Abstract
In plants, the actin cytoskeleton is a prime regulator of cell polarity, growth, and cytoplasmic streaming. Tip growth, as observed in root hairs, caulonema, and pollen tubes, is governed by many factors, including calcium gradients, exocytosis and endocytosis, reactive oxygen species, and the cytoskeleton. Several studies indicate that the polymerization of G-actin into F-actin also contributes to tip growth. The structure and function of F-actin within the apical dome is variable, ranging from a dense meshwork to sparse single filaments. The presence of multiple F-actin structures in the elongating apices of tip-growing cells suggests that this cytoskeletal array is tightly regulated. We recently reported that sublethal concentrations of fluorescently labeled cytochalasin could be used to visualize the distribution of microfilament plus ends using fluorescence microscopy, and found that the tip region of the growing root hair cells of a legume plant exhibits a clear response to the nodulation factors secreted by Rhizobium.1 In this current work, we expanded our analysis using confocal microscopy and demonstrated the existence of highly dynamic fluorescent foci along Arabidopsis root hair cells. Furthermore, we show that the strongest fluorescence signal accumulates in the tip dome of the growing root hair and seems to be in close proximity to the apical plasma membrane. Based on these findings, we propose that actin polymerization within the dome of growing root hair cells regulates polar growth.
Keywords: Root hairs, actin polymerization sites, fluorescently labeled cytochalasin D, Arabidopsis
Root hair cells, pollen tubes, caulonema, and fungal hyphae are typical tip-growing cells. These cells exhibit highly polarized growth exclusively in the apical dome, which is enriched in cytoplasm and vesicles.1 The well-documented reverse fountain streaming of root hairs and pollen tubes relies on the actin cytoskeleton. Actin microfilaments have been visualized in root hair cells by microinjecting fluorescent phalloidin, a fungal toxin that binds to filamentous actin, or expressing actin-binding proteins fused to GFP, and have been shown to form long cables that run from the base of the root hair cell to the tip.2,3 It has been established that actin polymerization can generate a force able to sustain growth or intracellular movement such as that observed during Listeria infections.4-6 The presence of G-actin foci in the tips of root hairs and pollen tubes5,7 suggests that actin polymerization in the tip region is essential for polar growth. This dynamic pool of actin has also been implicated in endosome movement during polar growth.8 The increased concentration of G-actin in the tip could fuel continuous polymerization and facilitate the constant actin remodelling mediated by calcium- and pH-sensitive actin-binding proteins, such as ADF, gelsolin, and villin. Furthermore, using a low concentration of fluorescently labeled cytochalasin, we recently reported that free actin plus ends (i.e., growing or polymerizing ends) are located in the tip region of growing root hairs.9 Under such low, sublethal concentrations, the intensity of the actin polymerization signal in the tip increased in growing root hair cells treated with Rhizobium etli nodulation factors (NFs), declined during the swelling response, and was restored to normal levels when tip growth was reinitiated. This finding adds weight to the idea that actin polymerization plays a key role in the tip region of growing root hair cells. In this study, we further strengthened this idea by analyzing growing Arabidopsis root hairs treated with fluorescently labeled cytochalasin and visualized by confocal microscopy. This approach allowed the visualization of fluorescent foci that are distributed throughout the cytoplasm, but enriched in the subapical region. Importantly, we found that the signal was strongest in close proximity to the plasma membrane in the tip dome, and exhibited dynamics that seemed to be correlated with tip growth.
The visualization of actin plus ends using a sublethal concentration of fluorescently labeled cytochalasin
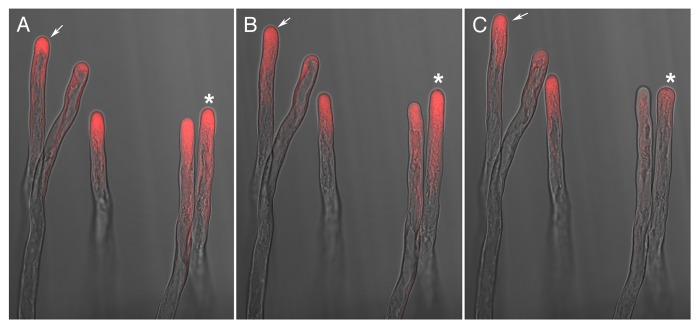
Arabidopsis root hairs were treated with a sublethal concentration of fluorescently labeled cytochalasin as reported9 and observed at low magnification (objective10X) under a confocal microscope. As reported previously, most of the fluorescence signal was focused in the tip region, and the root hairs continued to grow normally, as judged by the continuation of cytoplasmic streaming and robust growth rate. The morphology of the root hairs and the fluorescence distribution can be observed in Figure 1, which shows a merged image of the transmitted light and confocal images. The asterisk illustrates a root hair cell that was initially growing (Fig. 1A and B), but then stopped growing and underwent tip swelling (Fig. 1C). The other root hairs in the image exhibited continuous growth throughout the observation period.
Figure 1. The fluorescently labeled growing root hairs exhibit an apical distribution of the florescence signal. Root hairs from Arabidopsis were treated with fluorescently labeled cytochalasin and visualized under confocal microscopy. A, B, and C correspond to the same root hairs and the images were taken at 20 min intervals. Note that the root hair marked with an asterisk in panel A was still growing well after 20 min (panel B) in a similar way to the other root hairs; however, in panel C, growth of this root hair stopped and this non-growing stage was characterized by the loss of fluorescence signal. The root hair cell pointed with an arrow maintained tip growth throughout the observation period.
Confocal imaging reveals dynamic fluorescent foci in the cytoplasm and subapical region
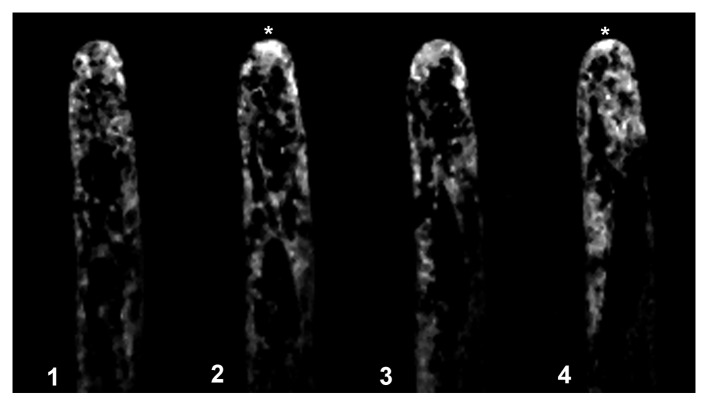
As reported using regular fluorescence microscopy,9 the distribution of the signal indicating actin plus ends is highly dynamic, with clear fluctuations in intensity and distribution in the tip region (Fig. 2). Confocal imaging, with its improved signal-to-noise ratio, allows close observation of these fluctuations, as visualized in the pseudocolored image presented in Figure 2, where red indicates a strong signal and purple a weak signal. In this Figure 2 note that panel 5–8 the fluorescent signal increase to the red level, indicating an increased accumulation of the plus en terminals, and then this level decrease to the initial values. Furthermore, we detected fluorescent foci throughout the cytoplasm. These foci were barely visible in the tip region, due to the high signal in this region. However, the images were subjected to low pass filtering using the macro option of Metamorph/Metafluor (Molecular Dynamics, USA) and the rendered images allowed the visualization of the fluorescent foci throughout the tip region, as depicted in Figure 3 (see arrow in panel 4 and 7). These fluorescent foci were highly dynamic, and frequently disappeared and reappeared in another location (Movie SV1). However, the fluorescence signal remained closely associated with the tip region.
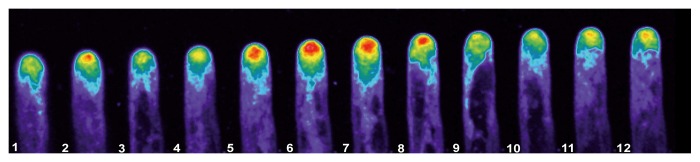
Figure 2. The fluorescently labeled growing root hairs exhibit a clear oscillation in the intensity of the intracellular fluorescence signal at the tip region. Fluorescently labeled growing root hairs were imaged at 10 s intervals. Note the increase in the fluorescence signal in panel 5–8 which return to the initial values in panel 9–12. The intensity of the fluorescence signal oscillated in rapidly growing root hairs, but not in non-growing root hair cells.
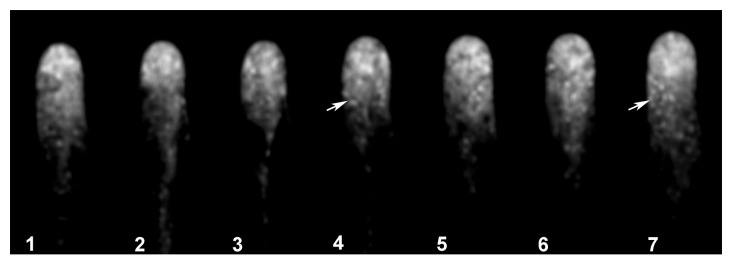
Figure 3. The fluorescently labeled root hairs exhibit highly dynamic and abundant fluorescent foci. Low pass filtering using the macro option of Metamorph/Metafluor (Molecular Dynamics, USA) revealed fluorescent foci in the fluorescently labeled root hairs as indicated by arrows. These fluorescent foci are more abundantly located in the apical region and are highly dynamic as depicted in the movie (Movie SV2).
Actin plus ends are closely associated with the tip dome
We subjected the time-lapse images of Arabidopsis root hairs treated with the fluorescent probe to Metamorph/Metafluor deconvolution analysis using the near neighbor application (Metamorph, Molecular Dynamics). With this approach, we were able to filter the image and visualize the fluorescent actin foci in greater detail. We found that the fluorescence signal was clearly localized to the tip dome, as depicted in Figure 4 and Movie SV2. The signal in the apex was very dynamic, exhibiting cycles of increased and decreased intensity as depicted in Figure 4, panel 2 and 4 (see asterisks). This fluorescent signal disappeared when the root hairs stopped growing.
Figure 4. The fluorescence signal is strong and highly dynamic in the apical dome of fluorescently labeled root hairs. Fluorescence images were subjected to deconvolution analysis using the near neighbor application (Metamorph, Molecular Dynamics). This processing revealed that the fluorescence signal was strongest in the apical dome in close proximity to the plasma membrane, that the signal was highly dynamic, and that the intensity of the signal oscillated as indicated by asterisks indicating when the signal is higher.
Using sublethal concentrations of fluorescent cytochalasin D (Fl-Cyt), we previously showed that the plus end terminals of actin filaments and thus actin polymerization support tip growth in P. vulgaris root hairs and are involved in the interaction with Rhizobium etli and specific NFs.9 In this work, we examined the distribution of polymerizing actin using confocal microscopy. Although the fluorescently tagged plus ends were mainly localized in the tip region, some actin foci were distributed in the cytoplasm. Time-lapse imaging demonstrated that these actin foci were highly dynamic and continuously generated. This result suggests that new plus ends are constantly generated in the tip region. Furthermore, the finding that the signal was appressed against the plasma membrane in the tip dome indicates that actin polymerization has a pivotal role in regulating and supporting polar growth.
Root hairs are highly polarized cellular structures resulting from the tip growth of specific root epidermal cells. The morphogenesis of these cells involves the interaction of many processes, including vesicle exocytosis, calcium homeostasis, and activation of reactive oxygen species (ROS), and the activity of several cell structures, such as (GPI)-anchored proteins with their associated lipid rafts and the cytoskeleton.10-14 The presence of a well-documented apical G-actin pool in root hairs and pollen tubes suggests that actin polymerization supports tip growth and is an important factor in this region.5,7 Actin organization in tip-growing cells is controlled by the tip-focused calcium gradient, pH, and ROS. These factors could have a significant role on the activity of several actin-binding proteins that mediate actin reorganization. Polar growth is characterized by the sustained transport of Golgi-derived vesicles carrying precursors for cell wall synthesis, which must be delivered to the apical region in a regulated fashion, and actin plays an important role in facilitating this transport, as demonstrated by studies that analyzed the effects of actin disrupting drugs.3-5,15,16 Our finding that actin plus ends are highly enriched in the apical dome suggests that actin polymerization is an important regulator of polar growth.
The tip-focused distribution of actin plus ends at the extreme apex suggests that actin polymerization contributes to key processes, such as endocytosis and exocytosis, and modulates calcium channel activity or even the activity of NADPH oxidases,17 which seem to be localized to membrane microdomains or lipid rafts in the apex of tip growing cells.18
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank the financial support from DGAPA IN-204409, IN202912 and CONACyT 58323 and 132155 to LC. Luis A. Bañuelos was supported by a scholarship from CONACyT. We also thank Olivia Santana and Noreide Nava for technical assistance.
References
- 1.Ketelaar T. The actin cytoskeleton in root hairs: all is fine at the tip. Curr Opin Plant Biol. 2013;16:749–56. doi: 10.1016/j.pbi.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Crdenas L, Vidali L, Domnguez J, Prez H, Snchez F, Hepler PK, Quinto C. Rearrangement of actin microfilaments in plant root hairs responding to rhizobium etli nodulation signals. Plant Physiol. 1998;116:871–7. doi: 10.1104/pp.116.3.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller DD, de Ruijter NCA, Bisseling T, Emons AM. The role of actin in root hair morphogenesis: studies with lipochito-oligosaccharide as a growth stimulator and cytochalasin as an actin perturbing drug. Plant J. 1999;17:141–54. doi: 10.1046/j.1365-313X.1999.00358.x. [DOI] [Google Scholar]
- 4.Vidali L, McKenna ST, Hepler PK. Actin polymerization is essential for pollen tube growth. Mol Biol Cell. 2001;12:2534–45. doi: 10.1091/mbc.12.8.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cárdenas L, Lovy-Wheeler A, Wilsen KL, Hepler PK. Actin polymerization promotes the reversal of streaming in the apex of pollen tubes. Cell Motil Cytoskeleton. 2005;61:112–27. doi: 10.1002/cm.20068. [DOI] [PubMed] [Google Scholar]
- 6.Zeile WL, Zhang F, Dickinson RB, Purich DL. Listeria’s right-handed helical rocket-tail trajectories: mechanistic implications for force generation in actin-based motility. Cell Motil Cytoskeleton. 2005;60:121–8. doi: 10.1002/cm.20050. [DOI] [PubMed] [Google Scholar]
- 7.He X, Liu YM, Wang W, Li Y. Distribution of G-actin is related to root hair growth of wheat. Ann Bot. 2006;98:49–55. doi: 10.1093/aob/mcl084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Voigt B, Timmers AC, Samaj J, Hlavacka A, Ueda T, Preuss M, Nielsen E, Mathur J, Emans N, Stenmark H, et al. Actin-based motility of endosomes is linked to the polar tip growth of root hairs. Eur J Cell Biol. 2005;84:609–21. doi: 10.1016/j.ejcb.2004.12.029. [DOI] [PubMed] [Google Scholar]
- 9.Zepeda I, Sánchez-López R, Kunkel JG, Bañuelos LA, Hernández-Barrera A, Sánchez F, Quinto C, Cárdenas L. Visualization of highly dynamic F-actin plus ends in growing phaseolus vulgaris root hair cells and their responses to Rhizobium etli nod factors. Plant Cell Physiol. 2014;55:580–92. doi: 10.1093/pcp/pct202. [DOI] [PubMed] [Google Scholar]
- 10.Baluska F, Salaj J, Mathur J, Braun M, Jasper F, Samaj J, Chua NH, Barlow PW, Volkmann D. Root hair formation: F-actin-dependent tip growth is initiated by local assembly of profilin-supported F-actin meshworks accumulated within expansin-enriched bulges. Dev Biol. 2000;227:618–32. doi: 10.1006/dbio.2000.9908. [DOI] [PubMed] [Google Scholar]
- 11.Fischer U, Men S, Grebe M. Lipid function in plant cell polarity. Curr Opin Plant Biol. 2004;7:670–6. doi: 10.1016/j.pbi.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 12.Jones MA, Raymond MJ, Smirnoff N. Analysis of the root-hair morphogenesis transcriptome reveals the molecular identity of six genes with roles in root-hair development in Arabidopsis. Plant J. 2006;45:83–100. doi: 10.1111/j.1365-313X.2005.02609.x. [DOI] [PubMed] [Google Scholar]
- 13.Sangiorgio V, Pitto M, Palestini P, Masserini M. GPI-anchored proteins and lipid rafts. Ital J Biochem. 2004;53:98–111. [PubMed] [Google Scholar]
- 14.Schindelman G, Morikami A, Jung J, Baskin TI, Carpita NC, Derbyshire P, McCann MC, Benfey PN. COBRA encodes a putative GPI-anchored protein, which is polarly localized and necessary for oriented cell expansion in Arabidopsis. Genes Dev. 2001;15:1115–27. doi: 10.1101/gad.879101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Torralba S, Raudaskoski M, Pedregosa AM, Laborda F. Effect of cytochalasin A on apical growth, actin cytoskeleton organization and enzyme secretion in Aspergillus nidulans. Microbiology. 1998;144:45–53. doi: 10.1099/00221287-144-1-45. [DOI] [PubMed] [Google Scholar]
- 16.Gibbon BC, Kovar DR, Staiger CJ. Latrunculin B has different effects on pollen germination and tube growth. Plant Cell. 1999;11:2349–63. doi: 10.1105/tpc.11.12.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaya H, Nakajima R, Iwano M, Kanaoka MM, Kimura S, Takeda S, Kawarazaki T, Senzaki E, Hamamura Y, Higashiyama T, et al. Ca2+-activated reactive oxygen species production by Arabidopsis RbohH and RbohJ is essential for proper pollen tube tip growth. Plant Cell. 2014;26:1069–80. doi: 10.1105/tpc.113.120642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu P, Li RL, Zhang L, Wang QL, Niehaus K, Baluska F, Samaj J, Lin JX. Lipid microdomain polarization is required for NADPH oxidase-dependent ROS signaling in Picea meyeri pollen tube tip growth. Plant J. 2009;60:303–13. doi: 10.1111/j.1365-313X.2009.03955.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.