Abstract
Gravity influences the growth direction of higher plants. Changes in the gravity vector (gravistimulation) immediately promote the increase in the cytoplasmic free calcium ion concentration ([Ca2+]c) in Arabidopsis (Arabidopsis thaliana) seedlings. When the seedlings are gravistimulated by reorientation at 180°, a transient two peaked (biphasic) [Ca2+]c-increase arises in their hypocotyl and petioles. Parabolic flights (PFs) can generate a variety of gravity-stimuli, and enables us to measure gravity-induced [Ca2+]c-increases without specimen rotation, which demonstrate that Arabidopsis seedlings possess a rapid gravity-sensing mechanism linearly transducing a wide range of gravitational changes into Ca2+ signals on a sub-second timescale. Hypergravity by centrifugation (20 g or 300 g) also induces similar transient [Ca2+]c-increases. In this review, we propose models for possible cellular processes of the garavi-stimulus-induced [Ca2+]c-increase, and evaluate those by examining whether the model fits well with the kinetic parameters derived from the [Ca2+]c-increases obtained by applying gravistimulus with different amplitudes and time sequences.
Keywords: calcium, gravistimulation, mechanosensitive channel, starch-statolish hypothesis, actin filament, parabolic flight, kinetics
Introduction
Plants respond to changes in the gravity vector (gravistimulation); plants sense gravity and orient their growth direction with respect to the gravity vector, known as gravitropism. Changes in the gravity vector are supposed to be transduced into certain intracellular signals such as reactive oxygen species,1 ionic gradient,cpH3,4 and cytoplasmic free calcium concentration ([Ca2+]c)2-5 in an early process of gravitropic response.6,7
Changes in the [Ca2+]c in gravity responses have repeatedly been reported in the field of plant biology; e.g., [Ca2+]c-increases in maize coleoptiles were observed by the [Ca2+]c indicator, fluo-3.8 Changes in the [Ca2+]c induced by gravistimulation have been studied in more detail in Arabidopsis (Arabidopsis thaliana) seedlings expressing the luminous Ca2+-reporting protein, apoaequorin.9-11 When the seedlings were gravistimulated by turning 180°, they show a transient biphasic [Ca2+]c-increase in their hypocotyls and petioles;9 a fast transient and a following slow [Ca2+]c-increases. These two Ca2+ changes have different characteristics. The first transient [Ca2+]c-increase (at 1g) depends on the rotational velocity but not the rotational angle, whereas the second slow [Ca2+]c increase depends on the rotational angle but not the velocity, suggesting that the first transient and second slow [Ca2+]c-increases are related to the rotational stimulation and the gravistimulation, respectively, and are mediated by distinct molecular mechanisms.9 Thus, the response to rotation may overlap the pure gravistimulus-induced response. In this review gravistimulation denotes the changes in the direction and magnitude of the gravity vector (some of them are shown in Figure 1).
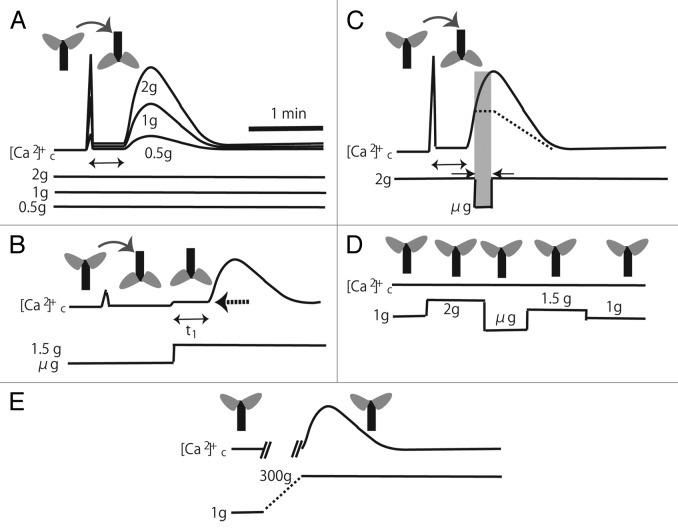
Figure 1. Schematic illustrations of the typical time course of gravistimulus-induced [Ca2+]c-increases. (A), Transient biphasic [Ca2+]c-increases by 180° rotation at 0.5, 1, and 2g conditions. (B), A slow [Ca2+]c-increase by transition from µg to 1.5g. Plants were rotated at µg before the gravity change, and a small transient [Ca2+]c-increase was detected. The aequorin signal during the delay (shown by double headed arrow, tl) is slightly higher (pointed by a dotted arrow) than the basal level. (C), A sudden gravitational decrease from 2g to µg does not attenuate the slow [Ca2+]c-increase induced by 180°-gravistimulation at 2g. The dotted line denotes a hypothetical [Ca2+]c change according to the model in panel (E) in Figure 2. (D), No apparent changes in [Ca2+]c is detected in plants in an upright position by the 1g-2g-µg-1.5g-1g change. (E), 300g-hypergravity stimulation induces a slow [Ca2+]c-increase with a similar time course. The gravitational acceleration increased from 1g to 300g gradually (dotted line). The accurate [Ca2+]c was not monitored during the gravitational acceleration increase due to the experimental setup13(not illustrated).
Recently, a gravistimulation-specific Ca2+ response in Arabidopsis seedlings has been demonstrated by separating gravity-vector-changes during rotation from gravistimulation by using the μg conditions provided by parabolic flights (PF).12 When Arabidopsis seedlings were rotated +180° under µg conditions, only the fast transient was observed and almost totally attenuated in a few seconds. Gravistimulation (transition from µg to 1.5g, see more detail in Figure 1) was then applied to pre-rotated specimens at the terminating phase of the PF. This gravistimulation without simultaneous rotation induced a slow [Ca2+]c-increase, which corresponds to the slow [Ca2+]c-increase observed in the ground experiments,9,10 and the amplitude of the slow [Ca2+]c-increase is not influenced by that of the fast transient. Interestingly, hypergravity stimulation (20g or 300g) by centrifugation induces a similar slow transient [Ca2+]c-increase.13
These accumulations of experimental data in a variety of conditions allow us to estimate the kinetic parameters of the slow [Ca2+]c-response, and to evaluate a variety of cellular mechanisms proposed to account for the gravistimulation-induced slow [Ca2+]c-increases. Here, a potential molecular process of the gravistimulus-induced [Ca2+]c-increase in hypocotyls and petioles of Arabidopsis seedlings is proposed and discussed with an updated knowledge of biophysics of cellular and molecular mechanism of cell mechano-sensing.14-16
Time Courses and Kinetic Parameters of [Ca2+]c-Increases to Various Gravistimuli
Typical time courses and kinetics parameters of the gravistimulation-induced [Ca2+]c-increases are schematically illustrated in Figure 1. Gravistimulation of seedlings by turning 180° induces a transient biphasic [Ca2+]c-increase (Fig. 1A). A wide variety of stimulation scenarios is possible with parabolic flights (PFs), e.g., sequential changes in the gravity intensity ranging from μg to 2g. Gravistimulation by 180° rotation at 0.5g induces a biphasic [Ca2+]c-increase but with smaller peaks than at 1g (Fig. 1A), while gravistimulation at 1.5g or 2g induces larger biphasic [Ca2+]c-increases with almost the same kinetics.12 The peak amplitude of the slow [Ca2+]c-increase is nearly linearly dependent on the magnitude of gravitational acceleration, suggesting that Arabidopsis seedlings possess a gravity-sensing mechanism that can transduce 0.5g to 2g gravitational acceleration into the amplitude of the slow [Ca2+]c-increase, while their kinetic parameters remain unchanged. This study12 revealed that the ampliude of the first peak is also garavity dependent as well as speed of rotation (for details, see “Discussion“ in the original paper12).
When seedlings were rotated under µg, a small transient [Ca2+]c-increase was detected. Gravitational acceleration from µg to 1.5g induced [Ca2+]c-increases with a time course similar to that of the slow [Ca2+]c-increase (Fig. 1B), demonstrating that the gravistimulation without rotation of specimen causes only the slow [Ca2+]c-increase. It also demonstarted that the [Ca2+]c increased with a delay between the transition (µg to 1.5g) and the onset of the slow [Ca2+]c-increase, named tl (tl ~20 s) (Fig. 1B). The aequorin signal during the delay (tl) was 10% higher than the basal level. This denotes that gravistimulation induces a slight [Ca2+]c-increase without delay prior to the initiation of a slow [Ca2+]c-transient (dotted arrow in Figure 1B).
The parabolic flight experiment also revealed that a sudden gravitational decrease from 2g to µg did not attenuate the slow [Ca2+]c-increase induced by 180°-gravistimulation at 2g (Fig. 1C). The rising phase of the response was not affected by the sudden g decrease (the shaded period in Figure 1C). This indicates that once the gravity sensing process is triggered, the [Ca2+]c-response proceeds irrespective of gravity changes.
The gravitational changes (e.g., 1g-2g-µg-1.5g) during the PF did not affect the [Ca2+]c in seedlings placed in the upright position (Fig. 1D). The plant may not generate active graviperception signals when growing at its preferred angle to gravity despite alterations in g force but rather in response to displacements from this angle, suggesting the plants adapt to the gravity in the preferred angle (e.g., upright position).
The [Ca2+]c transiently increases and decays exponentially during 20g to 300g-hypergravity stimulation (Fig. 1E), the time course of which resembles that shown in Figure 1B. The [Ca2+]c-response showed a strong desensitization. For example, [Ca2+]c-response was not elicited even 45 min after the first stimulation.13 Similar but less substantial desensitization to the repeated 180°-gravistimulation was also seen in 1g ground experiments.9
The potential mechanosensitive Ca2+-permeable channel (MSCC) inhibitors Gd3+ and La3+, the endomembrane Ca2+-permeable channel inhibitor ruthenium red (RR), and the phospholipase C (PLC) inhibitor U73122 profoundly attenuated the slow transient [Ca2+]c-increase, suggesting that it arises from Ca2+ influx via putative MSCCs in the plasma membrane and Ca2+ release from intracellular Ca2+ stores. The slow transient [Ca2+]c-increase was attenuated by actin-disrupting drugs cytochalasin B and latrunculin B, implying that actin filaments are involved in the activation of the MSCCs.9 The slow transient [Ca2+]c-increase is presumably induced in the following sequence based on the pharmacological experiments: 1) activation of plasma membrane MSCCs and PLC, 2) production of InsP3, and 3) InsP3-induced Ca2+-release (IICR) from ER (or vacuole).
Possible Cellular Mechanisms to Transduce Forces Generated by Gravity Vector Changes
“Starch-statolith” and “non-starch-statolith” hypotheses have been proposed to explain the gravity sensing.3,17,18 We briefly introduce these hypotheses and a reinforced “starch-statolith” hypothesis with knowledge of the mechano-sensing mechanism explored in mammalian endothelial cells with biophysical techniques.
“Starch-statolith” and “non-starch-statolith” hypotheses are schematically illustrated in Figure 2. The starch-statolith mechanism typically includes sedimentation of the high-density plastids, amyloplasts, which may cause 1) stress increase in the actin network, resulting in activation of MSCCs, which is followed by [Ca2+]c-increases or 2) membrane deformation and stress increase in the membrane, which may also activate MSCCs.19 The non-starch-statolith mechanism includes protoplast pressure model;20-22 cells are assumed to be a water-filled balloon inside a cardboard box, and cells determine the membrane to be in the “downward direction,” when pressure to the plasma membrane increases and stretches the membrane (Fig. 2G). MSCCs sense the tension increase in the membrane, and will be activated. Although the non-starch-statolith model is still controversial in higher plants, it could be supported by observations that a certain class of starchless mutants responds to gravity essentially in the same way as the wild type (ca. 70% of the wild type)23 and the elongation zone rather than the root cap containing amyloplasts contributes to gravity sensing in maize roots.24 Here, it should also be noted that the starchless plastids may also function as statolith.25
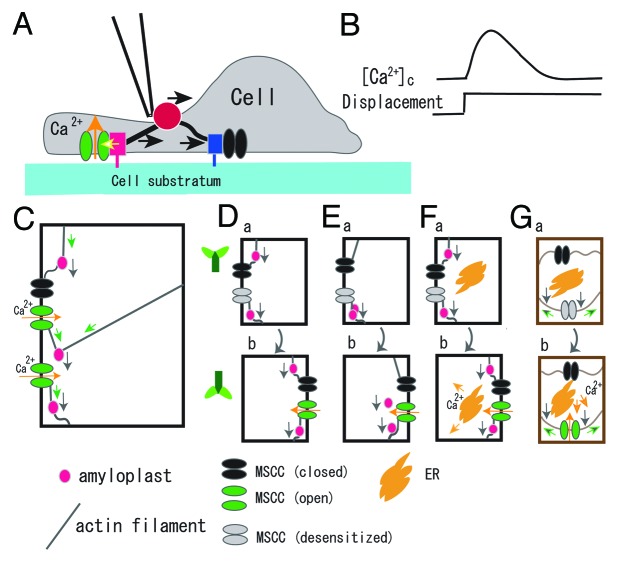
Figure 2. Schematic illustrations of the possible cellular process of sensing the tensile stress increase and the gravistimulus-induced slow [Ca2+]c-increase. (A), Displacement of the FN bead (ca. 1 μm for 10 s) generates tensile stress in the left-side actin stress fiber, activates MSCCs (a red box denotes an FA, and a yellow arrow designates stress that activates the MSCC colored green), while the compressive stress in the right side stress fiber is loaded to an FA (designated by a blue box), but not activate the MSCC colored black. The black arrow above a glass bead (red) indicates the direction of displacement. (B), A transient increase in [Ca2+]c (upper trace) induced by a displacement of the FN bead (lower trace). (C), Activation of MSCCs by the tensile increase in actin filaments. The gravitational force acting on amyloplasts (gray arrows) generates tensile stress (green arrows) and compressive stress designated by buckled lines of actin filaments. Open MSCCs are colored green and closed ones black. See text for more descriptions. (D), A “starch-statolith” model, which consist of amyloplasts, actin filaments, and MSCCs. A MSCC in upper panel (a) is desensitized (colored gray) to continuously applied tensile stress. The MSCC is activated (colored green) by a tensile tension increase when the plant is rotated 180° in panel (b). (E), A conventional “starch-statolith” model, which includes sedimentation of amyloplasts when the specimen rotated 180°. (F), A “starch-statolith” model with a signal transduction pathway that triggers Ca2+ release. (G), A “non-statolith-statolith” model. The cell wall (brown) and the plasma membrane (dark brown) are illustrated separately. Pressing the membrane (gray arrows) causes the stress increase in the membrane (green arrows). Ca2+ flux is shown by yellow arrows. The direction of plants is illustrated in the side of panel D.
“Starch-statolith” hypothesis is often referred to account for gravity-induced responses, and recent studies reinforced the “starch-statolith” hypothesis significantly. High-gradient magnetic field (HGMF) effectively mimics a gravitational field. Amyloplast displacement is induced under the HGMF, which in turn results in the organ curvature like the gravitropic response, and HGMF exploits differences in the diamagnetic susceptibilities between starch and cytoplasm.26,27 HGMF does not affect root gravitropism of starchless mutants, which indicates that HGMF is likely to act on amyloplasts.
The hypergravity condition has been used to examine the mechanism of gravitropism in roots and hypocotyls,28 and recently the hypergravity condition generated on a centrifuge microscope reveals that sedimentary movements of amyloplasts under hypergravity conditions are strongly correlated with gravitropic curvature in Arabidopsis stems even in the mutant which did not show amyloplast sedimentation at 1g.29 These studies28,29 strongly support and reinforce the “starch-statolith” hypothesis.
Arabidopsis seedlings may use MSCCs and actin fibers to detect changes in the direction of the gravity vector as mentioned above section. Thus, the gravistimulus-induced [Ca2+]c-increase may be accounted for by the “starch-statolith” hypothesis with MSCCs and actin fibers. To test the “starch-statolith” hypothesis it is indispensable to directly manipulate the actin fiber and examine the MSCCs activation. However, no useful experimental methodology had been available in plants.
By manipulating a bead attached on a dorsal surface of cultured human umbilical vein endothelial cells (HUVECs) with a micropipette, it has become possible to apply forces directly to actin fibers and to detect activation of MSCCs, which demonstrates that the actin stress fiber works as a force-transmitting molecular device to activate MSCCs with an extremely small magnitude of force16 in the range of the driving force of amyloplast sedimentation.18
In the newly developed method, glass beads conjugated with fibronectin (FN) were plated on cultured HUVECs. The beads adhered to the apical cell surface, leading to the formation of focal adhesions (FAs) underneath the beads. These FAs were connected to the pre-existing FAs at the basal cell surface via actin stress fibers (Fig. 2A). With this bead–apical-FA–stress-fiber–basal-FA linkage, it is possible to apply localized mechanical forces to the basal FAs by displacing the bead with a piezoelectric-driven glass pipette. Immediately after a displacement of the FN bead, the [Ca2+]c increased transiently (Fig. 2B). High speed imaging of the locus of the [Ca2+]c increase showed that MSCCs in the vicinity of the basal FAs were specifically activated by the tensile stress in the actin stress fibers, but MSCCs were not activated by compressive stress as shown in Figure 2A.16 This enables cells to sense the direction of force applied externally.
The time course of the [Ca2+]c-increase reflects the accumulated Ca2+ via the MSCCs activated by the mechanical force, and of the decline of the [Ca2+]c denotes the cessation of MSCCs activation and sequestration of Ca2+ to intracellular store sites and the extrusion of Ca2+ to extracellular space by Ca2+-pumps. When the bead was displaced and hold at the same position, the [Ca2+]c increased transiently, and returned to a resting [Ca2+]c level in a few minutes (Fig. 2B). Generally the rate of sequestration and extrusion of Ca2+ is low comparing with the rate of Ca2+ influx via Ca2+ channels.30 The Ca2+ influx via MSCCs would be nearly terminated at around the peak of [Ca2+]c-increase. This implies the inactivation of MSCCs whereas the bead was displaced continuously. This desensitization (i.e., inactivation of MSCCs to bead displacement) was confirmed by an observation that the mechano-stimuli induced [Ca2+]c-increase was attenuated by repeated stimulations. The mechanism of the desensitization is not identified at present, but decreases in the tension increase in the stress fiber during the repeated bead-displacement is known,15 which would result in less activation of MSCCs in HUVECs.
The high-speed imaging of the [Ca2+]c-increase shows tensile stress-increase in actin stress fibers is transmitted to FAs and activates the MSCCs in less than a few ms,16 suggesting the direct activation of MSCCs by the tension increase.
Recent time-lapse video analysis of the dynamic movement of the amyloplasts in an endodermal cell after gravistimulation by reorientation (90 degrees) revealed that some amyloplasts moved along a newly applied gravity direction and others did not.31 The amyloplasts that did not move are presumably tightly adhered to the actin filaments,32 and those moved in the direction of gravity will interact with actin filaments after sedimentation.
These ideas are included in the “starch-statolith” hypothesis to reinforce it in the following section. We use this “reinforced starch-statolith hypothesis” in Figure 2 (panels C to F) to explain the kinetics of the gravistimulus induced [Ca2+]c increase under different scenarios of gravistimulation as follows.
Possible Cellular Mechanisms That Explain the Cytoplasmic Calcium Increases by Gravistimuli in Hypocotyls and Petioles of Arabidopsis Seedlings
Among a wide variety of “starch-statolith” models of gravisensing,18,33,34 we choose models that include actin fibers as a force transmitting device, since the gravistimulus-induced slow [Ca2+]c-increase in hypocotyls and petioles is attenuated by actin-disrupting drugs.
The model in Panel C in Figure 2 shows activation of MCSSs by tension increases in actin filaments generated by gravitational force acting on the amyloplasts tightly adhered to the filaments. MSCCs connected to actin filaments with different angle are illustrated. The tensile stress increases activate two MSCCs (green channels), whereas the compressive stress does not (black channel) as illustrated in the case of HUVECs (panel A). This model can generate some simplified versions as depicted in panels D to G. Only two MSCCs are illustrated in these panels; one is activated and the other is not.
Three types of “starch-statolith” models are shown in panels D to F in Figure 2. The model in panel D shows amyloplasts tightly adhered to actin filaments. The model in panel E allows sedimentation of amyloplasts, which will introduce a delay between the onset of gravistimulation and the activation of MSCCs.7,18 The model in panel F includes intracellular organs (e.g., endoplasmic reticulum, ER, and vacuole in endodermal cells) that release Ca2+ in response to the second messengers, e.g., Ca2+ and inositol 1,4,5-trisphosphate (InsP3). ER serves as a major Ca2+ storage component, and is confined to the bottom of columella cells. A vacuole locates in the center of cells as well as occupies a major portion of the cell volume in endodermal cells.35
The model in panel E is often employed to account for the graviresponse in plants.34 The delay in Figure 1 (t1) is around 20 s, which is within the time range of amyloplasts sedimentation. This delay cannot be afforded by the model in panel D, because the force generated by the gravity will activate MSCCs immediately according to the biophysical analysis of force dependent MSCC activation,16 which will increase the [Ca2+]c within a few milliseconds.
Desensitization to the gravistimulation (Fig. 1D) can be accounted for by the desensitization of MSCCs (gray closed channels) to the continuously applied tensile stress generated by the gravitational force (Fig. Two D-F). The idea of desensitization to the mechanical stimulation is borrowed from the HUVECs studies (Fig. 2A and B). When plants are rotated 180°, newly generated tensile stress in the actin filament activates the MSCCs transiently (green MSCCs). The model in panel E also accounts for the hypergravity (> 20 g)-induced [Ca2+]c-increase. A large tensile stress will activate MSCCs and may induce a [Ca2+]c-increase with a similar time course.
The model in panel E, however, hardly accounts for the delay of the graviresponse in different gravitropic acceleration conditions (0.5g-2g, or 200g), because the delay owing to the sedimentation of amyloplasts must be strongly affected by the degree of gravity vector alteration; the delay should be shortened at higher gravitropic acceleration conditions and elongated at lower g conditions. According to the model in panel E, the sudden decrease in the gravity must affect the time course of the [Ca2+]c-increase as shown by the dotted line in Figure 1C (the [Ca2+]c will no longer elevate under μg, and it should decline due to the Ca2+ sequestration), because the sedimentation of amyloplasts must be terminated immediately due to that the small Reynolds number of the amyloplasts in the cytoplasm, < 10-6 36 if they sediment passively by the gravity.
We prefer the model in panel F, which includes a signal transduction pathway for triggering Ca2+ release subsequent to gravity perception via signals (e.g., InsP3 and Ca2+) that are presumably not affected by changes in gravity.12 This model is supported by a pharmacological study9 that shows the involvement of phospholipase C (PLC) and endomembrane Ca2+-permeable channels.
The delay (tl ~20 s) in the slow [Ca2+]c-increase can be explained by a slow production of InsP3 according to the following observations (1 and 2). (1) The minimum duration of gravistimulation needed to induce a detectable response is in a sub-second range.12 Within this time plants sense the change in gravity vector and trigger a signaling process (e.g., PLC). (2) The quantitative time-resolved measurements of cytosolic Ca2 release by photolysis of caged InsP3 shows that the [Ca2+]c increases less than a second by InsP3 increases,37 and pharmacological results9 suggest the major comportment of gravistimulus-induced [Ca2+]c-increase is PLC dependent. According to these (1 and 2), the delay in the gravistimulus-induced slow [Ca2+]c-increase is likely due to a slow onset of PLC dependent InsP3 production (ca. 20 s). The idea of such slow production of InsP3 (ca. 20 s) is not unusual because slow production of InsP3 is reported in muscle cells,38 and in human endothelial cells by shear stress.39
The hypothesis in panel F is summarized as follows; gravity changes (e.g., rotation) loaded onto the amyloplasts tightly adhered to the actin filaments generates tension increase immediately in the actin filament, which activate MSCCs, and PLC. PLC produces InsP3 with a considerable delay, and InsP3 causes Ca2+-release (IICR) from ER (or vacuole) in cell periphery.40,41 The Ca2+ influx through MSCCs increases the [Ca2+]c slightly without a delay, and InsP3 increases the [Ca2+]c profoundly with a delay (t1). This model fit well with that the gravistimulation-induced slight [Ca2+]c-increases prior to the slow large [Ca2+]c-increase (Fig. 1B). The slight [Ca2+]c-increases may also trigger the Ca2+-induced Ca2+-release.
This model gives a good fit to the results in Figure 1A, assuming that the delay t1 is not dependent on the magnitude of gravity change. Gravity stimulus at 0.5g will activate the above signaling process with less magnitude, and the same stimulus at 2g will activate it with greater magnitude. The [Ca2+]c-increases in proportion to the magnitude of gravity changes, and can be explained by the model with nearly the same kinetic parameters. The model also fits well Figure 1D, since MSCCs are desensitized to continuous stress increases as shown in Figure 2Fa. The MSCCs will be activated by large stress increase by the hypergravity stimulation as shown in Figure 1E.
The importance of this model is in giving a satisfactory fit to various aspects of the time course of the gravistimulus-induced slow [Ca2+]c-increase as mentioned above, while requiring only two additional components “desensitization of MSCCs” and “InsP3 production with delay.”
Note that all observations mentioned above do not exclude the “non-starch-statolith” model of graviresponse. One of the possible models is shown in Figure 2G; pressure increases to the cell membrane induced by the gravistimulus activate the MSCCs, PLC-dependent production of InsP3, and IICR. It may also work in parallel with the statolith sedimentation model.
Future perspective
The key components of our proposed “starch-statolith model” are mechano-sensitive (MS) channels and actin filaments.9 However, the molecular entities of plasma membrane MS channels responsible for the gravisensing have not been identified yet. Recent studies identified four types of plant MS channels; plant homologs of bacterial mechanosensitive channel of small conductance (MscS), MSL,42,43 Ca2+-permeable plasma membrane protein, MCA,44,45 two pore pottasium channel, TPK,46 and newly identified MS channel, Piezo.47 In Arabidopsis genome, 10 of MSL (MSL1 – 10), 2 of MCA (MCA1 and 2), 5 of TPK (AtTPK1 – 5), and 1 of Piezo (At2g48060) are present. Some of these MS channels in Arabidopsis are functionally characterized; MSL2 and 3 play a role in chloroplast division; MSL9 and 10 are plasma membrane MS anion channel; MCA1 and AtTPK4 are plasma membrane MS cation channels.42,43,48-50 Ca2+ uptake was altered in Arabidopsis seedling and yeast cells overexpressing MCA1-GFP proteins in the plasma membrane of Arabidopsis root cells. Seedlings of knockout line (mca1) lack the ability to penetrate their roots into a harder agar.44 These suggest that MCA may encode MS Ca2+-permeable channel. Among Arabidopsis TPKs, only AtTPK4 localizes in the plasma membrane but not permeate Ca2+. The function of the MSLs and Piezo remains to be demonstrated. The plasma membrane MS channels responsible for the gravistimulation-induced [Ca2+]c-increase, and the molecular process of activation and desensitization of MSCCs by tension is remained to be elucidated.
Recent studies using actin filaments disrupting drug latrunculin B have provided controversial results:51,52 root and shoot curvatures were enhanced in Arabidopsis by the treatment with the drug. Amyloplast sedimentation in Arabidopsis endodermal cells appears to be enhanced by latrunculin B treatment.31 However, it should be noted that the drug treatment affects the entire plant, and the effect of latrunculin B cannot be attributed to the effect of the drug to statocytes alone as pointed out,33 we should also keep in mind that actin filaments play multiple roles in cells such as endomembrane dynamics or trafficking.
A recent in vitro study reveals that the actin filament itself functions as a mechanosensor in human endothelial cells.14 A tiny force as small as 2 pN can be sensed by actin filaments, which affords an intriguing new hypothesis of gravity sensing by actin filaments; i.e., the sedimentation of amyloplasts increases the stress in the actin filaments,18 which may modulate the interaction between actin filaments and actin-binding proteins53 and lead to gravistimulus-induced responses.
The detail of signaling processes from tension increase in the actin fibers to the activation of MSCCs has not been explored, and the signaling molecules responsible for PLC activation is not identified at pressent. The processes from the [Ca2+]c-increase to the gravitropic response, which are highly likely auxin dependent,54 are also not identified yet, and left for future studies.
The durations of gravistimuli (especially those of parabolic flight experiments) reviewed here are mostly short (e.g., less than 20 s). Thus the analysis and interpretation of the effect of gravistimuli to the [Ca2+]c is limitted. Effects of gravistimuli with longer duration and different range to the [Ca2+]c will be required for understanting the pathway from gravi-sensing toward gravitopism. Mechanisms underlying the starch-statolith model have been examined in space flight experiments,55 which enables observation of gravitropism only made in an environment where plants are exposed to different magnitude of gravity for days or months.
The cellular and molecular mechanism of the gravity sensing is still enigmatic; “starch-statolith” and “non-starch-statolith” mechanisms are relevant for explaining the graviresponse. The gravisensing mechanism of shoots and roots might be considerably different. Therefore, the molecular characterization of gravisensing-deficient mutants33 and direct imaging of molecules crucial for the gravisensing in shoots and roots will accelerate the understanding of gravisensing machineries in plants.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research (C) (No. 20171720 to H.T.) and (A) (No. 20171720 to M.S.), and by a grant from the Japan Space Forum (to H.T. and M.S.). This work was also supported by a grant from TOYOBO Biotechnology Foundation (to M.T.) and Grant-in-Aid for JSPS Fellows (to M.T.), and Grant-in-Aid for JSPS Fellows for Research Abroad (to M.T.).
Glossary
- Abbrieviations
[Ca2+]c, cytoplasmic free calcium ion concentration
- PF
Parabolic flights
- MSCC
mechanosensitive Ca2+-permeable channel
- PLC
phospholipase C
- RR
ruthenium red
- IICR
InsP3-induced Ca2+-release
- InsP3
inositol 1,4,5-trisphosphate
- HGMF
High-gradient magnetic field
- HUVECs
human umbilical vein endothelial cells
- FN
fibronectin
- MscS
mechanosensitive channel of small conductance
- MS
mechano-sensitive
References
- 1.Joo JH, Bae YS, Lee JS. Role of auxin-induced reactive oxygen species in root gravitropism. Plant Physiol. 2001;126:1055–60. doi: 10.1104/pp.126.3.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sinclair W, Trewavas AJ. Calcium in gravitropism. A re-examination. Planta. 1997;203(Suppl 1):S85–90. doi: 10.1007/PL00008120. [DOI] [PubMed] [Google Scholar]
- 3.Fasano JM, Massa GD, Gilroy S. Ionic signaling in plant responses to gravity and touch. J Plant Growth Regul. 2002;21:71–88. doi: 10.1007/s003440010049. [DOI] [PubMed] [Google Scholar]
- 4.Toyota M, Gilroy S. Gravitropism and mechanical signaling in plants. Am J Bot. 2013;100:111–25. doi: 10.3732/ajb.1200408. [DOI] [PubMed] [Google Scholar]
- 5.Perera IY, Heilmann I, Chang SC, Boss WF, Kaufman PB. A role for inositol 1,4,5-trisphosphate in gravitropic signaling and the retention of cold-perceived gravistimulation of oat shoot pulvini. Plant Physiol. 2001;125:1499–507. doi: 10.1104/pp.125.3.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen R, Rosen E, Masson PH. Gravitropism in higher plants. Plant Physiol. 1999;120:343–50. doi: 10.1104/pp.120.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morita MT, Tasaka M. Gravity sensing and signaling. Curr Opin Plant Biol. 2004;7:712–8. doi: 10.1016/j.pbi.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Gehring CA, Williams DA, Cody SH, Parish RW. Phototropism and geotropism in maize coleoptiles are spatially correlated with increases in cytosolic free calcium. Nature. 1990;345:528–30. doi: 10.1038/345528a0. [DOI] [PubMed] [Google Scholar]
- 9.Toyota M, Furuichi T, Tatsumi H, Sokabe M. Cytoplasmic calcium increases in response to changes in the gravity vector in hypocotyls and petioles of Arabidopsis seedlings. Plant Physiol. 2008;146:505–14. doi: 10.1104/pp.107.106450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Plieth C, Trewavas AJ. Reorientation of seedlings in the earth’s gravitational field induces cytosolic calcium transients. Plant Physiol. 2002;129:786–96. doi: 10.1104/pp.011007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toyota M, Furuichi T, Tatsumi H, Sokabe M. Critical consideration on the relationship between auxin transport and calcium transients in gravity perception of Arabidopsis seedlings. Plant Signal Behav. 2008;3:521–4. doi: 10.4161/psb.3.8.6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toyota M, Furuichi T, Sokabe M, Tatsumi H. Analyses of a gravistimulation-specific Ca2+ signature in Arabidopsis using parabolic flights. Plant Physiol. 2013;163:543–54. doi: 10.1104/pp.113.223313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toyota M, Furuichi T, Tatsumi H, Sokabe M. Hypergravity stimulation induces changes in intracellular calcium concentration in Arabidopsis seedlings. Adv Space Res. 2007;39:1190–7. doi: 10.1016/j.asr.2006.12.012. [DOI] [Google Scholar]
- 14.Hayakawa K, Tatsumi H, Sokabe M. Actin filaments function as a tension sensor by tension-dependent binding of cofilin to the filament. J Cell Biol. 2011;195:721–7. doi: 10.1083/jcb.201102039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kiyoshima D, Kawakami K, Hayakawa K, Tatsumi H, Sokabe M. Force- and Ca²⁺-dependent internalization of integrins in cultured endothelial cells. J Cell Sci. 2011;124:3859–70. doi: 10.1242/jcs.088559. [DOI] [PubMed] [Google Scholar]
- 16.Hayakawa K, Tatsumi H, Sokabe M. Actin stress fibers transmit and focus force to activate mechanosensitive channels. J Cell Sci. 2008;121:496–503. doi: 10.1242/jcs.022053. [DOI] [PubMed] [Google Scholar]
- 17.Kondrachuk AV. Theoretical considerations of plant gravisensing. Adv Space Res. 2001;27:907–14. doi: 10.1016/S0273-1177(01)00187-9. [DOI] [PubMed] [Google Scholar]
- 18.Tatsumi H, Furuichi T, Nakano M, Toyota M, Hayakawa K, Sokabe M, Iida H. Mechanosensitive channels are activated by stress in the actin stress fibres, and could be involved in gravity sensing in plants. Plant Biol (Stuttg) 2014;16(Suppl 1):18–22. doi: 10.1111/plb.12095. [DOI] [PubMed] [Google Scholar]
- 19.Leitz G, Kang BH, Schoenwaelder MEA, Staehelin LA. Statolith sedimentation kinetics and force transduction to the cortical endoplasmic reticulum in gravity-sensing Arabidopsis columella cells. Plant Cell. 2009;21:843–60. doi: 10.1105/tpc.108.065052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wayne R, Staves MP, Leopold AC. The contribution of the extracellular matrix to gravisensing in characean cells. J Cell Sci. 1992;101:611–23. doi: 10.1242/jcs.101.3.611. [DOI] [PubMed] [Google Scholar]
- 21.Wayne R, Staves MP. A down-to-earth model of gravisensing Gravitatinal and Space Biology Bulletin 1997; 10:57-64. [PubMed]
- 22.Wayne R, Staves MP. A down to earth model of gravisensing or Newton’s Law of Gravitation from the apple’s perspective. Physiol Plant. 1996;98:917–21. doi: 10.1111/j.1399-3054.1996.tb06703.x. [DOI] [PubMed] [Google Scholar]
- 23.Caspar T, Pickard BG. Gravitropism in a starchless mutant of Arabidopsis: implications for the starch-statolith theory of gravity sensing. Planta. 1989;177:185–97. doi: 10.1007/BF00392807. [DOI] [PubMed] [Google Scholar]
- 24.Wolverton C, Mullen JL, Ishikawa H, Evans ML. Root gravitropism in response to a signal originating outside of the cap. Planta. 2002;215:153–7. doi: 10.1007/s00425-001-0726-9. [DOI] [PubMed] [Google Scholar]
- 25.Kiss JZ, Hertel R, Sack FD. Amyloplasts are necessary for full gravitropic sensitivity in roots of Arabidopsis thaliana. Planta. 1989;177:198–206. doi: 10.1007/BF00392808. [DOI] [PubMed] [Google Scholar]
- 26.Kuznetsov OA, Hasenstein KH. Intracellular magnetophoresis of amyloplasts and induction of root curvature. Planta. 1996;198:87–94. doi: 10.1007/BF00197590. [DOI] [PubMed] [Google Scholar]
- 27.Weise SE, Kuznetsov OA, Hasenstein KH, Kiss JZ. Curvature in Arabidopsis inflorescence stems is limited to the region of amyloplast displacement. Plant Cell Physiol. 2000;41:702–9. doi: 10.1093/pcp/41.6.702. [DOI] [PubMed] [Google Scholar]
- 28.Fitzelle KJ, Kiss JZ. Restoration of gravitropic sensitivity in starch-deficient mutants of Arabidopsis by hypergravity. J Exp Bot. 2001;52:265–75. doi: 10.1093/jexbot/52.355.265. [DOI] [PubMed] [Google Scholar]
- 29.Toyota M, Ikeda N, Sawai-Toyota S, Kato T, Gilroy S, Tasaka M, et al. Amyloplast displacement is necessary for gravisensing in Arabidopsis shoots as revealed by a centrifuge microscope. The Plant journal: for cell and molecular biology 2013; 76:648-60. [DOI] [PubMed]
- 30.Tatsumi H, Katayama Y. Regulation of the intracellular free calcium concentration in acutely dissociated neurones from rat nucleus basalis. J Physiol. 1993;464:165–81. doi: 10.1113/jphysiol.1993.sp019628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saito C, Morita MT, Kato T, Tasaka M. Amyloplasts and vacuolar membrane dynamics in the living graviperceptive cell of the Arabidopsis inflorescence stem. Plant Cell. 2005;17:548–58. doi: 10.1105/tpc.104.026138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakamura M, Toyota M, Tasaka M, Morita MT. An Arabidopsis E3 ligase, SHOOT GRAVITROPISM9, modulates the interaction between statoliths and F-actin in gravity sensing. Plant Cell. 2011;23:1830–48. doi: 10.1105/tpc.110.079442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morita MT. Directional gravity sensing in gravitropism. Annu Rev Plant Biol. 2010;61:705–20. doi: 10.1146/annurev.arplant.043008.092042. [DOI] [PubMed] [Google Scholar]
- 34.Perbal G. From ROOTS to GRAVI-1: Twenty Five Years for Understanding How Plants Sense Gravity. Microgravity Sci Technol. 2009;21:3–10. doi: 10.1007/s12217-008-9064-x. [DOI] [Google Scholar]
- 35.Morita MT, Kato T, Nagafusa K, Saito C, Ueda T, Nakano A, Tasaka M. Involvement of the vacuoles of the endodermis in the early process of shoot gravitropism in Arabidopsis. Plant Cell. 2002;14:47–56. doi: 10.1105/tpc.010216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Todd P. Gravity-dependent phenomena at the scale of the single cell. ASGSB bulletin: publication of the American Society for Gravitational and Space Biology 1989; 2:95-113. [PubMed]
- 37.Takeo T, Suga S, Wu J, Dobashi Y, Kanno T, Wakui M. Kinetics of Ca2+ release evoked by photolysis of caged InsP3 in rat submandibular cells. J Cell Physiol. 1998;174:387–97. doi: 10.1002/(SICI)1097-4652(199803)174:3<387::AID-JCP13>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 38.Jaimovich E, Reyes R, Liberona JL, Powell JA. IP(3) receptors, IP(3) transients, and nucleus-associated Ca(2+) signals in cultured skeletal muscle. Am J Physiol Cell Physiol. 2000;278:C998–1010. doi: 10.1152/ajpcell.2000.278.5.C998. [DOI] [PubMed] [Google Scholar]
- 39.Nollert MU, Eskin SG, McIntire LV. Shear stress increases inositol trisphosphate levels in human endothelial cells. Biochem Biophys Res Commun. 1990;170:281–7. doi: 10.1016/0006-291X(90)91271-S. [DOI] [PubMed] [Google Scholar]
- 40.Gilroy S, Read ND, Trewavas AJ. Elevation of cytoplasmic calcium by caged calcium or caged inositol triphosphate initiates stomatal closure. Nature. 1990;346:769–71. doi: 10.1038/346769a0. [DOI] [PubMed] [Google Scholar]
- 41.Knight H, Trewavas AJ, Knight MR. Cold calcium signaling in Arabidopsis involves two cellular pools and a change in calcium signature after acclimation. Plant Cell. 1996;8:489–503. doi: 10.1105/tpc.8.3.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haswell ES, Meyerowitz EM. MscS-like proteins control plastid size and shape in Arabidopsis thaliana. Curr Biol. 2006;16:1–11. doi: 10.1016/j.cub.2005.11.044. [DOI] [PubMed] [Google Scholar]
- 43.Haswell ES, Peyronnet R, Barbier-Brygoo H, Meyerowitz EM, Frachisse JM. Two MscS homologs provide mechanosensitive channel activities in the Arabidopsis root. Curr Biol. 2008;18:730–4. doi: 10.1016/j.cub.2008.04.039. [DOI] [PubMed] [Google Scholar]
- 44.Nakagawa Y, Katagiri T, Shinozaki K, Qi Z, Tatsumi H, Furuichi T, Kishigami A, Sokabe M, Kojima I, Sato S, et al. Arabidopsis plasma membrane protein crucial for Ca2+ influx and touch sensing in roots. Proc Natl Acad Sci U S A. 2007;104:3639–44. doi: 10.1073/pnas.0607703104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamanaka T, Nakagawa Y, Mori K, Nakano M, Imamura T, Kataoka H, Terashima A, Iida K, Kojima I, Katagiri T, et al. MCA1 and MCA2 that mediate Ca2+ uptake have distinct and overlapping roles in Arabidopsis. Plant Physiol. 2010;152:1284–96. doi: 10.1104/pp.109.147371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maathuis FJ. Vacuolar two-pore K+ channels act as vacuolar osmosensors. New Phytol. 2011;191:84–91. doi: 10.1111/j.1469-8137.2011.03664.x. [DOI] [PubMed] [Google Scholar]
- 47.Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, Dubin AE, Patapoutian A. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. 2010;330:55–60. doi: 10.1126/science.1193270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Veley KM, Marshburn S, Clure CE, Haswell ES. Mechanosensitive channels protect plastids from hypoosmotic stress during normal plant growth. Current biology: CB 2012; 22:408-13. [DOI] [PMC free article] [PubMed]
- 49.Furuichi T, Iida H, Sokabe M, Tatsumi H. Expression of Arabidopsis MCA1 enhanced mechanosensitive channel activity in the Xenopus laevis oocyte plasma membrane. Plant Signal Behav. 2012;7:1022–6. doi: 10.4161/psb.20783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Becker D, Geiger D, Dunkel M, Roller A, Bertl A, Latz A, Carpaneto A, Dietrich P, Roelfsema MR, Voelker C, et al. AtTPK4, an Arabidopsis tandem-pore K+ channel, poised to control the pollen membrane voltage in a pH- and Ca2+-dependent manner. Proc Natl Acad Sci U S A. 2004;101:15621–6. doi: 10.1073/pnas.0401502101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hou G, Mohamalawari DR, Blancaflor EB. Enhanced gravitropism of roots with a disrupted cap actin cytoskeleton. Plant Physiol. 2003;131:1360–73. doi: 10.1104/pp.014423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Palmieri M, Kiss JZ. Disruption of the F-actin cytoskeleton limits statolith movement in Arabidopsis hypocotyls. J Exp Bot. 2005;56:2539–50. doi: 10.1093/jxb/eri248. [DOI] [PubMed] [Google Scholar]
- 53.Uribe R, Jay D. A review of actin binding proteins: new perspectives. Mol Biol Rep. 2009;36:121–5. doi: 10.1007/s11033-007-9159-2. [DOI] [PubMed] [Google Scholar]
- 54.Vanneste S, Friml J. Calcium: the missing link in auxin action. Plants. 2013;2:650–75. doi: 10.3390/plants2040650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paul AL, Wheeler RM, Levine HG, Ferl RJ. Fundamental plant biology enabled by the space shuttle. Am J Bot. 2013;100:226–34. doi: 10.3732/ajb.1200338. [DOI] [PubMed] [Google Scholar]