25-27 The sHSPs play a major role in decreasing the intracellular level of reactive oxygen species (ROS), thereby protecting PSII function during stress.28 Spot no. 16 had identified to thioredoxin peroxidase which together with glutathione peroxidase might play dual and distinctive roles in ROS homeostasis, acting as a general scavenger and specifically relaying the ROS signal as an oxidative signal transducer in drought stress signaling.29,30 Spot no. 10 was identified as chalcone synthase which is a key enzyme of the flavonoid/isoflavonoid biosynthesis pathway and flavonoids act as an antioxidant which helps in neutralizing the effect of overproduced ROS in plant cell after drought stress.31,32 Spot no. 18 was identified as elicitor inducible protein, EIG-J7 which was reported to be expressed in response to a variety of stress conditions, including wounding, drought stress, viral infection and SA treatment.33,34 Spot no. 14 had the homology with ACC oxidase which is an important enzyme of ethylene biosynthetic pathway and it can be induced by stress.14,35 Spot no. 39 had identified to heme oxygenase I which earlier reported to play protective role in plants against oxidative stress.36
Spot no. 5, 19, 21, and 23 had the homology with ATPase β subunit, Chloroplast ATP Synthase (Delta subunit), ATP Synthase CF1 epsilon subunit and putative β -4 proteasome subunit respectively which is also supported by literature.37 Among the uniquely induced proteins in response to drought in NtGp11, spot no. 34 and 36 had identified to ATP Synthase CF1 α chain and Oxygen evolving complex photosystem II proteins. ATP synthase CF1 generally takes part in ATP biosynthetic processes and earlier it was found up-accumulated in response to drought stress in drought-tolerant sugarcane cultivars.38 It is a well-known fact that induction of stress tolerance is cost-intensive and stress tolerant plants accelerates its energy production which will eventually be utilized for generating defense-related metabolites.
Spot no. 8 and 9 had the homology with Rubisco activase which can be concluded that an altered expression of Rubisco activase might be crucial for continued CO2 fixation under drought stress39 protecting the plant photosynthetic capacity. The activation of complex metabolic pathways within the cells, including antioxidative pathways (especially ROS-scavenging systems) is required in plant cell that can contribute to continue growth under drought condition.8 Spot no. 1, 2, 6, and 7 were also found up-accumulated in response to drought stress in NtGp11 and showed homology with Rubisco large subunit protein. Previously similar pattern of the expression of Rubisco large subunit was reported in drought-tolerant varieties of wheat.40 The result could be due to the water loss in leaves subjected to drought causing an increase per fresh weight.
Among the down-accumulated proteins in NtGp11 in response to drought, spot no. 24, 25, and 33 were identified as chlorophyll a/b binding protein type I, Photosystem I assembly protein Ycf3, and light harvesting chlorophyll a/b binding protein respectively. Several studies have postulated that the these genes were downregulated in drought tolerant plants in response to stress.41,42 Downregulation of these proteins depicted that there has been a reduction in photosynthetic rate after stress treatment which might indicated that the NtGp11 plant curtailed some of its energy expenditure from normal metabolism which was utilized for the development of resistance against drought stress.
Spot no. 22 and 38 were identified as maturase and homeobox transcription factor KN3. Changes in expression level of these proteins indicated that the development of tolerance against abiotic stress require several changes at gene and protein regulation level.
These results clearly suggested that γ-ECS was upregulated at the transcription level by drought and salinity stress. Overexpression of γ-ECS resulted in enhanced GSH content in NtGp11 plants which ultimately enhanced tolerance to drought stress by enhancing germination rate, water recovery rate, chlorophyll, and proline content compare with wild-type plant, which demonstrated that GSH may be a positive regulator of drought tolerance. Taken together, we propose role of GSH in mitigating drought stress. Drought stress strongly induced γ-ECS activity in NtGp11, helped in enhancing GSH content in plant cell which in turn upregulated the activities of stress and defense, energy metabolism, carbon metabolism, gene regulatory proteins and enzymes which ultimately helped in minimizing the effect of drought in NtGp11 plant. However, many further studies are necessary to identify the direct GSH targets in response to drought stress.
Materials and Methods
Plant growth
The seeds of both wild-type and T2 generation of NtGp11 were surface sterilized and inoculated under white light in a 16 h light/8 h dark cycle at 22 °C in 1/2 Murashige-Skoog (1/2MS) media.43 For drought and salinity stress treatment 2 wk old seedlings were further transferred to 1/2MS media supplemented with 100, 150, and 200 mM mannitol and NaCl for an additional 72 h and the leaves were collected for biochemical analysis (proline and chlorophyll estimation), RNA, and protein isolation.
Drought and salinity stress analysis
The germination percentage of both wild-type and NtGp11 were measured daily after sowing it in ½ MS medium with different concentrations of mannitol and NaCl (data not shown). Proline content was measured in 500 mg wild-type and NtGp11 leaf tissue treated with 200 mM of mannitol and NaCl after 72 h according to Shan et al.44 In addition, leaf discs, ≈1 cm in diameter, were detached from healthy, fully expanded leaves of wild-type and NtGp11 of the same age. The discs were floated in solutions of 200 mM of mannitol for 72 h and then 100 mg of leaf tissue was immersed in 80% acetone for 48 h to extract the chlorophyll. Chlorophylls a and b were then subjected to spectrophotometric measurement. For the transpirational water loss assay, leaves (≈1.5 cm diameter) of wild-type and NtGp11 plants were detached and placed on an electronic balance at room temperature, and the change in fresh weight was recorded after 8 d. The rate of water loss was assumed to be equivalent to the loss of fresh weight of the samples. Additionally, water was withheld completely from 10-wk-old wild-type and NtGp11 plants sown in soil for 10 d, after which the plants were watered for 2 d to allow them to recover, and the water recovery was recorded. All the drought and salinity stress analyses were performed in replicates of 3.
Transcript analysis under stress condition
Total RNA was isolated using Trizol reagent (Invitrogen, USA) from the leaves of 2 wk old seedlings treated with 200 mM mannitol and NaCl for an additional 72 h. The first-strand cDNA was synthesized from total RNA using RevertAid H Minus First Strand cDNA Synthesis kit (Fermentas, USA) with oligo(dT) 18 primer. Semiquantitative RT-PCR was performed using 1 µg total RNA isolated from wild-type and NtGp11 plants. PCR amplification was performed for different stress related genes (Table 2) at different cycles of 94 °C for 30s, 30s at varying annealing temperatures for different genes, 72 °C for 1 min, with an initial denaturation at 94 °C for 1 min. Actin was used as a loading control. The PCR products were analyzed by 1% agarose gel electrophoresis. The relative mRNA expression for each transcript in respective gel was done by densitometric analysis using Quantity-One software (BioRad, USA). The expression of each gene was confirmed in at least 3 rounds of independent semi-quantitative RT-PCR reactions from the RNA isolated from leaf tissue of 3 biological replicates of independent stress treated wild-type and NtGp11 plants.
Table 2. List of primers used in the semi-quatitative RT-PCR.
| Genes | Forward Primer (5′- 3′) | Reverse Primer (5′-3′) | Amplicon Tm (°C) Size (bp) |
|---|---|---|---|
| Osmotin | ATGGGCAACTTGAGATCTTCTTT | CTACTTAGCCACTTCATCACTTC | 724 64 |
| HSP70 | ATGGCCGGAAAAGGAGAAG | AGTAAGGAGGGACACATCC | 681 62 |
| APX | ATGGGTAAGTGCTATCCCAC | AGCCTTGTCTGAAGGCAACT | 686 64 |
| EIG-C08 | ATGGCCAGCCAATCTAGCAAG | TCTTGATATCCTTCTCCATGCTA | 490 64 |
| GST | ATGGCGATCAAAGTCCATGG | TTATTTTTGCAGCTTCTCCAATC | 640 63 |
| γ ECS | AATATTTCTTCACGGAATTCCTC | AAGAGAGCTGTAGCAATAGGC | 700 64 |
| Thioredoxin peroxidase | ATGGCTTGCTCTGCTTCTTCTA | CCTGAAGAGTTCTCAATGTTTC | 700 62 |
| ACC-Oxidase | ATGGAGAACTTCCCAATTATCA | GCATCACACTCTTGTATTTCCC | 700 60 |
| Chalcone synthase | ATGGTGACCGTCGAGGAATTTC | CTAAGTAGCAACACTGTGGAGA | 700 64 |
| Heme oxygenase I | ATGGCTTCAATAACACCCTTAT | GAGAAAGGTCACCGTCCCATTT | 700 60 |
| Actin | ATGGCAGACGGTGAGGATATTCA | TGGCGCAACACGAAGTTCGTT | 300 64 |
Validation for the expression of γ-ECS by western blotting
Proteins were extracted after homogenizing leaves of 72 h 200 mM mannitol and 200 mM NaCl treated NtGp11 in 50 mM potassium phosphate buffer, pH 7.8, containing 0.15% (v/v) Triton X-100 at 0 °C. Protein samples were quantified by Bradford assay,45 using BSA as standard, resolved in 12% SDS-PAGE gels and transferred onto polyvinylidene difluoride membrane (Millipore, USA), blocked with 5% skimmed milk and γ-ECS protein bands were detected by using a rabbit polyclonal antibody raised against maize γ -ECS (Agrisera, Sweden) as the primary antibody and an anti-rabbit IgG conjugated to horseradish peroxidase (Sigma-Aldrich, USA) as the secondary antibody. Immunoreactive proteins were visualized using the SuperSignal West Pico (Pierce, USA) chemiluminescent reagent.
Isolation of total protein and 2-D electrophoresis from the protein isolated from drought stress treated plants
For the isolation of total proteins, leaves were collected from 2-wk-old seedlings of wild-type and NtGp11 plants after 72 h of 200 mM mannitol treatment. The collected leaf samples were dried and weighed. Protein was extracted using the phenol extraction procedure46,47 with minor modifications. Approximately 3 g of leaf tissue was ground in liquid nitrogen with PVPP (50 mg/g fresh weight) and suspended in extraction buffer (700 mM Sucrose,500 mM Tris–HCl, pH 7.5, 50 mM EDTA, 100 mM KCl, 2% (w/v) β-mercaptoethanol, and 1 mM PMSF), and an equal volume of ice-cold Tris–HCl, pH 7.5 saturated phenol was added. The mixture was shaken at 4 °C for 30 min. After centrifugation (5000 g, 4 °C, 30 min) the phenol phase was collected. This phenolic phase was extracted 3 times with the extraction buffer. Protein was precipitated from the finally collected phenol phase by adding 5 volumes of 100 mM ammonium acetate in methanol and overnight incubation at −20 °C. The protein pellets were rinsed twice with ice-cold methanol and acetone. The pellets were dried and dissolved in IEF resuspension buffer (9 M Urea, 4% (w/v) CHAPS, 0.5% (v/v) Triton X-100, 20 mM dithiothreitol (DTT) and 1% (w/v) Bio-Lyte (3/10) ampholyte (Bio-Rad Laboratories, Hercules, CA, USA). Proteins were quantified by the standard Bradford procedure45 using BSA as standard. The sample was diluted with the IEF resuspension buffer, and 1500 μg of total protein was loaded in 315 μl onto a 18-cm IPG strip (pH 4–7; Bio-Rad Laboratories) for passive rehydration. Programmed IEF was performed with the Protean IEF cell (Bio-Rad Laboratories) using the following conditions: 250 V for 15 min, 4000 V for 7 h, and 500 V for 5 min. Prior to second dimension migration, the strip was equilibrated twice, in Equilibration Buffers I and II respectively (Bio-Rad Laboratories), for 15 min each. The second dimension SDS-PAGE was performed with 12% resolving gels and 3.9% stacking gels (75 × 83 × 1.5 mm). After running for 35 min at 200 V, gels were stained with colloidal Coomassie brilliant blue G-250.
Image acquisition and analysis
The gel images were acquired using Versa-Doc image system (Bio-Rad Laboratories) and analyzed with PD Quest software version 8.0.1 (Bio-Rad Laboratories). A total of 2 2-DE gels resulting the mannitol treated wild-type and NtGp11 samples were analyzed.
Quantitative analyses were performed after normalizing the spot quantities. The normalized spot volumes (individual spot intensity/normalization factor) calculated for each gel based on the total quantity of valid spots were determined and used for statistical calculation for protein expression levels. Quantitative comparisons between the 2 sample sets were performed taking only into account statistically significant spots (P < 0.05), and a 2-fold increase or decrease was considered as a threshold for up-accumulation or down-accumulation, respectively. Qualitative comparisons were also done, in order to detect “on–off” protein differential expression and these spots will be referred there after as arising de-novo, present only in the treated sample. Automatic spot detection and matching was followed by a manual correction. Experimental molecular weights and iso-electric points were calibrated according to Bio-Rad standard 2-DE PAGE marker. Triplicate gels using independent protein preparations were analyzed for stress treated wild-type and NtGp11. The Student t-test (P < 0.05) was performed for statistical analysis.
In-gel digestion and mass spectrometric analysis
Selected spots from 2-DE gels were excised manually and digested with trypsin (in-gel trypsin digestion kit, Pierce, USA) following the manufacturer's protocol. The sample was desalted using Zip-Tip μ-C18 (Millipore, Billerica, MA USA), and analyzed using a 4800 MALDI TOF/TOF analyzer (Applied Biosystems, Foster City, CA USA). The samples were dissolved in a solvent consisting of 0.1% trifluoroacetate and 50% acetonitrile (ACN) in MilliQ Water. Then, 0.5 μl of sample solution was mixed with 0.5 μl of matrix solution (1mg/ml α-cyano-4-hydroxycinnamic acid dissolved in the aforementioned solvent), applied to a 384-MALDI sample target plate, and dried in air. Peptides were evaporated with a ND:YAG laser at 355 nm, using a delayed extraction approach. They were accelerated with 25 kV injection pulse for TOF analysis. Each spectrum was the cumulative average of 1000 laser shots. The MS/MS spectrum was collected in MS/MS 1 kV positive reflectron mode with fragments generated by post source decay (PSD). The MS/MS mass tolerance was set to ± 20 ppm. After processing, 10 MS/MS precursors were selected (Minimum signal to noise ratio-50). Before each analysis, the instrument was calibrated with the Applied Biosystems 4700 Proteomics Analyzer Calibration Mixture. Data interpretation was performed using the GPS Explorer Software (Applied Biosystems), and an automated database search was performed using the MASCOT program (Matrix Science Ltd, London, UK).
Validation of comparative proteomics analysis by semi-quantitative RT-PCR
In an independent experiment, total RNAs was isolated from leaf tissue of 3 biological replicates of wild-type and NtGp11 plants treated with 200 mM mannitol as mentioned previously. The differential expression of 6 selected genes viz. HSP70, glutathione peroxidase, thioredoxin peroxidase, ACC oxidase, chalcone synthase, and heme oxygenase I identified as being differentially accumulated in protein data were validated by applying semi-quantitative RT-PCR.
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by the Council of Scientific and Industrial Research (CSIR), New Delhi, India. We acknowledge the help and support provided by Dr Maitreyee Banerjee and the staffs of Greenhouse, West Bengal State Council of Science and Technology, Salt Lake, Kolkata, India. Research activities by Deepak Kumar, Ragini Sinha, Riddhi Dattta, and Aparupa Ghosh have been supported by fellowships from CSIR, Indian Council of Medical Research (ICMR), and Department of Science and Technology (DST), New Delhi, India, respectively.
References
- 1.Aroca R, Tognoni F, Irigoyen JJ, Sanchez-Diaz M, Pardossi A. Different root low temperature response of two maize genotypes differing in chilling sensitivity. Plant Physiol Biochem. 2001;39:1067–73. doi: 10.1016/S0981-9428(01)01335-3. [DOI] [Google Scholar]
- 2.Matsuo N, Ozawa K, Mochizuki T. Genotypic differences in root hydraulic conductance of rice (Oryza sativa L.) in response to water regimes. Plant Soil. 2009;316:25–34. doi: 10.1007/s11104-008-9755-5. [DOI] [Google Scholar]
- 3.Xu Z, Zhou G, Shimizu H. Plant responses to drought and rewatering. Plant Signal Behav. 2010;5:649–54. doi: 10.4161/psb.5.6.11398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu JK. Salt and drought stress signal transduction in plants. Annu Rev Plant Biol. 2002;53:247–73. doi: 10.1146/annurev.arplant.53.091401.143329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gill SS, Anjum NA, Hasanuzzaman M, Gill R, Trivedi DK, Ahmad I, Pereira E, Tuteja N. Glutathione and glutathione reductase: a boon in disguise for plant abiotic stress defense operations. Plant Physiol Biochem. 2013;70:204–12. doi: 10.1016/j.plaphy.2013.05.032. [DOI] [PubMed] [Google Scholar]
- 6.Mittler R, Vanderauwera S, Gollery M, Van Breusegem F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004;9:490–8. doi: 10.1016/j.tplants.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 7.Cruz de Carvalho MH. Drought stress and reactive oxygen species: Production, scavenging and signaling. Plant Signal Behav. 2008;3:156–65. doi: 10.4161/psb.3.3.5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noctor G, Mhamdi A, Foyer CH. The roles of reactive oxygen metabolism in drought: not so cut and dried. Plant Physiol. 2014;164:1636–48. doi: 10.1104/pp.113.233478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang X, Ma Y, Huang C, Li J, Wan Q, Bi Y. Involvement of glucose-6-phosphate dehydrogenase in reduced glutathione maintenance and hydrogen peroxide signal under salt stress. Plant Signal Behav. 2008;3:394–5. doi: 10.4161/psb.3.6.5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghanta S, Bhattacharyya D, Sinha R, Banerjee A, Chattopadhyay S. Nicotiana tabacum overexpressing γ-ECS exhibits biotic stress tolerance likely through NPR1-dependent salicylic acid-mediated pathway. Planta. 2011;233:895–910. doi: 10.1007/s00425-011-1349-4. a. [DOI] [PubMed] [Google Scholar]
- 11.Ghanta S, Bhattacharyya D, Chattopadhyay S. Glutathione signaling acts through NPR1-dependent SA-mediated pathway to mitigate biotic stress. Plant Signal Behav. 2011;6:607–9. doi: 10.4161/psb.6.4.15402. b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghanta S, Chattopadhyay S. Glutathione as a signaling molecule: another challenge to pathogens. Plant Signal Behav. 2011;6:783–8. doi: 10.4161/psb.6.6.15147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han Y, Chaouch S, Mhamdi A, Queval G, Zechmann B, Noctor G. Functional analysis of Arabidopsis mutants points to novel roles for glutathione in coupling H(2)O(2) to activation of salicylic acid accumulation and signaling. Antioxid Redox Signal. 2013;18:2106–21. doi: 10.1089/ars.2012.5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghanta S, Datta R, Bhattacharyya D, Sinha R, Kumar D, Hazra S, et al. Multistep involvement of glutathione with salicylic acid and ethylene to combat environmental stress. J Plant Physiol. 2014 doi: 10.1016/j.jplph.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Kocsy G, von Ballmoos P, Rüegsegger A, Szalai G, Galiba G, Brunold C. Increasing the glutathione content in a chilling-sensitive maize genotype using safeners increased protection against chilling-induced injury. Plant Physiol. 2001;127:1147–56. doi: 10.1104/pp.010107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morabito D, Guerrier G. The free oxygen radical scavenging enzymes and redox status in roots and leaves of Populus x Euramericana in response to osmotic stress, desiccation and rehydration. J Plant Physiol. 2000;157:74–80. doi: 10.1016/S0176-1617(00)80138-8. [DOI] [Google Scholar]
- 17.Pyngrope S, Bhoomika K, Dubey RS. Reactive oxygen species, ascorbate-glutathione pool, and enzymes of their metabolism in drought-sensitive and tolerant indica rice (Oryza sativa L.) seedlings subjected to progressing levels of water deficit. Protoplasma. 2013;250:585–600. doi: 10.1007/s00709-012-0444-0. [DOI] [PubMed] [Google Scholar]
- 18.Keyster M, Klein A, Ludidi N. Caspase-like enzymatic activity and the ascorbate-glutathione cycle participate in salt stress tolerance of maize conferred by exogenously applied nitric oxide. Plant Signal Behav. 2012;7:349–60. doi: 10.4161/psb.18967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rennenberg H, Steinkamp R, Kesselmeier J. 5-oxo-prolinase in Nicotiana tabacum: catalytic properties and subcellular localization. Physiol Plant. 1981;62:211–6. doi: 10.1111/j.1399-3054.1981.tb08496.x. [DOI] [Google Scholar]
- 20.Chen J, Goldsbrough PB. Increased activity of γ-glutamylcysteine synthetase in tomato cells selected for cadmium tolerance. Plant Physiol. 1994;106:233–9. doi: 10.1104/pp.106.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goel D, Singh AK, Yadav V, Babbar SB, Bansal KC. Overexpression of osmotin gene confers tolerance to salt and drought stresses in transgenic tomato (Solanum lycopersicum L.) Protoplasma. 2010;245:133–41. doi: 10.1007/s00709-010-0158-0. [DOI] [PubMed] [Google Scholar]
- 22.Faize M, Burgos L, Faize L, Piqueras A, Nicolas E, Barba-Espin G, Clemente-Moreno MJ, Alcobendas R, Artlip T, Hernandez JA. Involvement of cytosolic ascorbate peroxidase and Cu/Zn-superoxide dismutase for improved tolerance against drought stress. J Exp Bot. 2011;62:2599–613. doi: 10.1093/jxb/erq432. [DOI] [PubMed] [Google Scholar]
- 23.Scarpeci TE, Zanor MI, Valle EM. Investigating the role of plant heat shock proteins during oxidative stress. Plant Signal Behav. 2008;3:856–7. doi: 10.4161/psb.3.10.6021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le Martret B, Poage M, Shiel K, Nugent GD, Dix PJ. Tobacco chloroplast transformants expressing genes encoding dehydroascorbate reductase, glutathione reductase, and glutathione-S-transferase, exhibit altered anti-oxidant metabolism and improved abiotic stress tolerance. Plant Biotechnol J. 2011;9:661–73. doi: 10.1111/j.1467-7652.2011.00611.x. [DOI] [PubMed] [Google Scholar]
- 25.Cho EK, Hong CB. Over-expression of tobacco NtHSP70-1 contributes to drought-stress tolerance in plants. Plant Cell Rep. 2006;25:349–58. doi: 10.1007/s00299-005-0093-2. [DOI] [PubMed] [Google Scholar]
- 26.Sung DY, Guy CL. Physiological and molecular assessment of altered expression of Hsc70-1 in Arabidopsis. Evidence for pleiotropic consequences. Plant Physiol. 2003;132:979–87. doi: 10.1104/pp.102.019398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sung DY, Vierling E, Guy CL. Comprehensive expression profile analysis of the Arabidopsis Hsp70 gene family. Plant Physiol. 2001;126:789–800. doi: 10.1104/pp.126.2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heckathorn SA, Ryan SL, Baylis JA, Wang DF, Hamilton EW, Cundiff L, et al. In vivo evidence from an Agrostis stolonifera selection genotype that chloroplast small heat-shock proteins can protect photosystem II during heat stress. Funct Plant Biol. 2002;29:935–46. doi: 10.1071/PP01191. [DOI] [PubMed] [Google Scholar]
- 29.Miao Y, Lv D, Wang P, Wang XC, Chen J, Miao C, Song CP. An Arabidopsis glutathione peroxidase functions as both a redox transducer and a scavenger in abscisic acid and drought stress responses. Plant Cell. 2006;18:2749–66. doi: 10.1105/tpc.106.044230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun L, Ren H, Liu R, Li B, Wu T, Sun F, Liu H, Wang X, Dong H. An h-type thioredoxin functions in tobacco defense responses to two species of viruses and an abiotic oxidative stress. Mol Plant Microbe Interact. 2010;23:1470–85. doi: 10.1094/MPMI-01-10-0029. [DOI] [PubMed] [Google Scholar]
- 31.Dao TT, Linthorst HJ, Verpoorte R. Chalcone synthase and its functions in plant resistance. Phytochem Rev. 2011;10:397–412. doi: 10.1007/s11101-011-9211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hernández I, Alegre L, Munné-Bosch S. Drought-induced changes in flavonoids and other low molecular weight antioxidants in Cistus clusii grown under Mediterranean field conditions. Tree Physiol. 2004;24:1303–11. doi: 10.1093/treephys/24.11.1303. [DOI] [PubMed] [Google Scholar]
- 33.Chaves I, Pinheiro C, Paiva JA, Planchon S, Sergeant K, Renaut J, Graça JA, Costa G, Coelho AV, Ricardo CP. Proteomic evaluation of wound-healing processes in potato (Solanum tuberosum L.) tuber tissue. Proteomics. 2009;9:4154–75. doi: 10.1002/pmic.200700649. [DOI] [PubMed] [Google Scholar]
- 34.Takemoto D, Doke N, Kawakita K. Characterization of Elicitor-inducible Tobacco Genes Isolated by Differential Hybridization. J Gen Plant Pathol. 2001;67:89–96. doi: 10.1007/PL00013005. [DOI] [Google Scholar]
- 35.Zanetti ME, Terrile MC, Arce D, Godoy AV, Segundo BS, Casalongué C. Isolation and characterization of a potato cDNA corresponding to a 1-aminocyclopropane-1-carboxylate (ACC) oxidase gene differentially activated by stress. J Exp Bot. 2002;53:2455–7. doi: 10.1093/jxb/. [DOI] [PubMed] [Google Scholar]
- 36.Balestrasse KB, Noriega GO, Batlle A, Tomaro ML. Heme oxygenase activity and oxidative stress signaling in soybean leaves. Plant Sci. 2006;170:339–46. doi: 10.1016/j.plantsci.2005.09.001. [DOI] [Google Scholar]
- 37.Van Der Straeten D, Chaerle L, Sharkov G, Lambers H, Van Montagu M. Salicylic acid enhances the activity of the alternative pathway of respiration in tobacco leaves and induces thermogenicity. Planta. 1995;196:412–9. doi: 10.1007/BF00203637. [DOI] [Google Scholar]
- 38.Jangpromma N, Kitthaisong S, Lomthaisong K, Daduang S, Jaisil P, Thammasirirak S. A proteomics analysis of drought stress-responsive proteins as biomarker for drought-tolerant sugarcane cultivars. Am J Biochem Biotechnol. 2010;6:89–102. doi: 10.3844/ajbbsp.2010.89.102. [DOI] [Google Scholar]
- 39.Law RD, Crafts-Brandner SJ. High temperature stress increases the expression of wheat leaf ribulose-1,5-bisphosphate carboxylase/oxygenase activase protein. Arch Biochem Biophys. 2001;386:261–7. doi: 10.1006/abbi.2000.2225. [DOI] [PubMed] [Google Scholar]
- 40.Demirevska K, Zasheva D, Dimitrov R, Simova-Stoilova L, Stamenova M, Feller U. Drought stress effects on Rubisco in wheat: changes in the Rubisco large subunit. Acta Physiol Plant. 2009;31:1129–38. doi: 10.1007/s11738-009-0331-2. [DOI] [Google Scholar]
- 41.Guo P, Baum M, Grando S, Ceccarelli S, Bai G, Li R, von Korff M, Varshney RK, Graner A, Valkoun J. Differentially expressed genes between drought-tolerant and drought-sensitive barley genotypes in response to drought stress during the reproductive stage. J Exp Bot. 2009;60:3531–44. doi: 10.1093/jxb/erp194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hazen SP, Pathan MS, Sanchez A, Baxter I, Dunn M, Estes B, Chang HS, Zhu T, Kreps JA, Nguyen HT. Expression profiling of rice segregating for drought tolerance QTLs using a rice genome array. Funct Integr Genomics. 2005;5:104–16. doi: 10.1007/s10142-004-0126-x. [DOI] [PubMed] [Google Scholar]
- 43.Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–97. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- 44.Shan DP, Huang JG, Yang YT, Guo YH, Wu CA, Yang GD, Gao Z, Zheng CC. Cotton GhDREB1 increases plant tolerance to low temperature and is negatively regulated by gibberellic acid. New Phytol. 2007;176:70–81. doi: 10.1111/j.1469-8137.2007.02160.x. [DOI] [PubMed] [Google Scholar]
- 45.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 46.Isaacson T, Damasceno CMB, Saravanan RS, He Y, Catalá C, Saladié M, Rose JK. Sample extraction techniques for enhanced proteomic analysis of plant tissues. Nat Protoc. 2006;1:769–74. doi: 10.1038/nprot.2006.102. [DOI] [PubMed] [Google Scholar]
- 47.Sinha R, Chattopadhyay S. Changes in the leaf proteome profile of Mentha arvensis in response to Alternaria alternata infection. J Proteomics. 2011;74:327–36. doi: 10.1016/j.jprot.2010.11.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.