Abstract
Brain tumor-initiating cells (BTICs) become less tumorigenic when co-cultured with microglia/macrophages (M/Ms) isolated from subjects not affected by glioma, but not when exposed to the M/Ms of glioma patients. Microglial cells and macrophages from glioma patients, however, can be reactivated by non-toxic doses of amphotericin B to curb the growth of BTICs in vitro and in vivo.
Keywords: innate immunity, microglia, glioma stem cells, amphotericin, reactivation
Malignant gliomas, particularly Grade IV glioblastoma, account for 3–5% of all cancer-related deaths. Despite significant advances in multimodal therapeutic approaches, malignant gliomas remain largely untreatable, with median survival time not exceeding 15 mo.1 Glioblastoma is indeed associated with one of the worst 5-y survival rates of all human cancers. The growth of malignant gliomas is maintained by a rare population of cells that proliferate and undergo self-renewal, preserving the original tumor while seeding and generating relatively more differentiated lesions. These self-renewing precursors have been variously referred to as glioma stem cells, brain tumor stem cells, glioblastoma-derived cancer stem cells, or brain tumor-initiating cells (BTICs).2
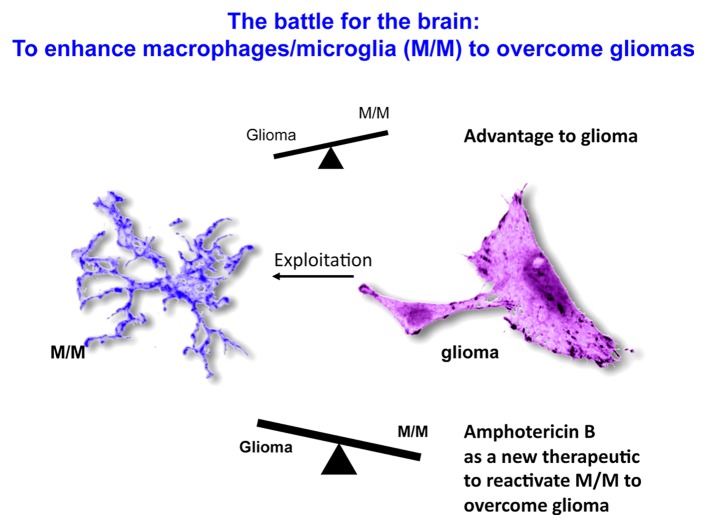
In situ, glioma cells are surrounded by monocytic cells, encompassing both central nervous system (CNS)-resident and tissue-infiltrating cells, such as the microglia and tumor-associated macrophages (TAMs), respectively. As it is difficult to differentiate microglial cells from macrophages in histological sections stained for various myeloid markers, these cells are collectively referred to as microglia/macrophages (M/Ms). A substantial literature has described the interactions between M/Ms and glioma cells. In physiological conditions, M/Ms operate as major antigen-presenting cells in the brain, not only activating T cell-mediated immune responses, but also exerting other immunological functions such as the phagocytic clearance of several threats. However, gliomas appear to suppress the immunological functions of M/Ms,3, 4 by producing immunosuppressive molecules such as interleukin-10, transforming growth factor β1 (TGFβ1) and various prostaglandins. Moreover, glioma cells commonly recruit M/Ms to produce growth and angiogenic factors. Nevertheless, there is evidence that M/Ms attempt to counteract the growth of gliomas. M/M-derived factors stimulate indeed the apoptotic demise of differentiated glioma cells in vitro and in vivo.5 Moreover, the microglia of glioma patients can suppress the proliferation of glioma cells in culture upon activation.6 This said, perhaps the most compelling evidence in support of the antineoplastic activity of M/Ms is the increased intracranial growth of gliomas in mice subjected to the genetic ablation of innate immune cells.7 These results suggest that M/Ms can engage in a “battle for the brain” (Fig. 1), although in most cases their activity appears to be co-opted by gliomas. Notably, M/Ms can be polarized to cytotoxic M1 or tumor-promoting M2 cells, depending upon the type of stimulus.8
Figure 1. The battle for the brain. Gliomas not only inhibit the pro-inflammatory and antigen-presenting capacity of microglia/macrophages (M/Ms), but also harness M/Ms for the production of trophic and angiogenic factors that support tumor growth. In some instances however, M/Ms appear to control the growth of glioma cells and to stimulate their apoptotic demise. Thus, enhancing the ability of M/Ms to suppress glioma cells, in particular brain-tumor initiating cells (BTICs), stands out as an important therapeutic goal. Our results suggest that amphotericin B may activate M/Ms to mediate robust antineoplastic effects against BTICs.
We recently set out to understand when M/Ms inhibit tumor growth in the course gliomagenesis and when they are co-opted by neoplastic lesions to promote tumor progression. We believed that this information would allow medications that activate M/Ms to be applied with an optimal timing to achieve superior antineoplastic effects. We found that M/Ms from individuals not affected by glioma, as well as their secretory products, not only reduced the growth of BTICs and their self-renewal in culture, but also caused them to differentiate into cells with characteristics of astrocytes and neurons. This interesting observation encouraged us to conduct in vivo studies. We found that the oncogenic potential of BTICs is significantly reduced if they are exposed to microglia-conditioned medium for 72 h prior to implantation into the murine brain. Thus, one component of the “battle for the brain” involves the inhibition of BTIC growth by M/Ms, demonstrating that these latter cells are capable of exerting an antineoplastic activity. In contrast, M/Ms isolated from glioblastoma patients not only failed to reduce the growth of autologous or heterologous BTICs, but also did not promote their differentiation. These data clearly demonstrate that the microglia, TAMs and circulating monocytes of glioma patients are deactivated.9 This encouraged us to search for a compound that would reprogram and/or reactivate these monocytic cells.
We sought to increase the activity of M/Ma using clinically employed drugs. Among 1040 distinct agents of this type, we identified amphotericin B (AmpB) which is primarily used to treat patients with life-threatening fungal infections, as a molecule capable of activating cultured M/Ms to secrete both M1 and M2 cytokines, and enhancing the ability of M/Ms to promote the differentiation of BTICs into cells with astrocytic or neuronal properties. The systemic administration of AmpB reduced the growth of patient-derived BTICs growing orthotopically in SCID-NOD mice and doubled the lifespan of these animals.9 This effect required M/Ms, as AmpB itself has no direct activity on BTICs and because the depletion of monocytic cells in vivo by clodronate-loaded liposomes abrogated the survival benefit afforded by AmpB. Moreover, The M/M-dependent antineoplastic activity of AmpB was observed in the absence of relevant side effects, and at 10% the serum concentration of AmpB needed to treat patients with fungal infections. This safety margin suggests that AmpB may represent a novel immunotherapeutic approach for patients with gliomas.
When we analyzed brain sections from AmpB-treated mice, we detected a massive activation of M/Ms in the proximity of malignant cells, as evidenced by increased expression levels of nitric oxide synthase 2, inducible (NOs2). As the initial experiments were based on patient-derived BTICs and hence necessarily on immunocompromised mice (to avoid cross-species tissue rejection), we generated BTICs from C57Bl/6 mice and implanted them into syngeneic, perfectly immunocompetent mice. Importantly, the administration of AmpB also prolonged the lifespan of tumor-bearing C57Bl/6 mice, an effects that was associated with reduced tumor growth. Finally, AmpB restored the capacity of M/Ms from glioblastoma patients to reduce the growth of autologous BTICs in vitro.9
To the best of our knowledge, our findings identify the first FDA-approved medication that has the capacity to reduce the oncogenic potential of BTICs. Our discovery may have a broad utility as the immunological activity of TAMs is compromised in most solid tumors.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Citation: Sarkar S, Yong VW. The battle for the brain: Brain tumor-initiating cells vs. microglia/macrophages. OncoImmunology 2014; 3:e28047; 10.4161/onci.28047
References
- 1.Chaichana KL, Jusue-Torres I, Navarro-Ramirez R, Raza SM, Pascual-Gallego M, Ibrahim A, Hernandez-Hermann M, Gomez L, Ye X, Weingart JD, et al. Establishing percent resection and residual volume thresholds affecting survival and recurrence for patients with newly diagnosed intracranial glioblastoma. Neuro Oncol. 2014;16:113–22. doi: 10.1093/neuonc/not137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 3.Li W, Graeber MB. The molecular profile of microglia under the influence of glioma. Neuro Oncol. 2012;14:958–78. doi: 10.1093/neuonc/nos116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu A, Wei J, Kong LY, Wang Y, Priebe W, Qiao W, Sawaya R, Heimberger AB. Glioma cancer stem cells induce immunosuppressive macrophages/microglia. Neuro Oncol. 2010;12:1113–25. doi: 10.1093/neuonc/noq082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hwang SY, Yoo BC, Jung JW, Oh ES, Hwang JS, Shin JA, Kim SY, Cha SH, Han IO. Induction of glioma apoptosis by microglia-secreted molecules: The role of nitric oxide and cathepsin B. Biochim Biophys Acta. 2009;1793:1656–68. doi: 10.1016/j.bbamcr.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 6.Kees T, Lohr J, Noack J, Mora R, Gdynia G, Tödt G, Ernst A, Radlwimmer B, Falk CS, Herold-Mende C, et al. Microglia isolated from patients with glioma gain antitumor activities on poly (I:C) stimulation. Neuro Oncol. 2012;14:64–78. doi: 10.1093/neuonc/nor182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galarneau H, Villeneuve J, Gowing G, Julien JP, Vallières L. Increased glioma growth in mice depleted of macrophages. Cancer Res. 2007;67:8874–81. doi: 10.1158/0008-5472.CAN-07-0177. [DOI] [PubMed] [Google Scholar]
- 8.Pyonteck SM, Akkari L, Schuhmacher AJ, Bowman RL, Sevenich L, Quail DF, Olson OC, Quick ML, Huse JT, Teijeiro V, et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med. 2013;19:1264–72. doi: 10.1038/nm.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarkar S, Döring A, Zemp FJ, Silva C, Lun X, Wang X, Kelly J, Hader W, Hamilton M, Mercier P, et al. Therapeutic activation of macrophages and microglia to suppress brain tumor-initiating cells. Nat Neurosci. 2014;17:46–55. doi: 10.1038/nn.3597. [DOI] [PubMed] [Google Scholar]