Abstract
Carotenoids are plastidial isoprenoids essential for the protection of photosynthetic tissues against excess light. They also serve as precursors of apocarotenoid hormones such as abscisic acid (ABA) and strigolactones. The first enzyme of the carotenoid pathway, phytoene synthase (PSY), is also the main rate-limiting step. Unlike that observed in most plants, PSY is encoded by a single gene in Arabidopsis thaliana. Whereas the PSY gene is induced by light in photosynthetic tissues, a root-specific upregulation of PSY expression by salt stress and ABA has been recently demonstrated. Here we report that transcription factors of the Phytochrome-Interacting Factor (PIF) family, previously shown to repress PSY expression in etiolated seedlings and mature leaves, do not influence PSY expression in roots. Together, our results suggest that organ-specific pathways regulate PSY expression and hence carotenoid production in response to different environmental cues.
Keywords: Arabidopsis, carotenoid, dark, PIF, phytoene synthase, root, salt
Carotenoids are isoprenoid metabolites with a strong interest as natural pigments in the industry. Additionally, they are a source of retinoids (including vitamin A) and health-promoting metabolites in the human diet.1,2 Plant carotenoids are synthesized in plastids. In chloroplasts of photosynthetic tissues, carotenoids contribute to light harvesting and photoprotection.3 In some plant species, they also accumulate at high levels in chromoplasts of flowers and fruits, contributing to their colors.4,5 Oxidative cleavage of carotenoids produce apocarotenoids with roles either as pigments and flavors that attract pollinators or seed-dispersing animals or as hormones such as abscisic acid (ABA) and strigolactones that regulate plant development and the interaction of plants with their environment.6-9
The first specific reaction of the carotenoid pathway is the production of phytoene from 2 geranylgeranyl diphosphate molecules catalyzed by the phytoene synthase (PSY) enzyme.10 The transcriptional regulation of PSY levels is a major factor controlling the production of carotenoids during deetiolation, when a burst in carotenoid biosynthesis takes place to protect the emerging photosynthetic apparatus against excess light.11,12 In deetiolating Arabidopsis thaliana plants, the expression of the single gene encoding PSY is directly controlled by transcription factors of the Phytochrome-Interacting Factor (PIF) family.12 In particular, PIF1 was shown to bind to the PSY promoter to downregulate its transcriptional activity in the dark. Other PIFs (PIF3, PIF4 and PIF5) were also found to contribute to repress PSY expression and hence carotenoid biosynthesis, resulting in low carotenoid levels in dark-grown (etiolated) seedlings.12 Upon illumination, PIFs are degraded following interaction with the photoactivated form of phytochromes,13,14 eventually resulting in a strong derepression of PSY expression and hence an increased production of carotenoids in deetiolating seedlings.12,15 PIFs also appear to have a role in regulating PSY expression and carotenoid accumulation in leaves from plants grown under different photoperiods.12 However, the role of these light-sensitive transcription factors in roots (organs that normally develop in the dark) remain virtually unexplored.
We recently reported that the Arabidopsis PSY gene is differentially regulated in shoot (photosynthetic) and root tissues.16 In the root, PSY expression is highest in the vascular area and it rapidly increases in response to salt stress. This induction results in an activated flux to the carotenoid pathway and an enhanced production of ABA precursors to fuel a sustained production of the hormone, in agreement with that proposed in other plants like maize (Zea mays) and rice (Oryza sativa).17-20 Also similar to that observed in other plant species, ABA treatment upregulates PSY gene expression in Arabidopsis roots.16 These results provide evidence of a conserved feedback mechanism to ensure the production of carotenoid precursors for ABA synthesis in the root. By contrast, neither salt nor ABA treatments have any effect on the expression of PSY in shoot tissues, where carotenoid levels are much higher and hence ABA synthesis can occur from available precursors.16
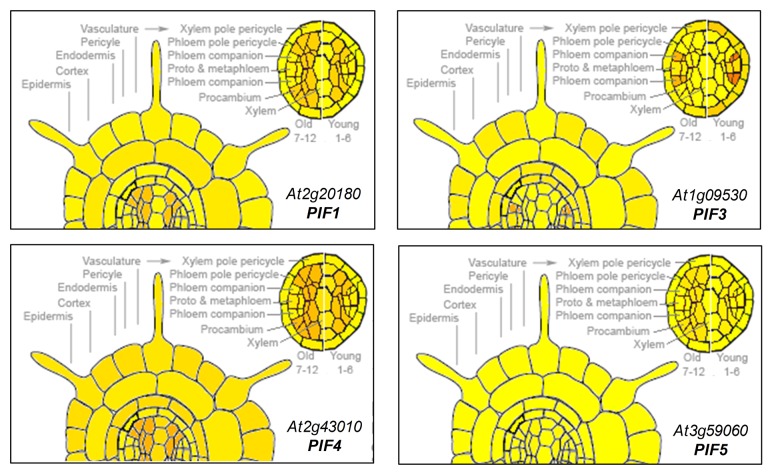
Besides directly repressing Arabidopsis PSY expression,12 PIF1 (also referred to as PIL5) has been shown to regulate the expression of genes involved in ABA synthesis and signaling during seed germination.21,22 We therefore reasoned that PIF1 and perhaps other light-sensitive PIFs might also act in the root to regulate PSY gene expression in response to salt stress and/or ABA signals. Based on the data available from the eFP browser, genes encoding PIF1, PIF3, PIF4, and PIF5 are expressed in the root stele (Fig. 1), similar to that observed for PSY.16 Because PIF protein stability is also a key determinant of their biological function, we next aimed to determine whether the accumulation of PIF proteins (and hence their putative impact on PSY expression) was light-dependent. For this experiment, we used available transgenic plants constitutively expressing a fusion of PIF3 to β-glucuronidase (GUS) previously shown to be useful as a reporter of PIF3 protein levels.25 These 35S:GUS-PIF3 plants, together with control 35S:GUS-GFP plants,11 were grown under long day (LD) conditions for 6 d and, in the subjective morning of day 7 (5h after the lights went on in the LD chamber), they were either transferred to constant dark or left under LD (Fig. 2A). Root samples were taken 24h later in the dark or in the light and stained for GUS activity. As expected, roots from control 35S:GUS-GFP plants showed similar GUS staining in all the tissues and light conditions (Fig. 2B). By contrast, roots from 35S:GUS-PIF3 plants collected in the light showed very little GUS staining, whereas those from dark-grown samples displayed a strong activity of the GUS-PIF3 protein in the vasculature (Fig. 2B). These results indicate that PIF3 (and, most likely, other PIFs) might accumulate under physiologically normal conditions (i.e., in roots growing underground in the dark). They also demonstrate that light prevents the accumulation of PIF3 in the root stele, suggesting that PIF stability in roots is regulated by light signals as previously reported for whole plants.13,14 The preferential accumulation of the GUS-PIF3 protein in the vascular tissue of dark-incubated 35S:GUS-PIF3 roots might suggest that this protein is not stable in other tissues of the root (since the 35S promoter is expected to produce the fusion protein in all the cell types, as observed in 35S:GUS-GFP roots). However, analysis of additional reporter lines would be necessary to draw any conclusion on this matter.
Figure 1. Electronic fluorescent pictographic (eFP) representations of the levels of transcripts encoding PIFs (PIF1, PIF3, PIF4, and PIF5) in Arabidopsis roots. Data were obtained from the Arabidopsis eFP browser at www.bar.utoront.ca23 and correspond to root material from 5- to 6-d-old seedlings collected by fluorescence-activated cell sorting.24
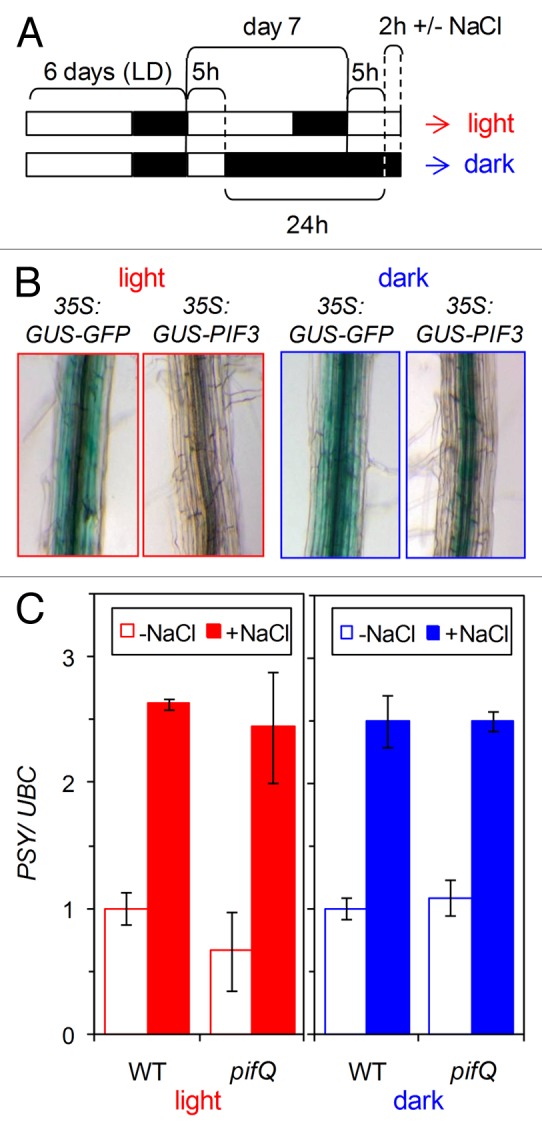
Figure 2. Analysis of PIF protein levels and their effect on PSY gene expression in roots. (A) Experimental setup. Seeds were germinated and grown on a mesh on top of solid Murashige and Skoog (MS) medium under long day (LD) photoperiod (8h of darkness and 16h under fluorescent white light at a photosynthetic photon flux density of 60 μmol m−2 s−1) at 22 °C. In the subjective morning of day 7 (5h after the lights went on in the LD chamber), the plates were either transferred to constant dark or left under LD for 24h. Then, samples were collected for GUS staining (B) or transferred to new plates containing solid MS medium either supplemented or not with 200 mM NaCl (C). Times and treatments were selected to compare conditions in which PIF proteins were either present (in the dark) or absent (after 5h of light) at the same moment of the day, hence preventing any possible circadian effect on PSY expression. (B) Representative images of roots from transgenic 35S:GUS-GFP and 35S:GUS-PIF3 seedlings grown as described in (A) and collected in the light or in the dark. Roots were separated from shoots and stained for GUS activity as described.11 The images correspond to the differentiation zone of the root. (C) Wild-type (WT) and mutant pifQ lines were grown and exposed for 2h to salt (+NaCl) or mock (-NaCl) treatments as described in (A). Then, root samples were collected for RNA extraction and qPCR analysis of PSY transcript levels using the UBC gene for normalization as described.16 Values are represented relative to those in mock-treated WT plants. Mean and standard deviation of n = 4 independent samples are shown. No statistically significant differences were found between WT and pifQ samples.
With this information in hand, we next evaluated whether PIFs contributed to the control of PSY gene expression in roots under normal conditions and in response to an abiotic (salt) stress. A quadruple mutant defective in PIF1, PIF3, PIF4, and PIF5, referred to as pifQ,26 and wild-type plants were grown together under LD and then either moved to the dark or left under LD conditions as described above (Fig. 2A). After 24h, the seedlings were transferred in the dark or in the light to fresh medium either supplemented or not with 200 mM NaCl and incubated for 2h (Fig. 2C). Analysis of PSY transcript levels showed no major differences between genotypes (wild-type vs. pifQ) or light conditions (dark vs. light). In response to salt treatment, the induction of PSY expression was also similar in wild-type and mutant roots, both in darkness and in the light (Fig. 2C). We therefore conclude that PIFs do not significantly contribute to regulate PSY expression in Arabidopsis roots.
In most plants, PSY is encoded by small gene families with members that show different expression profiles. In part due to the spatial distribution of gene expression, some isoforms are involved in the biosynthesis of carotenoids in chloroplast-containing photosynthetic tissues, whereas others participate in the production of carotenoids in the chromoplasts of the fruit, the amyloplasts of the seed, or the leucoplasts of the root.5 The genes encoding chloroplast PSY enzymes involved in photosynthesis are light-regulated, whereas those preferentially found in the root (such as maize and rice PSY3 isoforms) are not responsive to light but to salt, osmotic, or water stress, and specifically to ABA.17-20 The existence of a single gene encoding PSY in Arabidopsis implies that the same promoter must be able to differentially respond to signals coming from organs with distinct carotenoid requirements. This is particularly challenging when different signaling pathways converge in the same transcription factors. For example, PIFs modulate Arabidopsis gene expression in response to both light and hormone (including ABA) signals.13,14,27 However, we found that the Arabidopsis PSY gene is regulated by PIFs only in response to light signals in the shoot. Like other transcription factors of the bHLH type, PIFs preferentially bind to G-box sequences (CACGTG) in the promoter of target genes. Although both PSY and PIF-encoding genes are expressed in the same tissues of the root stele, it is possible that the G-box motifs of the PSY promoter are not available for PIF binding in the root. Strikingly, the G-box bound by PIF1 in the PSY promoter overlaps with other motifs recognized by transcription factors involved in ABA signaling.28,29 It is therefore possible that such transcription factors and PIFs might compete for a binding site on the PSY promoter in different plant tissues, including those of the root vascular system. This possibility, however, awaits experimental validation.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Rosa Rodriguez for excellent technical support. The work was mainly funded by research grants BIO2011–23680 from the Spanish Dirección General de Investigación (DGI, code BIO2011–23680) and European Union FP7 (TiMet, contract 245143) to M.R.C. DGI also provided funding through a FPI fellowship to M.A.R.S. The Generalitat de Catalunya provided a FI fellowship to A.R.V. and support grants (2009SGR-26 and XRB program). We are members of the IBERCAROT carotenoid network funded by CYTED (code 112RT0445).
References
- 1.Fraser PD, Bramley PM. The biosynthesis and nutritional uses of carotenoids. Prog Lipid Res. 2004;43:228–65. doi: 10.1016/j.plipres.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Botella-Pavía P, Rodríguez-Concepción M. Carotenoid biotechnology in plants for nutritionally improved foods. Physiol Plant. 2006;126:369–81. doi: 10.1111/j.1399-3054.2006.00632.x. [DOI] [Google Scholar]
- 3.Niyogi KK. PHOTOPROTECTION REVISITED: Genetic and Molecular Approaches. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:333–59. doi: 10.1146/annurev.arplant.50.1.333. [DOI] [PubMed] [Google Scholar]
- 4.Hirschberg J. Carotenoid biosynthesis in flowering plants. Curr Opin Plant Biol. 2001;4:210–8. doi: 10.1016/S1369-5266(00)00163-1. [DOI] [PubMed] [Google Scholar]
- 5.Howitt CA, Pogson BJ. Carotenoid accumulation and function in seeds and non-green tissues. Plant Cell Environ. 2006;29:435–45. doi: 10.1111/j.1365-3040.2005.01492.x. [DOI] [PubMed] [Google Scholar]
- 6.Nambara E, Marion-Poll A. Abscisic acid biosynthesis and catabolism. Annu Rev Plant Biol. 2005;56:165–85. doi: 10.1146/annurev.arplant.56.032604.144046. [DOI] [PubMed] [Google Scholar]
- 7.Van Norman JM, Sieburth LE. Dissecting the biosynthetic pathway for the bypass1 root-derived signal. Plant J. 2007;49:619–28. doi: 10.1111/j.1365-313X.2006.02982.x. [DOI] [PubMed] [Google Scholar]
- 8.Xie X, Yoneyama K, Yoneyama K. The strigolactone story. Annu Rev Phytopathol. 2010;48:93–117. doi: 10.1146/annurev-phyto-073009-114453. [DOI] [PubMed] [Google Scholar]
- 9.Walter MH, Strack D. Carotenoids and their cleavage products: biosynthesis and functions. Nat Prod Rep. 2011;28:663–92. doi: 10.1039/c0np00036a. [DOI] [PubMed] [Google Scholar]
- 10.Ruiz-Sola MA, Rodríguez-Concepción M. Carotenoid biosynthesis in Arabidopsis: a colorful pathway. Arabidopsis Book. 2012;10:e0158. doi: 10.1199/tab.0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodríguez-Villalón A, Gas E, Rodríguez-Concepción M. Phytoene synthase activity controls the biosynthesis of carotenoids and the supply of their metabolic precursors in dark-grown Arabidopsis seedlings. Plant J. 2009;60:424–35. doi: 10.1111/j.1365-313X.2009.03966.x. [DOI] [PubMed] [Google Scholar]
- 12.Toledo-Ortiz G, Huq E, Rodríguez-Concepción M. Direct regulation of phytoene synthase gene expression and carotenoid biosynthesis by phytochrome-interacting factors. Proc Natl Acad Sci U S A. 2010;107:11626–31. doi: 10.1073/pnas.0914428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leivar P, Quail PH. PIFs: pivotal components in a cellular signaling hub. Trends Plant Sci. 2011;16:19–28. doi: 10.1016/j.tplants.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castillon A, Shen H, Huq E. Phytochrome Interacting Factors: central players in phytochrome-mediated light signaling networks. Trends Plant Sci. 2007;12:514–21. doi: 10.1016/j.tplants.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Rodríguez-Villalón A, Gas E, Rodríguez-Concepción M. Colors in the dark: a model for the regulation of carotenoid biosynthesis in etioplasts. Plant Signal Behav. 2009;4:965–7. doi: 10.4161/psb.4.10.9672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruiz-Sola MA, Arbona V, Gómez-Cadenas A, Rodríguez-Concepción M, Rodríguez-Villalón A. A root specific induction of carotenoid biosynthesis contributes to ABA production upon salt stress in arabidopsis. PLoS One. 2014;9:e90765. doi: 10.1371/journal.pone.0090765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li F, Vallabhaneni R, Wurtzel ET. PSY3, a new member of the phytoene synthase gene family conserved in the Poaceae and regulator of abiotic stress-induced root carotenogenesis. Plant Physiol. 2008;146:1333–45. doi: 10.1104/pp.107.111120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Welsch R, Wüst F, Bär C, Al-Babili S, Beyer P. A third phytoene synthase is devoted to abiotic stress-induced abscisic acid formation in rice and defines functional diversification of phytoene synthase genes. Plant Physiol. 2008;147:367–80. doi: 10.1104/pp.108.117028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li F, Vallabhaneni R, Yu J, Rocheford T, Wurtzel ET. The maize phytoene synthase gene family: overlapping roles for carotenogenesis in endosperm, photomorphogenesis, and thermal stress tolerance. Plant Physiol. 2008;147:1334–46. doi: 10.1104/pp.108.122119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li F, Tsfadia O, Wurtzel ET. The phytoene synthase gene family in the Grasses: subfunctionalization provides tissue-specific control of carotenogenesis. Plant Signal Behav. 2009;4:208–11. doi: 10.4161/psb.4.3.7798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oh E, Yamaguchi S, Hu J, Yusuke J, Jung B, Paik I, Lee HS, Sun TP, Kamiya Y, Choi G. PIL5, a phytochrome-interacting bHLH protein, regulates gibberellin responsiveness by binding directly to the GAI and RGA promoters in Arabidopsis seeds. Plant Cell. 2007;19:1192–208. doi: 10.1105/tpc.107.050153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oh E, Kang H, Yamaguchi S, Park J, Lee D, Kamiya Y, Choi G. Genome-wide analysis of genes targeted by PHYTOCHROME INTERACTING FACTOR 3-LIKE5 during seed germination in Arabidopsis. Plant Cell. 2009;21:403–19. doi: 10.1105/tpc.108.064691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Winter D, Vinegar B, Nahal H, Ammar R, Wilson GV, Provart NJ. An “Electronic Fluorescent Pictograph” browser for exploring and analyzing large-scale biological data sets. PLoS One. 2007;2:e718. doi: 10.1371/journal.pone.0000718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brady SM, Orlando DA, Lee JY, Wang JY, Koch J, Dinneny JR, Mace D, Ohler U, Benfey PN. A high-resolution root spatiotemporal map reveals dominant expression patterns. Science. 2007;318:801–6. doi: 10.1126/science.1146265. [DOI] [PubMed] [Google Scholar]
- 25.Monte E, Tepperman JM, Al-Sady B, Kaczorowski KA, Alonso JM, Ecker JR, Li X, Zhang Y, Quail PH. The phytochrome-interacting transcription factor, PIF3, acts early, selectively, and positively in light-induced chloroplast development. Proc Natl Acad Sci U S A. 2004;101:16091–8. doi: 10.1073/pnas.0407107101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leivar P, Tepperman JM, Monte E, Calderon RH, Liu TL, Quail PH. Definition of early transcriptional circuitry involved in light-induced reversal of PIF-imposed repression of photomorphogenesis in young Arabidopsis seedlings. Plant Cell. 2009;21:3535–53. doi: 10.1105/tpc.109.070672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lau OS, Deng XW. Plant hormone signaling lightens up: integrators of light and hormones. Curr Opin Plant Biol. 2010;13:571–7. doi: 10.1016/j.pbi.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 28.Koussevitzky S, Nott A, Mockler TC, Hong F, Sachetto-Martins G, Surpin M, Lim J, Mittler R, Chory J. Signals from chloroplasts converge to regulate nuclear gene expression. Science. 2007;316:715–9. doi: 10.1126/science.1140516. [DOI] [PubMed] [Google Scholar]
- 29.Fujita Y, Fujita M, Shinozaki K, Yamaguchi-Shinozaki K. ABA-mediated transcriptional regulation in response to osmotic stress in plants. J Plant Res. 2011;124:509–25. doi: 10.1007/s10265-011-0412-3. [DOI] [PubMed] [Google Scholar]