Abstract
A successful honey bee forager tells her nestmates the location of good nectar and pollen with the waggle dance, a symbolic language that communicates a distance and direction. Because bees are adept at scouting out profitable forage and are very sensitive to energetic reward, we can use the distance that bees communicate via waggle dances as a proxy for forage availability, where the further the bees fly, the less forage can be found locally. Previously we demonstrated that bees fly furthest in the summer compared with spring or autumn to bring back forage that is not necessarily of better quality. Here we show that August is also the month when significantly more foragers return with empty crops (P = 7.63e-06). This provides additional support that summer may represent a seasonal foraging challenge for honey bees.
Keywords: Apis mellifera, forage availability, foraging dearth, foraging ecology, waggle dance
When a honey bee forager who has found a good source of food returns to her colony, she may recruit her nestmates to that location with a waggle dance, which encodes the vector from the hive to the forage.1,2 Honey bees are exceptional scouts—not only are they able to locate good forage over a large area,3-5 but they are very sensitive to relative energetic reward and will only recruit nestmates to the most profitable locations known at any given time.6 Researchers can eavesdrop on the waggle dance, specifically the distances communicated in the dance, as a tool to understand honey bee foraging ecology.7,8 Foragers will not recruit nestmates to long distances unnecessarily, as distance is a major consideration in a bee’s decision to dance.4 Distance can therefore act as a proxy for forage availability.
In Couvillon et al. (2014), we analyze 5097 dances to track seasonal changes in forage availability, as indicated by the dances of the economic honey bees, over a representative urban-rural landscape.9 We found that mean foraging distance / area increases between springs (493 min, 0.8km2) and summers (2156 min, 15.2km2) before decreasing again to autumns (1275 min, 5.1km2), which suggests that summer is the most challenging season, with the bees using an area 22 and 6 times greater compared with spring and autumn, respectively. Importantly, we also show that this increased distance is not necessarily because the bees bring back better forage: the sugar content in the nectar, which is a measure of quality as sweeter nectar contains more energy, was low in the summer months.9 It was during the measurements of nectar sugar content (Fig. Three of Couvillon et al. 2014) that we noticed an interesting phenomenon: many of the returning foragers possessed empty crops.
Our methods involved collecting and chilling 10 returning foragers without pollen loads from two study hives on 57 d from March to October.9 Once the bees were immobile, we would apply gentle pressure on their abdomen to cause them to regurgitate some of the nectar in their crop, which we would then measure for sugar concentration using a hand-held refractometre. If the bee’s crop was empty, we noted it down. If the bee regurgitated nectar, but the volume was too small for measurement with the refractometre, we would still consider the bee as possessing crop content. It is possible that our method of collecting bees, presumably foragers, returning at the entrance would sometimes also involve the erroneous collection of a few non-foraging bees. However, our methodology was consistent across the study and therefore does not affect our finding.
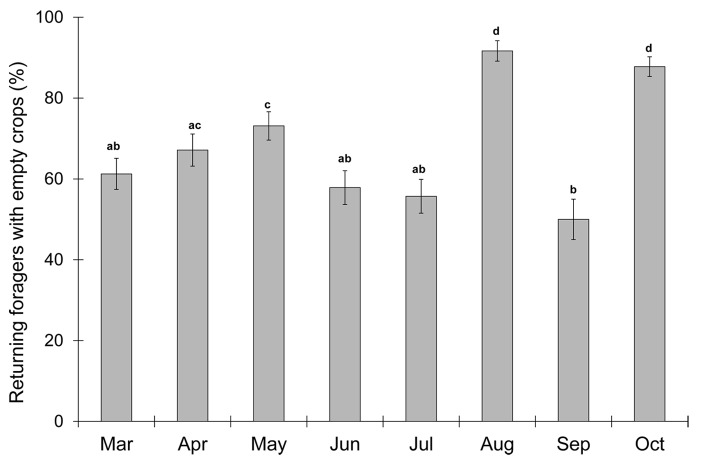
Overall, the number of bees returning with empty crops was surprisingly high, ranging from 50% (Fig. 1; September) to 92% (August). There is a significant effect of month in the proportion of foragers returning with empty crops (Fig. 1, n = 1140, GLM with Month as fixed factor compared with the null model GLM without Month and with binomial error, χ2 = -111.33, df = 7, P = 2.2e−16). Specifically, bees were found to possess empty crops most often in August (P = 7.63e-06), which is high summer and the season in which the bees are having to travel the furthest, and October (P = 1.47e-05), which is the end of the foraging year. Bees were least likely to possess an empty crop in September (P = 0.0079), which is the peak of the ivy bloom, a highly attractive plant to honey bees and other insect pollinators.10 There was no effect of hive in the proportion of bees with empty crops (χ2 = 0.07, P = 0.79), which allowed us to pool our data from the two hives per day (n = 20).
Figure 1. More honey bee foragers returned with empty crops in August and October and more possessed crop content in September. Bars display average across days per month, and error bars are standard error, as calculated for proportional data. Months that do not share letters are significantly different from each other.
Combined with the need for bees to collect summertime forage at greater distances, these data suggest that forage is less available in the summer. In the springtime, flowers are in abundance,11 and they compete for the bees; however, in the summertime, especially in a landscape dominated by intensively managed agricultural land with very few areas left with wildflowers,12-14 the bees compete for the flowers. Additionally, competition from other insects may also be greater in the summer (e.g., bumble bee colonies that reach peak population), all of which contributes to a landscape with fewer floral resources to provide good forage for flower-visiting insects.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was funded by grants from The Nineveh Charitable Trust (M.J.C.), Waitrose (K.A.F and S.K.L.Q). R.S. was funded by the Swiss National Science Foundation (Grant PA00P3_139731). Additional research funding was provided by Rowse Honey Ltd. Thanks to Professor Francis Ratnieks for his enthusiasm for the honey bee dance language.
References
- 1.von Frisch K. Die Tänze der Bienen. Österr zool Z. 1946;1:1–148. [Google Scholar]
- 2.von Frisch K. The Dance Language and Orientation of Bees. Cambridge, MA: Harvard University Press, 1967. [Google Scholar]
- 3.Seeley TD. The Wisdom of the Hive. Cambridge, Massachusetts: Harvard University Press, 1995. [Google Scholar]
- 4.Couvillon MJ, Schürch R, Ratnieks FLW. Dancing bees communicate a foraging preference for rural lands under High Level Agri-Environment Schemes. Curr Biol. doi: 10.1016/j.cub.2014.03.072. forthcoming. [DOI] [PubMed] [Google Scholar]
- 5.Visscher PK, Seeley TD. Foraging Strategy of Honeybee Colonies in a Temperate Deciduous Forest. Ecology. 1982;63:1790–801. doi: 10.2307/1940121. [DOI] [Google Scholar]
- 6.Seeley TD. Honey bee foragers as sensory units of their colonies. Behav Ecol Sociobiol. 1994;34:51–62. doi: 10.1007/BF00175458. [DOI] [Google Scholar]
- 7.Schürch R, Couvillon MJ, Burns DDR, Tasman K, Waxman D, Ratnieks FLW. Incorporating variability in honey bee waggle dance decoding improves the mapping of communicated resource locations. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2013;199:1143–52. doi: 10.1007/s00359-013-0860-4. [DOI] [PubMed] [Google Scholar]
- 8.Couvillon MJ. The dance legacy of Karl von Frisch. Insectes Soc. 2012;59:297–306. doi: 10.1007/s00040-012-0224-z. [DOI] [Google Scholar]
- 9.Couvillon MJ, Schürch R, Ratnieks FLW. Waggle dance distances as integrative indicators of seasonal foraging challenges. PLoS One. 2014;9:e93495. doi: 10.1371/journal.pone.0093495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garbuzov M, Ratnieks FLW. Ivy: an underappreciated key resource to flower visiting insects in autumn. Insect Conserv Diver 2013. [Google Scholar]
- 11.Clapham AR, Tutin TG, Warburg EF. Excursion Flora British Isles. Cambridge University Press, 1981. [Google Scholar]
- 12.Robinson RA, Sutherland WJ. Post-war changes in arable farming and biodiversity in Great Britain. J Appl Ecol. 2002;39:157–76. doi: 10.1046/j.1365-2664.2002.00695.x. [DOI] [Google Scholar]
- 13.Matson PA, Parton WJ, Power AG, Swift MJ. Agricultural intensification and ecosystem properties. Science. 1997;277:504–9. doi: 10.1126/science.277.5325.504. [DOI] [PubMed] [Google Scholar]
- 14.Wright CK, Wimberly MC. Recent land use change in the Western Corn Belt threatens grasslands and wetlands. Proc Natl Acad Sci U S A. 2013;110:4134–9. doi: 10.1073/pnas.1215404110. [DOI] [PMC free article] [PubMed] [Google Scholar]